Abstract
Among the various species from which induced pluripotent stem cells have been derived, nonhuman primates (NHPs) have a unique role as preclinical models. Their relatedness to humans and similar physiology, including central nervous system, make them ideal for translational studies. We review here the progress made in deriving and characterizing iPS cell lines from different NHP species. We focus on iPS cell lines from the marmoset, a small NHP in which several human disease states can be modeled. The marmoset can serve as a model for the implementation of patient-specific autologous cell therapy in regenerative medicine.
1. Induced Pluripotent Stem Cells in Regenerative Medicine
The aims of regenerative medicine are to restore healthy function to organs damaged by disease or aging. A major issue is the source of cells to be used in regenerative medicine. It is often thought to be desirable to use cells derived from the patient himself/herself, because this is hypothesized to avoid the need to administer drugs to suppress immune rejection of the transplanted cells. The possibility of using patient-specific cells in regenerative medicine was greatly expanded by the discovery of induced pluripotent stem cells (iPS cells) [1, 2]. iPS cells can be derived from any somatic cell, but have the properties of embryonic stem cells. Like embryonic cells, they can be used to generate any cell of the body that may be needed in regenerative medicine. It is widely thought that a form of autologous cell therapy will be possible, in which iPS cells would be derived from the patient's cells, in order to provide a source for cells that could be transplanted back to the patient to restore function to the heart, central nervous system, hematopoietic system, or other organs that are affected by disease or aging. The present experiments concern the development of nonhuman primate models for autologous cell therapy based on iPS cells.
2. Autologous versus Allogeneic Cells in Cell-Based Therapies
Any consideration of the implementation of regenerative medicine for human subjects must assess the source of the cells used in the therapy [3, 4]. Following the discovery of iPS cells, it was almost immediately realized that this discovery opened the way to autologous cell therapy. A review in 2007 stated: “If this method can be translated to humans, patient-specific stem cells could be made without the use of donated eggs or embryos” [5]. It is assumed that if the cells are accepted as “self” then they would represent the best possible functional outcome of a transplant: cells that function in their natural environment, without eliciting chronic immune or inflammatory reactions, and without the problems that would result from the use of immunosuppressive drugs. They would, in other words, be the “gold standard” for the best possible results of therapy based on cell transplantation. While allogeneic cells might produce an acceptable result for the patient, autologous cell transplants would provide the standard by which the results of allogeneic cells could be judged.
Shortly after the discovery of iPS cells, the technology was used in a tour-de-force study in which iPS cells were derived from a strain of mice that model human sickle cell anemia. The genetic defect was corrected in the iPS cells and they were transplanted back into mice of the same strain following differentiation to hematopoietic stem cells [6]. The symptoms in the treated mice were substantially ameliorated. This was the first demonstration of the potential power of iPS cell-based therapy. As these cells were derived from, and reintroduced into, mice of the same strain, they are an example of the use of syngeneic cells, rather than truly autologous cells. Subsequently, another study suggested that syngeneic iPS cells and their cell progeny may, in fact, elicit an immune response [7]. This unexpected finding has not yet received a satisfactory explanation. At the date of writing, the question of the immunogenicity of iPS cells and derivatives has only been addressed in mice, and not yet in more translationally relevant species, including primates.
Would therapeutic approaches based on the use of autologous cells be worth the considerable efforts of development and implementation that would be required? The answer at the moment is quite unknown. First, in the absence of suitable translational models, or actual clinical trials of iPS cell-based therapy, the advantages must remain theoretical. We do not know how much better, or not, therapy based on autologous cells would be in comparison to therapy based on allogeneic cells. Possibly, autologous cells will prove to be superior, but perhaps there will be little difference from allogeneic cells. In some therapies, the need for a very rapid treatment would preclude the use of autologous cells. For example, in stroke, due to the need for immediate treatment, “off-the-shelf” cells would be needed and iPS cells are unlikely to be useful. However, understanding whether immune-matched versus mismatched cells would have an advantage in a stroke model would be of great significance.
Second, it is extremely hard to predict how easily-implemented iPS cell-based therapy would eventually become. When iPS cells were first made from skin fibroblasts in 2006-2007, reprogramming was highly inefficient and laborious. Over the last 4 years, there has been astounding progress in terms of better, simpler protocols and increases in efficiency [8–11]. Given that there are no reasons to think that the process should not continue to undergo such improvement in efficiency, it is quite possible that the creation of iPS cells from a patient's cells would become quite routine and inexpensive at some time in the future. Similar dramatic improvements in efficiency and cost have been seen in other biomedical technologies, for example, DNA sequencing.
3. Importance of Nonhuman Primate Research in Regenerative Medicine
Before it would be possible to consider applying autologous cell therapy to human patients, the properties of iPS cells must be thoroughly explored in suitable animal models, in order to make sure that autologous cell therapy is both safe and effective. It has been generally recognized that clinically relevant experiments should be performed in a nonhuman primate (NHP) rather than a rodent. NHPs are thought be ideal for such preclinical trials because of their relatedness to humans and their similar physiology, particularly with respect to the central nervous system. Long-term studies of transplanted cell function (>3 years) will be possible in NHPs, but are impossible in rodents.
Thus there is a clear path from basic to translational studies in iPS cell-based regenerative medicine in NHPs. Of the various NHPs that could be used, the marmoset has several key advantages. The common marmoset (Callithrix jacchus) has the advantage of smaller size, more rapid breeding, and defined housing conditions. In contrast to humans, where uncontrolled environment and many comorbidities are confounding factors, marmosets can be housed in a defined environment and have few known comorbidities [12]. A variety of human diseases can potentially be modeled in marmosets [13–15]. A chemical-induced model of Parkinson's disease has also been developed in this species [16] and a stroke model [17] has been developed. Histological and MRI brain atlases are available [18]. The marmoset genome has been completed [19], and the marmoset is the first and so far only primate to have transgenic models that show germline transmission [20]. Although transgenics have also been created in the rhesus macaque, they have not passed the transgene to their offspring [21]. A genetic model of Parkinson's disease by overexpression of α-synuclein has been developed in the marmoset [20]. Finally, a spinal cord injury model in the marmoset has been used in tests of transplanted human neural stem cells for potential therapeutic effect [22, 23]. Our long-term goal is illustrated in Figure 1.
Figure 1.
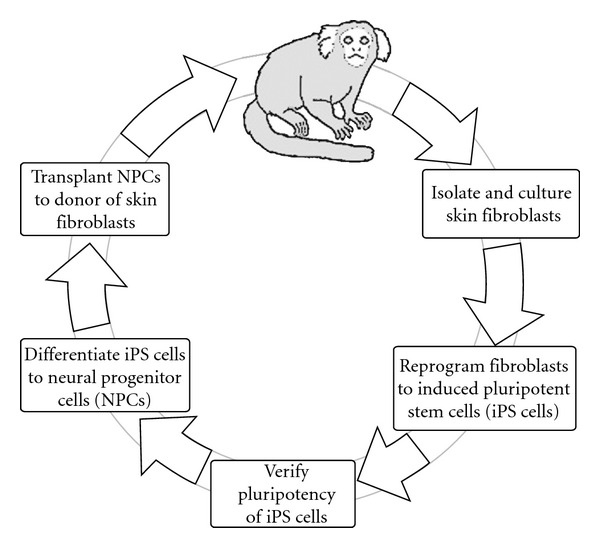
The marmoset as a preclinical model for patient-specific iPS cells in regenerative medicine. This scheme outlines progress to date and future studies of autologous cell transplantation using reprogramming and redifferentiation to a specific cell lineage. A skin biopsy is taken from an individual marmoset, and fibroblasts from the biopsy are grown in culture. Reprogramming factors are expressed in the cells; over a period of several weeks, clones of cells arise that may be iPS cells. Clones are isolated and screened to determine whether they are properly reprogrammed iPS cells; if so, they are expanded and cryopreserved. Neuronal progenitor cells (NPCs) are derived from these iPS cells via protocols described in the text. If the NPCs pass stringent tests of differentiation potential and safety, in the future they may be implanted into the CNS of the same individual from which the cells were originally derived.
4. Progress in NHP iPS Cell Research
Despite the importance of NHPs in regenerative medicine, there has yet been relatively little work on iPS cells derived from NHPs, in comparison to the extent of work on iPS cells derived from mice and humans. The first NHP iPS cells were derived from the rhesus macaque [24]. At the present time (September 2011), iPS cells have been derived from five NHP species (Table 1); three species of macaque (rhesus macaque, pigtailed macaque, and cynomolgus monkey), the common marmoset, and an endangered primate, the drill [24–33]. Common features of all reports on NHP iPS cells are: derivation by mixtures of retroviruses carrying transcription factor cDNAs, principally POU5F1, SOX2, KLF4, and MYC; maintenance of pluripotent characteristics over long-term growth in culture; ability to differentiate into cells and tissues of the three germ layers; a lack of malignant properties, despite the ability to form benign teratomas in immunodeficient mice [24–33].
Table 1.
Publications on nonhuman primate iPS cells.
| Species | Title of publication | cDNAs used for reprogramming | Origin of cDNAs |
|---|---|---|---|
| Rhesus macaque (Macaca mulatta) | Generation of induced pluripotent stem cells from adult rhesus monkey fibroblasts [24] | POU5F1, SOX2 KLF4 and MYC | Rhesus |
| Common marmoset (Callithrix jacchus) | Generation of induced pluripotent stem cells from newborn marmoset skin fibroblasts [25] | POU5F1, SOX2 KLF4 and MYC | Human |
| Common marmoset (Callithrix jacchus) | Generating induced pluripotent stem cells from common marmoset (Callithrix jacchus) fetal liver cells using defined factors, including Lin28 [26] | POU5F1, SOX2, KLF4, MYC, NANOG and LIN28 | Human |
| Rhesus macaque (Macaca mulatta) | Reprogramming Huntington monkey skin cells into pluripotent stem cells [27] | POU5F1, SOX2 and KLF4 | Rhesus |
| Pigtailed macaque (Macaca nemestrina) | Efficient generation of nonhuman primate induced pluripotent stem cells [28] | POU5F1, SOX2 KLF4 and MYC | Human |
| Cynomolgus monkey (Macaca fascicularis) | Development of histocompatible primate induced pluripotent stem cells for neural transplantation [29] | POU5F1, SOX2 KLF4 and MYC | Human |
| Rhesus macaque (Macaca mulatta) | Generation of pancreatic insulin-producing cells from rhesus monkey induced pluripotent stem cells [30] | POU5F1, SOX2 KLF4 and MYC | Rhesus |
| Pigtailed macaque (Macaca nemestrina) | Safeguarding nonhuman primate iPS cells with suicide genes [31] | POU5F1, SOX2 KLF4 and MYC | Human |
| Drill (Mandrillus leucophaeus) | Induced pluripotent stem cells from highly endangered species [32] | POU5F1, SOX2 KLF4 and MYC | Human |
| Cynomolgus monkey (Macaca fascicularis) | Induction of retinal pigment epithelial cells from monkey iPS cells [33] | POU5F1, SOX2 KLF4 and MYC | Human |
The table lists the publications (in order of publication, up to September 2011) that have reported the derivation and characterization of nonhuman primate iPS cells. All used mixtures of retroviruses, carrying the indicated cDNAs.
5. Marmoset iPS Cells: A Model for Autologous Cell Therapy
The eventual goal of our studies is to derive iPS cells from individual marmosets and implant the cells into the donor animal, following the directed differentiation of the iPS cells to specific cell lineages (Figure 1). Before such studies are possible, extensive in vitro investigations and studies in immunodeficient mice are needed.
We chose to derive marmoset iPS cells from skin fibroblasts because the fibroblast has been the most widely studied cell type for iPS cell generation, and because the use of small skin biopsies as a source of starting material is relevant to future clinical application of iPS cells and their derivatives. In initial experiments, we used fibroblasts derived from newborn marmoset skin [25]. Retroviruses encoding the human cDNAs for Oct4, Sox2, Klf4, and c-Myc [2] were prepared in Plat-A cells and were concentrated by Polybrene flocculation [34]. Following the infection of the cells with concentrated viruses, cultures were maintained in normal fibroblast growth conditions with the addition of valproic acid [35]. After 14–21 days, small colonies of altered morphology were noted in the confluent fibroblast cultures. These colonies comprised small rapidly dividing cells with high nuclear/cytoplasmic ratio and prominent nucleoli. When cultures containing such colonies were fixed and stained for alkaline phosphatase activity, most of the small colonies of altered morphology were found to be positive for alkaline phosphatase, a marker of pluripotency [36]. These colonies expanded rapidly, producing very dense patches of small cells. These cells have the morphological characteristics previously reported for human iPS cells [2].
Starting with a population of 4 × 105 marmoset fibroblasts, we obtained ~100 colonies of cells with iPS cell-like morphology. Colonies were isolated and expanded on feeder layers. Of those colonies that were isolated from the fibroblast cultures, 30 showed sustained growth and were able to be expanded to the point where they could be cryopreserved. Of these, 8 were selected for further study. Karyotypes were investigated by G banding and were found to be normal [25]. Following the initial expansion of marmoset iPS cell clones on feeder layers, we investigated if the cells could be grown under feeder-free conditions. Cells were replated on Matrigel-coated dishes in medium containing 20% fetal bovine serum and 40% MEF-conditioned medium and continued to grow rapidly. Cell populations were expanded under these conditions for further studies.
Marmoset iPS cell clones expressed pluripotency markers at levels that were comparable to that in a human embryonic stem cell line (I6) or exceeded that level [25]. In all 8 marmoset iPS cell clones, NANOG and SOX2 mRNA levels were higher than those in I6 cells, and levels of OCT4 were comparable to that of I6 cells. Levels of OCT4 mRNA were >100-fold higher in iPS cell clones than in the fibroblasts used for reprogramming, and levels of NANOG and SOX2 were >50-fold higher. We assessed the relative levels of vector and total mRNAs for OCT4 and SOX2, two of the factors used for reprogramming. We used primer pairs specific for reprogramming vectors (vector sequence 5′ primer and coding region 3′ primer). Vector OCT4 mRNA was present at 0.01% to 0.1% of that of total OCT4 mRNA, while vector SOX2 mRNA was present at 0.1% to 1% of the total SOX2 mRNA. These findings indicate that the viral genomes are appropriately silenced [38].
In order to assess the potential of marmoset iPS cell clones to differentiate to cells of all three germ layers, cells were transplanted into immunodeficient mice (subcutaneous injection in 50% Matrigel: [39, 40]). Teratomas from marmoset iPS cells contained a variety of tissue structures representing derivatives of all three germ layers. Because it has been reported that teratomas derived from incompletely reprogrammed cells formed tissues of ectodermal and mesodermal origin but not of endodermal origin [38] we performed histological studies of the development of mature structures of endodermal origin; we observed endodermal tissues, including simple columnar and pseudostratified epithelia, epithelia with goblet cells, and exocrine glandular structures [25]. Immunohistochemical studies were also performed; ectodermal tissue (developing neural tissue) was demonstrated by presence of βIII tubulin; mesodermal tissue by smooth muscle actin; endodermal tissue by α-fetoprotein.
Subsequently, we investigated the potential of a polycistronic vector for reprogramming (Figure 2). This retroviral vector has the features that (a) because expression of the reprogramming factors is driven by the 5′ LTR, expression is silenced during reprogramming, if cells have been properly reprogrammed [38]; (b) all factors are in one vector, thus avoiding the need for very high efficiency infection; (c) as a retroviral vector, only dividing cells are infected (this does not detract from the value of this type of vector, as iPS cells must arise from cells capable of cell division); (d) loxP sites enable future excision of the coding region when required. Marmoset iPS cells derived using this polycistronic retroviral vector exhibited the same characteristics of iPS cell clones derived by coinfection of the four factors. Therefore, cells derived by a 1 : 1 : 1 : 1 expression of the four reprogramming factors have properties that are basically the same as those derived by coinfection, in which the ratio of expression of the four factors is not necessarily equal and almost certainly varies from clone to clone.
Figure 2.
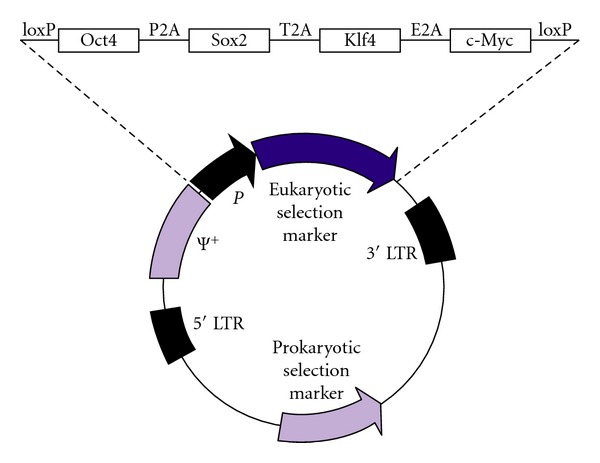
Retroviral reprogramming vector designed to deliver four reprogramming factors (Oct4, Sox2, Klf4, and c-Myc; OSKM) in a single virus using “self-cleaving” peptides, which support efficient polycistronic expression from a single promoter [8]. In this version, expression is driven by the 5′ LTR. Additionally, loxP sites are present just before and just after the OSKM coding region, enabling excision of the vector from the genome of the reprogrammed cells. This vector was constructed by replacing the internal promoter (P) and eukaryotic selection marker of retroviral vector pLXSN by the OSKM sequence from FUW-OSKM [8].
Despite the advantages of such retroviral vectors, it is likely that the use of integrating forms of viral vectors for reprogramming will be made obsolete by nonviral reprogramming methods using modified mRNA or modified proteins [9]. These methods avoid any genetic modification of the target cells during the reprogramming process.
Successful long-term expansion of marmoset iPS cells is critical for any extensive studies of the properties of the cells. Although we determined feeder-free conditions for growth of the cells, these conditions require fetal bovine serum and medium conditioned by a suitable cell type, such as mouse embryo fibroblasts. More recently, we have established that marmoset iPS cells can grow continuously and over long periods in defined medium without the addition of serum or of medium conditioned by another cell type. Several types of defined media support long-term marmoset iPS cell growth without loss of expression of pluripotency genes such as NANOG and OCT4/POU5F1. Both clones derived by coinfection and clones derived by infection with a polycistronic vector may be grown in defined medium (Figure 3).
Figure 3.
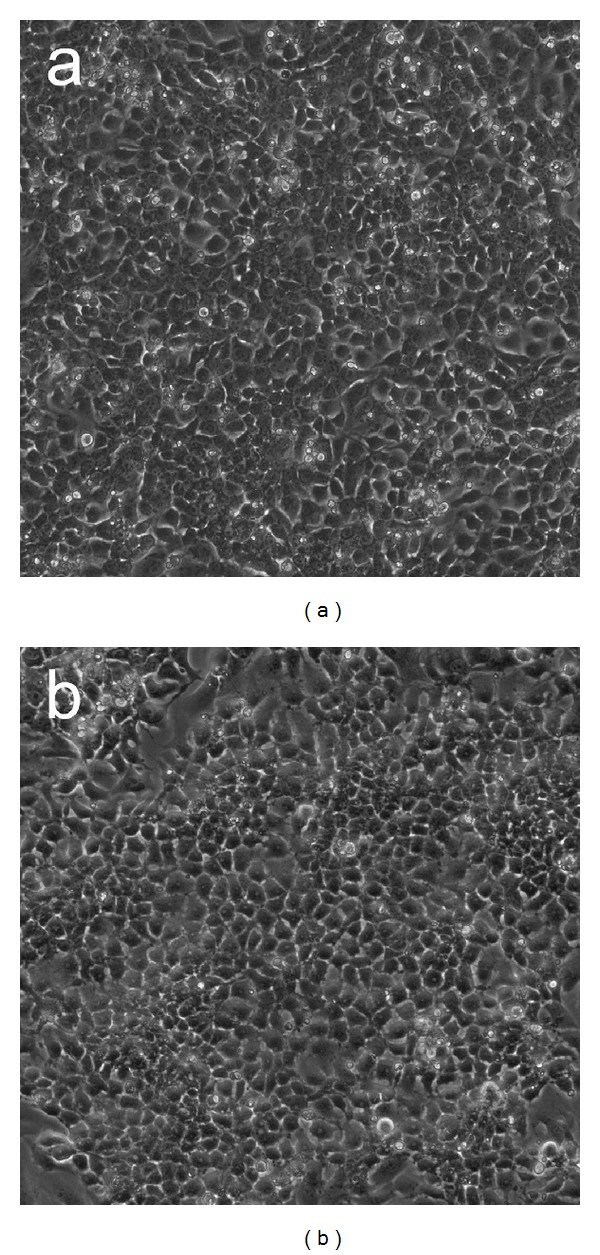
Marmoset iPS cells growing in feeder-free culture. (a) An iPS cell line derived by coinfection with four retroviruses (B8 cell line [25]). Cells are growing in defined xeno-free medium (Pluriton, Stemgent). (b) An iPS cell line derived by infection with a single retrovirus, encoding the OSKM reprogramming factors, illustrated in Figure 2.
In summary, by the criteria of morphology, growth requirements, expression of pluripotency factors, retroviral silencing, and the ability to generate teratomas with tissues of all three germ layers, we conclude that these lines of cells represent bona fide induced pluripotent stem cells.
6. Differentiation of Marmoset iPS Cells to Neural Progenitor Cells
In subsequent work, we investigated the potential of marmoset iPS cell lines to differentiate in vitro to cells of the neural lineage. Differentiation of iPS cells to neural progenitor cells (NPCs) has been extensively employed as a test of proper pluripotency; for example, this form of directed differentiation was used in a recent set of tests on a panel of well characterized human iPS cells [10, 11]. Protocols for NPC generation are of three general types: stromal cell-derived inducing activity (SDIA), a relatively poorly characterized mix of factors secreted by certain mesenchymal cells, such as the PA6 cell line [2, 41, 42]; embryoid body (EB) formation, followed by plating of the EBs on suitable surfaces in the presence of Neurobasal medium [43, 44]; and induction using small molecules, such as chemical inhibition of BMP/activin/nodal signaling via SMADs [45]. We have used each of these methods in marmoset iPS cells, and all of them produce NPC lines (Figure 4).
Figure 4.
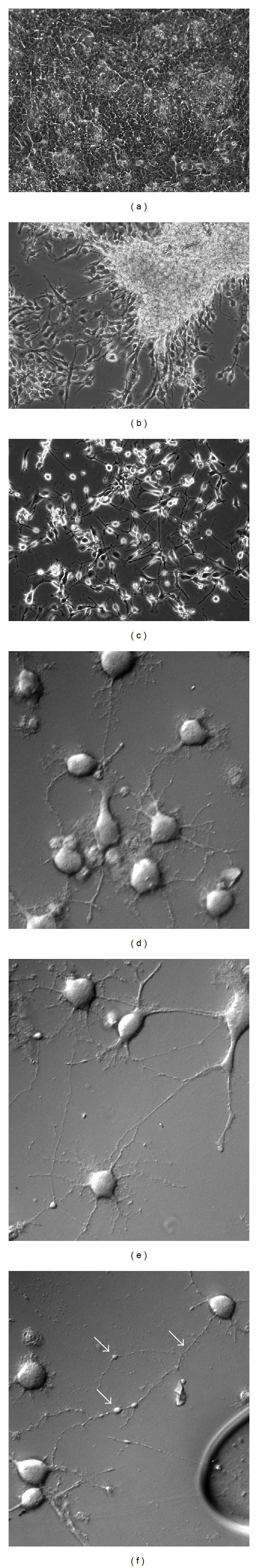
Derivation of neural progenitor cells (NPCs) from marmoset iPS cells and differentiation of NPCs to mature neurons. The series (a)–(c) shows the transition from undifferentiated iPS cells (a), to a line of NPCs (b), to mature neurons (c) (100x phase-contrast images). NPCs placed on a polylysine/laminin-coated glass surface stop dividing and form extensive axons and dendrites. Details of this further maturation are shown in series (d)–(f) (400x differential interference contrast images). Note particularly the varicosities of different sizes indicated by arrows in (f). These are sites of accumulation of cellular organelles and are precursors to the formation of synapses [37]. Their presence indicates the degree of maturity of these neurons.
7. Summary
In summary, iPS cells from NHPs have a unique importance in preclinical research leading to the implementation of regenerative medicine in human patients. We have derived and characterized iPS cells from the marmoset, a small NHP that can serve as a suitable model for autologous cell therapy involving iPS cells. Future studies will test the principles of autologous cell therapy in individual marmosets.
Acknowledgments
Work from the authors' laboratory was funded by the US National Institutes of Health (R21AG033286), by the US Department of Veterans Affairs (I01BX001454), by the Ted Nash Long Life Foundation, and by the Owens Medical Research Foundation. S. Farnsworth was supported by Grant T32DE014318 (Craniofacial Oral-biology Student Training in Academic Research).
References
- 1.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 2.Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 3.Agarwal S, Lensch MW, Daley GQ. Current prospects for the generation of patient-specific pluripotent cells from adult tissues. Regenerative Medicine. 2007;2(5):743–752. doi: 10.2217/17460751.2.5.743. [DOI] [PubMed] [Google Scholar]
- 4.Mason C, Dunnill P. Assessing the value of autologous and allogeneic cells for regenerative medicine. Regenerative Medicine. 2009;4(6):835–853. doi: 10.2217/rme.09.64. [DOI] [PubMed] [Google Scholar]
- 5.Rossant J. Stem cells: the magic brew. Nature. 2007;448(7151):260–261. doi: 10.1038/448260a. [DOI] [PubMed] [Google Scholar]
- 6.Hanna J, Wernig M, Markoulaki S, et al. Treatment of sickle cell anemia mouse model with iPS cells generated from autologous skin. Science. 2007;318(5858):1920–1923. doi: 10.1126/science.1152092. [DOI] [PubMed] [Google Scholar]
- 7.Zhao T, Zhang Z-N, Rong Z, Xu Y. Immunogenicity of induced pluripotent stem cells. Nature. 2011;474(7350):212–216. doi: 10.1038/nature10135. [DOI] [PubMed] [Google Scholar]
- 8.Carey BW, Markoulaki S, Hanna J, et al. Reprogramming of murine and human somatic cells using a single polycistronic vector. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(1):157–162. doi: 10.1073/pnas.0811426106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Warren L, Manos PD, Ahfeldt T, et al. Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell. 2010;7(5):618–630. doi: 10.1016/j.stem.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boulting GL, Kiskinis E, Croft GF, et al. A functionally characterized test set of human induced pluripotent stem cells. Nature Biotechnology. 2011;29(3):279–287. doi: 10.1038/nbt.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bock C, Kiskinis E, Verstappen G, et al. Reference maps of human ES and iPS cell variation enable high-throughput characterization of pluripotent cell lines. Cell. 2011;144(3):439–452. doi: 10.1016/j.cell.2010.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abbott DH, Barnett DK, Colman RJ, Yamamoto ME, Schultz-Darken NJ. Aspects of common marmoset basic biology and life history important for biomedical research. Comparative Medicine. 2003;53(4):339–350. [PubMed] [Google Scholar]
- 13.Mansfield K. Marmoset models commonly used in biomedical research. Comparative Medicine. 2003;53(4):383–392. [PubMed] [Google Scholar]
- 14.Tardif S, Bales K, Williams L, et al. Preparing New World monkeys for laboratory research. ILAR Journal. 2006;47(4):307–315. doi: 10.1093/ilar.47.4.307. [DOI] [PubMed] [Google Scholar]
- 15.Cyranoski D. Marmoset model takes centre stage. Nature. 2009;459(7246):p. 492. doi: 10.1038/459492a. [DOI] [PubMed] [Google Scholar]
- 16.Philippens IHCHM, ’t Hart BA, Torres G. The MPTP marmoset model of Parkinsonism: a multi-purpose non-human primate model for neurodegenerative diseases. Drug Discovery Today. 2010;15(23-24):985–990. doi: 10.1016/j.drudis.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 17.Bihel E, Pro-Sistiaga P, Letourneur A, et al. Permanent or transient chronic ischemic stroke in the non-human primate: behavioral, neuroimaging, histological, and immunohistochemical investigations. Journal of Cerebral Blood Flow and Metabolism. 2010;30(2):273–285. doi: 10.1038/jcbfm.2009.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Newman JD, Kenkel WM, Aronoff EC, Bock NA, Zametkin MR, Silva AC. A combined histological and MRI brain atlas of the common marmoset monkey, Callithrix jacchus. Brain Research Reviews. 2009;62(1):1–18. doi: 10.1016/j.brainresrev.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuhn RM, Karolchik D, Zweig AS, et al. The UCSC genome browser database: update 2009. Nucleic Acids Research. 2009;37(1):D755–D761. doi: 10.1093/nar/gkn875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sasaki E, Suemizu H, Shimada A, et al. Generation of transgenic non-human primates with germline transmission. Nature. 2009;459(7246):523–527. doi: 10.1038/nature08090. [DOI] [PubMed] [Google Scholar]
- 21.Schatten G, Mitalipov S. Developmental biology: transgenic primate offspring. Nature. 2009;459(7246):515–516. doi: 10.1038/459515a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iwanami A, Kaneko S, Nakamura M, et al. Transplantation of human neural stem cells for spinal cord injury in primates. Journal of Neuroscience Research. 2005;80(2):182–190. doi: 10.1002/jnr.20436. [DOI] [PubMed] [Google Scholar]
- 23.Yamane J, Nakamura M, Iwanami A, et al. Transplantation of galectin-1-expressing human neural stem cells into the injured spinal cord of adult common marmosets. Journal of Neuroscience Research. 2010;88(7):1394–1405. doi: 10.1002/jnr.22322. [DOI] [PubMed] [Google Scholar]
- 24.Liu H, Zhu F, Yong J, et al. Generation of induced pluripotent stem cells from adult rhesus monkey fibroblasts. Cell Stem Cell. 2008;3(6):587–590. doi: 10.1016/j.stem.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 25.Wu Y, Zhang Y, Mishra A, Tardif SD, Hornsby PJ. Generation of induced pluripotent stem cells from newborn marmoset skin fibroblasts. Stem Cell Research. 2010;4(3):180–188. doi: 10.1016/j.scr.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tomioka I, Maeda T, Shimada H, et al. Generating induced pluripotent stem cells from common marmoset (Callithrix jacchus) fetal liver cells using defined factors, including Lin28. Genes to Cells. 2010;15(9):959–969. doi: 10.1111/j.1365-2443.2010.01437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chan AWS, Cheng PH, Neumann A, Yang JJ. Reprogramming Huntington monkey skin cells into pluripotent stem cells. Cellular Reprogramming. 2010;12(5):509–517. doi: 10.1089/cell.2010.0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhong B, Trobridge GD, Zhang X, et al. Efficient generation of nonhuman primate induced pluripotent stem cells. Stem Cells and Development. 2011;20(5):795–807. doi: 10.1089/scd.2010.0343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Deleidi M, Hargus G, Hallett P, Osborn T, Isacson O. Development of histocompatible primate-induced pluripotent stem cells for neural transplantation. Stem Cells. 2011;29(7):1052–1063. doi: 10.1002/stem.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhu FF, Zhang PB, Zhang DH, et al. Generation of pancreatic insulin-producing cells from rhesus monkey induced pluripotent stem cells. Diabetologia. 2011;54(9):2325–2336. doi: 10.1007/s00125-011-2246-x. [DOI] [PubMed] [Google Scholar]
- 31.Zhong B, Watts KL, Gori JL, et al. Safeguarding nonhuman primate iPS cells with suicide genes. Molecular Therapy. 2011;19(9):1667–1675. doi: 10.1038/mt.2011.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Friedrich Ben-Nun I, Montague SC, Houck ML, et al. Induced pluripotent stem cells from highly endangered species. Nature Methods. 2011;8(10):829–831. doi: 10.1038/nmeth.1706. [DOI] [PubMed] [Google Scholar]
- 33.Okamoto S, Takahashi M. Induction of retinal pigment epithelial cells from monkey iPS cells. Investigative Ophthalmology & Visual Science. 2011;52(12):8785–8790. doi: 10.1167/iovs.11-8129. [DOI] [PubMed] [Google Scholar]
- 34.Wu Y, Melton DW, Zhang Y, Hornsby PJ. Improved coinfection with amphotropic pseudotyped retroviral vectors. Journal of Biomedicine and Biotechnology. 2009;2009:7 pages. doi: 10.1155/2009/901079. Article ID 901079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huangfu D, Osafune K, Maehr R, et al. Induction of pluripotent stem cells from primary human fibroblasts with only Oct4 and Sox2. Nature Biotechnology. 2008;26(11):1269–1275. doi: 10.1038/nbt.1502. [DOI] [PubMed] [Google Scholar]
- 36.O’Connor MD, Kardel MD, Iosfina I, et al. Alkaline phosphatase-positive colony formation is a sensitive, specific, and quantitative indicator of undifferentiated human embryonic stem cells. Stem Cells. 2008;26(5):1109–1116. doi: 10.1634/stemcells.2007-0801. [DOI] [PubMed] [Google Scholar]
- 37.Malkinson G, Spira ME. Clustering of excess growth resources within leading growth cones underlies the recurrent “deposition” of varicosities along developing neurites. Experimental Neurology. 2010;225(1):140–153. doi: 10.1016/j.expneurol.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 38.Chan EM, Ratanasirintrawoot S, Park IH, et al. Live cell imaging distinguishes bona fide human iPS cells from partially reprogrammed cells. Nature Biotechnology. 2009;27(11):1033–1037. doi: 10.1038/nbt.1580. [DOI] [PubMed] [Google Scholar]
- 39.Prokhorova TA, Harkness LM, Frandsen U, et al. Teratoma formation by human embryonic stem cells is site dependent and enhanced by the presence of Matrigel. Stem Cells and Development. 2009;18(1):47–54. doi: 10.1089/scd.2007.0266. [DOI] [PubMed] [Google Scholar]
- 40.Hentze H, Soong PL, Wang ST, Phillips BW, Putti TC, Dunn NR. Teratoma formation by human embryonic stem cells: evaluation of essential parameters for future safety studies. Stem Cell Research. 2009;2(3):198–210. doi: 10.1016/j.scr.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 41.Tabar V, Panagiotakos G, Greenberg ED, et al. Migration and differentiation of neural precursors derived from human embryonic stem cells in the rat brain. Nature Biotechnology. 2005;23(5):601–606. doi: 10.1038/nbt1088. [DOI] [PubMed] [Google Scholar]
- 42.Pomp O, Brokhman I, Ziegler L, et al. PA6-induced human embryonic stem cell-derived neurospheres: a new source of human peripheral sensory neurons and neural crest cells. Brain Research. 2008;1230:50–60. doi: 10.1016/j.brainres.2008.07.029. [DOI] [PubMed] [Google Scholar]
- 43.Zhang SC, Wernig M, Duncan ID, Brüstle O, Thomson JA. In vitro differentiation of transplantable neural precursors from human embryonic stem cells. Nature Biotechnology. 2001;19(12):1129–1133. doi: 10.1038/nbt1201-1129. [DOI] [PubMed] [Google Scholar]
- 44.Li XJ, Zhang SC. In vitro differentiation of neural precursors from human embryonic stem cells. Methods in Molecular Biology. 2006;331:169–177. doi: 10.1385/1-59745-046-4:168. [DOI] [PubMed] [Google Scholar]
- 45.Chambers SM, Fasano CA, Papapetrou EP, Tomishima M, Sadelain M, Studer L. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nature Biotechnology. 2009;27(3):275–280. doi: 10.1038/nbt.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]


