Abstract
Ultraviolet B (UVB) irradiation potently induces cytokines in the skin, including interleukin-1α (IL-1α) and tumor necrosis factor-α (TNF-α). The mechanism for TNF-α induction in UVB-irradiated keratinocytes is not clear. In the current study, we explored the effects of UVB and cytokines, alone or in combination in human keratinocytes. Keratinocytes were sham- or UVB-irradiated with 30 mJ/cm2, and then incubated in the absence or presence of IFN-α2b, TNF-α or IL-1α. UVB and IL-1α treatment synergistically enhanced TNF-α secretion and mRNA levels in human keratinocytes, similar to the findings reported previously in human fibroblasts. Exogenous recombinant TNF-α up-regulates its own mRNA level. However, addition of IFN-α2b did not show any additive effect on TNF-α mRNA induction. To understand the regulation of TNF-α mRNA by UVB, with or without IL-1α, we examined the transcription rate and half-life of TNF-α mRNA. Treatment of keratinocytes with IL-1α or UVB alone increased TNF-α gene transcription 4–5-fold over sham treatment, and TNF-α gene transcription increased 11-fold in cells treated with UVB plus IL-1α over sham. UVB with IL-1α did not enhance the half-life of TNF-α mRNA over that seen with UVB alone. In conclusion, TNF-α expression in primary keratinocytes is up-regulated transcriptionally by UVB and IL-1α.
Introduction
Tumor necrosis factor-α (TNF-α) plays an important role in photodamage and photoaging. Its secretion from keratinocytes (KCs) and dermal fibroblasts (Fbs) is specifically induced by UVB (290–320nm), but not by UVA (320–400nm), and it may therefore mediate harm from these more energetic UV wavelengths (Skov et al., 1998; Werth and Zhang, 1999). For example, TNF-α released after UVB exposure induces nearby endothelial cells and KCs to display cell adhesion molecules, thereby recruiting inflammatory cells that secrete elastases and collagenases, leading to damage and aging of the skin (Briscoe et al., 1992; Rijken et al., 2006; Strickland et al., 1997). TNF-α also promotes apoptosis, lymphocyte activation, and hyperproliferative skin disorders (Ashkenazi and Dixit, 1998; Banno et al., 2004; Kinkhabwala et al., 1990; van Hogerlinden et al., 2004). TNF-α is involved not only in the mediation of local inflammatory reactions within the epidermis but it may also enter the circulation and cause systemic effects (Kock et al., 1990; Kondo and Sauder, 1995).
Epidermal keratinocytes absorb the bulk of cutaneous UV exposure, and UVB is known to cause damage in the epidermis (Zhuang et al., 1999). Exposure of keratinocytes in vitro to UVB induces the synthesis of many cytokines, including tumor necrosis factor-α (TNF-α), interleukin-1α (IL-1α), IL-6, IL-8 and IL-10 (Brink et al., 2000; Clingen et al., 2001; Kock et al., 1990; Takashima and Bergstresser, 1996; Yarosh et al., 2000). Besides UVB irradiation, several growth factors and cytokines, including IL-1α, IL-1β and interferons also induce TNF-α expression in epidermal keratinocytes (Lisby and Hauser, 2002).
Of interest, dermal fibroblasts release small amounts of TNF-α when exposed to UVB alone or to IL-1α alone, but ~10-fold greater quantities when exposed to both UVB and IL-1α (Fujisawa et al., 1997; Werth and Zhang, 1999). The effect is mediated through a synergistic increase in TNF-α mRNA levels (Fujisawa et al., 1997). Thus, UVB may stimulate large amounts of TNF-α generation in skin by first inducing KCs to secrete IL-1α. Fibroblasts in the upper dermis become simultaneously exposed to KC-derived IL-1α via diffusion and to UVB through its penetration, thereby causing a massive, synergistic induction of TNF-α mRNA and protein in that location (Kondo et al., 1997). In addition, inflammatory cells in the skin are another source of IL-1α and TNF-α that may contribute to UVB-epidermal cytokine synergy in the production of TNF-α.
Nevertheless, possible synergy between UVB and cytokines in the induction of TNF-α production by KCs has been relatively unexplored. In the current study, we sought to determine if UVB, without or with IL-1α or other cytokines, can enhance TNF-α expression by human KCs, and then to define the molecular mechanisms.
Results
UVB and IL-1α, but not endogenous TNF-α, synergistically upregulate TNF-α mRNA in human keratinocytes
Keratinocytes were sham- or UVB-irradiated, then immediately afterwards incubated for up to 72h in the absence or presence of exogenous IL-1α. The viability of cells was measured by trypan blue. Trypan blue testing indicated that UVB-irradiated cells were 93–95% viable at 72h. Total cellular RNA was harvested at different time points after irradiation. TNF-α mRNA was quantified by real-time PCR assay. Cyclophilin A (PPIA) was used as an internal control. Our 72-hour time course study showed that the KCs treated with UVB alone or with exogenous IL-1α alone showed low levels of TNF-α mRNA (Figure 1a), consistent with prior literature (Fujisawa et al., 1997; Werth and Zhang, 1999). KCs treated with the UVB plus exogenous IL-1α strongly induced TNF-α mRNA, with maximal induction between 4–8 h (Figure 1b). Other experiments performed by ribonuclease protection technique also showed that TNF-α mRNA levels greatly increased starting at 3h in UVB + IL-1α-treated cells (data not shown). It is evident from Figure 1b that the TNF-α mRNA levels are maximally elevated at 8 h, followed by a gradual decline and returned to sham levels by 72 h despite the presence of high levels of TNF-α protein (as seen in Figure 5). TNF-α mRNA induction was greatly increased over UVB or sham + IL-1α treated cells (p < 0.001), indicating a powerful synergy between UVB and exogenous IL-1α in the induction of TNF-α mRNA in KCs (Figure 1b). Unlike fibroblasts, KCs already make their own IL-1α, but it is clear that addition of exogenous IL-1α exerts a strong effect on TNF-α mRNA production.
Figure 1. IL-1α synergistically increases TNF-α mRNA induction by UVB-irradiated neonatal KCs.
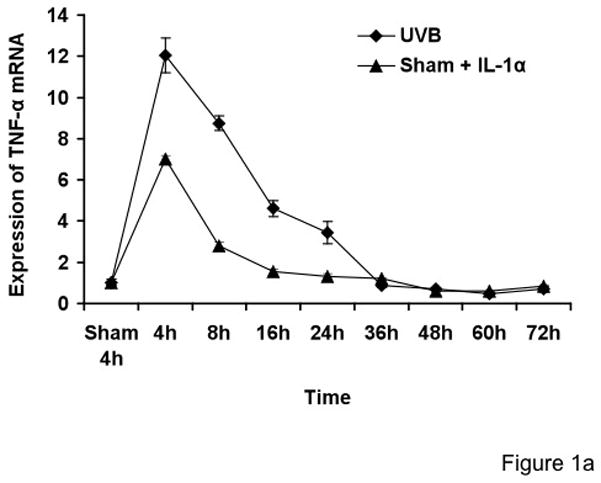
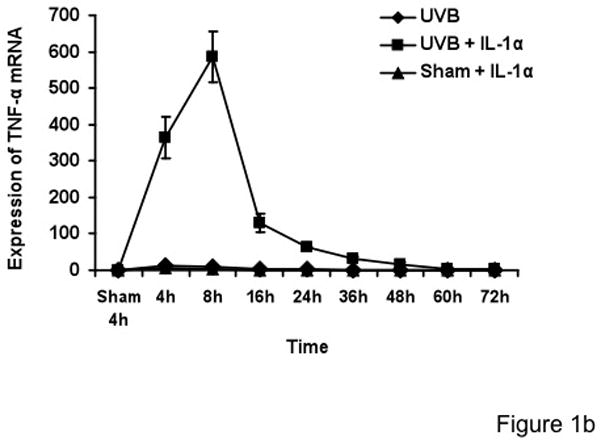
Cells were treated with sham, sham+IL-1α (10 ng/ml), UVB (30mJ/cm2) and UVB+IL-1α. RNA samples were collected at the indicated times and analyzed by real-time PCR. TNF-α mRNA cycle numbers were normalized to cyclophilin A (PPIA). Expression levels of TNF-α mRNA are indicated as “fold change” compared to control. UVB+IL-1α-treated cells showed synergistically increased TNF-α mRNA as compared to UVB or IL-1α alone (Figure 1a and b). The time-course study showed that there was no biphasic induction of TNF-α mRNA in keratinocytes up to 72h.
Figure 5. Synergistic increase of TNF-α protein in keratinocyte medium corresponds to TNF-α mRNA increase due to UVB and IL-1α response.
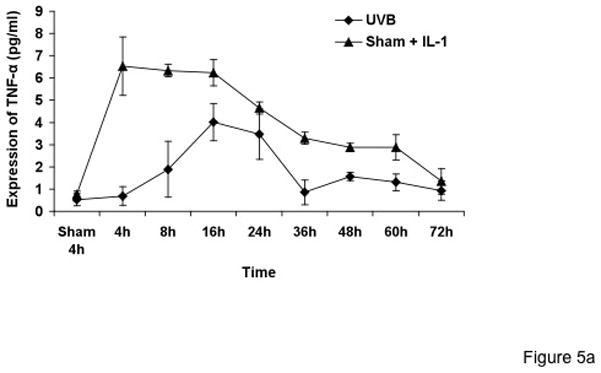
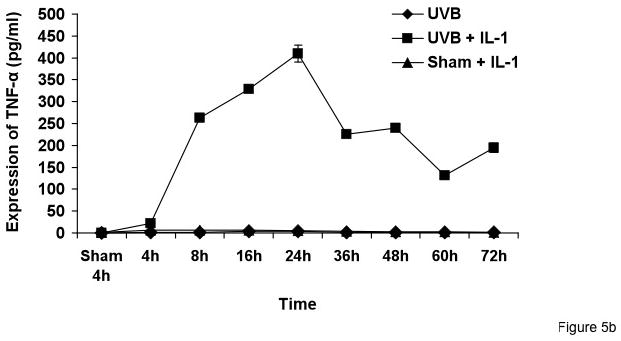
Cells were treated with sham, sham + IL-1α, UVB, and UVB+IL-1α for various time points. Supernatant samples were collected from sham, sham + IL-1α, UVB, and UVB+IL-1α-treated cells at the indicated time points. An equal amount of protein was used for TNF-α ELISA.
To rule out the possibility that TNF-α mRNA induction by UVB and exogenous IL-1α is not due to an immediate effect of TNF-α secreted from KCs at early time points, which triggers the synthesis of new TNF-α, we incubated the UVB + IL-1α-treated-KCs with TNF-α antibody to neutralize the effect of newly synthesized TNF-α. It is evident from our real-time PCR results that neutralization of TNF-α with TNF-α antibody at different doses (5, 10 and 100ng/ml) did not inhibit the induction of TNF-α mRNA relative to UVB + IL-1α treated-KCs (p= 0.48). This suggests that endogenous TNF-α secreted by KCs does not contribute to the synergistic increase of its own mRNA by UVB + IL-1α treated KCs (Figure 2).
Figure 2. Anti-TNF-α antibody has no effect on TNF-α mRNA synthesis in UVB-irradiated keratinocytes in the presence of IL-1α.
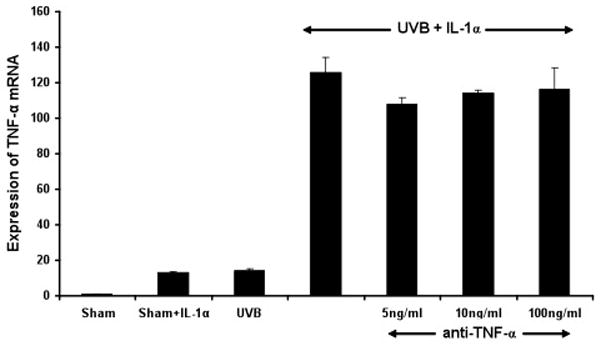
Controls included cells treated with sham, sham+IL-1α, UVB, and UVB+IL-1α. In the test group, UVB+ IL-1α-treated cells were also incubated with anti-TNF-α antibody (5, 10, 100 ng/ml). RNA samples were collected after 3h and analyzed by real-time PCR. Results were normalized to PPIA mRNA and compared to control groups. No significant difference in expression of TNF-α mRNA is observed between UVB+ IL-1α and UVB+ IL-1α + anti-TNF-α treated keratinocytes.
IFN-α does not upregulate TNF-α in UVB-irradiated keratinocytes
To examine whether IFNs and IL-1α synergistically affect TNF-α production in keratinocytes irradiated with sham or UVB, we stimulated cells with IFN-α2b, and IL-1α. Total cellular RNA was harvested 6h post-irradiation and processed for real-time PCR. IFN-α2b, alone or in combination with IL-1α, caused no additional upregulation of TNF-α mRNA in UVB-irradiated cells (p = 0.24) (Figure 3). TNF-α protein secretion was similarly unaffected by IFN-α2b under these conditions (data not shown).
Figure 3. IFN-α2b has no effect on TNF-α mRNA synthesis in sham- or UVB-irradiated keratinocytes in the presence or absence of IL-1α.
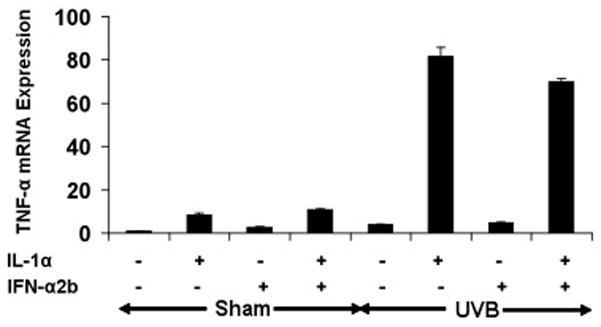
Sham- or UVB-irradiated keratinocytes were treated with IFN-α2b (2000u/ml) or IL-1α (10 ng/ml) alone or in combination with IFN-α2b + IL-1α. RNA sample were collected after 6 h and analyzed by real-time PCR. Results were normalized to PPIA mRNA and compared to control groups.
Upregulation of TNF-α by exogenous recombinant TNF-α in response to UVB in human keratinocytes
To examine whether exogenous TNF-α could induce its own synthesis in keratinocytes, we analyzed the effect of exogenous rTNF-α on the expression of TNF-α mRNA in sham- or UVB-irradiated keratinocytes. The addition of exogenous rTNF-α (5ng/ml) for 3h resulted in a 13-fold increase of TNF-α mRNA in sham- and 51-fold increase in UVB-irradiated keratinocytes, showing a synergistic effect of UVB + rTNF-α relative to rTNF-α alone (p<0.01) (Figure 4).
Figure 4. Exogenous rTNF-α increases TNF-α mRNA induction in UVB-irradiated neonatal keratinocytes.
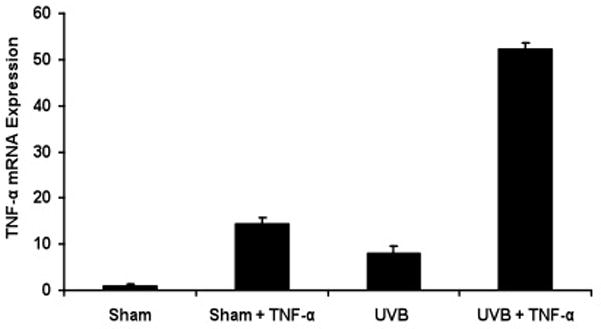
Cells were treated with sham, sham + rTNF-α (5ng/ml), UVB, and UVB + rTNF-α. RNA sample were collected 3h post irradiation and analyzed by real-time PCR. Results were normalized to PPIA (housekeeping gene control). Expression levels of TNF-α mRNA are indicated as “fold change” compared to control.
UVB and IL-1α synergistically upregulate TNF-α protein secretion from human keratinocytes
To correlate TNF-α mRNA induction with TNF-α protein production, medium from sham- or UVB-irradiated KCs, with or without IL-1α, was collected at different time points after irradiation and assayed for TNF-α by ELISA. Consistent with the mRNA data, TNF-α level in media were increased as early as 3h, with a maximal increase at 24h in UVB + IL-1α-treated cells. Cells treated with UVB + IL-1α showed significantly higher levels of TNF-α as compared to UVB or IL-1 α alone (p<0.0001) (Figure 5a and b).
Release of soluble TNF-α is not due to cleavage of membrane-bound TNF-α from keratinocytes
Induction of membrane-bound TNF-α has been previously reported, and so this was examined in our system (Yarosh et al., 2000). To examine whether the increased level of TNF-α in the medium of UVB-and IL-1α-treated cells is due to secreted TNF-α or release of membrane bound TNF-α in medium, we assayed TNF-α by ELISA in the membrane fraction of sham- or UVB-irradiated cells stimulated with IL-1α. Our results indicate that IL-1α decreased the low level of membrane-bound TNF-α in UVB-irradiated KCs after 12 h (p < 0.01) as compared to UVB or IL-1α-treated cells alone (from 15 pg/ng to 4 pg/ng of protein) (Figure 6). The supernatants collected from same experiment showed soluble TNF-α levels 536 pg/ml, increased from 2 pg/ml in untreated sham cells.
Figure 6. No significant changes were observed in the membrane bound TNF-α protein after UVB and IL-1α treatment.
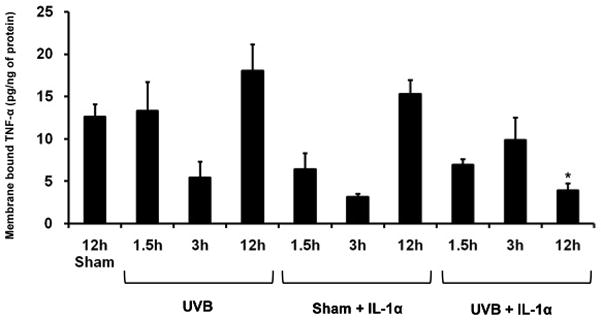
Cells were treated with sham, sham + IL-1α, UVB, and UVB+IL-1α for various time points. Membrane fractions were eluted and an equal amount of protein was used for TNF-α ELISA.
TNF-α transcription is induced by UVB irradiation and IL-1α
To determine whether TNF-α mRNA induction by UVB irradiation plus IL-1α results from increased transcription of the TNF-α gene, we performed a nuclear run-off assay. Nuclei from KCs treated with sham or UVB irradiation, with or without IL-1α, were isolated after 3 h, an early time point that shows large induction of TNF-α mRNA. In four independent experiments, the transcription rate of TNF-α gene increased to 4-fold of the untreated control value after IL-1α alone, 5-fold after UVB alone, and 11-fold after UVB + IL-1α (p<0.001) (Figure 7).
Figure 7. UVB irradiation and IL-1α enhances TNF-α gene transcription.
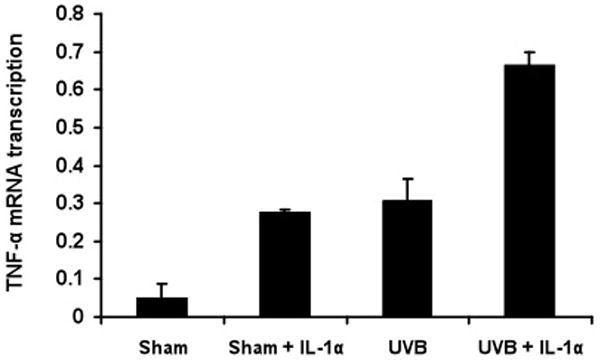
Nuclei were isolated 3 h after sham- or UVB-irradiation and stimulated with or without IL-1α. Nuclear run-off assay was performed to examine TNF-α and 18S (control) transcription. Detectable bands were assessed by densitometric analysis. UVB irradiation induced TNF-α gene transcription 5-fold. One of four experiments is shown, illustrating 11-fold induction of UVB+ IL-1α cells when compared with sham irradiated cells.
IL-1α does not alter the half-life of TNF-α mRNA
To determine whether TNF-α mRNA induction by UVB irradiation and IL-1α results in part from an increase in TNF-α mRNA half-life, we treated the KCs with actinomycin D, an inhibitor of RNA synthesis, and measured the disappearance of TNF-α mRNA by Northern analysis and real-time PCR (Figure 8). Because of its very long (3.8 days) half-life 18S was used for normalization (Yi et al., 1999). In four independent experiments, the t1/2 values for TNF-α mRNA were 75.3 ± 16.7 min in KC treated with UVB alone vs. 56.0 ± 4.5 min in KCs treated with the combination of UVB plus IL-1α (Figure 8; p=0.94 N.S). Thus, UVB plus IL-1α did not prolong the half-life of TNF-α mRNA. We detected very low level of TNF-α mRNA expression in sham, and IL-1α-treated cells. Therefore, after treating the cells with actinomycin D, TNF-α mRNA was not detectable up to 2h.
Figure 8. TNF-α mRNA stability is not induced after UVB irradiation and IL-1α stimulation.
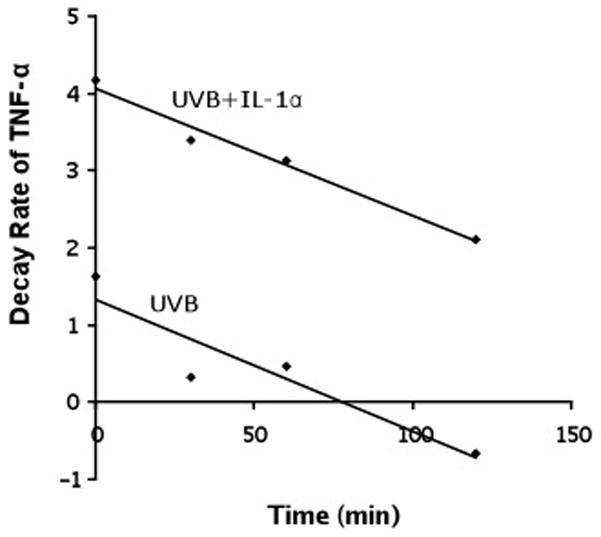
Keratinocytes were UVB or sham-irradiated and stimulated with IL-1α for 3 h. Actinomycin D (10 μg/ml) was added after 3h. Total RNA was extracted at different time points and TNF-α mRNA levels were determined by northern analysis and Real-time PCR. The half-life was calculated in UVB, and UVB+ IL-1α treated cells.
Discussion
Keratinocytes play an important role in the inflammatory response of the skin because of the induction of cytokines by UVB including TNF-α, IL-1 and IL-6 (Kirnbauer et al., 1991; Kock et al., 1990; Kondo et al., 1994). These UVB-induced cytokines act in a cascade fashion to induce inflammation, with initial release by keratinocytes or inflammatory cells in the skin, and subsequent synergizing with UV-irradiated keratinocytes to further increase their cytokine production (Takashima and Bergstresser, 1996).
This study has demonstrated a robust synergy between UVB and exogenous IL-1α in the induction of TNF-α gene transcription, mRNA levels, and protein secretion from neonatal KCs. To the best of our knowledge this is the first report of the role of UVB and exogenous IL-1α synergy in the production of TNF-α in neonatal KCs. There is also acute synergy between UVB and exogenous TNF-α in the induction of TNF-α mRNA in these cells. IFN-α2b did not further induce TNF-α expression in keratinocytes. Inflammatory cells release IL-1 and TNF-α in the skin, and our data suggest that interactions of these cytokines with keratinocytes can further upregulate epidermal cytokine production (Ansel et al., 1990; Ullrich, 1995). Other investigators have reported that after UVB irradiation alone, mouse epidermal keratinocytes increased TNF-α expression immediately, declined at 6 hours, rose again at 12 hours, before falling by 24 hours, giving a biphasic induction of TNF-α by UVB alone (Yarosh et al., 2000). Clingen et al (2000) showed an effect of UVB alone on induction of TNF-α mRNA after 6h and protein after 24h in human keratinocytes. They irradiated the cells through the bottom of Petri dishes, which is different from our irradiation procedure. We administered UVB through a UVC filter with the top of the dish removed. It is likely that the Petri dish blocks out some wavelengths of UVB and thus higher doses of more intense short wavelengths of UVB were potentially delivered in their system. In addition, it is not clear how far away the dishes were from the light source, and potentially there was a heat effects if the bulbs were close to the dishes. Higher doses of UVA (200 mJ/cm2 and 800 mJ/cm2 ) also induce the TNF-α mRNA expression in HaCat cells (Skiba et al., 2005), a finding not observed in primary keratinocytes (Werth and Zhang, 1999). Other studies reported synergy between IFN-γ and IL-1α in the induction of TNF-α in unirradiated human keratinocytes (Matsuura et al., 1998). UVB and IL-1α showed a synergistic effect in induction of TNF-α in dermal fibroblasts (Fujisawa et al., 1997; Werth and Zhang, 1999). The exact mechanism of this synergistic response has not been determined.
Cytokines may act in an autocrine manner and thereby regulate their own synthesis. Pro-inflammatory cytokines like IL-1α in human keratinocytes and vascular endothelial cells and IL-1β in thymic stromal cells exhibit the capacity to induce autocrine upregulation (Kameda and Sato, 1994; Lee et al., 1991; Tseng and Schuler, 1998; Warner et al., 1987). In addition, TNF-α is capable of increasing its own expression in ovarian tumor cells (Kulbe et al., 2007; Wu et al., 1993). Recombinant TNF-α upregulates its own mRNA synthesis in an autocrine manner in murine keratinocytes (Lisby and Hauser, 2002).
In the context of these previous studies, we sought to determine whether the endogenously enhanced levels of TNF-α found in response to UVB and IL-1α or exogenous rTNF-α is also capable of increasing its own synthesis in keratinocytes. Thus, we measured TNF-α expression levels up to 72h post irradiation and IL-1α stimulation. Our time course study revealed that neonatal keratinocytes did not show any biphasic response in upregulation of TNF-α message or protein, despite finding significantly higher levels of TNF-α protein present up to 72h. In our study, neutralization of TNF-α in medium by TNF-α antibody could not inhibit the early induction of TNF-α mRNA by UVB + IL-1α. These findings indicate that keratinocyte-derived TNF-α does not have an effect on TNF-α mRNA induction by UVB + IL-1α, suggesting that TNF-α protein is not synthesized by KCs early enough to have an autocrine effect. It is possible that mechanism, such as the previously described clustering and internalization of TNF receptors, may lessen the response to TNF-α protein over time (Higuchi and Aggarwal, 1994). The lack of effect of persistent TNF-α protein on continued TNF-α mRNA induction in the long-term experiments contrasts with the acute effects of exogenous human rTNF-α on TNF-α mRNA in UVB-irradiated KCs. We observed a synergy between rTNF-α and UVB in the expression of TNF-α mRNA (Figure 4). Our findings are in line with the results of Lisby and colleagues who demonstrated rTNF-α upregulates its own mRNA synthesis in an autocrine manner in HaCat cells. The autocrine effect of TNF-α is mediated by the signaling cascade comprising TNFR1, atypical protein kinase C (aPKC) and the transcription factor NF-kappaB (Lisby et al., 2007).
The mechanism by which UVB and IL-1α induce TNF-α in keratinocytes is not known. The expression of TNF-α is regulated at many levels, including transcription, post transcription, message turnover, protein production, and protein release (Huizinga et al., 1996; Kontoyiannis et al., 1999; Piecyk et al., 2000). Previous reports showed that TNF-α protein synthesis is likely due to an increase in release of the soluble form of TNF-α in response to septic stimuli. The soluble form of TNF-α is released by cleavage of membrane-bound TNF-α by TNF-α cleaving enzyme (Robertshaw and Brennan, 2005). Our results showed that there is slight decrease in membrane bound TNF-α in UVB + IL-1α cells relative to sham-treated keratinocytes. The decrease in membrane bound TNF-α ranges from 5–10 pg/ml which can not account for >200-fold induction of TNF-α observed in the supernatant of UVB + IL-1α-treated cells as compared to sham (see Figure 5b).
The UVB + IL-1α-mediated increased rate of TNF-α gene transcription in our system is consistent with the report that UV induces transcription of the TNF-α gene in macrophages (Bazzoni et al., 1994). Since TNF-α production has been described to be regulated both by transcriptional and post-transcriptional mechanisms, we tested in our system if one of the potential mechanisms for the synergistic induction of TNF-α by UVB and IL-1α is through increased message stability. One previous study in a transformed cell line treated with UV suggested an effect on both transcriptional and post-transcriptional mechanisms (Leverkus et al., 1998). In our study, the stability of TNF-α mRNA was not different in KCs, as indicated by no increase in half-life for TNF-α mRNA after UVB and IL-1α treatment. This suggests that message stability is not a major contributing factor for TNF-α induction by the combination of UVB + IL-1α. In other cell types treated with non-UV stimuli, both transcriptional and post-transcriptional regulation of TNF-α gene expression has been demonstrated (Han et al., 1991; Hel et al., 1998; Jacob and Tashman, 1993; Jongeneel, 1994). With these other systems, TNF-α mRNA binds to cytosolic proteins that specifically recognize the AUUUA rich structural elements and prevent the degradation of the message (Hel et al., 1998; Kim et al., 1996). Also, in virus stimulated astrocytes, TNF-α is regulated by both transcriptional and post-transcriptional mechanisms, suggesting various stimuli appear to induce TNF-α by more than one regulatory mechanism (Lieberman et al., 1990). UV irradiation has been reported to induce and activate the transcription factors AP-1 and NF-kB (Devary et al., 1993; Simon et al., 1994). The promoter of the TNF-α gene contains both AP-1 and NF-kB binding sites, major contenders for the upregulation of TNF-α gene transcription (Spriggs et al., 1992). In murine macrophages, LPS stimulates TNF-α gene transcription by translocation of NF-kB to the nucleus (Collart et al., 1990; Shakhov et al., 1990). However, the mechanism of UVB-induced TNF-α transcription has not yet been defined.
In summary, this data suggests that skin-derived IL-1α and TNF-α have further synergistic effects on the induction of TNF-α by UVB-irradiated keratinocytes. Our results demonstrate that the mechanistic effects of UVB and IL-1α on TNF-α induction are at a transcriptional level. Studies in our laboratory are dissecting the relative roles of these transcription factors on UVB-induced upregulation of the TNF-α gene.
Materials & Methods
Human Keratinocytes Culture
Normal neonatal human keratinocytes were obtained from foreskin and grown in MCDB 153 medium (Sigma M-7403, St Louis, MO) supplemented with 30 μM CaCl2, ethanolamine, phosphoethanolamine, bovine pituitary extract, epidermal growth factor, insulin, hydrocortisone, penicillin and streptomycin. Keratinocytes were plated in 100 mm dishes and grown to 60–90% confluence at 37°C in a humidified 5% CO2 atmosphere.
Cytokines and chemicals
Cytokines IL-1α, IFN-α2b, rTNF-α were purchased from R&D Systems, Inc. (Minneapolis, MN). All other chemicals were purchased from Fisher Scientific (Pittsburg, PA) or SIGMA (Saint Louis, MO).
Light source and radiometry
The UVB source was a bank of two FS 40 sunlamps (Light of America, Walnut, CA) with a peak irradiation of 313nm, equipped with a cellulose triacetate filter to remove wavelengths below 290nm, as previously described (Werth and Zhang, 1999). UVB doses were measured with an International Light UV IL-443 UVB meter. The filtered UVB light source measured by spectroradiometric measurement at the time of the experiments showed 0.64 % UVC, 44.51 % UVB, 19.43% UVA and 35.42% visible and near infrared (Vis + NIR).
UVB irradiation
An irradiation dose of 30 mJ/cm2 UVB was chosen based on cell viability, cytotoxicity assay with trypan- blue, and on our previous results (Werth and Zhang, 1999). Neonatal human keratinocytes were cultured and medium was replaced by PBS prior to irradiation. Cells receiving sham-irradiation treatment (0 mJ/cm2) went through the same procedure, but covered with aluminum foil. After irradiation, cells were immediately returned to MCDB complete medium with or without addition of the cytokines for different period of time according to the requirement of the different experiments. Conditioned medium was collected for TNF-α ELISA at different time points.
Extraction of total RNA from cultured keratinocytes
Depending upon our experimental requirements, cells at 60–90% confluency were irradiated with UVB or sham, followed by addition of cytokines. For short-term experiments we used 90% confluent cells and for longer three-day experiments we used 60–70% confluent cells. The viability of cells was measured by trypan blue up to 72h. After removal of medium, cells were washed with PBS and total RNA was extracted by adding 1 ml Trizol (Invitrogen, Carlsbad CA) directly to the dishes, followed by isopropanol precipitation and 70% ethanol wash. The RNA pellets were dissolved in DEPC-treated water. The RNA was quantified by measuring the optical density at 260 nm, with 260/280 (ratio >1.8) for evaluating purity.
Real-time Polymerase Chain Reaction
Total RNA (2 μg), from sham- or UVB-exposed human keratinocytes, with or without cytokine addition, was used for cDNA synthesis. cDNA was synthesized using SUPERSCRIPT First-Strand Synthesis for RT-PCR kit with random hexamers (Invitrogen life Technologies, Carlsbad CA). Oligonucleotide sequences and target-specific fluorescence-labeled DNA probes were designed on Primer Express software by Applied Biosystems. 2 μL of cDNA were amplified by PCR, in which 50 nM each of the forward and reverse gene-specific primers for TNF-α and human cyclophilin A (PPIA) were used. All PCR assays were performed in triplicates and read using an ABI PRISM 7000 sequence detection system. The cycle number was predetermined so that the products formed fell within the linear portion of the amplification curve.
Northern Blot Analysis
Ten micrograms of RNA were electrophoresed under formaldehyde denaturing conditions on 1.2% agarose containing 0.2 mol/l formaldehyde and transferred to Nytran membranes. The blots were probed with 32P-labeled TNF-α cDNA probe (ATCC no. 39894). Evidence that RNA was equally loaded and transferred was obtained by equivalent intensity of ethidium bromide staining of 18S and 28S rRNA bands. Furthermore, the human18S cDNA (ATCC no. 77242) probe was used as a control to verify the consistency and integrity of RNA loading.
TNF-α protein determination by ELISA
Conditioned media from UVB or sham-irradiated and IL-1α treated cells were collected at different time points. TNF-α was quantitated by commercial enzyme-linked immunosorbent assay (ELISA) (R&D Systems Inc., Minneapolis, MN). The protein level was measured by CB-Protein-Assay-Kit (G-Biosciences, St. Louis, MO) and volume was adjusted to 100 μl, so that equal amounts of protein were used for each sample.
Effect of TNF-α antibody on TNF-mRNA synthesis
Cells at 90% confluency were irradiated with sham, sham + IL-1α, UVB and UVB +IL-1α. Cells treated with UVB +IL-1α were incubated with TNF-α antibody at different doses (5, 10, 100 ng/ml). After 3 h the cells were harvested and total RNA was isolated as described above. The TNF-α expression level was determined by real-time PCR.
Isolation of plasma membrane
Cells were washed with PBS and incubated in a hypotonic lysis buffer (10 mM Tris, pH 7.4, 10 mM NaCl, 3 mM MgCl2, and 0.5% Nonidet P-40), causing them to swell. The cell suspension was homogenized by mechanical disruption using a needle and syringe. Intact cells, cell debris, nuclei and the major organelles were removed by centrifugation at 2000 × g for 5 min. The supernatant contains cytosolic proteins and microsomes, and plasma membranes. The supernatant was centrifuged at 12,000 × g and 4°C for 20 min. The pellet was stored in Tris buffer (50 mM Tris, pH 8.5, 5 mM MgCl2, and 40% glycerol) at −80 °C until use.
Nuclear Run-off Assay
The procedure was carried out as previously described (Li and Chaikof, 2002). Briefly, cells were irradiated with or without UVB and treated with IL-1α for 3h. Nuclei were isolated by centrifugation using sucrose gradient (Nuclei Pure Prep Nuclei Isolation kit from Sigma, Saint Louis, MO). Nuclear run-off reaction was performed by mixing the 200 μg of nuclei with an equal volume of reaction buffer (10 mM Tris.Cl, pH 8.0, 5 mM MgCl2, 5 mM dithiothreitol, 0.3 M KCl), 1.0 mM ATP, GTP and CTP, 5 μl of [α-32P] UTP (3000 Ci/mmol) and incubated at 30 °C for 1h with gentle shaking. The reaction was stopped by addition of DNAse I (10 units). The newly synthesized 32P-UTP labeled nuclear RNA was extracted with phenol/chloroform/isoamyl alcohol followed by isopropanol precipitation and 70% ethanol wash. Equal counts of 32P-UTP labeled RNA were hybridized to slot blots containing 2 μg of target inserts per slot onto a nitrocellulose filter (Schleicher & Schuell, Keene, NH) for 36h at 65 °C. The filters were then washed and exposed to phosphor screen (Molecular Dynamics). The signals were detected using the PhosphorImager Typhoon 9400 (Amersham Biosciences, Piscataway, NJ). Each band was quantitated using ImageQuant.
Inhibition of mRNA synthesis and mRNA half-life determination
The selective mRNA synthesis inhibitor actinomycin D was used to block TNF-α mRNA production, and the decay of TNF-α message was calculated by Northern and Real-Time PCR analysis using 18S as a control for Northern and PPIA as a control for Real-time PCR analysis. Cells were irradiated with UVB or sham, followed by addition of IL-1α. After 3 h, cells were treated with actinomycin D (10μg/ml). The cells were harvested at different time points after actinomycin D treatment and total RNA was isolated as described above.
Statistical analysis
Comparisons of several groups were performed simultaneously by initially using analysis of variance (ANOVA). When the ANOVA indicated differences amongst the groups, pairwise comparisons of each experimental group versus the control group were performed using the Dunnett q′ statistic. When comparing between groups, the Student-Newman-Keuls test was used after performing ANOVA. Unless otherwise indicated, summary statistics are reported as means SEM, n=3. Absent error bars in graphical displays of summary statistics indicate SEM values smaller than the drawn symbols.
Acknowledgments
This study was supported by a Veterans Affairs Merit Review Grant (Dr. Werth).
Abbreviations
- UVB
Ultraviolet B
- IL-1
interleukin-1
- TNF-α
Tumor necrosis factor-α
- KCs
keratinocytes
- IFN-α2b
Interferon α2b
- rTNF-α
recombinant tumor necrosis factor-α
- ELISA
enzyme-linked immunosorbent assay
References
- Ansel J, Perry P, Brown J, Damm D, Phan T, Hart C, et al. Cytokine modulation of keratinocyte cytokines. J Invest Dermatol. 1990;94:101S–107S. doi: 10.1111/1523-1747.ep12876053. [DOI] [PubMed] [Google Scholar]
- Ashkenazi A, Dixit VM. Death receptors: signaling and modulation. Science. 1998;281:1305–1308. doi: 10.1126/science.281.5381.1305. [DOI] [PubMed] [Google Scholar]
- Banno T, Gazel A, Blumenberg M. Effects of tumor necrosis factor-alpha (TNF alpha) in epidermal keratinocytes revealed using global transcriptional profiling. J Biol Chem. 2004;279:32633–32642. doi: 10.1074/jbc.M400642200. [DOI] [PubMed] [Google Scholar]
- Bazzoni F, Kruys V, Shakhov A, Jongeneel CV, Beutler B. Analysis of tumor necrosis factor promoter responses to ultraviolet light. J Clin Invest. 1994;93:56–62. doi: 10.1172/JCI116984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brink N, Szamel M, Young AR, Wittern KP, Bergemann J. Comparative quantification of IL-1beta, IL-10, IL-10r, TNFalpha and IL-7 mRNA levels in UV-irradiated human skin in vivo. Inflamm Res. 2000;49:290–296. doi: 10.1007/PL00000209. [DOI] [PubMed] [Google Scholar]
- Briscoe DM, Cotran RS, Pober JS. Effects of tumor necrosis factor, lipopolysaccharide, and IL-4 on the expression of vascular cell adhesion molecule-1 in vivo. Correlation with CD3+ T cell infiltration. J Immunol. 1992;149:2954–2960. [PubMed] [Google Scholar]
- Clingen PH, Berneburg M, Petit-Frere C, Woollons A, Lowe JE, Arlett CF, et al. Contrasting effects of an ultraviolet B and an ultraviolet A tanning lamp on interleukin-6, tumour necrosis factor-alpha and intercellular adhesion molecule-1 expression. Br J Dermatol. 2001;145:54–62. doi: 10.1046/j.1365-2133.2001.04281.x. [DOI] [PubMed] [Google Scholar]
- Collart MA, Baeuerle P, Vassalli P. Regulation of tumor necrosis factor alpha transcription in macrophages: involvement of four kappa B-like motifs and of constitutive and inducible forms of NF-kappa B. Mol Cell Biol. 1990;10:1498–1506. doi: 10.1128/mcb.10.4.1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devary Y, Rosette C, DiDonato JA, Karin M. NF-kappa B activation by ultraviolet light not dependent on a nuclear signal. Science. 1993;261:1442–1445. doi: 10.1126/science.8367725. [DOI] [PubMed] [Google Scholar]
- Fujisawa H, Wang B, Kondo S, Shivji GM, Sauder DN. Costimulation with ultraviolet B and interleukin-1 alpha dramatically increase tumor necrosis factor-alpha production in human dermal fibroblasts. J Interferon Cytokine Res. 1997;17:307–313. doi: 10.1089/jir.1997.17.307. [DOI] [PubMed] [Google Scholar]
- Han J, Huez G, Beutler B. Interactive effects of the tumor necrosis factor promoter and 3′-untranslated regions. J Immunol. 1991;146:1843–1848. [PubMed] [Google Scholar]
- Hel Z, Di Marco S, Radzioch D. Characterization of the RNA binding proteins forming complexes with a novel putative regulatory region in the 3′-UTR of TNF-alpha mRNA. Nucleic Acids Res. 1998;26:2803–2812. doi: 10.1093/nar/26.11.2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi M, Aggarwal BB. TNF induces internalization of the p60 receptor and shedding of the p80 receptor. J Immunol. 1994;152:3550–3558. [PubMed] [Google Scholar]
- Huizinga TW, Brinkman BM, Verweij CL. Regulation of tumor necrosis factor-alpha production: basic aspects and pharmacological modulation. J Rheumatol. 1996;23:416–418. [PubMed] [Google Scholar]
- Jacob CO, Tashman NB. Disruption in the AU motif of the mouse TNF-alpha 3′ UTR correlates with reduced TNF production by macrophages in vitro. Nucleic Acids Res. 1993;21:2761–2766. doi: 10.1093/nar/21.11.2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jongeneel CV. Regulation of the TNF alpha gene. Prog Clin Biol Res. 1994;388:367–381. [PubMed] [Google Scholar]
- Kameda K, Sato K. Regulation of IL-1 alpha expression in human keratinocytes: transcriptional activation of the IL-1 alpha gene by TNF-alpha, LPS, and IL-1 alpha. Lymphokine and cytokine research. 1994;13:29–35. [PubMed] [Google Scholar]
- Kim YU, Rus HG, Fisher SN, Pitha PM, Shin ML. Binding of a protein to an AU-rich domain of tumour necrosis factor alpha mRNA as a 35 kDa complex and its regulation in primary rat astrocytes. Biochem J. 1996;316 ( Pt 2):455–460. doi: 10.1042/bj3160455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinkhabwala M, Sehajpal P, Skolnik E, Smith D, Sharma VK, Vlassara H, et al. A novel addition to the T cell repertory. Cell surface expression of tumor necrosis factor/cachectin by activated normal human T cells. J Exp Med. 1990;171:941–946. doi: 10.1084/jem.171.3.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirnbauer R, Kock A, Neuner P, Forster E, Krutmann J, Urbanski A, et al. Regulation of epidermal cell interleukin-6 production by UV light and corticosteroids. J Invest Dermatol. 1991;96:484–489. doi: 10.1111/1523-1747.ep12470181. [DOI] [PubMed] [Google Scholar]
- Kock A, Schwarz T, Kirnbauer R, Urbanski A, Perry P, Ansel JC, et al. Human keratinocytes are a source for tumor necrosis factor alpha: evidence for synthesis and release upon stimulation with endotoxin or ultraviolet light. J Exp Med. 1990;172:1609–1614. doi: 10.1084/jem.172.6.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo S, Kooshesh F, Sauder DN. Penetration of keratinocyte-derived cytokines into basement membrane. J Cell Physiol. 1997;171:190–195. doi: 10.1002/(SICI)1097-4652(199705)171:2<190::AID-JCP9>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Kondo S, Sauder DN. Keratinocyte-derived cytokines and UVB-induced immunosuppression. J Dermatol. 1995;22:888–893. doi: 10.1111/j.1346-8138.1995.tb03939.x. [DOI] [PubMed] [Google Scholar]
- Kondo S, Sauder DN, Kono T, Galley KA, McKenzie RC. Differential modulation of interleukin-1 alpha (IL-1 alpha) and interleukin-1 beta (IL-1 beta) in human epidermal keratinocytes by UVB. Exp Dermatol. 1994;3:29–39. doi: 10.1111/j.1600-0625.1994.tb00263.x. [DOI] [PubMed] [Google Scholar]
- Kontoyiannis D, Pasparakis M, Pizarro TT, Cominelli F, Kollias G. Impaired on/off regulation of TNF biosynthesis in mice lacking TNF AU-rich elements: implications for joint and gut-associated immunopathologies. Immunity. 1999;10:387–398. doi: 10.1016/s1074-7613(00)80038-2. [DOI] [PubMed] [Google Scholar]
- Kulbe H, Thompson R, Wilson JL, Robinson S, Hagemann T, Fatah R, et al. The inflammatory cytokine tumor necrosis factor-alpha generates an autocrine tumor-promoting network in epithelial ovarian cancer cells. Cancer Res. 2007;67:585–592. doi: 10.1158/0008-5472.CAN-06-2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SW, Morhenn VB, Ilnicka M, Eugui EM, Allison AC. Autocrine stimulation of interleukin-1 alpha and transforming growth factor alpha production in human keratinocytes and its antagonism by glucocorticoids. J Invest Dermatol. 1991;97:106–110. doi: 10.1111/1523-1747.ep12478503. [DOI] [PubMed] [Google Scholar]
- Leverkus M, Yaar M, Eller MS, Tang EH, Gilchrest BA. Post-transcriptional regulation of UV induced TNF-alpha expression. J Invest Dermatol. 1998;110:353–357. doi: 10.1046/j.1523-1747.1998.00154.x. [DOI] [PubMed] [Google Scholar]
- Li L, Chaikof EL. Quantitative nuclear run-off transcription assay. Biotechniques. 2002;33:1016–1017. doi: 10.2144/02335bm09. [DOI] [PubMed] [Google Scholar]
- Lieberman AP, Pitha PM, Shin ML. Protein kinase regulates tumor necrosis factor mRNA stability in virus-stimulated astrocytes. J Exp Med. 1990;172:989–992. doi: 10.1084/jem.172.3.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisby S, Faurschou A, Gniadecki R. The autocrine TNFalpha signalling loop in keratinocytes requires atypical PKC species and NF-kappaB activation but is independent of cholesterol-enriched membrane microdomains. Biochem Pharmacol. 2007;73:526–533. doi: 10.1016/j.bcp.2006.11.006. [DOI] [PubMed] [Google Scholar]
- Lisby S, Hauser C. Transcriptional regulation of tumor necrosis factor-alpha in keratinocytes mediated by interleukin-1beta and tumor necrosis factor-alpha. Exp Dermatol. 2002;11:592–598. doi: 10.1034/j.1600-0625.2002.110612.x. [DOI] [PubMed] [Google Scholar]
- Matsuura K, Otsuka F, Fujisawa H. Effects of interferons on tumour necrosis factor alpha production from human keratinocytes. Cytokine. 1998;10:500–505. doi: 10.1006/cyto.1997.0326. [DOI] [PubMed] [Google Scholar]
- Piecyk M, Wax S, Beck AR, Kedersha N, Gupta M, Maritim B, et al. TIA-1 is a translational silencer that selectively regulates the expression of TNF-alpha. Embo J. 2000;19:4154–4163. doi: 10.1093/emboj/19.15.4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rijken F, Kiekens RC, van den Worm E, Lee PL, van Weelden H, Bruijnzeel PL. Pathophysiology of photoaging of human skin: focus on neutrophils. Photochem Photobiol Sci. 2006;5:184–189. doi: 10.1039/b502522b. [DOI] [PubMed] [Google Scholar]
- Robertshaw HJ, Brennan FM. Release of tumour necrosis factor alpha (TNFalpha) by TNFalpha cleaving enzyme (TACE) in response to septic stimuli in vitro. Br J Anaesth. 2005;94:222–228. doi: 10.1093/bja/aei021. [DOI] [PubMed] [Google Scholar]
- Shakhov AN, Collart MA, Vassalli P, Nedospasov SA, Jongeneel CV. Kappa B-type enhancers are involved in lipopolysaccharide-mediated transcriptional activation of the tumor necrosis factor alpha gene in primary macrophages. J Exp Med. 1990;171:35–47. doi: 10.1084/jem.171.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon MM, Aragane Y, Schwarz A, Luger TA, Schwarz T. UVB light induces nuclear factor kappa B (NF kappa B) activity independently from chromosomal DNA damage in cell-free cytosolic extracts. J Invest Dermatol. 1994;102:422–427. doi: 10.1111/1523-1747.ep12372194. [DOI] [PubMed] [Google Scholar]
- Skiba B, Neill B, Piva TJ. Gene expression profiles of TNF-alpha, TACE, furin, IL-1beta and matrilysin in UVA- and UVB-irradiated HaCat cells. Photodermatol Photoimmunol Photomed. 2005;21:173–182. doi: 10.1111/j.1600-0781.2005.00162.x. [DOI] [PubMed] [Google Scholar]
- Skov L, Hansen H, Allen M, Villadsen L, Norval M, Barker JN, et al. Contrasting effects of ultraviolet A1 and ultraviolet B exposure on the induction of tumour necrosis factor-alpha in human skin. Br J Dermatol. 1998;138:216–220. doi: 10.1046/j.1365-2133.1998.02063.x. [DOI] [PubMed] [Google Scholar]
- Spriggs DR, Deutsch S, Kufe DW. Genomic structure, induction, and production of TNF-alpha. Immunology series. 1992;56:3–34. [PubMed] [Google Scholar]
- Strickland I, Rhodes LE, Flanagan BF, Friedmann PS. TNF-alpha and IL-8 are upregulated in the epidermis of normal human skin after UVB exposure: correlation with neutrophil accumulation and E-selectin expression. J Invest Dermatol. 1997;108:763–768. doi: 10.1111/1523-1747.ep12292156. [DOI] [PubMed] [Google Scholar]
- Takashima A, Bergstresser PR. Impact of UVB radiation on the epidermal cytokine network. Photochem Photobiol. 1996;63:397–400. doi: 10.1111/j.1751-1097.1996.tb03054.x. [DOI] [PubMed] [Google Scholar]
- Tseng YH, Schuler LA. Transcriptional regulation of interleukin-1beta gene by interleukin-1beta itself is mediated in part by Oct-1 in thymic stromal cells. J Biol Chem. 1998;273:12633–12641. doi: 10.1074/jbc.273.20.12633. [DOI] [PubMed] [Google Scholar]
- Ullrich SE. The role of epidermal cytokines in the generation of cutaneous immune reactions and ultraviolet radiation-induced immune suppression. Photochem Photobiol. 1995;62:389–401. doi: 10.1111/j.1751-1097.1995.tb02359.x. [DOI] [PubMed] [Google Scholar]
- van Hogerlinden M, Rozell BL, Toftgard R, Sundberg JP. Characterization of the progressive skin disease and inflammatory cell infiltrate in mice with inhibited NF-kappaB signaling. J Invest Dermatol. 2004;123:101–108. doi: 10.1111/j.0022-202X.2004.22706.x. [DOI] [PubMed] [Google Scholar]
- Warner SJ, Auger KR, Libby P. Human interleukin 1 induces interleukin 1 gene expression in human vascular smooth muscle cells. J Exp Med. 1987;165:1316–1331. doi: 10.1084/jem.165.5.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werth VP, Zhang W. Wavelength-specific synergy between ultraviolet radiation and interleukin-1 alpha in the regulation of matrix-related genes: mechanistic role for tumor necrosis factor-alpha. J Invest Dermatol. 1999;113:196–201. doi: 10.1046/j.1523-1747.1999.00681.x. [DOI] [PubMed] [Google Scholar]
- Wu S, Boyer CM, Whitaker RS, Berchuck A, Wiener JR, Weinberg JB, et al. Tumor necrosis factor alpha as an autocrine and paracrine growth factor for ovarian cancer: monokine induction of tumor cell proliferation and tumor necrosis factor alpha expression. Cancer Res. 1993;53:1939–1944. [PubMed] [Google Scholar]
- Yarosh D, Both D, Kibitel J, Anderson C, Elmets C, Brash D, et al. Regulation of TNFalpha production and release in human and mouse keratinocytes and mouse skin after UV-B irradiation. Photodermatol Photoimmunol Photomed. 2000;16:263–270. doi: 10.1034/j.1600-0781.2000.160606.x. [DOI] [PubMed] [Google Scholar]
- Yi X, Tesmer VM, Savre-Train I, Shay JW, Wright WE. Both transcriptional and posttranscriptional mechanisms regulate human telomerase template RNA levels. Mol Cell Biol. 1999;19:3989–3997. doi: 10.1128/mcb.19.6.3989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang L, Wang B, Shinder GA, Shivji GM, Mak TW, Sauder DN. TNF receptor p55 plays a pivotal role in murine keratinocyte apoptosis induced by ultraviolet B irradiation. J Immunol. 1999;162:1440–1447. [PubMed] [Google Scholar]


