Abstract
ETV6-RUNX1 fusion is the most common genetic aberration in childhood acute lymphoblastic leukemia (ALL). To evaluate whether outcomes for this drug-sensitive leukemia are improved by contemporary risk-directed therapy, we studied clinical features, response and adverse events of 168 children with newly diagnosed ETV6-RUNX1-positive ALL on St Jude Total Therapy studies XIIIA (N=36), XIIIB (N=38) and XV (N=94). Results were compared to 494 ETV6-RUNX1-negative B-precursor ALL patients. ETV6-RUNX1 was associated with age 1-9 years, pre-treatment classification as low-risk and lower levels of minimal residual disease (MRD) on day 19 of therapy (p<0.001). Event-free survival (EFS) or overall survival (OS) did not differ between patients with or without ETV6-RUNX1 in Total XIIIA or XIIIB. By contrast, in Total XV, patients with ETV6-RUNX1 had significantly better EFS (p=0.04; 5-year estimate, 96.8±2.4% versus 88.3±2.5%) and OS (p=0.04; 98.9±1.4% versus 93.7±1.8%) than those without ETV6-RUNX1. Within the ETV6-RUNX1 group, the only significant prognostic factor associated with higher OS was the treatment protocol Total XV (versus XIIIA or XIIIB) (p=0.01). Thus, the MRD-guided treatment schema including intensive asparaginase and high-dose methotrexate in the Total XV study produced significantly better outcomes than previous regimens and demonstrated that nearly all children with ETV6-RUNX1 ALL can be cured.
Keywords: leukemia, ETV6-RUNX1, TEL-AML1
Introduction
The t(12;21) (p13.1;q22) translocation causes the fusion of the ETS variant 6 (ETV6) and Runt-related transcription factor 1 (RUNX1) genes (formerly TEL and AML1 respectively). It is the most common genetic abnormality in childhood acute lymphoblastic leukemia (ALL), occurring in approximately 25% of cases with a precursor-B phenotype.(1) ETV6-RUNX1-positive ALL is thought to arise pre-natally and may be preceded by a pre-leukemic phase.(2) The presence of ETV6-RUNX1 alters differentiation and enhances self renewal of hematopoietic progenitor cells, particularly of B-lineage.(3) The expression of ETV6-RUNX1 in human cord blood progenitor cells reportedly caused the expansion of a candidate preleukemic population that had a growth advantage in the presence of transforming growth factor-beta.(4) A second hit, commonly a deletion of the non-translocated ETV6 gene, is often present at leukemic transformation,(5) but additional genomic alterations have been uncovered by high-resolution single nucleotide polymorphism arrays.(6) ETV6-RUNX1-positive ALL cells have distinct biologic features and are reported to have an increased in vitro sensitivity to anti-leukemic drugs such as L-asparaginase, doxorubicin, etoposide and dexamethasone compared to leukemic cells of other cytogenetic subtypes.(7, 8)
Since its identification in 1995,(9) the presence of ETV6-RUNX1 has been associated with a relatively low rate of relapse in multiple studies.(10-12) Moreover, relapses tend to occur late and have a better salvage rate than other ALL subtypes.(13) A Children's Oncology Group (COG) study indicated that the presence of ETV6-RUNX1 was an independent predictor of favorable outcome.(10) However, in a study from the Dana Farber Cancer Institute (DFCI) Consortium, ETV6-RUNX1 status was not an independent prognostic factor after accounting for age, initial leukocyte count and treatment group.(14) Thus, it is not clear whether the ETV6-RUNX1 fusion has independent prognostic significance in the context of current risk-adapted therapy and whether the outcome of children with ETV6-RUNX1-positive ALL can be further improved by contemporary therapeutic strategies. We addressed these outstanding questions and determined the relation between ETV6-RUNX1 and presenting clinical and biologic features, early treatment response and clinical outcome among children with B-precursor ALL treated on three successive Total Therapy studies.
Materials and Methods
Patients
From 1991 to 2007, 763 children with B-precursor ALL were enrolled on three institutional protocols for newly diagnosed ALL: Total Therapy studies XIIIA,(15) XIIIB(16) and XV.(17) The Total XIV study (August 1998 to July 1999) terminated early after accrual of 39 patients with B-precursor ALL (including 7 with the ETV6-RUNX1 fusion). The low accrual in Total XIV precludes meaningful analyses, and patients on this study are not included in this report. ETV6-RUNX1 status was not evaluated in 101 of the 763 patients (13%) with B-precursor ALL, the majority of whom were treated in the two earlier Total Therapy studies (27 on XIIIA, 55 on XIIIB, and 19 on XV). These patients did not differ with regard to age, race, presenting leukocyte count, risk group allocation, minimal residual disease (MRD) levels after remission induction or outcome from the 662 patients who were evaluated for ETV6-RUNX1 status (Supplementary table S1, online only). There was a slight female preponderance and a higher number of patients with central nervous system (CNS) disease among patients not evaluated for the fusion.
The treatment protocols were approved by the institutional review board of St Jude Children's Research Hospital, Memphis, TN and Cook Children's Medical Center, Fort Worth, TX (Total XV). Informed consent was obtained from the parent or guardian and assent obtained from the patient when appropriate.
Diagnostic tests
The diagnosis of ALL and lineage discrimination was made by morphology and immunophenotypic analyses. Genetic subtypes were identified by conventional cytogenetics, fluorescent in situ hybridization and real-time polymerase chain reaction as previously described.(18) MRD was measured by flow cytometric analysis of leukemia-associated markers or polymerase chain reaction of antigen receptor genes at protocol defined time-points as previously described.(17, 19)
Therapy
Details of the therapeutic protocols have been described previously.(15-17) Table S2 (supplement, online only) lists the key differences in risk stratification and treatment in the 3 studies. Briefly, in Total XIIIA (1991-1994), in addition to early intensification of systemic chemotherapy for all patients, intrathecal therapy was intensified for patients with higher risk ALL and those with any blasts in the cerebrospinal fluid.(15) In Total XIIIB (1994-1998), a detailed risk stratification scheme was used, resulting in more patients being treated on the lower-risk arm than in Total XIIIA.(16) Dexamethasone was used instead of prednisone post-remission to improve systemic and CNS disease control.(20) Asparaginase was not given during continuation therapy due to the increased incidence of secondary acute myeloid leukemia noted when it was used in combination with etoposide.(21) In Total XV (2000-2007), risk stratification was further refined by including MRD measurements on day 19 and at the end of remission induction therapy (Day 46).(17) Patients with the ETV6-RUNX1 fusion or hyperdiploidy without CNS or testicular disease and a satisfactory early MRD decline (<1% on day 19 and <0.01% on day 46) were classified as being low-risk for relapse regardless of age and leukocyte count. The dose and intensity of asparaginase, anthracyclines and high-dose methotrexate differed by risk group. Prophylactic CNS radiation was not used.
Statistical analyses
To compare differences in clinical features between ETV6-RUNX1-positive and negative patients, the exact chi-square and Fisher exact test were used. Overall survival (OS) and event free survival (EFS) were estimated by the Kaplan-Meier method and compared by the Mantel-Haenszel test(22). Cox proportional hazard regression models were used to identify independent prognostic factors.
Results
Patient characteristics
The ETV6-RUNX1 fusion was detected in 168 (25.4%) of 662 evaluable patients with B-precursor ALL. Thirty-six patients were enrolled in Total XIIIA, 38 in Total XIIIB and 94 in Total XV. As shown in Table 1, there was no association between the presence of ETV6-RUNX1 and gender, race or presenting leukocyte count. However, ETV6-RUNX1 was significantly related with favorable age (1-9 years; p<0.001), CNS1 status (no blasts in cerebrospinal fluid; p=0.0002) and low-risk allocation (p<0.001). Of all patients assigned to the lower-risk arm, 34.9% were ETV6-RUNX1-positive versus 16.6% of those stratified to the standard and high-risk arms. In the most recent study, Total XV, patients with ETV6-RUNX1-positive ALL without CNS or testicular involvement who demonstrated a very good early MRD reduction on therapy were treated on the lower-risk arm of the trial regardless of age and presenting leukocyte count. Thus, less intensive therapy was given to the majority (89.4%) of ETV6-RUNX1-positive patients versus 46.8% of ETV6-RUNX1-negative patients.
Table 1. Presenting features and early response according to ETV6-RUNX1 status in patients with B-precursor ALL.
| Clinical Features | All patients [N (%)] N=662 | ETV6-RUNX1 positive [N (%)] N=168 | ETV6-RUNX1 negative [N (%)] N=494 | P-value | |
|---|---|---|---|---|---|
| Age Groups | < 1 year | 12 (1.8) | 0 (0.0) | 12 (2.4) | <.0001 |
| 1-10 years | 498 (75.2) | 157 (93.5) | 341 (69.0) | ||
| > 10 years | 152 (23.0) | 11 (6.5) | 141 (28.5) | ||
| Initial WBC | <50 ×109/L | 527 (79.6) | 137 (81.5) | 390 (79.0) | 0.51 |
| ≥50 ×109/L | 135 (20.4) | 31 (18.5) | 104 (21.0) | ||
| Race | White | 529 (79.9) | 128 (76.2) | 401 (81.2) | 0.22 |
| Black | 97 (14.7) | 27 (16.1) | 70 (14.2) | ||
| Other | 36 (5.4) | 13 (7.7) | 23 (4.7) | ||
| Gender | Male | 361 (54.5) | 88 (52.4) | 273 (54.9) | 0.53 |
| Female | 301 (45.5) | 80 (47.6) | 221 (45.1) | ||
| NCI/Rome Risk group | Standard risk | 401 (60.6) | 127 (75.6) | 274 (55.5) | <.0001 |
| High risk | 261 (39.4) | 41 (24.4) | 220 (44.5) | ||
| SJ Risk Group | Low risk | 318 (48.0) | 111 (66.1) | 207 (41.9) | <.0001 |
| Standard/High risk | 344 (52.0) | 57 (33.9) | 287 (58.1) | ||
| CNS status | CNS1 | 472 (71.3) | 141 (83.9) | 331 (67.0) | 0.0002 |
| CNS2 | 141 (21.3) | 20 (11.9) | 121 (24.5) | ||
| CNS3 | 9 (1.4) | 0 (0.0) | 9 (1.8) | ||
| Traumatic with blasts | 40 (6.0) | 7 (4.2) | 33 (6.7) | ||
| MRD day 19 | <0.01% | 199 (42.9) | 63 (59.4) | 136 (38.0) | <.0001 |
| ≥0.01 to <1% | 179 (38.6) | 39 (36.8) | 140 (39.1) | ||
| ≥1% | 86 (18.5) | 4 (3.8) | 82 (22.9) | ||
| MRD day 46 | <0.01% | 376 (80.2) | 97 (88.2) | 279 (77.7) | 0.01 |
| ≥0.01 to <1% | 77 (16.4) | 13 (11.8) | 64 (17.8) | ||
| ≥1% | 16 (3.4) | 0 (0.0) | 16 (4.5) | ||
| Treatment protocol | Total XIIIA | 114 (17.2) | 36 (21.4) | 78 (15.8) | 0.19 |
| Total XIIIB | 144 (21.8) | 38 (22.6) | 106 (21.4) | ||
| Total XV | 404 (61.0) | 94 (55.9) | 310 (62.8) | ||
WBC: white blood cell; NCI: National Cancer Institute; SJ: St Jude; CNS: central nervous system; MRD: Minimal residual disease
Response and outcome
MRD measurements on day 19 of induction therapy were available for 464 patients (67 from Total XIIIA/B and 397 from Total XV) and on Day 46 for 469 patients (69 from Total XIIIA/B and 400 from Total XV). Patients with the ETV6-RUNX1 fusion had significantly lower rates of MRD positivity than ETV6-RUNX1-negative patients at both time points (Table 1). None of the ETV6-RUNX1-positive patients had MRD ≥1% at the end of remission induction (day 46) compared to 16 ETV6-RUNX1-negative patients (4.5%). Nine of the 94 patients with the ETV6-RUNX1 fusion in Total XV switched from the provisional low-risk arm to the standard-risk arm of the trial because of ≥1% MRD on day 19 or ≥0.01% MRD on day 46. Among 310 ETV6-RUNX1-negative patients with B-precursor ALL, 27 patients switched from low-risk to standard-risk, 1 from low-risk to high-risk and 10 from standard-risk to high risk (p=0.33).
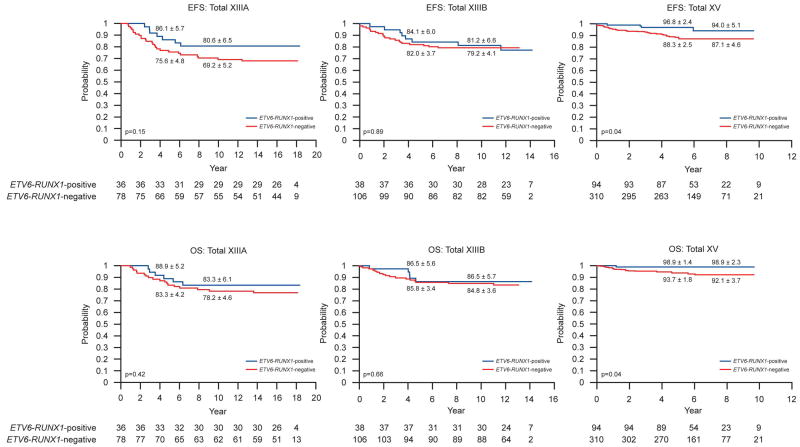
Figure 1 illustrates the EFS and OS for patients in each study. The EFS and OS did not differ between patients with or without ETV6-RUNX1 in Total XIIIA or XIIIB. By contrast, patients with ETV6-RUNX1 had significantly better EFS (p=0.04) and OS (p=0.04) than those without the gene fusion in Total XV: 5-year EFS estimates were 96.8± 2.4% vs. 88.3 ± 2.5%; 5-year OS, 98.9 ± 1.4% vs. 93.7 ± 1.8%.
Figure 1.
Kaplan-Meier estimates of EFS and OS in ETV6-RUNX1-positive versus negative patients in St Jude Total studies XIIIA, XIIIB and XV. Rates at 5 years, 10 years (for Total XIIIA and Total XIIIB) and 8 years (for Total XV) are reported as means ± standard errors.
Treatment failures
A total of 19 patients with ETV6-RUNX1 had major adverse events. The causes of failure and distribution among three studies are shown in Table 2. There were no induction failures or induction deaths among patients with ETV6-RUNX1. Two patients died in complete remission, 1 from pseudomonas sepsis during continuation therapy and 1 from a car accident while off therapy. Six patients developed secondary acute leukemia (4 in Total XIIIA and 2 in Total XIIIB). Eleven patients relapsed, with isolated marrow relapse (n=8), combined marrow and CNS relapse (n=1) and isolated CNS relapse (n=2). Three of these 11 patients (2 with marrow and 1 with isolated CNS relapse) developed a second hematologic relapse 9 months, 2.5 years and 2 years after completing retrieval therapy respectively. Of the 11 relapses, 4 occurred during or within 6 months of completion of therapy while 7 occurred later. The median time to relapse for ETV6-RUNX1-positive patients was 38 months (range 6-139 months) as compared to 34 months (range 3-119 months) in ETV6-RUNX1-negative patients (p=0.13). In the most recent Total XV study, marrow relapses in four patients were the causes of treatment failure (three are alive in complete remission).
Table 2. Details of events for patients in Total XIIIA, XIIIB and XV according to ETV6-RUNX1 status.
| Event | Total XIIIA | Total XIIIB | Total XV | All patients | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ETV6-RUNX1 | Total | ETV6-RUNX1 | Total | ETV6-RUNX1 | Total | ETV6-RUNX1 | Total | |||||
| Positive | Negative | Positive | Negative | Positive | Negative | Positive | Negative | |||||
| Induction death | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 1 |
| No response | 0 | 0 | 0 | 0 | 2 | 2 | 0 | 3 | 3 | 0 | 5 | 6 |
| Hematologic relapse | 1 | 10 | 11 | 3 | 9 | 12 | 4 | 11 | 15 | 8 | 30 | 36 |
| Central nervous system relapse | 0 | 1 | 1 | 2 | 2 | 5 | 0 | 5 | 5 | 2 | 9 | 11 |
| Testicular relapse | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 1 |
| Combined relapse | 1 | 4 | 5 | 0 | 0 | 0 | 0 | 4 | 4 | 1 | 8 | 9 |
| Death in complete remission | 1 | 3 | 4 | 1 | 5 | 6 | 0 | 8 | 8 | 2 | 16 | 18 |
| Secondary leukemia | 4 | 7 | 11 | 2 | 3 | 5 | 0 | 1 | 1 | 6 | 11 | 17 |
| Total | 7 | 25 | 32 | 8 | 22 | 30 | 4 | 34 | 38 | 19 | 81 | 100 |
Prognostic features
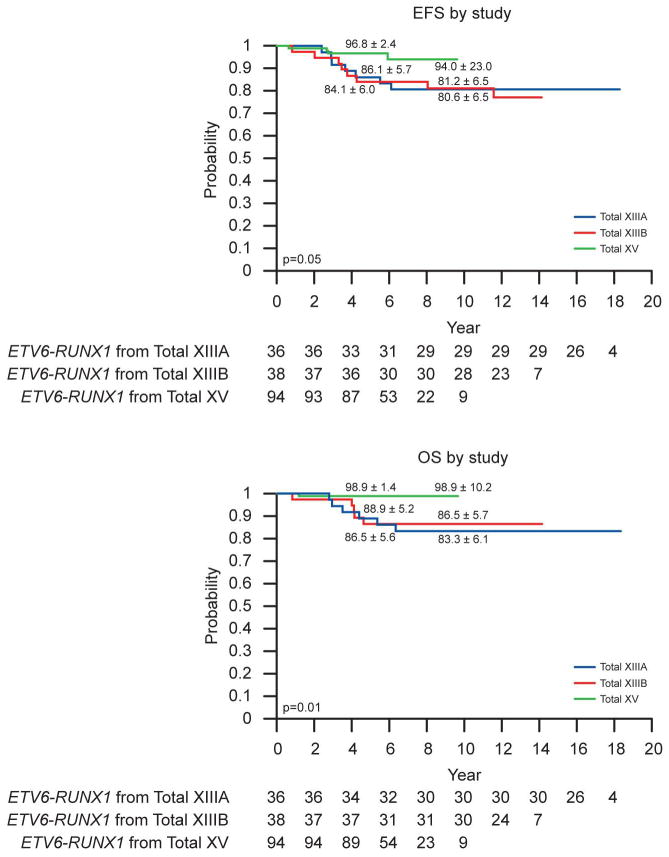
With the exception of treatment, there were no significant predictors of outcome within the ETV6-RUNX1 subset (Table 3). Only therapy received on Total XV (vs. Total XIIIA or XIIIB) was independently associated with an improved outcome: EFS (p=0.05) and OS (p=0.01) (Figure 2).
Table 3. Potential prognostic variables assessed for relation to outcome in patients with ETV6-RUNX1 ALL.
| Features | Total number of patients | 5-year EFS ± SE (%) | p-value | 5-year OS ± SE (%) | p-value | |
|---|---|---|---|---|---|---|
| Age group | 1-10 years | 157 | 91.3 ± 2.6 | 0.10 | 93.6 ± 2.3 | 0.14 |
| > 10 years | 11 | 90.9 ± 9.1 | 90.9 ± 9.1 | |||
| Initial WBC | <50 ×109/L | 137 | 90.8 ± 2.8 | 0.77 | 92.7± 2.5 | 0.89 |
| ≥50 ×109/L | 31 | 92.9 ± 5.4 | 96.8 ± 3.7 | |||
| Race | Other | 40 | 88.4 ± 5.6 | 0.71 | 93.7 ± 4.4 | 0.57 |
| White | 128 | 91.9 ± 2.8 | 93.3 ± 2.5 | |||
| Gender | Male | 88 | 90.6 ± 3.5 | 0.21 | 93.7 ± 2.9 | 0.77 |
| Female | 80 | 92.0 ± 3.6 | 93.2 ± 3.3 | |||
| NCI/Rome Risk Group | Standard risk | 127 | 90.1 ± 3.0 | 0.88 | 92.2 ± 2.7 | 0.96 |
| High risk | 41 | 94.6 ± 4.1 | 97.6 ± 2.8 | |||
| SJ Risk Group | Low risk | 111 | 92.4 ± 3.1 | 0.22 | 94.7 ± 2.6 | 0.13 |
| Standard/High risk | 57 | 89.2 ± 4.3 | 91.0 ± 3.9 | |||
| CNS status | CNS1 | 141 | 91.1± 2.8 | 0.47 | 93.8± 2.3 | 0.95 |
| Others | 27 | 92.3 ± 5.9 | 91.8 ± 6.0 | |||
| MRD day 19 | Negative (<0.01%) | 63 | 96.3 ± 5.9 | 0.08 | 100 ± 0.0 | 0.09 |
| Positive* (≥0.01%) | 43 | 93.0 ± 4.7 | 94.4 ± 4.3 | |||
| MRD day 46 | Negative (<0.01%) | 97 | 95.6 ± 2.6 | 0.30 | 99.0 ± 1.3 | 0.12 |
| Positive* (≥0.01%) | 13 | 92.3 ± 8.1 | 90.9 ± 8.7 | |||
| Treatment protocol | Total XIIIA | 36 | 86.1 ± 5.7 | 0.05 | 88.9 ± 5.2 | 0.01 |
| Total XIIIB | 38 | 84.1 ± 6.0 | 86.5 ± 5.6 | |||
| Total XV | 94 | 96.8 ± 2.4 | 98.9 ± 1.4 | |||
Due to small numbers, data from patients with MRD ≥0.01% to <1% and MRD ≥ 1% were combined. Four of 43 patients had ≥1% MRD on Day 19 and 0/13 on day 46
WBC: white blood cell; NCI: National Cancer Institute; SJ: St Jude; CNS: central nervous system; MRD: Minimal residual disease; EFS: Event-free survival; OS: overall survival
Figure 2.
Kaplan-Meier estimates of EFS and OS in ETV6-RUNX1-positive patients by study. Rates at 5 years and 10 years are reported as means ± standard errors
Discussion
Stepwise refinements in risk classification and treatment strategies have led to improved cure rates for children with ALL. In general, over the past 5 decades, the intensity of treatment regimens has continued to increase, albeit with multiple short and long-term adverse events. As insights are gained into host pharmacogenetics and the heterogeneity of response to chemotherapeutic agents in various leukemia subtypes, and therapy is guided by sensitive and specific methods of MRD monitoring, tailoring the type and intensity of chemotherapeutic agents becomes feasible to improve cure rates and decrease morbidity. The results presented here indicate that the benefits of this approach also extend to a “low-risk” leukemia subtype, ETV6-RUNX1 ALL as demonstrated in the Total XV study which provided greater benefit to children with ETV6-RUNX1-positive ALL than prior treatment protocols. The interpretation of this result should take into consideration the retrospective nature of the study, potential for compounding variables such as improvements in supportive care and the relatively small number of patients in the earlier Total XIII studies that may have introduced a sampling bias. Though the follow-up time is shorter on Total XV and relapses are known to occur later in this particular ALL subtype, the EFS and OS in the first 8 years are significantly better in Total XV compared to those of the previous studies. Modifications that may have contributed to the improved outcome in Total XV include the incorporation of MRD for risk assignment, intensification of asparaginase (compared to Total XIIIB), increased intensity of high-dose methotrexate during the consolidation phase (4 doses of 2.5 to 5 g/m2 versus 2 doses of 2 g/m2 in Total XIII studies) and avoidance of agents with high risk of causing secondary leukemia. Total XV therapy provided improvement in outcome for the entire cohort of patients treated on the study,(17) but it was especially advantageous for patients with ETV6-RUNX1 ALL. With this improved treatment, conventional risk factors such as age and leukocyte count had no prognostic significance among patients with ETV6-RUNX1 ALL treated in Total XV. Thus, In the ETV6-RUNX1-positive subgroup, 5 patients who were 10 years or older, and 17 patients 1-9 years old with leukocyte count 50 × 109/L or greater at presentation received lower-risk therapy in Total XV. Only one of these patients relapsed. She was an 11-year old girl with a presenting leukocyte count of 80 × 109/L who was unable to receive optimal therapy due to invasive fungal infection early during the remission induction phase. She developed an early hematologic relapse and subsequently died with refractory disease.
In Total XV, the 5-year EFS rate of 96.8% for the 94 ETV6-RUNX1 patients is superior to the EFS rates reported by various ALL study groups in the past 5 years (that included more than 50 patients with this genetic abnormality).(10, 14, 23, 24) Investigators from the AIEOP-BFM 2000 study reported an excellent outcome for the subset of ETV6-RUNX1-positive patients (58% of all patients) with negative MRD at days 33 and 78 of therapy (5-year EFS 94.9%), but patients with low level of MRD positivity at either time point (41% of patients) had 5-year EFS of 81.7% and outcome was poorer in the small fraction (1%) of patients with MRD levels ≥ 0.1% on day 78 (5-year EFS, 54.9%). Although the MRD cut-off values and time points of measurement were different in our trials, only a minority of our patients (11.8%) had positive MRD at low levels (≥0.01% to < 1%) on day 46, possibly due to more effective remission induction regimens. MRD did not demonstrate additional prognostic relevance in ETV6-RUNX1 ALL in our study, but this could be due to the fact that MRD results were used to change risk group allocation in 9 ETV6-RUNX1 patients (9.6%) on Total XV from low to standard risk, with consequent administration of more intensive therapy (similarly 12.2% of ETV6-RUNX1-negative patients were switched to a higher-risk arm). The Children's Oncology Group has recently reported excellent outcomes for NCI standard-risk patients(25). Five-year EFS and OS estimates were 93.2 ± 2.2% and 98.6 ± 1.0 % for ETV6-RUNX1-positive patients. Intravenous escalating methotrexate did not provide greater benefit than oral methotrexate for ETV6-RUNX1 patients in this study. It remains to be seen if NCI high-risk patients (approximately 25% of ETV6-RUNX1 patients) also have an equally good outcome in the Children's Oncology Group study. In comparison, for the subgroup of ETV6-RUNX1-positive patients classified as NCI standard-risk in Total XV (N=71), 5-year EFS and OS estimates were 97.1 ± 2.5% and 100% respectively. EFS and OS estimates for the NCI high-risk patients in Total XV (N=23) were 95.7 ± 2.5%. In the DFCI ALL Consortium study 95-01, 5-year EFS for all ETV6-RUNX1-positive patients was 89% compared with 80% for ETV6-RUNX1-negative patients.(14) The 5-year OS was 97%, which is comparable to that of ETV6-RUNX1-positive patients in the Total XV study (98.9%).
The Total XV regimen intensified asparaginase (higher cumulative doses compared to Total XIIIB), dexamethasone (post remission) and vincristine, which are known to be preferentially cytotoxic to blasts bearing the ETV6-RUNX1 fusion.(8, 26) By impairing mesenchymal cell function, vincristine can potentially enhance the efficacy of asparaginase(27, 28), which in turn can increase systemic exposure of dexamethasone(29). In the DFCI study, asparaginase and steroid therapy was intensified as well, but prednisone was used instead of dexamethasone for pre- and post-remission therapy. ETV6-RUNX1-positive lymphoblasts are also known to accumulate lower amounts of methotrexate polyglutamates than ETV6-RUNX1-negative lymphoblasts,(30) thus patients with ETV6-RUNX1 ALL on Total XV might have benefitted from the increased intensity of high-dose methotrexate (2.5 g/m2 for low-risk and 5 g/m2 for standard and high-risk cases) administered during the consolidation phase. In addition, measures such as avoiding cranial radiotherapy and limiting the use of epipodophyllotoxins and alkylating agents in Total XV decreased the chances of adverse events such as secondary malignancies (none versus 6 in the earlier studies). This reduction in secondary malignancies contributed to the improvement of overall outcome of patients on Total XV. Even though the majority of patients with ETV6-RUNX1-positive ALL were treated on the low-risk arm of Total XV, we do not suggest that they receive an overall reduction in therapy. In fact, all patients received two reinduction courses but patients treated in the low-risk arm received lower cumulative doses of cyclophosphamide and anthracyclines, drugs associated with late sequelae. In conclusion, nearly all children with ETV6-RUNX1 ALL can be cured with risk-directed therapy including intensive asparaginase, vincristine, dexamethasone and high-dose methotrexate.
Supplementary Material
Acknowledgments
Supported in part by grants (CA21765, CA60419, CA36401 and GM92666) from the National Institutes of Health and by American Lebanese Syrian Associated Charities (ALSAC).
Footnotes
Conflicts of Interest: The authors declare no competing financial conflicts of interest
Supplementary Information accompanies the paper on the Leukemia website (http://www.nature.com/leu)”
References
- 1.Pui CH, Relling MV, Downing JR. Acute lymphoblastic leukemia. N Engl J Med. 2004 Apr 8;350(15):1535–1548. doi: 10.1056/NEJMra023001. [DOI] [PubMed] [Google Scholar]
- 2.Wiemels JL, Cazzaniga G, Daniotti M, Eden OB, Addison GM, Masera G, et al. Prenatal origin of acute lymphoblastic leukaemia in children. Lancet. 1999 Oct 30;354(9189):1499–1503. doi: 10.1016/s0140-6736(99)09403-9. [DOI] [PubMed] [Google Scholar]
- 3.Morrow M, Horton S, Kioussis D, Brady HJ, Williams O. TEL-AML1 promotes development of specific hematopoietic lineages consistent with preleukemic activity. Blood. 2004 May 15;103(10):3890–3896. doi: 10.1182/blood-2003-10-3695. [DOI] [PubMed] [Google Scholar]
- 4.Ford AM, Palmi C, Bueno C, Hong D, Cardus P, Knight D, et al. The TEL-AML1 leukemia fusion gene dysregulates the TGF-beta pathway in early B lineage progenitor cells. J Clin Invest. 2009 Apr;119(4):826–836. doi: 10.1172/JCI36428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raynaud S, Cave H, Baens M, Bastard C, Cacheux V, Grosgeorge J, et al. The 12;21 translocation involving TEL and deletion of the other TEL allele: two frequently associated alterations found in childhood acute lymphoblastic leukemia. Blood. 1996 Apr 1;87(7):2891–2899. [PubMed] [Google Scholar]
- 6.Mullighan CG, Goorha S, Radtke I, Miller CB, Coustan-Smith E, Dalton JD, et al. Genome-wide analysis of genetic alterations in acute lymphoblastic leukaemia. Nature. 2007 Apr 12;446(7137):758–764. doi: 10.1038/nature05690. [DOI] [PubMed] [Google Scholar]
- 7.Frost BM, Forestier E, Gustafsson G, Nygren P, Hellebostad M, Jonsson OG, et al. Translocation t(12;21) is related to in vitro cellular drug sensitivity to doxorubicin and etoposide in childhood acute lymphoblastic leukemia. Blood. 2004 Oct 15;104(8):2452–2457. doi: 10.1182/blood-2003-12-4426. [DOI] [PubMed] [Google Scholar]
- 8.Ramakers-van Woerden NL, Pieters R, Loonen AH, Hubeek I, van Drunen E, Beverloo HB, et al. TEL/AML1 gene fusion is related to in vitro drug sensitivity for L-asparaginase in childhood acute lymphoblastic leukemia. Blood. 2000 Aug 1;96(3):1094–1099. [PubMed] [Google Scholar]
- 9.Golub TR, Barker GF, Bohlander SK, Hiebert SW, Ward DC, Bray-Ward P, et al. Fusion of the TEL gene on 12p13 to the AML1 gene on 21q22 in acute lymphoblastic leukemia. Proc Natl Acad Sci U S A. 1995 May 23;92(11):4917–4921. doi: 10.1073/pnas.92.11.4917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rubnitz JE, Wichlan D, Devidas M, Shuster J, Linda SB, Kurtzberg J, et al. Prospective analysis of TEL gene rearrangements in childhood acute lymphoblastic leukemia: a Children's Oncology Group study. J Clin Oncol. 2008 May 1;26(13):2186–2191. doi: 10.1200/JCO.2007.14.3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rubnitz JE, Downing JR, Pui CH, Shurtleff SA, Raimondi SC, Evans WE, et al. TEL gene rearrangement in acute lymphoblastic leukemia: a new genetic marker with prognostic significance. J Clin Oncol. 1997 Mar;15(3):1150–1157. doi: 10.1200/JCO.1997.15.3.1150. [DOI] [PubMed] [Google Scholar]
- 12.Borkhardt A, Cazzaniga G, Viehmann S, Valsecchi MG, Ludwig WD, Burci L, et al. Incidence and clinical relevance of TEL/AML1 fusion genes in children with acute lymphoblastic leukemia enrolled in the German and Italian multicenter therapy trials. Associazione Italiana Ematologia Oncologia Pediatrica and the Berlin-Frankfurt-Munster Study Group. Blood. 1997 Jul 15;90(2):571–577. [PubMed] [Google Scholar]
- 13.Forestier E, Heyman M, Andersen MK, Autio K, Blennow E, Borgstrom G, et al. Outcome of ETV6/RUNX1-positive childhood acute lymphoblastic leukaemia in the NOPHO-ALL-1992 protocol: frequent late relapses but good overall survival. Br J Haematol. 2008 Mar;140(6):665–672. doi: 10.1111/j.1365-2141.2008.06980.x. [DOI] [PubMed] [Google Scholar]
- 14.Loh ML, Goldwasser MA, Silverman LB, Poon WM, Vattikuti S, Cardoso A, et al. Prospective analysis of TEL/AML1-positive patients treated on Dana-Farber Cancer Institute Consortium Protocol 95-01. Blood. 2006 Jun 1;107(11):4508–4513. doi: 10.1182/blood-2005-08-3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pui CH, Mahmoud HH, Rivera GK, Hancock ML, Sandlund JT, Behm FG, et al. Early intensification of intrathecal chemotherapy virtually eliminates central nervous system relapse in children with acute lymphoblastic leukemia. Blood. 1998 Jul 15;92(2):411–415. [PubMed] [Google Scholar]
- 16.Pui CH, Sandlund JT, Pei D, Campana D, Rivera GK, Ribeiro RC, et al. Improved outcome for children with acute lymphoblastic leukemia: results of Total Therapy Study XIIIB at St Jude Children's Research Hospital. Blood. 2004 Nov 1;104(9):2690–2696. doi: 10.1182/blood-2004-04-1616. [DOI] [PubMed] [Google Scholar]
- 17.Pui CH, Campana D, Pei D, Bowman WP, Sandlund JT, Kaste SC, et al. Treating childhood acute lymphoblastic leukemia without cranial irradiation. N Engl J Med. 2009 Jun 25;360(26):2730–2741. doi: 10.1056/NEJMoa0900386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mathew S, Shurtleff SA, Raimondi SC. Novel cryptic, complex rearrangements involving ETV6-CBFA2 (TEL-AML1) genes identified by fluorescence in situ hybridization in pediatric patients with acute lymphoblastic leukemia. Genes Chromosomes Cancer. 2001 Oct;32(2):188–193. doi: 10.1002/gcc.1182. [DOI] [PubMed] [Google Scholar]
- 19.Coustan-Smith E, Sancho J, Behm FG, Hancock ML, Razzouk BI, Ribeiro RC, et al. Prognostic importance of measuring early clearance of leukemic cells by flow cytometry in childhood acute lymphoblastic leukemia. Blood. 2002 Jul 1;100(1):52–58. doi: 10.1182/blood-2002-01-0006. [DOI] [PubMed] [Google Scholar]
- 20.Bostrom BC, Sensel MR, Sather HN, Gaynon PS, La MK, Johnston K, et al. Dexamethasone versus prednisone and daily oral versus weekly intravenous mercaptopurine for patients with standard-risk acute lymphoblastic leukemia: a report from the Children's Cancer Group. Blood. 2003 May 15;101(10):3809–3817. doi: 10.1182/blood-2002-08-2454. [DOI] [PubMed] [Google Scholar]
- 21.Pui CH, Relling MV, Behm FG, Hancock ML, Boyett JM, Raimondi SC, et al. L-asparaginase may potentiate the leukemogenic effect of the epipodophyllotoxins. Leukemia. 1995 Oct;9(10):1680–1684. [PubMed] [Google Scholar]
- 22.Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep. 1966 Mar;50(3):163–170. [PubMed] [Google Scholar]
- 23.Conter V, Bartram CR, Valsecchi MG, Schrauder A, Panzer-Grumayer R, Moricke A, et al. Molecular response to treatment redefines all prognostic factors in children and adolescents with B-cell precursor acute lymphoblastic leukemia: results in 3184 patients of the AIEOP-BFM ALL 2000 study. Blood. 2010 Apr 22;115(16):3206–3214. doi: 10.1182/blood-2009-10-248146. [DOI] [PubMed] [Google Scholar]
- 24.Moorman AV, Ensor HM, Richards SM, Chilton L, Schwab C, Kinsey SE, et al. Prognostic effect of chromosomal abnormalities in childhood B-cell precursor acute lymphoblastic leukaemia: results from the UK Medical Research Council ALL97/99 randomised trial. Lancet Oncol. 2010 May;11(5):429–438. doi: 10.1016/S1470-2045(10)70066-8. [DOI] [PubMed] [Google Scholar]
- 25.Matloub Y, Bostrom BC, Hunger SP, Stork LC, Angiolillo A, Sather H, et al. Escalating intravenous methotrexate improves event-free survival in children with standard-risk acute lymphoblastic leukemia: a report from the Children's Oncology Group. Blood. 2011 Jul 14;118(2):243–251. doi: 10.1182/blood-2010-12-322909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krishna Narla R, Navara C, Sarquis M, Uckun FM. Chemosensitivity of TEL-AML1 fusion transcript positive acute lymphoblastic leukemia cells. Leuk Lymphoma. 2001 May;41(5-6):615–623. doi: 10.3109/10428190109060352. [DOI] [PubMed] [Google Scholar]
- 27.Fung KL, Liang RH, Chan GC. Vincristine but not imatinib could suppress mesenchymal niche's support to lymphoid leukemic cells. Leuk Lymphoma. 2010 Mar;51(3):515–522. doi: 10.3109/10428190903406798. [DOI] [PubMed] [Google Scholar]
- 28.Iwamoto S, Mihara K, Downing JR, Pui CH, Campana D. Mesenchymal cells regulate the response of acute lymphoblastic leukemia cells to asparaginase. J Clin Invest. 2007 Apr;117(4):1049–1057. doi: 10.1172/JCI30235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang L, Panetta JC, Cai X, Yang W, Pei D, Cheng C, et al. Asparaginase may influence dexamethasone pharmacokinetics in acute lymphoblastic leukemia. J Clin Oncol. 2008 Apr 20;26(12):1932–1939. doi: 10.1200/JCO.2007.13.8404. [DOI] [PubMed] [Google Scholar]
- 30.Whitehead VM, Payment C, Cooley L, Lauer SJ, Mahoney DH, Shuster JJ, et al. The association of the TEL-AML1 chromosomal translocation with the accumulation of methotrexate polyglutamates in lymphoblasts and with ploidy in childhood B-progenitor cell acute lymphoblastic leukemia: a Pediatric Oncology Group study. Leukemia. 2001 Jul;15(7):1081–1088. doi: 10.1038/sj.leu.2402165. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.