Abstract
The cornea is highly sensitive to ultraviolet B (UVB) light-induced oxidative stress, a process that results in the production of inflammatory mediators which have been implicated in tissue injury. In the present studies, we characterized the inflammatory response of human corneal epithelial cells to UVB (2.5 - 25 mJ/cm2). UVB caused a dose-dependent increase in the generation of reactive oxygen species in the cells. This was associated with increases in mRNA expression of the antioxidants Cu,Zn superoxide dismutase (SOD), Mn-SOD, catalase and heme oxygenase-1 (HO-1), as well as the glutathione S-transferases (GST), GSTA1-2, GSTA3, GSTA4, GSTM1, mGST2. UVB also upregulated expression of the proinflammatory cytokines, IFNγ, IL-1β, TGFβ and TNFα, and enzymes important in prostaglandin (PG) biosynthesis including cyclooxygenase-2 (COX-2) and the PG synthases mPGES-2, PGDS, PGFS and thromboxane synthase, and in leukotriene biosynthesis including 5-lipoxygenase (5-LOX), and the epidermal and platelet forms of 12-LOX and 15-LOX-2. UVB was found to activate JNK and p38 MAP kinases in corneal epithelial cells; ERK1/2 MAP kinase is constitutively active, but its activity was increased following UVB treatment. Inhibition of p38 blocked UVB-induced expression of TNFα, COX-2, PGDS and 15-LOX-2, while JNK inhibition suppressed TNFα and HO-1. These data indicate that UVB modulates corneal epithelial cell expression of antioxidants and proinflammatory mediators by distinct mechanisms. Alterations in expression of these mediators are likely to be important in regulating inflammation and protecting the cornea from UVB-induced oxidative stress.
Keywords: ultraviolet light, ocular, oxidative stress, prostaglandins, leukotrienes
1. Introduction
The higher energy wavelengths of ultraviolet light from sunlight including UVB (290-320 nm) are known to cause oxidative stress and ocular injury [1, 2]. The corneal epithelium serves as a protective barrier, absorbing UVB light and minimizing damage to the interior eye including the lens and retina. However, in excessive amounts, UVB light can injure the cornea, a process that causes aberrant epithelial cell growth and differentiation, as well as cell death via necrosis and apoptosis [3, 4]. This can result in corneal opacities or photokeratitis [5-7]. UVB-induced corneal injury is thought to be initiated by oxidative stress due to the localized generation of reactive oxygen species (ROS) by corneal epithelial cells [8, 9]. ROS, including superoxide anion, hydrogen peroxide and hydroxyl radicals, can damage DNA, resulting in mutations [10]. ROS can also oxidize proteins and lipids, leading to the generation of highly toxic electrophilic species including malondialdehyde and 4-hydroxynonenal, which can initiate inappropriate or altered cellular signal transduction pathways and contribute to toxicity [10].
A critical step in limiting oxidative damage involves upregulation of antioxidants that remove or detoxify ROS [11]. UVB light has been shown to upregulate the antioxidant enzymes, glutathione peroxidase, heme oxygenase-1 (HO-1) and the glutathione S-transferases GST1A-2 and GSTM1 in mouse keratinocytes [12] and glutathione peroxidase, GSTA4 and GSTP1 in human and rat skin [13, 14]. In a rabbit eye model, reduced corneal expression of antioxidants including catalase, glutathione peroxidase and superoxide dismutase (SOD) was observed following UVB light treatment, a process thought to accelerate oxidative stress and tissue injury [15]. UVB-induced oxidative stress in the skin is also associated with the initiation of an inflammatory response characterized by the generation of cytokines, growth factors and lipid-derived eicosanoids [16]. Previous studies in mouse and human keratinocytes and corneal epithelial cells showed that exposure to UVB light stimulated production of proinflammatory cytokines and growth factors including tumor necrosis factor-α (TNF-α), interleukin (IL)-1β, tumor growth factor β (TGFβ) and interferon-γ (IFNγ) [7]. In skin keratinocytes, UVB light increased the release of PGE2 and leukotriene B4, a process associated with upregulation of cyclooxygenase-2 (COX-2) and prostaglandin and leukotriene synthases, enzymes mediating the generation of prostaglandins and leukotrienes [17-19].
At present, little is known about the mechanisms by which corneal epithelial cells respond to UVB light-induced oxidative stress and this represents the focus of the present studies. We found that human corneal epithelial cells were highly responsive to UVB light, which effectively upregulated mRNA expression of proinflammatory cytokines, growth factors and enzymes important in mediating prostaglandin and leukotriene biosynthesis, as well as antioxidants. Moreover, these responses were regulated by mitogen activated protein kinases. Coordinate regulation of the production of inflammatory mediators is likely important in mediating UVB light induced corneal inflammation and tissue injury. Alterations in antioxidants may be a key adaptive mechanism of controlling oxidative stress and protecting against ROS-induced cytotoxicity following exposure to UVB light.
2. Materials and Methods
2.1. Chemicals and reagents
Rabbit polyclonal antibodies to p38, phospho-p38, JNK, phospho-JNK, ERK 1/2, and phospho-ERK 1/2 were purchased from Cell Signaling Technology (Beverly, MA), and horseradish peroxidase-labeled goat anti-rabbit secondary antibodies and detergent-compatible protein assay reagents from Bio-Rad Laboratories (Hercules, CA). M-MLV Reverse Transcriptase was from Promega (Madison, WI) and the Western Lightning enhanced chemiluminescence kit (ECL) from Perkin Elmer Life Sciences, Inc. (Boston, MA). SYBR Green Master Mix and other PCR reagents were from Applied Biosystems (Foster City, CA). SP600125 was purchased from Calbiochem (La Jolla, CA). Cell culture reagents, 2′,7′-dichlorofluorescein-diacetate (DCFH-DA) and keratinocyte growth medium with supplement kits were purchased from Invitrogen Corp (Carlsbad, CA). Tri reagent, SB203580, protease inhibitor cocktail and all other chemicals were Sigma (St. Louis, MO) unless otherwise indicated.
2.2. Cells and UVB light treatments
The human corneal epithelial (HCE) cell line was kindly provided by Kaoru Araki-Sasaki, Kinki Central Hospital (Hyogo, Japan) and grown in keratinocyte serum-free medium (K-SFM), supplemented with fetal bovine serum (5%), gentamicin (0.05 %), epidermal growth factor (0.05 μg/ml) and bovine pituitary extract (0.05 mg/ml). The cells were originally isolated from donor corneas and immortalized using a recombinant SV40-adenovirus [20]. HCE cells were exposed to UVB light using a bank of 2 Westinghouse FS40BL light bulbs, calibrated with an International Light IL-1700 UV-radiometer as previously described [12, 21]. Briefly, cells (2 × 105) were seeded into 6 well culture dishes and allowed to grow 3-4 days until approximately 90% confluent. Cells were then exposed to UVB (2.5 - 25 mJ/cm2) in phosphate-buffered saline (PBS); PBS was then removed and the cells were refed with medium. The times of exposure to UVB light were 30 sec, 1 min, 2 min and 5 min. In control experiments, cells were mock irradiated for 5 min. In some experiments, the p38 MAP kinase inhibitor, SB203580 (10 μM), or the JNK kinase inhibitor, SP600125 (20 μM), was added to the medium and the cells preincubated at 37°C for 3 h prior to UVB treatment. Cell viability following UVB treatment was assessed by measuring cell growth as determined by protein content of the cultures. Low doses of UVB light (2.5-10 mJ/cm2) had no effects of cell growth. At 25 mJ/cm2, we found a 15% inhibition of cell growth.
2.3. Western blotting
Cell lysates were prepared from control and UVB light treated cells using SDS-lysis buffer (10 mM Tris-base and 1% SDS, pH 7.6 supplemented with a protease inhibitor cocktail consisting of 4-(2-aminoethyl)benzenesulfonyl fluoride, aprotinin, bestatin hydrochloride, N-(trans-epoxysuccinyl)-L-leucine 4-guanidinobutylamide, EDTA and leupeptin) as previously described [12]. Protein lysates (20 μg) were separated on 10% SDS-polyacrylamide gels and then transferred to nitrocellulose. After incubation in blocking buffer (5% dry milk Tris-buffered saline with 0.1% Tween 20), the membranes were incubated overnight at 4°C with primary antibodies followed by horseradish peroxidase-conjugated secondary antibodies for 1 hr at room temperature. Protein expression was visualized using ECL reagents.
2.4. Measurement of ROS production
Intracellular hydrogen peroxide production was measured using DCFH-DA and flow cytometry as previously described [22]. Briefly, the cells were incubated with DCFH-DA (5 μM) in PBS for 15 min, washed with PBS, exposed to UVB light (2.5-25 mJ/cm2), and then immediately analyzed on a Beckman Coulter Cytomics FC 500 flow cytometer (Miami, FL). Control cells were mock irradiated. In preliminary experiments we found similar DCF fluorescence in cells mock irradiated for 30 sec, 1 min, 2 min and 5 min. Therefore, we used a 5 min mock irradiation for control experiments. Data were analyzed using Beckman Counter CXP software.
2.5. Real-time PCR
RNA was isolated from the cells using Tri Reagent (Sigma) following the manufacturers protocol. RNA was converted to cDNA using M-MLV reverse transcriptase. The cDNA was diluted 1:10 in RNase-DNase-free water for PCR analysis. For each gene, a standard curve was generated from serial dilutions of cDNA. All values were normalized to β-actin (n = 3). The control was assigned a value of one and changes in gene expression calculated relative to this control. Real-time PCR was performed on an ABI Prism 7900 Sequence Detection System (Life Technolgies, Carlsbad, CA) using 96-well optical reaction plates. SYBR-Green was used for detection of the fluorescent signal and the standard curve method was used for relative quantitative analysis. The primer sequences for the genes were generated using Primer Express software (Life Technologies) and the oligonucleotides were synthesized by Integrated DNA Technologies, Inc. (Coralville, IA). The forward and reverse (5′-3′) primer sequences used are listed in Table 1.
Table 1. Realtime PCR primer sequences.
| Gene | Forward (5′ → 3′) | Reverse (5′ → 3′) |
|---|---|---|
| β-actin | AAAGACCTGTACGCCAACAC | GTCATACTCCTGCTTGCTGAT |
| catalase | CGGAGATTCAACACTGCCAA | GAATGCCCGCACCTGAGTAA |
| COX-1 | GTACCGCAAGAGGTTTGGCA | ACGAGCTCCTGGAAGGAGG |
| COX-2 | GCCTGATGATTGCCCGACT | GCTGGCCCTCGCTTATGATCT |
| cPLA2 | GATTGCATTCTGCACGTGATG | CCACCTGAACCCAATATGGC |
| FLAP | AAGTGGAGCACGAAAGCAGG | CGGTCCTCTGGAAGCTCCTC |
| GSTA1-2 | TTGATGTTCCAGCAAGTGCC | CACCAGCTTCATCCCATCAAT |
| GSTA3 | TTCTGCCCTTATGTCGACCTG | TGATCAAGGCAATCTTGGCAT |
| GSTA4 | GCTCCACTATCCCAACGGAA | AAAACCCATCTCACGGACTCC |
| GSTM1 | AGCAACGCCATCTTGTGCTAC | CTCCCCACACAGGTTGTGC |
| GSTP1 | CTATGGGAAGGACCAGCAGG | CCGTCATTCACCATGTCCAC |
| HO-1 | GCTCAAAAAGATTGCCCAGA | GCGGTAGAGCTGCTTGAACT |
| IL-1β | CACTACAGCAAGGGCTTCAGG | GTCCATGGCCACAACAACTG |
| IFNγ | GGCTTTTCAGCTCTGCATCG | TCCTGGCAGTAACAGCCAAGA |
| 5-LOX | TCGAGTTCCCCTGCTACCG | TCAGGACAACCTCGACATCG |
| 12-LOX-epi | ACCGACCTCTTGTCGGGAAC | CCCCACAATGGTCAGTGAGAT |
| 12-LOX-plt | AGATGGAGCCCAATGGGAAG | GAGGCTGAATCTGGATGACCA |
| 15-LOX-1 | GAGATCACTGAAATCGGGCTG | GACAGGAAACCCTCGGTCCT |
| 15-LOX-2 | TGATATTCACCTGCTCCGCC | CAAACTGCCCTGCACTGACA |
| LTA4 hydrolase | TGAAGTTTACCCGGCCCTTA | GGATTTGTCAAAGGCAGCA |
| LTC4 synthase | AGTACTTCCCGCTGTTCCTCG | GAAAGAAGATGCCGGCGAC |
| mGST1 | GCCCACCTGAATGACCTTGA | GAGGCCAATTCCAAGAAATGG |
| mGST2 | GAACTCGATCCTGCTGGCTG | CTTTGCTGACAGGCCGAGA |
| mGST3 | CCACCCGCGTATAGCTTCTG | ACTCGTCCAACAATCCAGGC |
| mPGES-1 | CACCGGAACGACATGGAGAC | GACGAAGCCCAGGAAAAGG |
| mPGES-2 | GATGTACGTGGTGGCCATCA | CTCTTCTTCCGCAGCCTCAC |
| PGDS | CAAGGCTGGTGACTTTACGGA | AGTTAGCGACGGCAGGAATG |
| PGFS | CTCTGTACCACCTGGGAGGC | GGCCAATCCTGCATCCTTAC |
| PGIS | AGAGTATCCTTTGGCAAGCGG | GGAGAGTGGTCGTCTGCGAG |
| Cu,Zn-SOD | GTCGTAGTCTCCTGCAGCGTC | CTGGTTCCGAGGACTGCAA |
| Mn-SOD | TCTGGACAAACCTCAGCCCT | GCAACTCCCCTTTGGGTTCT |
| TGFβ | GAAGGGAGACAATCGCTTTAGC | TGTAGACTCCTTCCCGGTTGAG |
| TNFα | CCTGCCCCAATCCCTTTATT | CCCTAAGCCCCCAATTCTCT |
| TXAS | CATCCATAATGGTCCCACTGG | CGTCTCGGTTCTTATTGGGC |
2.6. Statistical analysis
Data were evaluated using the Student's t-test and differences were considered statistically significant at p < 0.05.
3. Results
3.1. UVB light-induced oxidative stress in HCE cells
In initial studies, we determined if UVB light causes oxidative stress in corneal epithelial cells by quantifying its ability to generate intracellular hydrogen peroxide. Using the hydroperoxy-sensitive probe DCFH-DA in conjunction with flow cytometry, we found that HCE cells generated low basal levels of hydrogen peroxide (Fig. 1). UVB (2.5 - 25 mJ/cm2) treatment resulted in a dose-dependent increase in hydrogen peroxide production; a 20-30-fold increase in hydrogen peroxide production was evident after exposure to the maximal UVB dose tested.
Figure 1. UVB induces oxidative stress in human corneal epithelial cells.
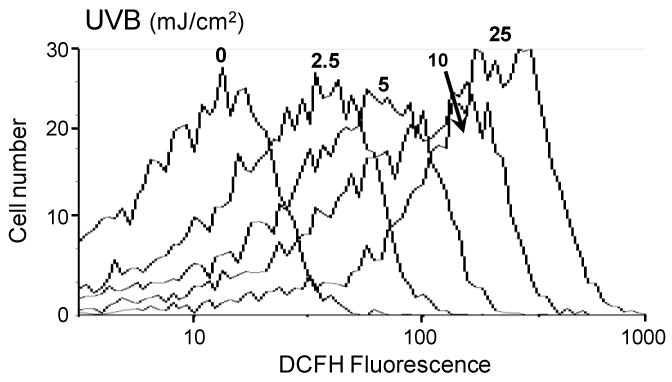
HCE cells were incubated with DCFH-DA (5 μM) for 15 min. Cells were then rinsed with PBS, exposed to 2.5, 5, 10 or 25 mJ/cm2 UVB light and analyzed by flow cytometry for DCF fluorescence. Control cells were treated with DCFH-DA and mock irradiated. The panel shows that UVB light increased DCF fluorescence intensity which is directly proportional to hydrogen peroxide production. Increases in fluorescence intensity are presented on a log scale.
3.2. UVB light alters mRNA expression of antioxidant enzymes
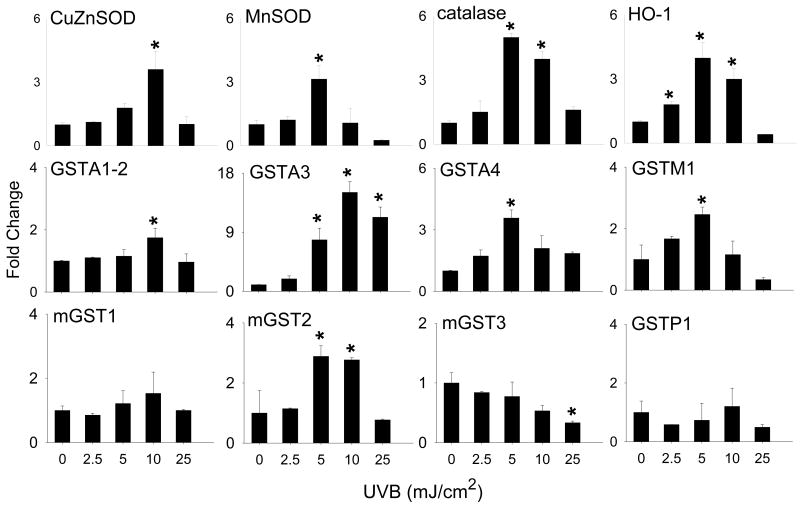
We next examined the effects of UVB light on expression of primary antioxidant enzymes. UVB light treatment of HCE cells resulted in increased mRNA expression of Cu,Zn-SOD, Mn-SOD, catalase and HO-1 (Fig. 2). For each of these genes, maximal effects were observed at 5-10 mJ/cm2. At the highest dose of UVB (25 mJ/cm2), mRNA expression was reduced to near basal levels. The glutathione S-transferases (GST) consists of a class of secondary intracellular antioxidant enzymes that function to eliminate oxidized macromolecules and xenobiotics via glutathione conjugation and include both cytosolic (GSTA, GSTM and GSTP families) and microsomal (mGST1, mGST2 and mGST3) enzymes [23-26]. Significant increases in mRNA expression of the GSTA family including GSTA1-2, GSTA3 and GSTA4, as well as GSTM1 and mGST2 were noted, in HCE cells following UVB light treatment (Fig. 2). In contrast, a 2-fold decrease in mGST3 expression was evident after exposure of the cells to 25 mJ/cm2 UVB light. No major changes were observed in mRNA expression for GSTP1 or mGST1 at any dose of UVB tested.
Figure 2. Effects of UVB light on antioxidant enzyme gene expression.
HCE cells were exposed to UVB (2.5 - 25 mJ/cm2) or control. After 24 hr, RNA was extracted and analyzed by real-time PCR. Data are presented as fold change in gene expression relative to controls. Each bar is the mean ± SE (n = 3). *Significantly different from control (p < 0.05).
3.3. UVB light alters mRNA expression of enzymes generating eicosanoids
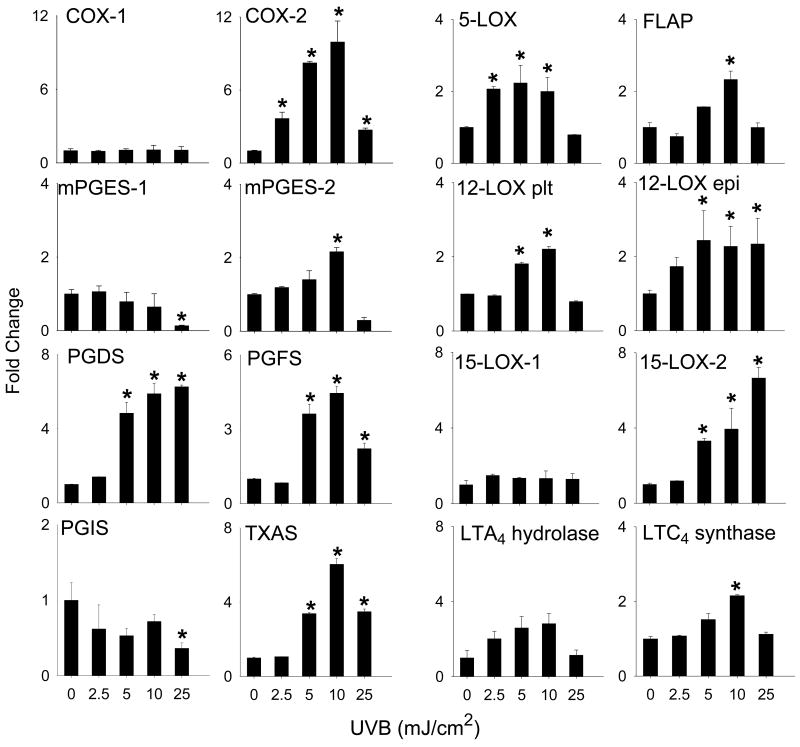
Arachidonic acid, released from the phospholipid membrane by phospholipase A2, is oxidized by cyclooxygenase (COX) and lipoxygenase (LOX) enzymes to generate prostaglandins (PG) and leukotrienes, respectively [19]. UVB light treatment of HCE cells resulted in a dose-related increase in expression of mRNA for COX-2, microsomal PGE2 synthase (mPGES-2), PGD2 synthase (PGDS), PGF2 synthase (PGFS), and thromboxane synthase (TXAS) (Fig. 3). Maximal increases in expression of these genes were observed at 10 mJ/cm2 UVB. In contrast, at the highest dose of UVB tested, a 2-fold decrease in mPGES-1 and PGI2 synthase (PGIS) expression was evident (Fig. 3). UVB light had no effect on COX-1 mRNA expression.
Figure 3. Effects of UVB light on expression of the eicosanoid biosynthetic enzymes.
HCE cells were exposed to UVB (2.5 - 25 mJ/cm2) or control. After 24 hr, RNA was extracted and analyzed by real-time PCR. Data are presented as fold change in gene expression relative to controls. Each bar is the mean ± SE (n = 3). *Significantly different from control (p < 0.05).
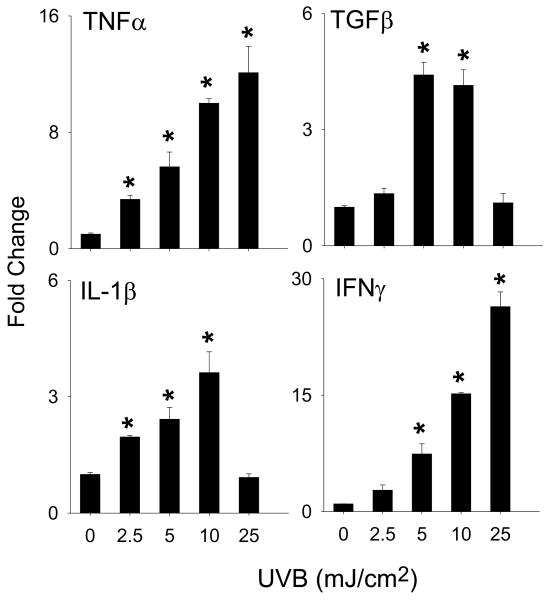
We next determined if UVB light altered expression of the LOX family of enzymes. Increases in mRNA expression for 15-LOX-2, LTC4 synthase, 12-LOX, 5-LOX, FLAP and LTA4 hydrolase were observed in HCE cells after UVB treatment (Fig. 3). While increases in expression of LTC4 synthase, 5-LOX, FLAP and LTA4 hydrolase were evident after exposure to 5-10 mJ/cm2 UVB, 15-LOX-2 and epidermal type 12-LOX increased continuously over the dose range tested (2.5 to 25 mJ/cm2) (Fig. 3). Cytokine expression was also analyzed as a marker of UVB-induced inflammation. UVB caused a dose-dependent increase in IFNγ, TNFα, TGFβ and IL-1β mRNA expression with maximal effects at 10-25 mJ/ cm2 (Fig. 4).
Figure 4. Effects of UVB light on cytokine expression.
HCE cells were exposed to UVB (2.5-25 mJ/cm2) or control. After 24 h RNA was extracted and analyzed by real-time PCR. Data are presented as fold change in gene expression relative to controls. Each bar is the mean ± SE (n = 3). *Significantly different from control (p < 0.05).
3.4. Role of MAP kinase signaling in regulating expression of antioxidants and inflammatory proteins
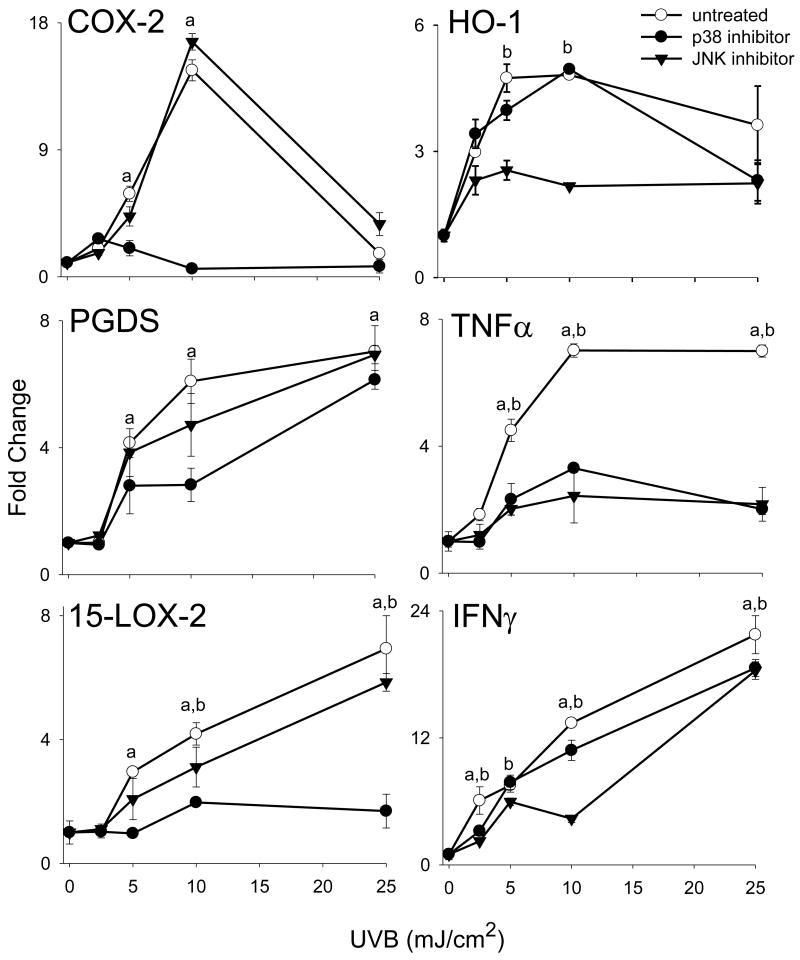
In our next series of studies, we analyzed the role of MAP kinases in UVB-induced alterations in gene expression. UVB treatment (10-25 mJ/cm2) resulted in a dose-dependent activation of JNK and p38 MAP kinase expression in HCE cells, as measured by increases in the phosphorylated forms of the enzymes (Fig. 5). In contrast, relatively small increases in activated ERK1/2 were detected at doses of (2.5-15 mJ/cm2 UVB (Fig. 5). HCE cells also constitutively expressed activated phosphorylated ERK1/2 kinases, but not JNK or p38 kinase. To determine if JNK and p38 MAP kinases mediated UVB-induced changes in mRNA expression in HCE cells, we used specific enzyme inhibitors. Treatment of the cells with the p38 MAP kinase inhibitor, SB203580, markedly suppressed UVB-induced expression of COX-2, 15-LOX-2 and TNFα while expression of PGDS and IFN-γ was decreased. In contrast, inhibition of JNK kinase using SP600125 markedly suppressed expression of HO-1 and TNFα and decreased PGDS, 15-LOX-2 and IFN-γ (Fig. 6).
Figure 5. Effects of UVB on MAP kinase expression in HCE cells.
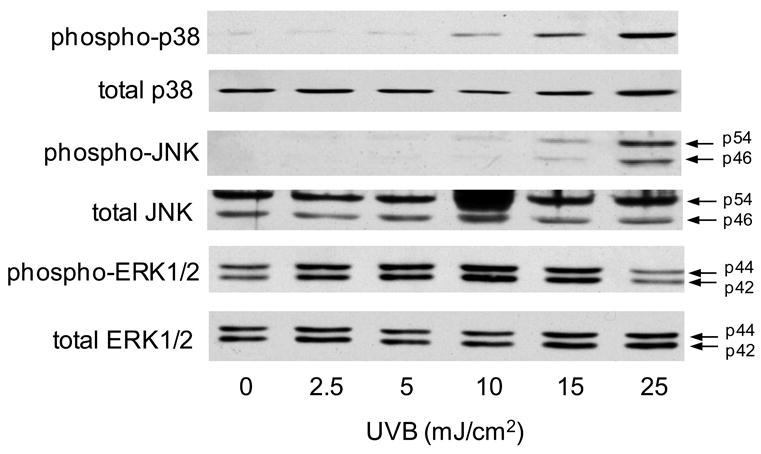
HCE cells were exposed to UVB (2.5-25 mJ/cm2) or control. After 15 min, cells were lysed and analyzed for total and phosphorylated p38, JNK, and ERK1/2 MAP kinases by Western blotting. One representative blot from three experiments is shown.
Figure 6. Effects of p38 and JNK MAP kinase inhibition on UVB-induced gene expression.
Cells were incubated with SB203580 (10 μM), a p38 MAP kinase inhibitor, or SP600125 (20 μM), a JNK MAP kinase inhibitor, for 3 hr and then exposed to UVB (2.5 - 25 mJ/cm2) or control. After 24 h, RNA was extracted and analyzed by real-time PCR. Data are presented as fold change in gene expression relative to untreated controls. Differences were considered statistically significant at p < 0.05: ap38 kinase inhibitor treatment versus control, bJNK kinase inhibitor treatment versus control.
4. Discussion
ROS are key mediators of many of the cytotoxic effects of UVB light in epithelial cells [27, 28]. ROS generated by UVB can trigger oxidative stress including lipid peroxidation, protein oxidation and DNA damage, as well as cytokine and growth factor signaling leading to changes in gene expression [29, 30]. Consistent with previous studies in mouse and human keratinocytes [12, 31], we found that UVB light readily stimulates the formation of intracellular ROS in human corneal epithelial cells, as measured by the production of hydrogen peroxide. These findings are in accord with reports that UVB alters expression of markers of oxidative stress in human and rabbit corneal epithelial cells, and in rabbit corneal tissue [32-34].
We also found that UVB upregulates expression of both primary and secondary antioxidant enzymes, which most likely reflects an adaptive cytoprotective response to oxidative stress [35, 36]. Thus, UVB increased mRNA expression of Mn-SOD and Cu,Zn-SOD, HO-1 and catalase. Mn-SOD is a mitochondrial-associated enzyme, while Cu,Zn-SOD is cytosolic. Coordinate regulation of these antioxidants, as well as catalase, is presumably key in protecting different intracellular compartments in corneal epithelial cells from UVB-induced increases in formation of superoxide anion and hydrogen peroxide, respectively [37]. We have previously shown that mRNA expression for catalase and Cu,Zn-SOD are not affected by UVB in mouse keratinocytes, while Mn-SOD is suppressed [12]. Differences in expression of these enzymes in cornea and skin may be due to unique characteristics of epithelial cells in different tissues. Increased expression of these enzymes in the cornea may be required to protect against ROS-induced damage to the lens and/or retina at higher fluences of UVB [9, 15]. HO-1, the rate-limiting enzyme in the catabolism of the prooxidant heme, functions as an antioxidant/antiinflammatory protein and is induced in many cells by oxidative stress [38]. Our findings of increased mRNA expression for HO-1 in corneal epithelial cells is consistent with earlier studies showing that UVB induces HO-1 mRNA and protein in keratinocytes [12]. In the rabbit cornea, HO-1 has been shown to attenuate corneal inflammation and accelerate wound healing after injury. Increased expression of HO-1 in human corneal epithelial cells following UVB treatment may be important not only in limiting oxidative stress and photokeratitis, but also in mitigating UVB-induced tissue injury [39].
The GST enzymes are secondary antioxidants that conjugate reduced glutathione to electrophilic compounds, decreasing their potential for inducing cytotoxicity [23]. Preferred substrates have been identified for different classes of GSTs. For example, GSTP1 and the GSTA family of enzymes are known to detoxify lipid peroxidation products, a reaction important in breaking radical-forming chain reactions, while GSTM1 protects against protein oxidation [23-25]. Total GST activity has been reported to increase in the skin following UVB treatment [23, 40, 41]. UVB also increases mRNA expression of GSTA1-2 and GSTA4 in primary cultures of undifferentiated and differentiated mouse keratinocytes [12]. Similarly, the present studies demonstrate UVB upregulated mRNA's for these GSTs, as well as for GSTA3 and GSTM1 in corneal epithelial cells. Microsomal GSTs consist of a class of enzymes that function to protect against microsomal lipid peroxidation. UVB exposure resulted in increased expression of mGST2 which may play a role in mitigating UVB-induced oxidative stress in the microsomal compartment of the corneal cells. Consistent with earlier findings in mouse keratinocytes [12], we found that mGST3 decreased following UVB treatment of corneal epithelial cells. mGST3 has been reported to mediate glutathione conjugation to leukotriene A4, a reaction important in generating proinflammatory leukotrienes (see further below) [24]. In both corneal epithelial cells and keratinocytes, decreases in mGST3 may limit production of proinflammatory lipid mediators and contribute to the resolution of inflammation following UVB treatment.
As highly active arachidonic acid-derived mediators, prostaglandins are known to regulate inflammation and evidence suggests that they may contribute to UVB-induced tissue injury [42]. For example, in human skin and rabbit eyes, toxic doses of UVB light stimulate production of PGE2, PGF2α and PGD2 [18, 43]. Moreover, in both of these tissues, non-specific inhibitors of COX-2 reduce prostaglandin biosynthesis and prevent tissue injury. Hence, treatment of human skin with diclofenac, which inhibits UVB light-induced erythema, also reduces dermal levels of PGE2, while treatment of rabbits with indomethacin, which reduces UVB light-induced corneal injury, suppresses ocular levels of PGE2 and PGF2α [6, 44]. Our studies demonstrate that UVB light induces mRNA for enzymes that generate prostaglandin biosynthesis in corneal epithelial cells including COX-2, mPGES-2, PGDS and PGFS and are consistent with earlier studies on the effects of UVB on rabbit ocular tissue [18, 43]. Previous studies have shown that both rat and rabbit corneas have the capacity to synthesize thromboxanes [45, 46]. Our data show that UVB light can upregulate mRNA expression of thromboxane synthase. Taken together, these data suggest that arachidonic acid-derived thromboxanes as well as prostaglandins may be important mediators of UVB light toxicity in the cornea.
Leukotrienes and their metabolites are pleiotropic autocoids that regulate inflammation and wound healing in the cornea [47, 48]. We found that UVB light treatment of corneal epithelial cells increased expression of mRNA for 5-LOX and its activator protein FLAP, an important pathway for generating 5-hydroperoxyeicosatetraenoic acid and its dehydration product LTA4 from arachidonic acid. LTC4 synthase is key for the generation of the cysteinyl leukotrienes (LTC4, LTD4 and LTE4) while LTA4 hydrolase mediates the conversion of LTA4 to LTB4. We found that UVB light also upregulates LTC4 synthase, an enzyme important in mediating LTC4 biosynthesis. Earlier work has shown that corneal inflammation is associated with increased production of LTB4 [49]. Both LTB4 and the cysteinyl leukotrienes are proinflammatory and function by binding to specific G protein coupled receptors on target cells. The actions of the leukotrienes in inflammation are mutifactorial. For example, both LTB4 and the cysteinyl leukotrienes stimulate chemokine production and endothelial cell-leukocyte adhesion, while LTB4 is a potent neutrophil chemoattractant [48]. Active neutrophil infiltration into the cornea is known to be associated with infection and injury and increased production of LTB4 may be an important mechanism mediating this process.
UVB light was also found to increase cornea epithelial mRNA expression for the platelet and epithelial forms of 12-LOX, as well as 15-LOX-2, enzymes mediating the generation of anti-inflammatory and protective lipid mediators, including the lipoxins and the docosahexaenoic acid-derived protectins and resolvins [50, 51]. Recent studies have shown that lipid mediators such as lipoxin A4 and the neuroprotectin D1 are generated in the cornea where they inhibit chemokine formation and promote corneal wound healing [52, 53]. The importance of these enzymes in corneal epithelial function is also evident by the findings that mice deficient in 12/15-LOX synthesize reduced amounts of lipoxin A4, and exhibit defects in corneal reepithelialization and neutrophil recruitment following wounding [52]. The precise mechanisms by which lipoxin A4 and neuroprotectin D1 promote wound healing in the cornea is not known and may involve enhanced epithelial cell proliferation and/or stimulation of pro-survival repair pathways which lead to inhibition of apoptosis [52, 54].
It is well recognized that MAP kinase signaling cascades regulate the action of many growth factors and inflammatory mediators [55]. Earlier studies demonstrated that UVB is a potent activator of MAP kinase signaling, a process thought to be due to direct or indirect activation of growth factor and/or cytokine receptors and subsequent down-stream signaling, referred to as “the UV response” [56, 57]. Our laboratory and others have shown that UVB is a potent inducer of MAP kinase signaling in keratinocytes [12, 21]. These enzymes control expression of genes important in regulating cell growth and differentiation as well as oxidative stress and inflammation [56]. The present studies show that corneal epithelial cells are also highly responsive to UVB-induced MAP kinase signaling, specifically JNK and p38 MAP kinases. These enzymes regulate UVB-induced mRNA for enzymes that generate both eicosanoids and inflammatory mediators, although their expression is regulated by distinct mechanisms. Thus, while both COX-2 and 15-LOX-2 expression are controlled by p38 MAP kinase, JNK regulates HO-1 and IFNγ and both kinases regulate TNFα. Generally similar results have been reported in UVB treated mouse and human skin and/or keratinocytes [21, 58-62].
In summary, our data demonstrate that corneal epithelial cells are highly sensitive to UVB light-induced oxidative stress. This is associated with increased mRNA expression for antioxidants, as well as proinflammatory mediators including cytokines and enzymes that generate prostaglandins and leukotrienes. UVB light also upregulates expression of enzymes that can generate mediators that downregulate inflammation and promote wound healing including lipoxin A4 and neuroprotectin D1. Coordinate regulation of these mediators in the cornea is likely important in initiating, as well as controlling the resolution of inflammation and the repair of damaged cells and tissues. Whether or not the genes induced by UVB in the cornea contribute to its biological actions will depend on levels of expression of functional proteins and on production of both pro- and anti-inflammatory mediators. Further studies are needed to more clearly define the mediators produced by the corneal epithelial cells and their roles in modulating the responses of the cornea to UVB light.
Acknowledgments
This research was supported by the CounterACT Program, National Institutes of Health Office of the Director, and the National Institute of Arthritis and Musculoskeletal and Skin Diseases, Grant number U54AR055073. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the federal government. This work was also supported in part by National Institutes of Health grants CA100994, AR055073, CA093798, CA132624, ES004738, ES005022, GM034310, EY009056, AI084138 and AI51214.
Abbreviations
- COX-1
Cyclooxygenase-1
- COX-2
cyclooxygenase-2
- FLAP
5-lipoxygenase activating protein
- GST
glutathione S-transferase
- mGST
microsomal GST
- HO-1
heme oxygenase-1
- LTA4 hydrolase
leukotriene A4 hydrolase
- LTC4 synthase
leukotriene C4 synthase
- LOX
lipoxygenase
- 5-LOX
5-lipoxygenase
- 12-LOX-epi
12-lipoxygenase-epidermal type
- 12-LOX-plt
12-lipoxygenase platelet-type
- IFN
interferon
- IL
interleukin
- PGE2
prostaglandin E2
- mPGES-1
microsomal PGE2 synthase-1
- mPGES-2
microsomal PGE2 synthase-2
- PGD2
prostaglandin D2
- PGDS
PGD2 synthase
- PGF2
prostaglandin F2
- PGFS
PGF2 synthase
- PGI2
prostaglandin I2
- PGIS
PGI2 synthase
- PGH2
prostaglandin H2
- SOD
superoxide dismutase
- TXA2
thromboxane A2
- TXAS
TXA2 synthase
- TGFβ
tumor growth factor beta
- TNFα
tumor necrosis factor alpha
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kolozsvari L, Nogradi A, Hopp B, Bor Z. UV absorbance of the human cornea in the 240-400-nm range. Invest Ophthal Vis Sci. 2002;43:2165–8. [PubMed] [Google Scholar]
- 2.Podskochy A. Protective role of corneal epithelium against ultraviolet radiation damage. Acta Ophthalmol Scand. 2004;82:714–7. doi: 10.1111/j.1600-0420.2004.00369.x. [DOI] [PubMed] [Google Scholar]
- 3.Rogers CS, Chan LM, Sims YS, Byrd KD, Hinton DL, Twining SS. The effects of sub-solar levels of UV-A and UV-B on rabbit corneal and lens epithelial cells. Exp Eye Res. 2004;78:1007–14. doi: 10.1016/j.exer.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 4.Estil S, Olsen WM, Huitfeldt HS, Haaskjold E. UVB-induced formation of (6-4) photoproducts in the rat corneal epithelium. Acta Ophthalmol Scand. 1997;75:120–3. doi: 10.1111/j.1600-0420.1997.tb00104.x. [DOI] [PubMed] [Google Scholar]
- 5.Cullen AP. Photokeratitis and other phototoxic effects on the cornea and conjunctiva. Int J Toxicol. 2002;21:455–64. doi: 10.1080/10915810290169882. [DOI] [PubMed] [Google Scholar]
- 6.Andley UP, Fritz C, Morrison AR, Becker B. The role of prostaglandins E2 and F2α in ultraviolet radiation-induced cortical cataracts in vivo. Invest Ophthal Vis Sci. 1996;37:1539–48. [PubMed] [Google Scholar]
- 7.Kennedy M, Kim KH, Harten B, Brown J, Planck S, Meshul C, et al. Ultraviolet irradiation induces the production of multiple cytokines by human corneal cells. Invest Ophthalmol Vis Sci. 1997;38:2483–91. [PubMed] [Google Scholar]
- 8.Cejkova J, Stipek S, Crkovska J, Ardan T, Midelfart A. Reactive oxygen species (ROS)-generating oxidases in the normal rabbit cornea and their involvement in the corneal damage evoked by UVB rays. Histo Histopath. 2001;16:523–33. doi: 10.14670/HH-16.523. [DOI] [PubMed] [Google Scholar]
- 9.Cejkova J, Stipek S, Crkovska J, Ardan T, Platenik J, Cejka C, et al. UV rays, the prooxidant/antioxidant imbalance in the cornea and oxidative eye damage. Physiol Rev. 2004;53:1–10. [PubMed] [Google Scholar]
- 10.Halliwell B, Gutteridge JMC. Free radicals in biology and medicine. Oxford: Oxford University Press; 2007. [Google Scholar]
- 11.Droge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 12.Black AT, Gray JP, Shakarjian MP, Laskin DL, Heck DE, Laskin JD. Distinct effects of ultraviolet B light on antioxidant expression in undifferentiated and differentiated mouse keratinocytes. Carcinogenesis. 2008;29:219–25. doi: 10.1093/carcin/bgm242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Desmots F, Rissel M, Loyer P, Turlin B, Guillouzo A. Immunohistological analysis of glutathione transferase A4 distribution in several human tissues using a specific polyclonal antibody. J Histochem Cytochem. 2001;49:1573–80. doi: 10.1177/002215540104901211. [DOI] [PubMed] [Google Scholar]
- 14.Hiratsuka A, Saito H, Hirose K, Watabe T. Marked expression of glutathione S-transferase A4-4 detoxifying 4-hydroxy -2(E)-nonenal in the skin of rats irradiated by ultraviolet B-band light (UVB) Biochem Biophys Res Commun. 1999;260:740–6. doi: 10.1006/bbrc.1999.0971. [DOI] [PubMed] [Google Scholar]
- 15.Cejkova J, Stipek S, Crkovska J, Ardan T. Changes of superoxide dismutase, catalase and glutathione peroxidase in the corneal epithelium after UVB rays. Histochemical and biochemical study. Histo Histopath. 2000;15:1043–50. doi: 10.14670/HH-15.1043. [DOI] [PubMed] [Google Scholar]
- 16.Hruza LL, Pentland AP. Mechanisms of UV-induced inflammation. J Invest Dermatol. 1993;100:35S–41S. doi: 10.1111/1523-1747.ep12355240. [DOI] [PubMed] [Google Scholar]
- 17.Nakaguma H, Takahashi H. Remarkable elevation of leukotriene B4 in rat skin after induction of UV photodermatitis. Inflammation. 1990;14:195–203. doi: 10.1007/BF00917458. [DOI] [PubMed] [Google Scholar]
- 18.Miller CC, Hale P, Pentland AP. Ultraviolet B injury increases prostaglandin synthesis through a tyrosine kinase-dependent pathway. Evidence for UVB-induced epidermal growth factor receptor activation. J Biol Chem. 1994;269:3529–33. [PubMed] [Google Scholar]
- 19.Funk CD. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science. 2001;294:1871–5. doi: 10.1126/science.294.5548.1871. [DOI] [PubMed] [Google Scholar]
- 20.Araki-Sasaki K, Ohasi Y, Sasabe T, Hayashi K, Watanabe H, Tano Y, et al. An SV40-immortalized human corneal epithelial cell line and its characterization. Invest Ophthal Vis Sci. 1995;36:614–21. [PubMed] [Google Scholar]
- 21.Black AT, Gray JP, Shakarjian MP, Mishin V, Laskin DL, Heck DE, et al. UVB light upregulates prostaglandin synthases and prostaglandin receptors in mouse keratinocytes. Toxicol Appl Pharmacol. 2008;232:14–24. doi: 10.1016/j.taap.2008.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heck DE, Laskin DL, Gardner CR, Laskin JD. Epidermal growth factor suppresses nitric oxide and hydrogen peroxide production by keratinocytes. J Biol Chem. 1992;267:21277–80. [PubMed] [Google Scholar]
- 23.Hayes JD, Flanagan JU, Jowsey IR. Glutathione transferases. Annu Rev Pharmacol Toxicol. 2005;45:51–88. doi: 10.1146/annurev.pharmtox.45.120403.095857. [DOI] [PubMed] [Google Scholar]
- 24.Hayes JD, McLellan LI. Glutathione and glutathione-dependent enzymes represent a co-ordinately regulated defense against oxidative stress. Free Radical Res. 1999;31:273–300. doi: 10.1080/10715769900300851. [DOI] [PubMed] [Google Scholar]
- 25.Hayes JD, Pulford DJ. The glutathione S-transferase supergene family: regulation of GST and the contribution of the isoenzymes to cancer chemoprotection and drug resistance. Crit Rev Biochem Mol Biol. 1995;30:445–600. doi: 10.3109/10409239509083491. [DOI] [PubMed] [Google Scholar]
- 26.Mosialou E, Oiemonte F, Andersson C, Vos RM, van Bladeren PJ, Morgenstern R. Microsomal glutathione transferase: lipid-derived substrates and lipid dependence. Arch Biochem Biophys. 1995;320:210–6. doi: 10.1016/0003-9861(95)90002-0. [DOI] [PubMed] [Google Scholar]
- 27.Black HS. Potential involvement of free radical reactions in ultraviolet light-mediated cutaneous damage. Photochem Photobiol. 1987;46:213–21. doi: 10.1111/j.1751-1097.1987.tb04759.x. [DOI] [PubMed] [Google Scholar]
- 28.Tyrrell RM. Ultraviolet radiation and free radical damage to skin. Biochem Soc Symp. 1995;61:47–53. doi: 10.1042/bss0610047. [DOI] [PubMed] [Google Scholar]
- 29.Bender K, Blattner C, Knebel A, Iordanov M, Herrlich P, Rahmsdorf HJ. UV-induced signal transduction. J Photochem Photobiol. 1997;37:1–17. doi: 10.1016/s1011-1344(96)07459-3. [DOI] [PubMed] [Google Scholar]
- 30.Halliday GM. Inflammation, gene mutation and photoimmunoesuppression in response to UVR-induced oxidative damage contributes to photocarcinogenesis. Mutat Res. 2005;571:107–20. doi: 10.1016/j.mrfmmm.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 31.Sander CS, Chang H, Hamm F, Elsner P, Thiele JJ. Role of oxidative stress and the antioxidant network in cutaneous carcinogenesis. Int J Dermatol. 2004;43:326–35. doi: 10.1111/j.1365-4632.2004.02222.x. [DOI] [PubMed] [Google Scholar]
- 32.Pauloin T, Dutot M, Joly F, Warnet JM, Rat P. High molecular weight hyaluronan decreases UVB-induced apoptosis and inflammation in human epithelial corneal cells. Mol Vis. 2009;15:577–83. [PMC free article] [PubMed] [Google Scholar]
- 33.Larrosa M, Lodovici M, Morbidelli L, Dolara P. Hydrocaffeic and p-coumaric acids, natural phenolic compounds, inhibit UV-B damage in WKD human conjunctival cells in vitro and rabbit eye in vivo. Free Radic Res. 2008;42:903–10. doi: 10.1080/10715760802510077. [DOI] [PubMed] [Google Scholar]
- 34.Lodovici M, Raimondi L, Guglielmi F, Gemignani S, Dolara P. Protection against ultraviolet B-induced oxidative DNA damage in rabbit corneal-derived cells (SIRC) by 4-coumaric acid. Toxicology. 2003;184:141–7. doi: 10.1016/s0300-483x(02)00572-3. [DOI] [PubMed] [Google Scholar]
- 35.Franco AA, Odom RS, Rando TA. Regulation of antioxidant enzyme gene expression in response to oxidative stress and during differentiation of mouse skeletal muscle. Free Radic Biol Med. 1999;27:1122–32. doi: 10.1016/s0891-5849(99)00166-5. [DOI] [PubMed] [Google Scholar]
- 36.Scandalios JG. Oxidative stress: molecular perception and transduction of signals triggering antioxidant defenses. Braz J Med Biol Res. 2005;38:995–1014. doi: 10.1590/s0100-879x2005000700003. [DOI] [PubMed] [Google Scholar]
- 37.Zelko IN, Mariani TJ, Folz RJ. Superoxide dismutase multigene family: a comparison of the CuZn-SOD (SOD1), Mn-SOD (SOD2), and EC-SOD (SOD3) gene structures, evolution, and expression. Free Radic Biol Med. 2002;33:337–49. doi: 10.1016/s0891-5849(02)00905-x. [DOI] [PubMed] [Google Scholar]
- 38.Maines MD, Panahian N. The heme oxygenase system and cellular defense mechanisms. Do HO-1 and HO-2 have different functions? Adv Exp Med Biol. 2001;502:249–72. doi: 10.1007/978-1-4757-3401-0_17. [DOI] [PubMed] [Google Scholar]
- 39.Patil K, Bellner L, Cullaro G, Gotlinger KH, Dunn MW, Schwartzman ML. Heme oxygenase-1 induction attenuates corneal inflammation and accelerates wound healing after epithelial injury. Invest Ophthalmol Vis Sci. 2008;49:3379–86. doi: 10.1167/iovs.07-1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Das M, Bickers DR, Santella RM, Mukhtar H. Altered patterns of cutaneous xenobiotic metabolism in UVB-induced squamous cell carcinoma in SKH-1 hairless mice. J Biol Chem. 1985;84:532–6. doi: 10.1111/1523-1747.ep12273527. [DOI] [PubMed] [Google Scholar]
- 41.Sultana S, Alam A, Khan N, Sharma S. Inhibition of benzoyl peroxide and ultraviolet-B radiation induced oxidative stress and tumor promotion markers by cycloartenol in murine skin. Redox Rep. 2003;8:105–12. doi: 10.1179/135100003125001422. [DOI] [PubMed] [Google Scholar]
- 42.Wang D, Dubois RN. Eicosanoids and cancer. Nat Rev Cancer. 2010;10:181–93. doi: 10.1038/nrc2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Andley UP, Becker B, Hebert JS, Reddan JR, Morrison AR, Pentland AP. Enhanced prostglandin synthesis after ultraviolet-B exposure modulates DNA synthesis of lens epithelial cells and lowers intraocular pressure in vivo. Invest Ophthal Vis Sci. 1996;37:142–53. [PubMed] [Google Scholar]
- 44.Zhan H, Zheng H. The role of topical cyclo-oxygenase-2 inhibitors in skin cancer: treatment and prevention. Am J Clin Dermatol. 2007;8:195–200. doi: 10.2165/00128071-200708040-00002. [DOI] [PubMed] [Google Scholar]
- 45.Leahy KM, Ornberg RL, Wang Y, Zweifel BS, Koki AT, Masferrer JL. Cyclooxygenase-2 inhibition by celecoxib reduces proliferation and induces apoptosis in angiogenic endothelial cells in vivo. Cancer Res. 2002;62:625–31. [PubMed] [Google Scholar]
- 46.Bazan HE, Birkle DL, Beuerman R, Bazan NG. Cryogenic lesion alters the metabolism of arachidonic acid in rabbit cornea layers. Invest Ophthalmol Vis Sci. 1985;26:474–80. [PubMed] [Google Scholar]
- 47.Bazan HE. Cellular and molecular events in corneal wound healing: significance of lipid signalling. Exp Eye Res. 2005;80:453–63. doi: 10.1016/j.exer.2004.12.023. [DOI] [PubMed] [Google Scholar]
- 48.Kenchegowda S, Bazan HE. Significance of lipid mediators in corneal injury and repair. J Lipid Res. 2009;51:879–91. doi: 10.1194/jlr.R001347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tjebbes GW, van Delft JL, van Haeringen NJ. Production of lipid mediators in experimental keratitis of rabbit eye. J Lipid Mediat. 1993;8:87–93. [PubMed] [Google Scholar]
- 50.Serhan CN, Yacoubian S, Yang R. Anti-inflammatory and proresolving lipid mediators. Annu Rev Pathol. 2008;3:279–312. doi: 10.1146/annurev.pathmechdis.3.121806.151409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liclican EL, Gronert K. Molecular circuits of resolution in the eye. Scientific World Journal. 2010;10:1029–47. doi: 10.1100/tsw.2010.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gronert K, Maheshwari N, Khan N, Hassan IR, Dunn M, Laniado Schwartzman M. A role for the mouse 12/15-lipoxygenase pathway in promoting epithelial wound healing and host defense. J Biol Chem. 2005;280:15267–78. doi: 10.1074/jbc.M410638200. [DOI] [PubMed] [Google Scholar]
- 53.Biteman B, Hassan IR, Walker E, Leedom AJ, Dunn M, Seta F, et al. Interdependence of lipoxin A4 and heme-oxygenase in counter-regulating inflammation during corneal wound healing. FASEB J. 2007;21:2257–66. doi: 10.1096/fj.06-7918com. [DOI] [PubMed] [Google Scholar]
- 54.Bazan NG, Calandria JM, Serhan CN. Rescue and repair during photoreceptor cell renewal mediated by docosahexaenoic acid-derived neuroprotectin D1. J Lipid Res. 2010;51:2018–31. doi: 10.1194/jlr.R001131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kyriakis JM, Avruch J. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol Rev. 2001;81:807–69. doi: 10.1152/physrev.2001.81.2.807. [DOI] [PubMed] [Google Scholar]
- 56.Bode AM, Dong Z. Mitogen-activated protein kinase activation in UV-induced signal transduction. Sci STKE. 2003;2003:RE2. doi: 10.1126/stke.2003.167.re2. [DOI] [PubMed] [Google Scholar]
- 57.Rosette C, Karin M. Ultraviolet light and osmotic stress: activation of the JNK cascade through multiple growth factor and cytokine receptors. Science. 1996;274:1194–7. doi: 10.1126/science.274.5290.1194. [DOI] [PubMed] [Google Scholar]
- 58.Chen W, Tang Q, Gonzales MS, Bowden GT. Role of p38 MAP kinases and ERK in mediating ultraviolet-B induced cyclooxygenase-2 gene expression in human keratinocytes. Oncogene. 2001;20:3921–6. doi: 10.1038/sj.onc.1204530. [DOI] [PubMed] [Google Scholar]
- 59.Einspahr JG, Bowden GT, Alberts DS, McKenzie N, Saboda K, Warneke J, et al. Cross-validation of murine UV signal transduction pathways in human skin. Photochem Photobiol. 2008;84:463–76. doi: 10.1111/j.1751-1097.2007.00287.x. [DOI] [PubMed] [Google Scholar]
- 60.Bashir MM, Sharma MR, Werth VP. TNF-alpha production in the skin. Arch Dermatol Res. 2009;301:87–91. doi: 10.1007/s00403-008-0893-7. [DOI] [PubMed] [Google Scholar]
- 61.Matsuura K, Otsuka F, Fujisawa H. Effects of interferons on tumour necrosis factor alpha production from human keratinocytes. Cytokine. 1998;10:500–5. doi: 10.1006/cyto.1997.0326. [DOI] [PubMed] [Google Scholar]
- 62.Yoshizumi M, Nakamura T, Kato M, Ishioka T, Kozawa K, Wakamatsu K, et al. Release of cytokines/chemokines and cell death in UVB-irradiated human keratinocytes, HaCaT. Cell Biol Int. 2008;32:1405–11. doi: 10.1016/j.cellbi.2008.08.011. [DOI] [PubMed] [Google Scholar]