Abstract
Context
Cognitive impairment and functional disability are major determinants of caregiving needs and societal healthcare costs. Although the incidence of severe sepsis is high and rising, especially among older adults, the magnitude of patients’ long-term cognitive and functional limitations after sepsis is unknown.
Objective
Determine the change in cognitive impairment and physical functioning among patients who survive severe sepsis, controlling for their pre-sepsis functioning.
Design
Prospective cohort.
Setting
The Health and Retirement Study (HRS) conducted interviews with a nationally representative cohort of older Americans every 2 years during 1998–2006. There were 9,223 HRS respondents with linked Medicare claims who had a baseline cognitive and functional assessment 1998–2004.
Patients
1,520 hospitalizations for severe sepsis occurred in 1,194 patients, as ascertained from Medicare claims linked to the HRS. Of this group, 516 individuals survived 623 episodes of severe sepsis and had at least 1 follow-up survey, and were analyzed here. A comparison group included 5,574 respondents who experienced a non-sepsis general hospitalization in the study period, of whom 4,517 survived to at least 1 follow-up survey for analysis here.
Interventions
None
Main Outcomes Measures
Personal interviews with respondents and proxies were used to assess cognitive impairment using the validated modified-TICS and IQCODE questionnaires. Disability was measured by the number of ADLs and IADLs for which patients needed assistance. We used within-person regression to identify the association of sepsis with changes in the trajectory of disability with up to 8 years of post-sepsis follow-up.
Results
Survivors’ mean age at hospitalization was 76.9 years. The prevalence of moderate/severe cognitive impairment increased 10.6 percentage points among patients who survived severe sepsis, an odds ratio of 3.33, (95% CI: 1.53, 7.25) in multivariable regression. Likewise, a high rate of new functional limitations was seen following sepsis in those with no limits before sepsis (mean 1.57 new limitations [95% CI: 0.99, 2.15], where no effect would be 0 new limitations) and in those with mild/moderate limitations before sepsis (mean 1.50 new limitations [95% CI: 0.87, 2.12]). In contrast, non-sepsis general hospitalizations were associated with no change in moderate/severe cognitive impairment (OR: 1.15, 95% CI: 0.80, 1.67, differences versus sepsis p=0.012) and much smaller changes in functional limitations (mean 0.48 [95% CI: 0.39, 0.56] and 0.43 [95% CI: 0.23, 0.63] new limitations among those with no and mild/moderate limits before hospitalization, respectively; differences versus sepsis p<0.001 and p=0.001). The declines in cognitive and physical function persisted for up to 8 years of follow-up.
Conclusion
Severe sepsis in this older population was independently associated with substantial and persistent new cognitive impairment and functional disability among survivors. The magnitude of these new deficits was large, likely resulting in a pivotal downturn in patients' ability to live independently. Identifying modifiable components of hospital and rehabilitation care to prevent these disabilities would be valuable for patients and their families.
Please Note: This is the manuscript as accepted by JAMA, but it does not contain the significant copy-editing and content clarification provided by JAMA. The final version as published in JAMA - which should be considered the definitive version - is available at: http://jama.ama-assn.org/content/304/16/1787.abstract or doi: 10.1001/jama.2010.1553.
Cognitive impairment and physical disability are major health burdens and drivers of health care costs. The onset of disability is associated with worsened mortality 1 and substantial increases in medical costs over subsequent years,2 including a disproportionate strain on Medicaid and Medicare. Both cognitive and physical disability impose yet further burdens on families and informal caregivers.3 Irreversible cognitive and physical impairment following acute illnesses (such as severe sepsis) are particularly feared outcomes, and weigh heavily on patient decision-making.4
Hundreds of thousands of patients endure severe sepsis each year in the U.S alone.5 It has been suspected that many are discharged with a new—but poorly defined—constellation of cognitive and functional impairments, 6 which may explain their reduced quality of life.7 Even hospitalizations for less severe illness often result in a period of functional disability 8 and may hasten the progression of dementia.9, 10 Long-term cognitive and functional declines have been shown among survivors of other critical illnesses, but these declines may be at least partially preventable.11–14 Although severe sepsis is the most common non-cardiac cause of critical illness,5, 15 the long-term impact of severe sepsis on cognitive and physical functioning is unknown.
We studied whether an incident episode of severe sepsis increased the odds of subsequent worsened cognitive impairment and functional disability among those who survive. We took advantage of an ongoing cohort study that is nationally representative of older Americans, and which included detailed information from personal surveys and Medicare claims. This provided a unique opportunity to examine the long-term impact of severe sepsis within a large representative cohort with well-characterized cognitive and physical functioning, before—and up to 8 years after—incident disease.
METHODS
Data Source
The Health and Retirement Study (HRS) is an ongoing cohort nationally representative of community-dwelling Americans over the age of 50. Begun in 1992, over 27,000 individuals have contributed 200,000 hours of data-collection interviews. Every two years, the cohort has been re-interviewed about a wide array of topics, including detailed questions about their functional status. The HRS achieved a very high follow-up rate, including the use of proxies when respondents could not complete the survey on their own; reinterview rates routinely exceeded 90–95%.16 16,772 individuals in the HRS have consented for linkage of their HRS data with Medicare.
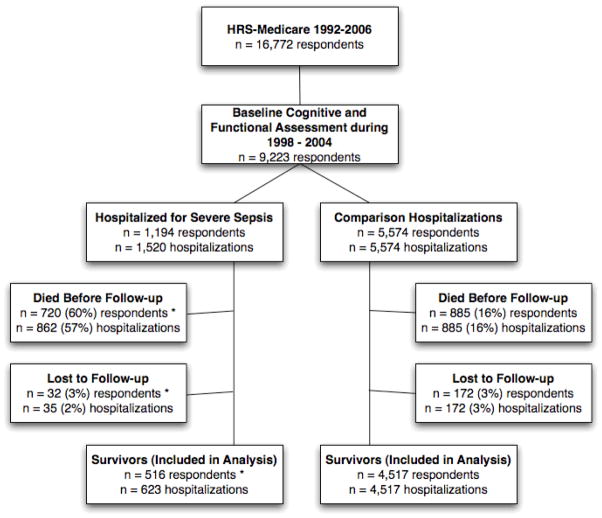
We studied all respondents with at least one HRS interview during 1998–2004 in which cognitive and physical functioning were assessed, and for whom there were subsequent claims-based data on a hospitalization for severe sepsis during 1998–2005 (Figure 1). All patients were followed through death or the 2006 HRS survey. Our primary analyses focus on hospitalizations which patients survived until at least one follow-up interview–the “survivors” cohort.
Figure 1. Patient Cohorts.
Note that this is a hospitalization-level analysis. As such, in the severe sepsis cohort, a single respondent might contribute a hospitalization to the Survivor cohort in one hospitalization, but be Lost to Follow-up after a future hospitalization. Thus the categorization of hospitalizations as included vs. excluded are mutually exclusive, but the categorizations of respondents (*) are not. The comparison hospitalizations were all first hospitalizations.
Characteristics of the hospitalizations for severe sepsis were abstracted from the Medicare claims, including organ dysfunction score (the sum of number of organ failures of cardiovascular, neurologic, hematologic, hepatic, renal or respiratory failure).5, 17 Self-reported race and ethnicity were included only in the descriptive statistics, as they may be of interest to some readers.
Definition of Severe Sepsis
We relied on a claims-based definition of severe sepsis, which has been widely used and clinically validated.5 This definition requires evidence of both an infection and new-onset organ dysfunction during a single hospitalization. If a patient had more than one distinct septic hospitalization, each hospitalization was included. We conducted our cohort analyses for patients who received and did not receive mechanical ventilation in order to ensure that our results were not simply the result of ARDS.
As a comparison, we conducted parallel analyses in a cohort of 5,574 hospitalizations. These were first hospitalizations for members of the linked HRS-Medicare cohort which included neither severe sepsis nor critical care use, and for which baseline survey and at least one follow-up interview were available. We refer to these comparisons as “non-sepsis general hospitalizations.”
Definition of Functional Status
At each wave of the HRS, patients or their proxies were asked if they required assistance with any of 6 Activities of Daily Living (ADLs: walking, dressing, bathing, eating, getting into and out of bed, and toileting) or 5 Instrumental Activities of Daily Living (IADLs: preparing a hot meal, shopping for groceries, making telephone calls, taking medicines, and managing money). As others have done, we totaled the number of ADLs and IADLs to create a total deficiency score, running from 0 (requiring no assistance) to 11 (requiring assistance on all ADLs and IADLs).18 The HRS asked proxies to evaluate the functional status of patients who could not answer for themselves; proxies could answer these questions with high reliability.18 For some analyses, a baseline of functioning was used, defined as the last survey prior to severe sepsis. It was decided a priori that patients would be divided into 3 groups based on their baseline functioning: “no limits” having 0 deficiencies, “mild/moderate limitations” having 1–3 deficiencies, and “severe limitations” having 4 or more deficiencies.
Definition of Cognitive Impairment
The HRS assessed cognitive function in 2 ways during biennial personal interviews. For those age 65 and above, a 35-point scale was administered that included tests of memory, serial seven subtractions, naming, and orientation. 19, 20 For self-respondents under age 65, the HRS administered a more limited 27-point scale that excluded the orientation measures.
For patients aged 65 and above who were unable to be interviewed themselves, the validated Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE) 21 was administered to proxies. The IQCODE was not administered to proxies representing respondents younger than 65, but the following questions were used to determine cognitive function for these younger respondents: “How would you rate [the respondent’s] memory at the present time?” and “How would you rate [the respondent] in making judgments and decisions?” The response options for both of these questions were: excellent, very good, good, fair, or poor.
We defined cut-points on the cognitive assessments for mild and moderate/severe cognitive impairment based on prior studies with the HRS data,3, 22, 23 as well as the methods used for the Aging, Demographics, and Memory Study (ADAMS), a supplemental study of dementia in the HRS. 24 These cut-points defined a level of cognitive impairment that was generally consistent with mild and moderate / severe dementia in the ADAMS. Further detail on the HRS cognitive measures is available.25
Analyses
For analyses of functional status, our primary outcome was measured by a combined ADL and IADL score. For unadjusted analyses, we grouped patients by the number of surveys they had completed since the occurrence of severe sepsis; for example, we compared all patients at their last survey before hospitalization with severe sepsis to patients at their first survey after severe sepsis, and so on. For multivariable models, we use longitudinal models to examine the association between the timing of severe sepsis and the timing of cognitive and functional changes; these models use only within-person variation over time in functional status to estimate the impact of severe sepsis, and control for characteristics of the patient that did not change over time—in essence, patients served as their own controls.26 Specifically, we constructed latent growth curve models using a hospitalization-level fixed effect, sometimes also called conditional models.26 These results controlled for not only the functional status of the patient before his or her episode of severe sepsis, but also for their functional trajectory. All of these sequential evaluations were included in the analysis. In these models, time from admission with severe sepsis to survey interview was measured to the day as a continuous variable. Additional information about the statistical approach is presented in the eMethods. Fixed effects models were estimated using xtreg, fe in Stata 10.1. We also replicated our findings using random effects models (using xtmixed) and ordered logistic regression models (where the number of I/ADL limitations was treated as ordinal categories, using GLLAMM, with random intercepts) in Stata, and our findings were similar across modeling strategies. These analyses were not conducted according to a fully pre-specified protocol.
For analyses of cognitive functioning, our primary outcome was level of cognitive impairment. Unadjusted analyses were conducted as for functional status. For multivariable analyses, we used conditional logistic regression to analyze the impact of severe sepsis on moderate/severe cognitive impairment among survivors, using clogit in Stata 10.1. As for functional status, these analyses used only within-person variation over time to estimate the effect of severe sepsis, controlling for time-invariant characteristics of the respondent.
All analyses were conducted with the hospitalization as the unit of analysis unless otherwise indicated. Two-sided significance testing was used throughout, and a p value of 0.05 was considered statistically significant.
Human Subjects
This work was approved by the University of Michigan Institutional Review Board. Patients provided informed consent on enrollment in the HRS, and again for linkage to Medicare claims.
RESULTS
There were 1,520 identified episodes of severe sepsis among 1,194 respondents in the HRS for the years 1998 – 2005, from a cohort of 9,223 respondents. (Figure 1) Detail about the entire population of severe sepsis hospitalizations is presented in eTable 1. 90 day mortality after severe sepsis was 41.3% (95% CI: 38.8%, 43.8%); 5-year mortality was 81.9% (95% CI: 79.8%, 84.0%). 5-year survival curves, including those showing increased mortality for those with cognitive and functional deficits before sepsis, are presented in eFigure 1. 516 individuals survived 623 episodes of severe sepsis and had at least 1 follow-up survey; these hospitalizations by survivors are our primary cohort for analysis. Their demographics are reported in Table 1, grouped by their baseline functional status. Mean age was 76.9 years upon admission. 20.4% of severe sepsis hospitalizations involved major surgery and 19.7% involved mechanical ventilation during their sepsis hospitalization. Their mean Charlson score was 1.88 for the sepsis hospitalization. 86.8% had normal cognition at baseline, 7.1% were mildly cognitively impaired and 6.1% were moderately/severely cognitively impaired. Patients were followed for up to 4 surveys (7.8 years) of data prior to severe sepsis, and up to 4 surveys (8.3 years) afterwards.
Table 1. Demographics of Study Cohort of Survivors, by Baseline Physical Functioning (n= 623).
Data for the entire cohort of incident severe sepsis hospitalizations are in eTable 1, and on risk factors for cognitive impairments are presented in eTable 2.
| No Limits | Mild / Moderate Limits | Severe Limits | |
|---|---|---|---|
| n | 269 | 195 | 159 |
| Male (%) | 143 (53%) | 92 (47%) | 46 (29%) |
| Black (%) | 49 (18%) | 41 (21%) | 38 (24%) |
| Hispanic (%) | 19 (7%) | 12 (6%) | 13 (8%) |
| Age at Sepsis (mean (SD), years) | 75.8 (7.5) | 76.7 (9.5) | 79.1 (9.6) |
| Length of Stay (mean (SD), days) | 11.4 (10.7) | 11.3 (11.2) | 8.5 (6.3) |
| Required Mechanical Ventilation | 64 (23%) | 32 (16%) | 27 (17%) |
| Required Dialysis | 9 (3.4%) | 6 (3.1%) | 12 (7.6%) |
| Used an Intensive Care Unit | 137 (51%) | 75 (38%) | 57 (36%) |
| Underwent Major Surgery | 73 (27%) | 39 (20%) | 15 (9%) |
| Charlson Score (mean (SD)) | 1.69 (1.42) | 1.96 (1.64) | 2.11 (1.41) |
| Organ Dysfunction Score (mean (SD)) | 1.15 (0.39) | 1.16 (0.45) | 1.11 (0.34) |
| Acute Cardiovascular Dysfunction | 60 (22%) | 62 (32%) | 45 (28%) |
| Acute Neurologic Dysfunction | 19 (7%) | 20 (10%) | 17 (11%) |
| Acute Hematologic Dysfunction | 61 (23%) | 34 (17%) | 27 (17%) |
| Acute Hepatic Dysfunction | 2 (1%) | 0 (0%) | 1 (1%) |
| Acute Renal Dysfunction | 103 (38%) | 79 (41%) | 60 (38%) |
| Acute Respiratory Dysfunction | 64 (24%) | 32 (16%) | 27 (17%) |
| Baseline Cognition Normal | 254 (94%) | 182 (93%) | 105 (66%) |
| Baseline Mild Cognitive Impairment | 15 (5.6%) | 9 (4.6%) | 20 (12.6%) |
| Baseline Moderate/Severe Cognitive Impairment | 0 (0.0%) | 4 (2.1%) | 34 (21.4%) |
| Baseline ADL Deficiencies (mean (SD)) | 0 (0) | 1.3 (0.9) | 4.0 (1.7) |
| Baseline IADL Deficiencies (mean (SD)) | 0 (0) | 0.5 (0.7) | 3.0 (1.5) |
| Proxy Respondent at Baseline | 9 (3%) | 22 (11%) | 59 (37%) |
| Proxy Respondent at First Post-Sepsis Survey | 46 (17%) | 47 (24%) | 87 (55%) |
Cognitive Outcomes
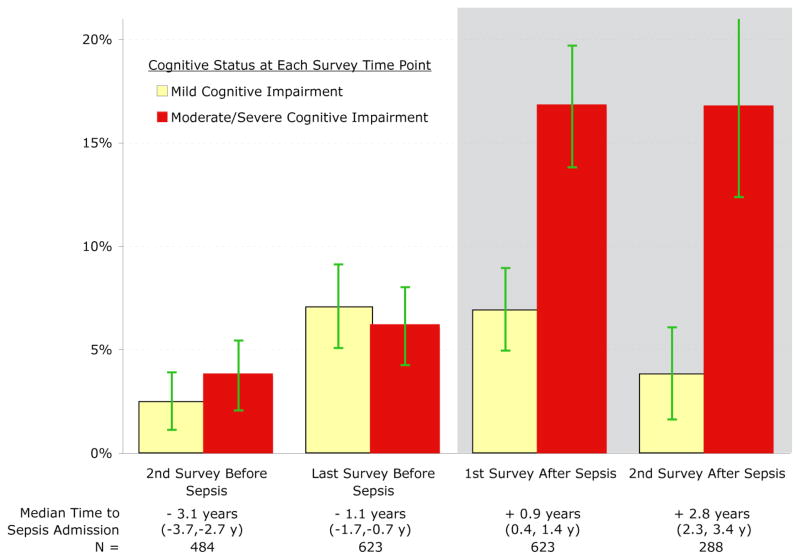
Incident severe sepsis was associated with a clinically and statistically significant increase in moderate/severe cognitive impairment among survivors. For example, 6.1% (95% CI: 4.2%, 8.0%) of eventual survivors had moderate/severe cognitive impairment at the survey just before severe sepsis, and the prevalence increased to 16.7% (95% CI: 13.8%, 19.7%) at the first survey after severe sepsis (Figure 2, p<0.001 by χ2). In conditional logistic regression, with each patient serving as his or her own control, the incidence of severe sepsis remained highly associated with progression to moderate/severe cognitive impairment (OR: 3.33, 95% CI: 1.53, 7.25, Table 2). There was no association between severe sepsis and the net prevalence of mild cognitive impairment in adjusted or unadjusted analyses, as approximately equal numbers of previously normal patients developed mild cognitive impairment after severe sepsis as patients with pre-sepsis mild cognitive impairment developed moderate/severe cognitive impairment after severe sepsis.
Figure 2. Cognitive Impairment among Survivors of Severe Sepsis at Each Survey Time Point.
The (unadjusted) percentage of surviving patients suffering from cognitive impairment at each time point is shown, on the white background before sepsis and on the grey background after sepsis. 95% confidence intervals for the proportions are shown.
Interpretive Example: These data demonstrate that in comparison to relatively stable rates before severe sepsis, the prevalence of moderate/severe cognitive impairment among eventual survivors increased from 6.1% (95% CI: 4.2%, 8.0%) before severe sepsis to 16.7% (95% CI: 13.8%, 19.7%) at the first survey after severe sepsis (p<0.001 by χ2). (See also Table 2.)
Table 2. Severe Sepsis and Moderate/Severe Cognitive Impairment Among Survivors.
Results of latent growth curve regression with individual-level fixed effects, controlling for all time-invariant characteristics of the patient. Confidence intervals are in parentheses. The absence of association would be indicated by an odds ratio of 1.
| Odds Ratio | 95% CI | p-value | |
|---|---|---|---|
| Before Sepsis (per additional year) | 1.35 | (1.11, 1.65) | p = 0.002 |
| Effect of Sepsis | 3.34 | (1.53, 7.25) | p = 0.002 |
| After Sepsis (per additional year) | 1.68 | (1.28, 2.21) | p < 0.001 |
Interpretation Example: With each passing year, patients were modestly more likely to develop moderate/severe cognitive impairment. After severe sepsis, survivors had a 3.3-fold greater odds of having moderate/severe cognitive impairment than before sepsis. (See also Figure 2.)
Functional Outcomes
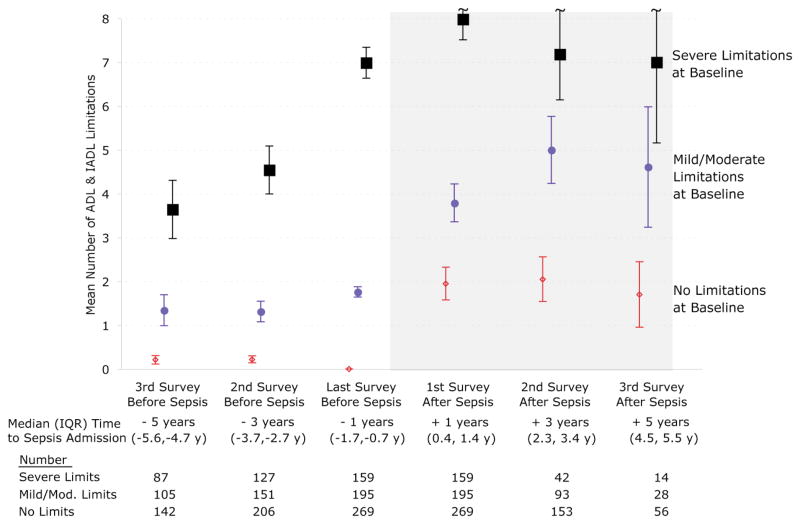
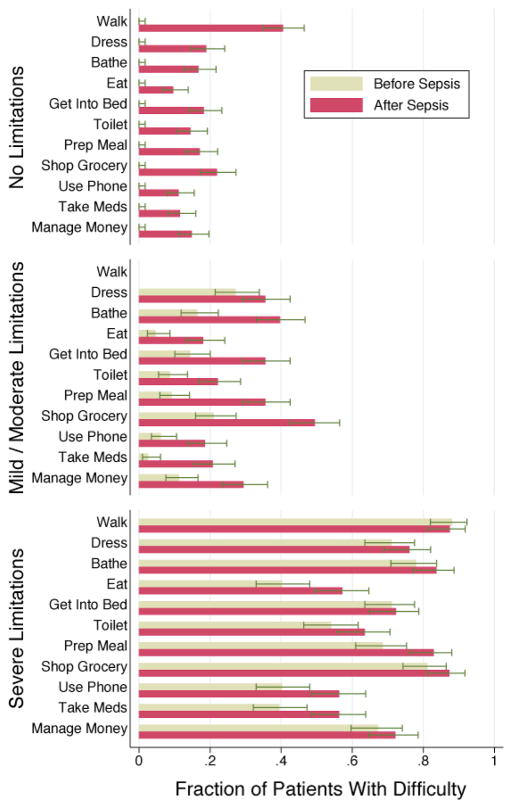
Survivors of hospitalization for severe sepsis were at greater risk of additional functional limitations at their next survey. Sepsis was associated with the development of new I/ADL limitations; this was a substantial worsening in their trajectory relative to before sepsis. The negative effects of severe sepsis were greater in those patients with better baseline physical functioning (Figure 3). The new functional deficits were not concentrated in any particular subset of the functioning measures (Figure 4).
Figure 3. Functional Trajectories by Baseline Functioning.
The mean number (unadjusted) of functional limitations of surviving cohort members is shown; surveys before sepsis are on the white background and interviews after sepsis are on the gray background. 95% confidence intervals for the means are shown.
Interpretive Example: In the groups which had no limitations or mild/moderate limitations prior to sepsis, their trajectory had been stable before sepsis but they developed approximately 2 new limitations after severe sepsis. In contrast, patients with severe limitations at baseline, who had been steadily acquiring limitations over years prior to severe sepsis, had a modest increase from a baseline of 6.99 to 7.98 at their first survey post-sepsis. (See also Table 3.)
Figure 4. Change in Individual ADLs and IADLs.
The proportion of patients with difficulty in each of the activities of daily living and instrumental activities of daily living is shown. The last survey before hospitalization is compared to the first survey after severe sepsis. Patients are grouped by functional status as in Figure 3.
Interpretive Example: No single ADL or IADL accounted for the worsened functional status among survivors of severe sepsis. Instead, there was a wide range of new difficulties across the array of activities.
The independent effects of severe sepsis on long-term disability persisted in multivariable analyses with each patient’s pre-sepsis functional trajectory serving as his or her own control. Table 3 shows that severe sepsis was associated with the development of 1.57 (95% CI: 0.99, 2.15) new limitations among patients with no functional limitations before sepsis. Patients with mild/moderate limitations before sepsis had a similar increase of 1.50 new I/ADL limitations (95% CI: 0.87, 2.12). For those with mild/moderate limitations at baseline, not only was sepsis associated with an acute increase in the number of functional limitations, but sepsis also heralded a more rapid rate of developing further limitations thereafter, at 0.51 new limitations per year (p=0.007 for difference versus baseline). In contrast, patients with already poor functioning did not experience a statistically significant change in functioning with severe sepsis, although the regressions may be limited by ceiling effects in measurement of functioning.
Table 3. Acquisition of New Functional Limitations Before and After Sepsis Among Survivors, by Functional Class at Baseline.
Results of latent growth curve regression with individual-level fixed effects, controlling for all time-invariant characteristics of the patient. The within-patient R2 were 0.25 for the no limitation group, 0.37 for those with mild/moderate baseline limitations, and 0.45 for those with severe baseline limitations. Confidence intervals are in parentheses. The absence of association would be indicated by the acquisition of 0 new functional limitations.
| Functional Class at Baseline | |||
|---|---|---|---|
| No Limits | Mild/Moderate Limits | Severe Limits | |
| n = 269 | n = 195 | n = 159 | |
| Before Sepsis (per year) | −0.020 (−0.046,0.086) | 0.11 (0.01,0.21) | 0.84 (0.73,0.92) |
| p-value | p = 0.545 | p = 0.027 | p < 0.001 |
| Effect of Sepsis | 1.57 (0.99,2.15) | 1.50 (0.87,2.12) | 0.04 (−0.74,0.81) |
| p-value | p < 0.001 | p < 0.001 | p = 0.927 |
| After Sepsis (per year) | 0.19 (−0.03,0.41) | 0.51 (0.24,0.77) | 0.16 (−0.19,0.50) |
| p-value | p = 0.093 | p < 0.001 | p = 0.369 |
Interpretive Example: Patients with mild/moderate limitations at baseline were acquiring 0.11 new limitations per year prior to severe sepsis. They acquired 1.50 new limitations at the time of their hospitalization for severe sepsis. In addition, each year after sepsis, they acquired 0.51 new limitations per year, a statistically significant increase relative to their rate before sepsis. (See also Figure 3.)
59.3% (95% CI: 55.5%, 63.2%) of severe sepsis hospitalizations were associated with worsened cognitive and/or physical function in survivors at the first post-sepsis survey. The association of severe sepsis with increased functional limitations remained clinically meaningful and statistically significant in regression when controlling for changes in level of cognitive impairment after severe sepsis. (1.30 [95% CI: 0.86, 1.74] new limitations for those with no limitations at baseline; 1.20 [95% CI: 0.62, 1.79] new limitations for those with mild/moderate limitations at baseline.) The increased risk of moderate/severe cognitive impairment remained clinically meaningful but was attenuated in the regressions when controlling for contemporaneous changes in levels of physical functioning after severe sepsis (OR: 1.73, 95% CI: 0.83, 3.6).
Comparison to Other Hospitalizations
The changes in physical and cognitive functioning noted after severe sepsis were worse than those seen after non-sepsis general hospital admissions in a cohort of 4,517 survivors of 5,574 hospitalizations. Thus, patients who did not develop severe sepsis and who had no functional limitations prior to their hospitalization developed an average of 0.48 (95% CI: 0.39, 0.57, n=2,852, difference versus severe sepsis: p<0.001, see eTable 3) new functional limitations, and patients with mild/moderate functional limitations at baseline developed 0.46 (95% CI: 0.23, 0.63, n=1,124, difference versus severe sepsis: p<0.001, see eTable 3) new functional limitations after a non-sepsis general hospitalization. Again in contrast to the effect seen with sepsis, non-sepsis hospitalizations were not associated with a clinically or statistically significant increase in the odds of moderate/severe cognitive impairment (OR: 1.15, 95% CI: 0.80, 1.67, n=4,517, difference versus severe sepsis: p=0.012, see eTable 4).
Subgroup and Sensitivity Analyses
We replicated our analyses in several subgroups to examine their robustness. For example, the effects of severe sepsis were quite similar in the 500 survivors who had severe sepsis but did not require mechanical ventilation. The regression demonstrated a similarly increased odds (OR: 4.0, 95% CI: 1.71, 9.31) of developing moderate/severe cognitive impairment after severe sepsis among patients who were not mechanically ventilated. Similarly, in the 205 survivors with no limitations at baseline, severe sepsis without mechanical ventilation was associated with the development of 1.56 new functional limitations (95% CI: 0.91, 2.22) in multivariable fixed-effects models. For 163 patients with mild/moderate limitations, severe sepsis without mechanical ventilation was associated with 1.65 new functional limitations (95% CI: 1.01, 2.28).
A potential threat to the validity of the results is that patients may have suffered some other cause of cognitive and functional decline between their baseline survey and their sepsis hospitalization. Therefore, we reanalyzed data for the smaller subset of 276 survivors who were never hospitalized between their baseline survey and their severe sepsis admission. We found consistent results, albeit with larger standard errors. In this subpopulation, severe sepsis was associated with increased odds of moderate/severe cognitive impairment (OR: 2.49, 95% CI: 0.99, 6.26). In the 128 patients with no functional limitations at baseline and no intercurrent hospitalizations, severe sepsis was associated with the development of 1.46 new functional limitations (95% CI: 0.76, 2.15). In the 86 patients with mild to moderate functional limitations at baseline and no intercurrent hospitalizations, severe sepsis was associated with the development of 1.34 new functional limitations (95% CI: 0.34, 2.34).
Further sensitivity analyses were performed, and yielded consistent results. The associations between severe sepsis and functional and cognitive impairment were substantively similar in those aged 65 and above at baseline cognitive assessment, and who therefore were assessed using a single instrument before and after severe sepsis. (See eTables 5 and 6.) The patterns observed for functional limitations were similar in a larger cohort of 2,043 hospitalizations (including 829 hospitalizations among 684 survivors) for severe sepsis followed for up to 14 years during the period 1992–2006. (See eTable 7.) Examining only the subset of 516 first sepsis admissions for each survivor—so that no patient appeared in the analysis more than once—yielded nearly identical results. (See eTables 8 and 9.)
DISCUSSION
In this nationally representative cohort of older Americans, we have demonstrated for the first time that severe sepsis is independently associated with enduring cognitive and functional limitations. Severe sepsis is independently associated with a tripling in the odds of moderate/severe cognitive impairment (OR: 3.33). Further, severe sepsis was independently associated with the acquisition of 1.5 new functional limitations in patients with no, mild or moderate pre-existing functional limitations. These new disabilities were substantially larger than those seen after non-sepsis general hospital admissions. Cognitive and functional declines of the magnitude seen after severe sepsis are associated with significant increases in caregiver time, nursing home admission, depression, and mortality. 3, 27–30 These data argue that the burden of sepsis survivorship is a substantial, under-recognized public health problem with major implications for patients, families and the health care system.
Our findings, and the nationally representative data from the HRS, allow us to make an estimate of the overall public health burden of sepsis on “brain health” among older adults in the US. Given published dementia 31 and sepsis 5 incidence rates for those age 65+ in the U.S., our results suggest that nearly 20,000 new cases per year of moderate/severe cognitive impairment in the elderly may be attributable to sepsis. Thus, an episode of severe sepsis, even when survived, may represent a sentinel event in the lives of patients and their families, resulting in new and often persistent disability.3, 22 This level of severe cognitive impairment has been associated with an additional 40 hours per week of informal care provided by families, 3 analogous to an additional full-time job for many families of sepsis survivors. If causally related, this represents a substantial public health burden of accelerated or de novo brain dysfunction, and one that has received almost no attention, even in the face of the dramatically rising incidence of severe sepsis. 15 In marked contrast to Alzheimer’s disease and some other forms of dementia, onset and acceleration of cognitive impairment due to sepsis is likely at least partially preventable in many patients. These benefits might be achieved by raising the standard of care for patients who develop sepsis—both sepsis-specific care as well other ICU practices such as sedation management and early physical and cognitive rehabilitation—and by avoiding sepsis altogether.32 Improving the prevention and management of sepsis may warrant a place in the broader “brain health” and disability agendas.
Although an observational study can never prove causation, there are multiple plausible causal pathways by which sepsis and its treatment may lead to significant declines in physical and cognitive function. The literature on ICU-acquired weakness and chronic illness myopathy and polyneuropathy suggests that there is a direct inflammatory and hypoperfusion-mediated degradation of muscle fibers and neurons 33–35 which may be exacerbated by prolonged immobility 36 and lack of physical therapy. 37 Similarly, frank hypotension or relative hypoperfusion may directly contribute to brain injury and subsequent cognitive impairment. 38–40 Inflammation—a cardinal component of the pathophysiology of sepsis—is hypothesized to contribute to both vascular dementia and Alzheimer’s disease. 6, 10, 41 Delirium, an acute form of brain dysfunction characterized by inattention, is common in sepsis, preventable and treatable. 42, 43 Delirium has been associated with increased cognitive decline among patients with Alzheimer’s disease9, 44 as well as with increased rates of long-term cognitive impairment in mechanically ventilated patients. 45 Basic biological research to understand these mechanisms is clearly warranted. Equally pressing is the need for innovative clinical trials of both sepsis-specific therapy and improved life support. Our results suggest that such trials should look beyond short-term mortality to long-term cognitive and functional outcomes of crucial interest to patients. 46
We conducted analyses that address several possible limitations. The regression used only within-person variation in order to estimate the association with severe sepsis; thus, characteristics of the survivors that did not change over time cannot explain the timing of changes in functional status. The different cognitive and physical function outcomes between the survivors of severe sepsis and survivors of the comparison general hospitalizations suggest that the sepsis results were not simply due to the aging of the cohort or the mere fact of hospitalization, processes shared equally by both groups. These different outcomes also suggest that our results cannot be attributed solely to asymmetric censoring, one form of a potential bias known as “truncation by death.”47 However, as patients with worse cognitive and physical functioning have greater mortality (see eFigure 1),22, 30 there may be some conservative bias in our results (towards the null). This form of truncation by death results if patients with the worst cognitive and physical declines after sepsis do not survive long enough for a follow-up survey. To the extent that such truncation by death is present, our results are biased towards the null and the full effect of severe sepsis on cognitive and physical functioning would be even greater.
Our study has several limitations. Unlike prior studies that have focused on acute functional decline in the peri-hospitalization period,1, 8, 48–50 the present results demonstrate only long-term effects; short-term deficits (e.g., less than 6–12 months) are likely greater, with at least some patients recovering some function prior to their next HRS biennial survey. The neuropsychological battery that we used provided an assessment of global cognitive function, but did not allow detailed study of individual cognitive domains, nor did it establish a definitive clinical diagnosis of dementia. Importantly, we used cognitive categories and cut-off scores that have shown good correlation with clinical dementia51 and expected outcomes of dementia3, 27 in prior studies. We used a claims-based definition of severe sepsis. While this is not the same as prospective clinical assessment, it is the same approach used in recent landmark epidemiologic studies. 5, 15 Our data were restricted to fee-for-service Medicare patients aged 65 and above; the impact of severe sepsis in younger patients may be different.5 We have shown that these deteriorations were temporally associated with severe sepsis and independent of other stable patient characteristics, but we have not conclusively proven that it was severe sepsis rather than other co-occurring events that led to these declines. Although this is the largest study to date of severe sepsis and our outcomes of interest, our study was not powered to examine interactions, such as the extent to which the changes after sepsis varied with the number of organ failures or type of inciting organism. Medicare claims lack the information necessary to disentangle whether particular acute interventions are associated with differing long-term outcomes. Finally, we demonstrated the association of severe sepsis with functioning under the treatment regimes in effect in a range of U.S. hospitals at a particular point in time. New treatments for sepsis, or changes in life support or other hospital practices, may modify the long-term cognitive and functional impacts of severe sepsis, even if these deficits are not the an explicit target of care.
In summary, in this large nationally representative cohort of older adults, we found that the odds of acquiring moderate/severe cognitive impairment were 3.3 times as high following an episode of sepsis, with an additional mean increase of 1.5 functional limitations per person among those with no, mild or moderate pre-existing functional limitations. Thus, sepsis is often a sentinel event in the lives of older patients, initiating major and enduring cognitive and functional declines with lasting implications for patients’ independence, for their loved ones, and for the societal institutions charged with supporting them. Future research to identify mechanisms leading from sepsis to cognitive impairment and functional disability—and interventions to prevent or slow these accelerated declines—is especially important now given the aging of the population.
Supplementary Material
Acknowledgments
This work was supported by NIH grants K08 HL091249, R01 AG027010 and R01 AG030155, the Society of Critical Care Medicine’s 2010 Vision Grant, and by pilot support from the Michigan Institute for Clinical and Health Research (MICHR), UL1RR024986. The National Institute on Aging provides funding for the Health and Retirement Study (U01 AG09740) which is performed at the Institute for Social Research, University of Michigan. Dr. Ely was supported by the National Institutes of Health (AG001023), Veterans Affairs Merit Award and Tennessee Valley Geriatric Research, Education and Clinical Center (GRECC). Consultative support was provided by the Measurement Core of the Michigan Diabetes Center (NIDDK P60 DK-20572). The funders played no role in the design, interpretation, or decision to publish the analysis presented here.
We thank Mohamed Kabeto, MS, Ryan McCammon, AB, Lili Deng, MD, MA, and Tish Shapiro, MA, all at the University of Michigan, for their expert programming; they were financially compensated for their work. We further thank Robert Hyzy, MD, and Rodney Hayward, MD, of the University of Michigan for insightful critiques; they received no compensation for their work. We thank the Contributing Editor and anonymous reviewers for their helpful suggestions.
Footnotes
The authors report no conflict of interest. TJI had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Rozzini R, Sabatini T, Cassinadri A, et al. Relationship between functional loss before hospital admission and mortality in elderly persons with medical illness. Journal fo Gerontology: A Biol Sci Med Sci. 2005 Sep;60(9):1180–1183. doi: 10.1093/gerona/60.9.1180. [DOI] [PubMed] [Google Scholar]
- 2.Fried TR, Bradley EH, Williams CS, Tinetti ME. Functional Disability and Health Care Expenditures for Older Persons. Archives of Internal Medicine. 2002;161:2602–2607. doi: 10.1001/archinte.161.21.2602. [DOI] [PubMed] [Google Scholar]
- 3.Langa KM, Chernew ME, Kabeto MU, et al. National Estimates of the Quantity and Cost of Informal Caregiving for the Elderly with Dementia. Journal of General Internal Medicine. 2001;16(11):770–778. doi: 10.1111/j.1525-1497.2001.10123.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fried TR, Bradley EH, Towle VR, Allore H. Understanding the Treatment Preferences of Seriously Ill Patients. New England Journal of Medicine. 2002;346(14):1061–1066. doi: 10.1056/NEJMsa012528. [DOI] [PubMed] [Google Scholar]
- 5.Angus D, Linde-Zwirble W, Lidicker J, Clermont G, Carcillo J, Pinsky M. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Critical Care Medicine. 2001;29(7):1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Yende S, Angus DC. Long-term Outcomes from Sepsis. Current Infectious Disease Reports. 2007;9:382–386. doi: 10.1007/s11908-007-0059-3. [DOI] [PubMed] [Google Scholar]
- 7.Winters BD, Eberlein M, Leung J, Needham DM, Pronovost PJ, Sevransky JE. Long-term mortality and quality of life in sepsis: a systematic review. Critical Care Medicine. 2010 May;38(5):1276–1283. doi: 10.1097/CCM.0b013e3181d8cc1d. [DOI] [PubMed] [Google Scholar]
- 8.Boyd CM, Landefeld CS, Counsell SR, et al. Recovery of activities of daily living in older adults after hospitalization for acute medical illness. Journal of the American Geriatrics Society. 2008 Dec;56(12):2171–2179. doi: 10.1111/j.1532-5415.2008.02023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fong TG, Jones RN, Shi P, et al. Delirium accelerates cognitive decline in Alzheimer disease. Neurology. 2009;72:1570–1575. doi: 10.1212/WNL.0b013e3181a4129a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holmes C, Cunningham C, Zotova E, et al. Systemic inflammation and disease progression in Alzheimer disease. Neurology. 2009;73(10):768–774. doi: 10.1212/WNL.0b013e3181b6bb95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Herridge MS, Cheung AM, Tansey CM, et al. One-Year Outcomes in Survivors of the Acute Respiratory Distress Syndrome. New England Journal of Medicine. 2003;348(8):683–693. doi: 10.1056/NEJMoa022450. [DOI] [PubMed] [Google Scholar]
- 12.Chelluri L, Pinsky M, Donahoe M, Grenvik A. Long-term outcome of critically ill elderly patients requiring intensive care. JAMA. 1993;269(24):3119–3123. [PubMed] [Google Scholar]
- 13.Hopkins RO, Weaver LK, Collingridge D, Parkinson RB, Chan KJ, Orme JJF. Two-Year Cognitive, Emotional, and Quality-of-Life Outcomes in Acute Respiratory Distress Syndrome. American Journal of Respiratory and Critical Care Medicine. 2005;171(4):340–347. doi: 10.1164/rccm.200406-763OC. [DOI] [PubMed] [Google Scholar]
- 14.Kaarlola A, Tallgren M, Pettila V. Long-term survival, quality of life, and quality-adjusted life-years among critically ill elderly patients. Critical Care Medicine. 2006 Aug;34(8):2120–2126. doi: 10.1097/01.CCM.0000227656.31911.2E. [DOI] [PubMed] [Google Scholar]
- 15.Martin GS, Mannino DM, Eaton S, Moss M. The Epidemiology of Sepsis in the United States from 1979 through 2000. New England Journal of Medicine. 2003;348(16):1546–1554. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 16.Health and Retirement Study. [Accessed 27 October 2009];Sample Sizes and Response Rates. 2008 Available at http://hrsonline.isr.umich.edu/sitedocs/sampleresponse.pdf.
- 17.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Medical Care. 2005 Nov;43(11):1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 18.Fonda S, Herzog AR. Documentation of Physical Functioning Measured in the Heath and Retirement Study and the Asset and Health Dynamics among the Oldest Old Study. Vol. 2004 Ann Arbor, Michigan: Survey Research Center; Dec 21, 2004. [Google Scholar]
- 19.Herzog AR, Wallace RB. Measure of Cognitive Functioning in the AHEAD Study. Journal of Gerontology, Series B. 1997;52B(Special Issue):37–48. doi: 10.1093/geronb/52b.special_issue.37. [DOI] [PubMed] [Google Scholar]
- 20.Welsh KA, Breitner JCS, Magruder-Habib KM. Detection of Dementia in the Ederly Using Telephone Screening of Cognitive Status. Neuropsychology and Behavioral Neurology. 1996;6:103–110. [Google Scholar]
- 21.Jorm AF. The Informant Questionnaire on cognitive decline in the elderly (IQCODE): a review. Int Psychogeriatr. 2004 Sep;16(3):275–293. doi: 10.1017/s1041610204000390. [DOI] [PubMed] [Google Scholar]
- 22.Langa KM, Larson EB, Karlawish JH, et al. Trends in the prevalence and mortality of cognitive impairment in the United States: Is there evidence of a compression of cognitive morbidity? Alzheimer's & Dementia. 2008;4:134–144. doi: 10.1016/j.jalz.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alzheimer's Association. [Accessed on 26 August 2010];Early Onset Dementia: A National Challenge, a Future Crisis. 2006 Available at: http://www.alz.org/national/documents/report_earlyonset_full.pdf.
- 24.Langa KM, Plassman BL, Wallace RB, et al. The Aging, Demographics, and Memory Study: Study Design and Methods. Neuroepidemiology. 2005;25:181–191. doi: 10.1159/000087448. [DOI] [PubMed] [Google Scholar]
- 25.Ofstedal MB, Fisher GG, Herzog AR. [Accessed 25 August 2010];Documentation of Cognitive Functioning Measures in the Health and Retirement Study. 2005 available at http://hrsonline.isr.umich.edu/sitedocs/userg/dr-006.pdf.
- 26.Allison PD. Fixed Effects Regression Models. Thousand Oaks: Sage; 2009. [Google Scholar]
- 27.Banaszak-Holl J, Fendrick AM, Foster NL, et al. Cognitive function and chronic medical conditions as predictors of nursing home admission in a nationally representative sample of older Americans. Alzheimer's Disease and Associated Disorders. 2004;18(2):83–89. doi: 10.1097/01.wad.0000126619.80941.91. [DOI] [PubMed] [Google Scholar]
- 28.Zivin K, Llewellyn D, Lang I, et al. Depression among older adults in the US and England. American Journal of Geriatric Psychiatry. 2010 doi: 10.1097/JGP.0b013e3181dba6d2. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yaffe K, Fox P, Newcomer R, et al. Patient and caregiver characteristics and nursing home placement in patients with dementia. JAMA. 2002;287(16):2090–2097. doi: 10.1001/jama.287.16.2090. [DOI] [PubMed] [Google Scholar]
- 30.Mehta KM, Yaffe K, Langa KM, Sands L, Whooley MA, Covinsky KE. Additive effects of cognitive function and depressive symptoms on mortality in elderly community-living adults. Journal fo Gerontology: A Biol Sci Med Sci. 2003 May;58(5):M461–467. doi: 10.1093/gerona/58.5.m461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ziegler-Graham K, Brookmeyer R, Johnson E, Arrighi HM. Worldwide variation in the doubling time of Alzheimer’s disease incidence rates. Alzheimer's & Dementia. 2008;4:316–323. doi: 10.1016/j.jalz.2008.05.2479. [DOI] [PubMed] [Google Scholar]
- 32.Saint S, Savel RH, Matthay MA. Enhancing the safety of critically ill patients by reducing urinary and central venous catheter-related infections. American Journal of Respiratory & Critical Care Medicine. 2002 Jun 1;165(11):1475–1479. doi: 10.1164/rccm.2110035. [DOI] [PubMed] [Google Scholar]
- 33.Sharshar T, Bastuji-Garin S, Stevens RD, et al. Presence and severity of intensive care unit-acquired paresis at time of awakening are associated with increased intensive care unit and hospital mortality. Critical Care Medicine. 2009 Dec;37(12):3047–3053. doi: 10.1097/CCM.0b013e3181b027e9. [DOI] [PubMed] [Google Scholar]
- 34.Callahan LA, Supinski GS. Sepsis-Induced Myopathy. Critical Care Medicine. 2009;37(10S):S354–S367. doi: 10.1097/CCM.0b013e3181b6e439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schweickert WD, Hall J. ICU-Acquired Weakness. Chest. 2007;131:1541–1549. doi: 10.1378/chest.06-2065. [DOI] [PubMed] [Google Scholar]
- 36.Needham DM. Mobilizing Patients in the Intensive Care Unit: Improving Neuromuscular Weakness and Physical Function. JAMA. 2008;300(14):1685–1690. doi: 10.1001/jama.300.14.1685. [DOI] [PubMed] [Google Scholar]
- 37.Schweickert WD, Pohlman MC, Pohlman AS, et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. The Lancet. 2009;373:1874–1882. doi: 10.1016/S0140-6736(09)60658-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kuo H-K, Sorond F, Iloputaife I, Gagnon M, Milberg W, Lipsitz LA. Effect of Blood Pressure on Cognitive Functions in Elderly Persons. Journal of Gerontology, Series A Biological Sciences and Medical Sciences. 2004;59:1191–1194. doi: 10.1093/gerona/59.11.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Langa KM, Foster NL, Larson EB. Mixed dementia: emerging concepts and therapeutic implications. JAMA. 2004 Dec 15;292(23):2901–2908. doi: 10.1001/jama.292.23.2901. [DOI] [PubMed] [Google Scholar]
- 40.Mikkelsen ME, Lanken PE, Biester R, et al. Conservative Fluide Strategy is Associated with Neurocognitive Deficits in Survivors of Acute Lung Injury. Paper presented at: American Thoracic Society; May 21, 2008; Toronto, Ontario. 2008. [Google Scholar]
- 41.Kuo HK, Yen CJ, Chang CH, Kuo CK, Chen JH, Sorond F. Relation of C-reactive protein to stroke, cognitive disorders, and depression in the general population: systematic review and meta-analysis. Lancet Neurology. 2005 Jun;4(6):371–380. doi: 10.1016/S1474-4422(05)70099-5. [DOI] [PubMed] [Google Scholar]
- 42.Sprung CL, Peduzzi PN, Shatney CH, et al. Impact of encephalopathy on mortality in the sepsis syndrome. The Veterans Administration Systemic Sepsis Cooperative Study Group. Critical Care Medicine. 1990;18(8):801–806. doi: 10.1097/00003246-199008000-00001. [DOI] [PubMed] [Google Scholar]
- 43.Ely EW, Shintani A, Truman B, et al. Delirium as a Predictor of Mortality in Mechanically Ventilated Patients in the Intensive Care Unit. JAMA. 2004;19(14):1753–1762. doi: 10.1001/jama.291.14.1753. [DOI] [PubMed] [Google Scholar]
- 44.Jackson JC, Gordon SM, Hart RP, Hopkins RO, Ely EW. The Association Between Delirium and Cognitive Decline: A Review of the Empirical Literature. Neuropsychology Review. 2004;14(2):87–98. doi: 10.1023/b:nerv.0000028080.39602.17. [DOI] [PubMed] [Google Scholar]
- 45.Girard TD, Jackson JC, Pandharipande PP, et al. Delirium as a predictor of long-term cognitive impairment in survivors of critical illness. Critical Care Medicine. 2010;38(7):1513–1520. doi: 10.1097/CCM.0b013e3181e47be1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Iwashyna TJ. Survivorship will be the defining challenge of critical care in the 21st century. Annals of Internal Medicine. 2010 Aug 3;153(3):204–205. doi: 10.7326/0003-4819-153-3-201008030-00013. [DOI] [PubMed] [Google Scholar]
- 47.McConnell S, Stuart EA, Devaney B. The truncation-by-death problem: what to do in an experimental evaluation when the outcome is not always defined. Evaluation Review. 2008 Apr;32(2):157–186. doi: 10.1177/0193841X07309115. [DOI] [PubMed] [Google Scholar]
- 48.Boyd CM, Ricks M, Fried LP, et al. Functional Decline and Recovery of Activities of Daily Living in Hospitalized, Disabled Older Women: The Women’s Health and Aging Study I. Journal of the American Geriatrics Society. 2009;57(10):1757–1766. doi: 10.1111/j.1532-5415.2009.02455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Boyd CM, Xue QL, Guralnik JM, Fried LP. Hospitalization and development of dependence in activities of daily living in a cohort of disabled older women: the Women's Health and Aging Study I. Journal fo Gerontology: A Biol Sci Med Sci. 2005 Jul;60(7):888–893. doi: 10.1093/gerona/60.7.888. [DOI] [PubMed] [Google Scholar]
- 50.Covinsky KE, Palmer RM, Fortinsky RH, et al. Loss of Independence in Activities of Daily Living in Older Adults Hospitalized with Medical Illnesses: Increased Vulnerability with Age. Journal of the American Geriatrics Society. 2003;51:451–458. doi: 10.1046/j.1532-5415.2003.51152.x. [DOI] [PubMed] [Google Scholar]
- 51.Plassman BL, Langa KM, Fisher GG, et al. Prevalence of Dementia in the United States: The Aging, Demographics, and Memory Study. Neuroepidemiology. 2007;29(1–2):125–132. doi: 10.1159/000109998. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.