Abstract
The four colony stimulating factors (CSFs) are glycoproteins regulating the generation and some functions of infection-protective granulocytes and macrophages. Recombinant G-CSF and GM-CSF have now been used to elevate dangerously low white blood cell levels in many millions of cancer patients following chemotherapy. These CSFs also release haematopoietic stem cells to the peripheral blood (PBSC) and these cells have now largely replaced bone marrow as more effective populations for transplantation to cancer patients with treatment-induced bone marrow damage.
When I began my career in leukaemia research in 1954, most workers in the field were searching for human leukaemia viruses. My interest in the quite different field of blood cell regulators had been aroused by work with tumours of endocrine target tissues, such as the thyroid or breast1. In elegant studies, Furth had shown that if mice were subjected to a sustained imbalance in hormones favouring cell proliferation, tumour development occurred in a stepwise fashion in the target tissues2,3. When thinking about how leukaemia might initiate, I was intrigued by the ideas of Furth in his 1954 essay: “On the basis of events with other regulated cells it can be postulated that a permanent disturbance of the homeostatic balance might result in leukaemias in which the proliferating cells are essentially unaltered, and which could be controlled at their inception by restoration of the deranged equilibrium of the regulatory forces.”3. In the context of leukaemia, although commonsense said that regulators must exist to control white blood cells, unfortunately nothing was known about the possible nature of these regulators.
Discovery, purification and cloning of the CSFs
Prior to the 1960s, many investigators had performed experiments in intact animals to discover possible regulators of white blood cell homeostasis, but nothing of substance had been observed. The situation changed dramatically in 1965–66 when two groups simultaneously developed methods for growing colonies of white blood cells from mouse bone marrow or spleen cells in semi-solid agar and later in methylcellulose cultures4,5. The colonies, as initially grown, contained maturing neutrophilic granulocytes (hereafter simply called granulocytes or neutrophils) and/or macrophages. The remarkable features of these colonies were that they were clones derived from single precursor cells (later termed progenitor cells (Box 1)) and that the formation, number and size of colonies were absolutely dependent on the amount of cells, tissue extracts, or medium conditioned by various tissues that were added to the cultures4,6. The culture system clearly was dependent on the presence of an unknown active factor(s) (given the operational term, colony-stimulating factor, CSF)7 that was needed to stimulate cell division. Subsequent efforts succeeded in growing comparable colonies from human marrow cells, using underlayers of white blood cells as ‘feeder layers’ that provided a source of the as yet unknown CSF8.
Box 1.
The Road Map of Haematopoiesis
-
Stratified Hierarchy of Haematopoiesis
Three sequential classes of increasingly numerous ancestors exist in the bone marrow that generate maturing blood cells28. A major separation occurs into cells committed to the formation of myeloid cells and those committed to the formation of T and B lymphocytes. Dendritic cells can be derived from both groups157. Cells committed to one or other group can have their lineage commitment switched artificially by overexpression of genes such as GATA-1 or PU.1158.
-
Responsiveness to Regulators
Committed myeloid progenitor cells and their progeny can respond to a single CSF regulator but proliferation is enhanced synergistically by combining regulators. Less mature precursors require costimulation by multiple regulators28.
-
Common Ancestors
Many granulocyte and macrophage precursors have common ancestral cells as do many erythroid and megakaryocyte precursors.
-
Heterogeneity of Individual Cells
Within each maturation category of granulocytes and macrophages there is wide heterogeneity between individual cells in quantitative responsiveness to CSF stimulation and some cells respond better or only to one particular CSF28.
Initial studies indicated that CSF was probably not a virus that had transformed marrow cells (at that time only transformed cells were believed capable of proliferation in agar medium), was not some trivial nutritional material, and was probably a protein. Efforts to purify CSF occupied many laboratories during 1968–1985. Initially human urine was used as source material9, then mouse organ or cell line conditioned medium and eventually comparable media from human cells or human tumour cell conditioned media were used. The task proved to be formidable. It slowly became inescapable that there was not a single CSF but, in fact, there were four quite different glycosylated CSF proteins each with differing colony stimulating activity. The task of separating and purifying these four CSFs was rendered much more difficult by variable glycosylation of the CSFs and the minute amounts of CSF in tissues. The four CSFs were given working names that indicated the most numerous type of colony stimulated – GM-CSF (also known as CSF2), stimulating granulocyte and macrophage colony formation; M-CSF (also termed CSF1), stimulating macrophage colony formation; G-CSF (also known as CSF3), stimulating granulocyte colony formation; and multi-CSF (now more commonly termed interleukin 3, IL3) stimulating a broad range of haematopoietic cell colony types. In two instances, purification of more than half a million-fold was required to achieve pure CSF. It required the introduction and application of high-performance liquid chromatography to lead to eventual success in these purification attempts. Purification of murine GM-CSF10 and M-CSF11 was reported in 1977, IL3 in 198212 and G-CSF in 198313. Purification of human CSFs corresponding to the four murine factors followed the purifications of murine CSFs, with the investigators making better use of human tumour cell lines as superior sources of CSFs14–18.
The molecular biology cloning of the cDNAs for all four CSFs, both mouse and human, from libraries using either sequence-based probes or expression screening, was achieved between 1984–1986 and were some of the earliest successes of molecular biology16, 19–27. This was followed by the then difficult task of achieving expression of active protein either in bacterial, yeast or mammalian cell systems, but eventually adequate expression systems were developed.
Biology of the CSFs
The CSFs are 18–70 kDa glycoproteins and unlike the comparable erythroid regulator, erythropoietin (EPO), the CSFs are active in vivo in both glycosylated and non-glycosylated forms. The half-lives of glycosylated CSFs are longer than non-glycosylated CSFs, but are still only a matter of 1 to 6 hrs28. Unexpectedly, CSFs were found in studies between 1966 and 1984 to be the products or potential products of most tissues and cell types in the body28. Normal levels of production were very low, even in the most active tissues, but CSF production was markedly inducible by microorganisms, endotoxin or foreign cells, which could increase production up to 1,000-fold within hours28. The CSFs can therefore be viewed as highly labile agents that are produced rapidly and to high levels in the presence of an inducing agent. M-CSF differs from this in that it is produced in higher concentrations in a much more stable manner. The lability and the short lifespan of CSF molecules makes the CSFs able to function as a highly responsive control system regulating haematopoietic cells.
CSFs are secreted and enter the circulation in active form. The CSFs, at times, resemble hormones, except for the multiplicity of cell types able to produce CSFs, but at other times CSFs are produced and act in a paracrine fashion in local microenvironments. Specific membrane receptors exist that are unique for each CSF and are displayed in small numbers on all maturation stages of cells in the granulocyte and monocyte-macrophage lineages from committed progenitor cells to post-mitotic mature cells in the peripheral blood and tissues (Box 1). The CSFs are removed from the circulation by binding to the specific membrane receptors displayed on granulocytic and macrophage cells, then, after internalisation of the CSF-receptor complex, are degraded28,29. Degradation and/or clearance of CSFs also occurs in the liver and kidney28.
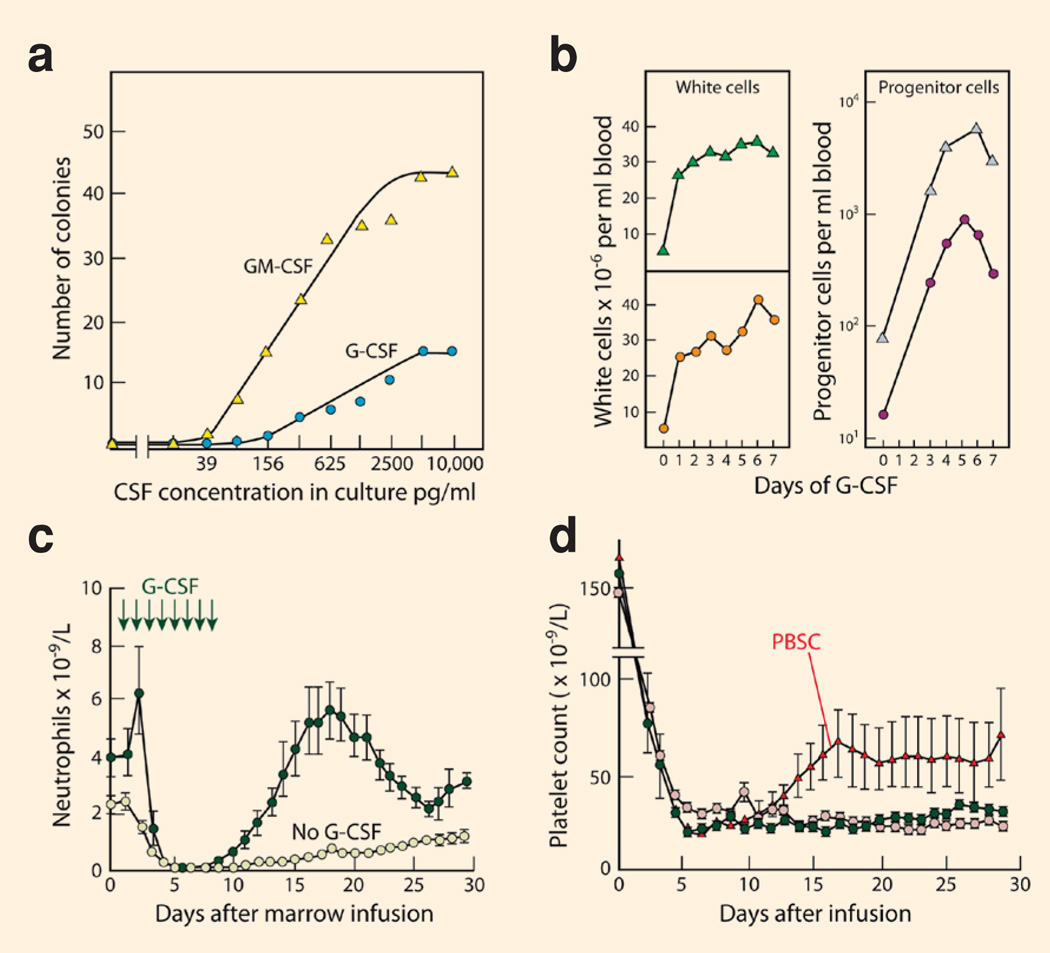
The CSFs proved to be notable because of their multiple actions on haematopoietic cells (Box 2). The CSFs are mandatory to stimulate the division of every appropriate lineage-committed haematopoietic progenitor cell and its progeny. Haematopoietic progenitors exhibit great heterogeneity in their responsiveness to CSF stimulation, resulting in the characteristic sigmoid dose response curves shown in Figure 1a as more and more progenitor cells are stimulated to commence proliferation11–13, 28 (Box 1). Individual progenitor cells also vary in their proliferative activity but, in general, as CSF concentrations are increased, cell cycle times are shortened and there is a progressive increase in the number of progeny cells in each colony. The cells in developing granulocyte-macrophage colonies exhibit progressive maturation with time so higher CSF concentrations achieve higher numbers of mature progeny cells28.
Box 2.
Operating through a single type of membrane receptor on responding cells, the CSFs are able to elicit a surprising range of biological responses. They are necessary to initiate in a dose-responsive manner every cell division in responding cells28. They prevent cell death from apoptosis36–39. They can, arguably, initiate lineage-commitment and maturation in appropriate haematopoietic subpopulations42–44. Finally, they have powerful effects on the survival and functional activity of mature cells47–51. These pleotropic effects are made possible by distinct regions of CSF-activated cytoplasmic domains in the receptors52–59.
Figure 1. The biological actions of the CSFs.
(a) CSF-stimulated colony formation in vitro by lineage-committed progenitor cells in mouse bone marrow, the sigmoid dose response indicating heterogeneity in the responsiveness of the progenitor cells. Adapted from Fig. 7.1, page 111 of REF. 28; (b) In man, injected G-CSF not only increases peripheral blood neutrophil levels but increases peripheral blood progenitor cells 100-fold. Data shown are from two patients injected with 10µg/Kg G-CSF daily for 7 days. Adapted from Fig. 5, page 4443 of REF. 156; (c) In transplanted cancer patients, the injection of G-CSF accelerates the recovery of neutrophil levels following chemotherapy, allowing a shorter duration of hospitalisation. Adapted from Fig. 1, page 893 of REF. 159; (d) Transplantation of CSF-mobilised peripheral blood stem cells augments the rate of recovery of platelet levels following chemotherapy compared with control patients receiving marrow transplants. Adapted from Fig. 4, page 643 of REF. 136.
When marrow cultures were more carefully analysed and purified native or recombinant CSFs became available for more general use, it was recognised between 1977 and 1987 that agar cultures of bone marrow could sustain the development not only of granulocyte and macrophage colonies, but also colonies of eosinophils, megakaryocytes, mast cells, erythroid cells, blast cells and T and B lymphocytes. It also became apparent that the prefixes used to describe the CSFs under-represented their action. GM-CSF also stimulated eosinophil colony formation and, at high concentrations, megakaryocyte colony formation30; G-CSF had a minor capacity to stimulate some granulocyte-macrophage colonies31. M-CSF could stimulate granulocyte colony formation by some progenitor cells28 and IL3 could stimulate colony formation by progenitors of blast cells, granulocytes, macrophages, megakaryocytes, eosinophils, mast cells and erythroid cells32. When used in combination with an agent like stem cell factor (SCF, also known as KIT ligand), the CSFs could co-stimulate the proliferation of the earliest haematopoietic cells (see Box 1)33–35.
It became apparent in 1967 that the CSFs were necessary for the survival of the progenitor (colony-forming) cells and their progeny in culture36–38 and in 1990 withdrawal of CSF was shown to lead to death from apoptosis39. This initially prompted some to postulate that the CSFs had only survival effects and that cells were then able to proliferate spontaneously. This improbable suggestion was discounted by the persisting dependency on CSF for proliferation of cell lines whose survival had been ensured by overexpression of BCL240 and by the characterisation of some of the intracellular mitotic signals including JAK-STAT and cyclin activations initiated when CSFs interact with their membrane receptors41.
More controversial have been the findings that CSFs appear able to initiate maturation events in leukaemic cell lines13,42, and possibly, at times, to dictate commitment decisions in granulocyte-macrophage precursors in vitro 43, 44. These actions require further elucidation because some maturation was observed in a BCL2-immortalized cell line in the absence of CSFs45 and CSFs can stimulate cell proliferation in unusual cell types following insertion and expression of CSF receptors into such cells46. This type of stimulation, however, does not alter the phenotype of the cells responding to the CSF.
Finally, CSFs clearly have the capacity to stimulate the functional activity of mature cells. For example, GM-CSF can act on mature neutophils to exhibit chemotaxis, enhance oxidative metabolism, enhance antibody-dependent phagocytosis and killing of microorganisms and produce a variety of regulatory proteins. Similar actions have been documented on eosinophils and monocytes. These actions have been noted both in vitro and in vivo. A similar range of actions has been documented for G-CSF, M-CSF and IL-3 acting on mature neutrophils or monocyte-macrophages28, 47–51.
It was initially puzzling how a single agent, acting at very low molar concentrations on the few hundred receptors present on responding cells could induce such diverse changes52. This problem was made more complex by the recognition that only a single type of receptor existed for each CSF and that receptors for all four CSFs can co-exist on most granulocytic and macrophage cells. The problem was resolved in the early 1990s when the specific membrane receptors for each CSF were characterised and cloned. The membrane receptors in simplest form, such as for G-CSF, are homodimers53 but the receptor chains can be arranged in more complex form for agents such as GM-CSF where the heterodimeric receptor is arranged as a dodecamer complex54. All CSF receptors exhibit specific regions in their cytoplasmic domain of their signalling chains that are able to initiate the different signalling events that are required to induce a varied range of biological responses55–59.
In the period following the discovery of the CSFs, other regulators of haematopoietic populations were discovered, resulting in a confusing picture of further potential redundancies or interactions in the control system. For example, granulocyte colony formation in vitro can be stimulated by G-CSF, GM-CSF, M-CSF, IL3, SCF, IL-6 and weakly by IL1128. In particular, the CSFs appeared to have many biological actions that were potentially overlapping or redundant and it required gene knockout studies in mice in the mid 1990s to establish that each CSF did, in fact, have actions that were exclusive to that CSF.
For example, G-CSF was clearly responsible for the formation of the 75% of granulocytes under basal conditions60. GM-CSF, by contrast, did not appear to influence mature cell numbers. Instead, it was essential for the functional activity of macrophages, particularly those in the lung. In mice, the absence of GM-CSF or its receptor leads to alveolar proteinosis – a lung disease due to failure of local macrophages to eliminate surfactant61,62 and the same disease state has been noted in those humans producing neutralising autoantibodies against GM-CSF63. M-CSF was necessary for the formation and function of major macrophage populations and, strangely, was necessary also for tooth eruption and successful pregnancy64–66. IL3 was expected to be a regulator of major importance, but mice lacking IL3 receptors showed no obvious changes in haematopoiesis67,68. Later studies in mice showed that IL3 did have significant actions in allowing satisfactory mast cell and basophil responses to parasites69 and hapten-specific delayed-type hypersensitivity responses70.
What has emerged from knockout studies on the CSFs and other regulators is that each regulator does have some unique actions in vivo but, importantly, the body design often calls for synergistic actions between two or more regulators on many haematopoietic cell. For example, the dramatic effects of G-CSF in vivo (see below) require the synergistic action of SCF71 and strong synergy is observable on granulocyte-macrophage progenitor cells in vitro between GM-CSF and M-CSF or G-CSF and on less mature blast colony-forming cells between G-CSF and SCF or SCF and IL-634,72. Conversely, some combinations are inhibitory. For example, G-CSF inhibits megakaryocyte colony formation stimulated by SCF and EPO73.
One picture that has emerged from the culture of mouse bone marrow cells is that lineage-committed progenitor cells can respond to single regulators but that more immature cells require two or more regulators acting in concert before proliferation occurs (Box 1). There are exceptions, and, for example, optimal proliferation of mature megakaryocyte progenitor cells requires SCF, IL-3 and EPO74, as does the proliferation of subsets of apparently lineage-committed progenitor cells that co-fractionate after FACS separation with stem cell and CFU-S (colony-forming unit, spleen, early progeny of stem cells) populations75.
In vivo actions of the CSFs in mice
By the early 1980s the growing evidence for the existence of multiple regulators for haematopoietic tissues raised the spectre that complex interactions between these regulators might dampen or prevent any one agent from eliciting measurable responses in vivo.
This was not the case with the CSFs when recombinant murine CSFs became available for testing in the mid 1980s. Injection of CSFs in mice elicited responses that were qualitatively similar to the actions observed in vitro. Twice daily subcutaneous injections of G-CSF within four days elicited major rises in blood neutrophil levels following increased production of granulocytes in the bone marrow76. Intraperitoneal injections of GM-CSF in mice had lesser effects on circulating white cell levels but strongly elevated peritoneal macrophage numbers and proliferative activity77. Subcutaneous injections of murine IL3 increased bone marrow cellularity and particularly the numbers of mast cells in various tissues78,79. It was also evident that GM-CSF and IL3 injections in mice increased the phagocytic activity of mature macrophages for antibody-coated erythrocytes77,78.
An obvious question to pose was whether CSF injections could enhance resistance in mice to serious fungal or bacterial infections of the type encountered in cancer patients following chemotherapy. This question was examined in mice at the time of the earliest clinical trials on CSFs. In particular, G-CSF injections were tested in multiple infectious disease models, and found to clearly enhance resistance to and survival from a variety of infectious organisms80–84. An important conclusion from these studies was that CSF administration prior to challenge with infectious agents was highly effective, whereas if CSF was administered after infections were initiated, the protective effects were minimal and were only significant if combined with antibiotics.
Do excessive levels of CSF induce toxic effects in mice?
Excess GM-CSF levels either in transgenic mice or in mice repopulated by marrow cells engineered to overexpress GM-CSF, caused excess numbers of granulocytes and macrophages to develop and induced a variety of fatal inflammatory lesions in the lung, muscles, bowel and peritoneal cavity85,86. Similarly, in mice repopulated by marrow cells expressing excess IL3 levels, hyperproliferation of haematopoietic and mast cell populations occurred. This was associated with uncontrollable itching and scratching probably because of mast cell degranulation in the skin87. Although repopulation of mice with marrow cells expressing excess levels of G-CSF induced excessive granulopoiesis and very high granulocyte levels, these caused no apparent tissue damage88. In subsequent studies, this was radically altered by knocking out the suppressor of cytokine signaling 3 (Socs3) gene. SOCS3 is one of the SOCS family of cytoplasmic suppressors of cytokine-initiated receptor signalling and suppresses signalling from activated G-CSF receptors89. Mice lacking Socs3 are hyper-responsive to G-CSF and administration of even normal doses of G-CSF caused hind limb paralysis and death within days from massive accumulations of neutrophils in the spinal cord, liver, lungs and marrow90. The lack of toxicity of G-CSF in mice and presumably humans is therefore dependent on the modulating effects of SOCS3.
CSFs and myeloid leukaemia
From studies in the early 1970s on the clonal culture of primary human myeloid leukaemic cells, it was established that chronic myeloid leukaemia (CML) cells formed large, apparently normal, granulocyte or granulocyte-macrophage colonies in vitro91. In contrast, acute myeloid leukaemia (AML) cells often failed to proliferate or at best formed small clusters of progeny in vitro92. The striking observation was that all CML and most AML cells remained wholly dependent on stimulation by CSF-containing material for proliferation in vitro, the concentrations of CSF required being unexceptional. This situation did not change when purified CSFs later became available93.
This suggested that the CSFs, at a minimum, might be co-factors in the development of myeloid leukaemia, if for no other reason than that they could supply the proliferative and survival stimuli for the clonal expansion of emerging leukaemia cells in vivo.
Do sustained excess levels of CSF lead to myeloid leukaemia development? This question has only been posed for GM-CSF. While lifelong excess GM-CSF levels in transgenic mice were not leukaemogenic94, these mice were more susceptible to leukaemic transformation by the Moloney leukaemia virus95. Repopulation of mice for a period of a few months with marrow cells engineered to produce excess levels of G-CSF or IL3 did not lead to leukaemia development87,88. On this basis, the original simple hypothesis of leukaemia development that led to the search for the CSFs, seemed not to be sustained.
Despite these negative data, at least GM-CSF and IL3 can function as oncogenes in haematopoietic cells. In the initial study, GM-CSF cDNA was inserted in vitro into FDC-P1 cells, which is a mouse immortalised haematopoietic cell line. These cells have remained CSF-dependent in culture for the past 25 years and remain non-leukaemic. However, after transfection with GM-CSF cDNA, the FDC-P1 cells were immediately transformed to cells that showed factor-independent growth in vitro and behaved as leukaemic cells when transplanted in vivo96.
In a related series of studies, non-leukaemic FDC-P1 cells were injected into pre-irradiated recipients. The injected cells had therefore not themselves been subject to irradiation but were in a host better able to support the survival of these factor-dependent cells. The cells remained dormant for up to 1 year but eventually most mice developed leukaemia97. In each case the leukaemic cells were derived from injected FDC-P1 cells that had acquired the autocrine capacity to produce either GM-CSF or IL398. Analyses showed that this autocrine capacity to produce CSF was determined by the activating insertion of intracisternal A particles in variable locations upstream of one or other CSF gene98. It is unresolved why extrinsically applied CSF in high concentrations does not transform immortalized cells such as FDC-P1 after decades in culture, whereas autocrine production of the same CSF leads to immediate transformation. However, the drawback to the FDC-P1 cell experiments was that the molecular basis of the original immortalization of this cell line was never clearly established.
In a more informative experiment, normal bone marrow cells were co-transfected with homeobox B8 (HOXB8, also known as HOX-2.4) and IL3 cDNAs. HOX-2.4 modulates self-renewal and again, there was immediate transformation of the transfected cells to growth factor independence in vitro and to leukaemogenicity in vivo99. This has given rise to the concept that, at least in mice, myeloid leukaemia development requires two types of change: an imbalance of lineage commitment at cell division favouring self-generation or immortalization in extreme form, and the acquisition of a capacity for autocrine growth stimulation.
There is no reason at present to suppose that myeloid leukaemia development in humans differs in principle from that in the mouse. The various leukaemia-associated genes affected by translocation or mutation that have been detected in one or other type of AML presumably achieve one or other of the two changes needed in leukaemogenesis100. Autocrine production of GM-CSF has been reported in some cases of AML101. However, what is of interest in view of the murine data is that autocrine production of CSF by myeloid leukaemic cells does not appear to be as common in humans as it is in the mouse. It has been reported that, in early CML development, transient autocrine production of IL3 and G-CSF occurs102 and the presence of activating mutations in the transmembrane for the extracellular domains of CSF receptors remains a possibility in some AML populations103. More commonly, however, autocrine proliferative stimulation in human AML seems to be achieved by other mechanisms, such as activation of cellular MYC or Ras genes.
A curious outcome of the ability of CSFs to enforce maturation in responding haematopoietic cells is seen in the action of CSFs on some myeloid leukaemia populations. The purification of murine G-CSF was originally monitored using in part an assay system in which proliferation of WEHI-3B myelomonocytic leukaemia cells was suppressed by G-CSF enforced maturation13. A dramatic example of this type of action was also observed with the murine GB-2 leukaemia cell line. When these undifferentiated cells were cultured in agar with various CSFs they formed well-differentiated colonies with the correct maturation pattern for the CSFs used42. Finally, it could be argued that when CSFs are used to stimulate the growth of human CML colonies in vitro which then show normal maturation, the CSF has induced this normal maturation. Unfortunately, responses to the maturation action of CSFs appear to be an uncommon feature of human AML populations and little evidence for a similar therapeutic action of CSFs has been observed in patients with AML receiving injections of CSF.
Clinical trials of CSFs and therapeutic applications
Results of the first clinical trials of CSFs were published in 1988 and 1989. In general these tests were performed following chemotherapy. Similar haematopoietic responses to those in the mouse were noted in these preliminary clinical trials on CSFs in so far as these could be monitored in the peripheral blood and marrow104–107. G-CSF injections elicited clear dose-responsive elevations of blood neutrophil levels and GM-CSF elicited somewhat smaller responses. Responses were maintained for as long as CSF injections were continued and from extended studies on children with abnormally low blood neutrophil levels, no loss of responsiveness was noted after repeated daily G-CSF injections108,109.
Based on these responses and the minimal toxicity associated with the injection of G-CSF or GM-CSF, the licensing of these agents for clinical research was prompt. For example, G-CSF was registered in the US in 1991 for use in the prophylaxis of febrile neutropenia in cancer patients following chemotherapy. Registration of both agents followed in other countries and the indications for clinical use were progressively widened. A less favourable outcome followed trials of M-CSF and IL3. Intravenous infusion of M-CSF in metastatic cancer patients was associated with a fall in platelet levels possibly due to macrophage activation110. Similarly, subcutaneous injections of IL3 in some relapsed lung cancer patients after chemotherapy did increase neutrophil levels but also led to adverse responses, some of which may have been due to mast cell activation111. Neither agent has entered clinical use because of the risk of unacceptable side-effects.
Leaving aside the possible special role that CSFs may have in the biology of myeloid leukaemia, the CSFs have had a major impact on the treatment of cancer in two situations: cytotoxic drug-induced neutropenia, and the common need to replace aplastic bone marrow with transplanted haematopoietic cells.
Treating chemotherapy-induced neutropenia
The most common complication of the treatment of cancer with chemotherapy is the development of neutropenia due to bone marrow damage. Low neutrophil levels are associated with a heightened risk of infection112 and a substantial proportion (60%) of patients with febrile neutropenia syndrome (low neutrophil levels plus fever) develop infections. This usually requires hospitalisation and intensive antibiotic therapy. Perhaps of more importance for those patients where the chemotherapy is potentially curative, episodes of neutropenia, with or without infections, disrupt scheduled chemotherapy resulting in either dose reduction or loss of treatment cycles.
The initial clinical trials of subcutaneously-injected recombinant human G-CSF and GMCSF were in a miscellany of cancer patients following chemotherapy and the results showed that administration of either agent could elevate neutrophil levels even after chemotherapy104–107. This resulted in a reduction in the duration and severity of the chemotherapy-induced neutropenia (Fig. 1c). In subsequent trials, it was documented in patients with small cell lung cancer and non-Hodgkin’s lymphoma that the use of G-CSF or GM-CSF reduced episodes of drug reduction and the frequency of infections113–117. Analysis showed that use of G-CSF allowed patients with chemotherapy-sensitive cancers, such as non-Hodgkin’s lymphoma or early stage breast cancer, to avoid dose reductions or delays in their chemotherapy and confirmed that this had an impact on patient survival118. GM-CSF was also used effectively to enhance haematopoietic regeneration after bone marrow transplantation119,120 and was initially licensed for clinical use for this purpose.
To date, approximately nine million patients have received G-CSF therapy. These were most often cancer patients in whom cytotoxic drugs had been used. Overall, meta-analyses of multiple controlled trials involving G-CSF have found that G-CSF reduces febrile neutropenia by 46%, the risk of infection-related mortality by 45% and the risk of early mortality from all causes by 40%118. Toxic side-effects of G-CSF have been minor, most commonly, slowly developing bone pain as marrow populations expand118. This may be related to the recent report that sensory nerves have receptors for G-CSF and GM-CSF and that both CSFs can sensitise these nerves to mechanical stimuli121.
Experience with the use of GM-CSF has been similar, if less extensive than with G-CSF, In the context of providing supporting treatment for patients on chemotherapy, clinical attention has properly been focussed on responding neutrophil levels because of the landmark study linking low neutrophil levels with infections112 and here G-CSF has a stronger action than has GM-CSF. The subtle differences in biological actions between G-CSF and GM-CSF, such as the special actions of GM-CSF on macrophages and dendritic cells, have not yet had much impact on the manner in which the two agents are used clinically.
A notable advance in the use of CSFs for post-chemotherapy neutropenia, was the development of polyethylene glycol-conjugated G-CSF (pegylated G-CSF, pegfilgrastim), which was approved for clinical use in 2002 following Phase II trials on cancer patients receiving chemotherapy122,123. The biological actions of this modified CSF are similar to those of G-CSF, but the larger size of the pegylated molecule prevents renal clearance and greatly increases the life-span of the molecule, a single injection of pegylated G-CSF being the equivalent of a series of daily injections of G-CSF. Studies have shown the efficacy of pegylated CSF in allowing full dose chemotherapy, particularly in elderly patients who would otherwise have been restricted to less toxic, mild to moderate chemotherapy118.
Currently, international guidelines recommend the use of G-CSF as primary prophylaxis when there is an increased risk of febrile neutropenia of greater than or equal to 20%, although the broader use of prophylactic CSF has been suggested118,124. How extensively a non-toxic agent is used is based in part on economic criteria and, when the costs of CSFs are reduced with the introduction of generic CSFs, the occasions on which CSFs may usefully be employed should increase.
From the biological point of view, current clinical practice is probably suboptimal because it has made no use of the powerful synergies to be obtained by combining CSFs or CSFs with other agents; and has made insufficient use of the fact that CSFs function best when used prophylactically before infections initiate and on bone marrow with reasonable cellularity.
As an aside, and for completeness, there are, of course, less common types of non-cancer patients, such as those with chronic neutropenia or cyclic neutropenia, where the use of G-CSF has been highly effective in preventing infections and has been administered for years without loss of activity or major adverse long-term effects 125,126.
It is of interest how the development of the CSFs paralleled that of erythropoietin (EPO), the corresponding regulator of erythroid cell populations. The existence of EPO was recognised long before that of the CSFs but, even so, EPO was purified in 1977127, cloned in 1985128 and was approved for clinical use in 1989. It is now used routinely in patients with anaemia associated with chronic renal disease and often in cancer patients with anaemia – in both situations to reduce the number of blood transfusions and increase survival and the quality of life129,130. In both types of patient, to avoid cardiovascular complications, EPO-induced rises in haemoglobin levels need to be restricted to below 120g/l.
Haematopoietic Transplantation
During the first clinical trials of G-CSF in cancer patients in 1988 an unexpected observation was made that the patients developed a 100-fold rise in the frequency of colony-forming progenitor cells in the peripheral blood (Fig. 1b)131. Rises in haematopoietic progenitor cell numbers were also noted in subjects injected with GM-CSF132,133. Although slightly delayed compared with the rises in mature neutrophil levels, the rises were of such a magnitude that it became an intriguing possibility that CSF-induced cells in the peripheral blood might be used for transplantation in place of harvested bone marrow cells.
Subsequent studies in mice in 1990 showed that G-CSF could also elicit rises in haematopoietic stem cells in the blood134. With this supporting information, clinical trials were initiated using peripheral blood cells harvested after injections of GM-CSF or G-CSF. Both types of peripheral blood stem cells (PBSC) achieved successful haematopoietic engraftment135,136.
The PBSCs generated were found to result in more rapid rates of restoration of peripheral blood neutrophil levels than those achieved by harvested bone marrow cells and to equal the recovery of cells in patients receiving marrow plus CSF137. In addition, CSF-induced PBSCs unexpectedly allowed a more rapid recovery of platelet levels (Fig. 1d)136,138. Thrombocytopenia, requiring treatment with platelet transfusions, is an important reason for continued hospitalisation of cancer patients with myelosuppression following chemotherapy. It is now accepted that the superiority of CSF-elicited PBSCs in transplantation is probably due to the ability to harvest higher numbers of stem and CD34+ progenitor cells than by routine bone marrow aspiration. Chemotherapy itself can elevate PBSCs139 and higher levels of harvested PBSCs can be obtained by combining CSF with chemotherapy. However, CSF alone usually achieves satisfactory yields of PBSCs and chemotherapy cannot be used when normal donors are providing PBSCs for allografting to patients.
The high cell yields possible after daily injection of CSF or a single injection of pegylated CSF are of great importance when low or medium intensity chemotherapy is used in the treatment of elderly patients. High numbers of haematopoietic progenitor and stem cells are required in these patients to effect adequate engraftment. This is not because of any failure to empty bone marrow niches in the recipient with the less severe chemotherapy. Populations of grafted cells with stable chimaerism can be achieved in normal mice simply by increasing the numbers of transplanted cells to a significant fraction of the resident cells in the recipient140. This same principle appears to be operative in elderly patients receiving low dose chemotherapy when the high numbers of harvested PBSCs allow adequate engraftment.
CSF-mobilised PBSCs have now become the dominant cell populations used in transplantations to cancer and other patients. Particularly for normal donors of PBSCs, the safety of G-CSF and GM-CSF is a matter of importance. Extensive clinical experience has shown that CSF induction is a safe procedure without any immediate or long-term consequences141.
Immunotherapy
Dendritic cells are key cellular components of immune responses because of their capacity to capture, process and present antigens to initiate responses in T lymphocytes. GM-CSF was observed to be a major regulator of dendritic cell development in vitro142–145, and this has led to study of the positive influence of GM-CSF on immune responses. With the increasing availability of tumour-specific peptides, there has been much interest in the possibility that GM-CSF might be able to enhance specific immune responses against tumour cells. The cancers most frequently considered in this context are melanomas and cancers of the kidney, lung and prostate – cancers for which some evidence exists that host responses can occasionally have significant anti-tumour effects. GM-CSF has variously been co-injected with tumour peptides, injected as a GM-CSF-peptide complex or transfected into sterilised autologous or similar tumour cells using retroviral or adenoviral vectors146. GM-CSF proved to be the most potent of ten candidate gene products in tests to detect enhanced responses elicited by transfected tumour cells147. Evidence of enhanced local immune responses within tumours has been obtained in a proportion of patients148–154. To date, positive clinical responses have been restricted to a small subset of injected patients and it is unclear whether these have been superior to those obtained with tumour peptides alone146,155. These are ongoing studies and it is too early to decide whether the use of GM-CSF in immunisation strategies will prove of clinical value.
Conclusions
It has been a long journey since the first agar cultures of marrow cells in 1966. The CSFs have emerged as key regulators of major haematopoietic lineages and two have been in clinical use for two decades to stimulate neutrophil and macrophage production and function, particularly in cancer patients. The use of CSFs to elicit peripheral blood stem cells has revolutionised haematopoietic transplantation, making it simpler, more efficient and more widely applicable in the clinic. Despite this progress, it is still early days in the clinical exploitation of the CSFs to further manipulate haematopoiesis to improve the management of cancer patients.
Although autocrine production of CSF can be involved as one of the steps in the development of myeloid leukaemia, the maturation-inducing effects of CSFs can, conversely, suppress some myeloid leukaemia populations. The clinical application of this complex biology again awaits future developments.
Timeline Table.
 |
Acknowledgments
The author is indebted to grant support from the Cancer Council of Victoria; The National Institutes of Health, Bethesda Grant No. CA22556; and the National Health and Medical Research Council, Canberra. Program Grant 461219.
Glossary of Terms
- Conditioned medium
medium harvested after incubation of cultured cells or tissues.
- Transformed
usually indicating an irreversible change from normal to neoplastic cells
- Lineage
a sub family of one type of haematopoietic cell ranging from immature to mature cells.
- Commitment
the change, usually, irreversible, when a multipotential cell generates or becomes a cell expressing membrane markers and a gene programme restricting the cell to a particular lineage.
- Maturation
the sequence of morphological and biochemical changes during which immature cells generate or become mature cells.
- Synergy
enhanced cellular responses when two or more regulators interact on target cells.
- SOCS family
a family of cytokine (regulator)-induced cytoplasmic inhibitors of signalling from regulator-activated membrane receptors.
- Immortalization
a change rendering cells capable of proliferation for prolonged (perhaps unlimited) time periods, the cells usually not being neoplastic.
- Neutropenia
abnormally low blood neutrophil levels.
- Thrombocytopenia
abnormally low blood platelet levels.
- Aplasia
severely reduced cellular content.
References
- 1.Metcalf D. Foundations in Cancer Research. Hemopoietic regulators and leukemia development: A personal retrospective. Advances in Cancer Research. 1994;6:41–91. doi: 10.1016/s0065-230x(08)60398-x. [DOI] [PubMed] [Google Scholar]
- 2.Furth J. Conditioned and autonomous neoplasms: A review. Cancer Res. 1953;13:477–492. [PubMed] [Google Scholar]
- 3.Furth J. The concept of conditioned and autonomous neoplasms. Leuk. Res. Ciba Found. Symp. 1954:38–41. [Google Scholar]
- 4.Bradley TR, Metcalf D. The growth of mouse bone marrow cells in vitro. Australian Journal of Experimental Biology and Medical Science. 1966;44:287–300. doi: 10.1038/icb.1966.28. [DOI] [PubMed] [Google Scholar]
- 5.Ichikawa Y, Pluznik DH, Sachs L. In vitro control of the development of macrophage and granulocyte colonies. Proceedings of the National Academy of Sciences, USA. 1966;56:488–495. doi: 10.1073/pnas.56.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pluznik DH, Sachs L. The induction of clones of normal“mast” cells by a substance in conditioned medium. Experimental Cell Research. 1966;43:553–563. doi: 10.1016/0014-4827(66)90026-7. [DOI] [PubMed] [Google Scholar]
- 7.Robinson WA, Metcalf D, Bradley TR. Stimulation by normal and leukaemic mouse sera of colony formation in vitro by mouse bone marrow cells. Journal of Cellular and Comparative Physiology. 1967;69:83–92. [Google Scholar]
- 8.Pike BL, Robinson WA. Human bone marrow colony growth in agar-gel. J. Cell Physiol. 1970;76:77–84. doi: 10.1002/jcp.1040760111. [DOI] [PubMed] [Google Scholar]
- 9.Stanley ER, Metcalf D. Partial purification of some properties of the factor in normal and leukaemic human urine stimulating mouse bone marrow colony growth in vitro. Australian Journal of Experimental Biology and Medical Science. 1969;47:467–483. doi: 10.1038/icb.1969.51. [DOI] [PubMed] [Google Scholar]
- 10.Burgess AW, Camakaris J, Metcalf D. Purification and properties of colony-stimulating factor from mouse lung conditioned medium. Journal of Biological Chemistry. 1977;252:1998–2003. [PubMed] [Google Scholar]
- 11.Stanley ER, Heard PM. Factors regulating macrophage production and growth: Purification and some properties of the colony stimulating factor from medium conditioned by mouse L cells. Journal of Biological Chemistry. 1977;252:4305–4312. [PubMed] [Google Scholar]
- 12.Ihle JN, Keller J, Henderson L, Klein F, Palaszynski E. Procedures for the purification of interleukin 3 to homogeneity. Journal of Immunology. 1982;129:2431–2436. [PubMed] [Google Scholar]
- 13.Nicola NA, Metcalf D, Matsumoto M, Johnson GR. Purification of a factor inducing differentiation in murine myelomonocytic leukemia cells: Identification as granulocyte colony-stimulating factor. Journal of Biological Chemistry. 1983;258:9017–9023. [PubMed] [Google Scholar]
- 14.Gasson JC, et al. Purified human granulocyte-macrophage colony-stimulating factor: Direct action on neutrophils. Science (Washington) 1984;266:1339–1342. doi: 10.1126/science.6390681. [DOI] [PubMed] [Google Scholar]
- 15.Welte KE, et al. Purification and biochemical characterization of human pluripotent hematopoietic colony-stimulating factor. Proceedings of the National Academy of Sciences, USA. 1985;82:1526–1530. doi: 10.1073/pnas.82.5.1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wong GG, et al. Human GM-CSF: Molecular cloning of the complementary DNA and purification of the natural and recombinant proteins. Science (Washington) 1985;228:810–815. doi: 10.1126/science.3923623. [DOI] [PubMed] [Google Scholar]
- 17.Nomura H, et al. Purification and characterization of human granulocyte colony-stimulating factor (G-CSF) EMBO Journal. 1986;5:871–876. doi: 10.1002/j.1460-2075.1986.tb04297.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zenke G, et al. Purification and characterization of natural human interleukin-3. Lymphokine and Cytokine Research. 1991;10:329–335. [PubMed] [Google Scholar]
- 19.Fung M-C, et al. Molecular cloning of cDNA for murine interleukin-3. Nature (London) 1984;307:233–237. doi: 10.1038/307233a0. [DOI] [PubMed] [Google Scholar]
- 20.Yokota T, et al. Isolation and characterisation of a mouse cDNA clone that expresses mast cell growth factor activity in monkey cells. Proceedings of the National Academy of Sciences, USA. 1984;81:1070–1074. doi: 10.1073/pnas.81.4.1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gough NM, et al. Molecular cloning of cDNA encoding a murine haematopoietic growth regulator, granulocyte-macrophage colony stimulating factor. Nature (London) 1984;309:763–767. doi: 10.1038/309763a0. [DOI] [PubMed] [Google Scholar]
- 22.DeLamarter JF, et al. Nucleotide sequence of a cDNA encoding murine CSF-1 (macrophage-CSF) Nucleic Acids Research. 1987;15:2389–2390. doi: 10.1093/nar/15.5.2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cantrell MA, et al. Cloning, sequence and expression of a human granulocyte/macrophage colony stimulating factor. Proceedings of the National Academy of Sciences, U.S.A. 1985;82:6250–6254. doi: 10.1073/pnas.82.18.6250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nagata S, et al. Molecular cloning and expression of cDNA for human granulocyte colony-stimulating factor. Nature (London) 1986;319:415–418. doi: 10.1038/319415a0. [DOI] [PubMed] [Google Scholar]
- 25.Souza LM, et al. Recombinant human granulocyte colony-stimulating factor: Effects on normal and leukemic myeloid cells. Science (Washington) 1986;232:61–65. doi: 10.1126/science.2420009. [DOI] [PubMed] [Google Scholar]
- 26.Kawasaki ES, et al. Molecular cloning of complementary DNA encoding human macrophage-specific colony-stimulating factor (CSF-1) Science (Washington) 1985;230:291–296. doi: 10.1126/science.2996129. [DOI] [PubMed] [Google Scholar]
- 27.Yang Y-C, et al. Human IL-3 (Multi-CSF): Identification by expression cloning of a novel hematopoietic growth factor related to murine IL-3. Cell. 1986;47:3–10. doi: 10.1016/0092-8674(86)90360-0. [DOI] [PubMed] [Google Scholar]
- 28.Metcalf D, Nicola NA. The hematopoietic colony-stimulating factors: From Biology to Clinical Applications. Cambridge, UK: Cambridge University Press; 1995. pp. 1–327. [Google Scholar]
- 29.Tushinski RJ, et al. Survival of mononuclear phagocytes depends on a lineage-specific growth factor that the differentiated cells selectively destroy. Cell. 1982;28:71–81. doi: 10.1016/0092-8674(82)90376-2. [DOI] [PubMed] [Google Scholar]
- 30.Metcalf D, et al. In vitro actions on hemopoietic cells of recombinant murine GM-CSF purified after production in Escherichia coli: Comparison with purified native GM-CSF. Journal of Cellular Physiology. 1986;128:421–431. doi: 10.1002/jcp.1041280311. [DOI] [PubMed] [Google Scholar]
- 31.Metcalf D, Nicola NA. Proliferative effects of purified granulocyte colony-stimulating factor (G-CSF) on normal mouse hematopoietic cells. Journal of Cellular Physiology. 1983;116:198–206. doi: 10.1002/jcp.1041160211. [DOI] [PubMed] [Google Scholar]
- 32.Metcalf D, Begley CG, Nicola N, Johnson GR. Quantitative responsiveness of murine hemopoietic populations in vitro and in vivo recombinant Multi-CSF (IL-3) Experimental Hematology. 1987;15:288–295. [PubMed] [Google Scholar]
- 33.Li CL, Johnson GR. Rhodamine 123 reveals heterogeneity within murine Lin−, Sca-1+ hemopoietic stem cells. Journal of Experimental Medicine. 1992;175:1443–1447. doi: 10.1084/jem.175.6.1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Metcalf D, Nicola NA. Direct proliferative actions of stem cell factor on murine bone marrow cells in vitro: Effects of combination with colony-stimulating factors. Proceedings of the National Academy of Sciences, U.S.A. 1991;88:6239–6243. doi: 10.1073/pnas.88.14.6239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meunch MD, Schneider JG, Moore MAS. Interaction amongst colony stimulating factors, IL-1β, IL-IL-6 and kit-ligand in the regulation of primitive murine hematopoietic cells. Experimental Hematology. 1992;20:339–349. [PubMed] [Google Scholar]
- 36.Metcalf D, Foster R. Behavior on transfer of serum stimulated bone marrow colonies. Proceedings of the Society for Experimental Biology and Medicine. 1967;126:758–762. [Google Scholar]
- 37.Paran M, Sachs L. The continuous requirement for inducers for the development of macrophage and granulocyte colonies. Journal of Cellular Physiology. 1968;72:247–250. doi: 10.1002/jcp.1040720312. [DOI] [PubMed] [Google Scholar]
- 38.Begley CG, et al. Purified colony stimulating factors enhance the survival of human neutrophils and eosinophils in vitro: A rapid and sensitive microassay for colony stimulating factors. Blood. 1986;68:162–166. [PubMed] [Google Scholar]
- 39.Williams GT, Smith CA, Spooncer E, Dexter TM, Taylor DR. Haemopoietic colony stimulating factors promote cell survival by suppressing apoptosis. Nature (London) 1990;343:76–79. doi: 10.1038/343076a0. [DOI] [PubMed] [Google Scholar]
- 40.Vaux DL, Cory S, Adams JM. Bcl-2 gene promotes haematopoietic cell survival and cooperates with c-myc to immortalize pre-B cells. Nature. 1988;335:440–442. doi: 10.1038/335440a0. [DOI] [PubMed] [Google Scholar]
- 41.Roussel MF, Sherr CJ. Signal transduction by the macrophage colony-stimulating factor receptor. Current Opinion in Hematology. 1993;1:11–18. [Google Scholar]
- 42.Laâbi Y, Metcalf D, Mifsud S, Di Rago L. Differentiation commitment and regulator-specific granulocyte-macrophage maturation in a novel pro-β murine leukemic cell line. Leukemia. 2000;14:1785–1795. doi: 10.1038/sj.leu.2401931. [DOI] [PubMed] [Google Scholar]
- 43.Rieger MA, et al. Hematopoietic cytokines can instruct lineage choice. Science. 2009;325:217–218. doi: 10.1126/science.1171461. [DOI] [PubMed] [Google Scholar]
- 44.Metcalf D. Lineage commitment of hemopoietic progenitor cells in developing blast cell colonies: Influence of colony stimulating factors. Proceedings of the National Academy of Sciences, U.S.A. 1991;88:11310–11314. doi: 10.1073/pnas.88.24.11310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fairbairn LJ, Cowling GJ, Reipert BM, Dexter TM. Suppression of apoptosis allows differentiation and development of a multipotent haemopoietic stem cell line in the absence of added growth factors. Cell. 1993;74:823–832. doi: 10.1016/0092-8674(93)90462-y. [DOI] [PubMed] [Google Scholar]
- 46.McArthur GA, Rohrschneider LR, Johnson GR. Induced expression of c-fms in normal hematopoietic cells shows evidence for both conservation and lineage restriction of signal transduction in response to macrophage colony-stimulating factor. Blood. 1994;83:972–981. [PubMed] [Google Scholar]
- 47.Demetri GD, Griffin JD. Granulocyte colony-stimulating factor and its receptor. Blood. 1991;78:2791–2808. [PubMed] [Google Scholar]
- 48.Gasson JC. Molecular physiology of granulocyte-macrophage colony-stimulating factor. Blood. 1991;77:1131–1145. [PubMed] [Google Scholar]
- 49.Hollingshead LM, Goa KL. Recombinant granulocyte colony-stimulating factor (rG-CSF): A review of its pharmacological properties and prospective role in neutropenic conditions. Drugs. 1991;42:300–330. doi: 10.2165/00003495-199142020-00009. [DOI] [PubMed] [Google Scholar]
- 50.Grant SM, Heel RC. Recombinant granulocyte-macrophage colony-stimulating factor (rGM-CSF): A review of its pharmacological properties and prospective role in the management of myelosuppression. Drugs. 1992;43:516–560. doi: 10.2165/00003495-199243040-00008. [DOI] [PubMed] [Google Scholar]
- 51.Hamilton JA. Colony-stimulating factors in inflammation and autoimmunity. Nature Reviews Immunology. 2008;8:533–544. doi: 10.1038/nri2356. [DOI] [PubMed] [Google Scholar]
- 52.Nicola NA. Structural and functional characteristics of receptors for colony-stimulating factors (CSFs) In: Quesenberry PJ, Asano S, Saito K, editors. Hemopoietic Growth Factors. Amsterdam: Excerpta Medica; 1991. pp. 101–120. [Google Scholar]
- 53.Fukunaga R, Ishizaka-Ikeda E, Nagata S. Purification and characterization of the receptor for murine granulocyte colony-stimulating factor. Journal of Biological Chemistry. 1990;265:14008–14015. [PubMed] [Google Scholar]
- 54.Hansen G, et al. The structure of the GM-CSF receptor complex reveals a distinct mode of cytokine receptor activation. Cell. 2008;134:496–507. doi: 10.1016/j.cell.2008.05.053. [DOI] [PubMed] [Google Scholar]
- 55.Dong F, et al. Distinct cytoplasmic regions of the human granulocyte colony-stimulating factor receptor involved in induction of proliferation and maturation. Molecular and Cellular Biology. 1993;13:7774–7778. doi: 10.1128/mcb.13.12.7774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sakamaki K, Miyajima I, Kitamura T, Miyajima A. Critical cytoplasmic domains of the common beta subunit of the human GM-CSF, Il-3 and and IL-5 receptors for growth signal transduction and tyrosine phosphorylation. EMBO Journal. 1992;11:3541–3549. doi: 10.1002/j.1460-2075.1992.tb05437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nicholson SE, Novak U, Zeigler SF, Layton JE. Distinct regions of the granulocyte colony-stimulating factor receptor are required for tyrosine phosphorylation of the signalling molecules JAK2, Stat3, and p42, p44MAPK. Blood. 1995;10:3698–3704. [PubMed] [Google Scholar]
- 58.Brown AL, Peters M, D’Andrea RJ, Gonda TJ. Constitutive mutants of the GM-CSF receptor reveal multiple pathways leading to myeloid cell survival, proliferation, and granulocyte-macrophage differentiation. Blood. 2004;103:507–516. doi: 10.1182/blood-2003-05-1435. [DOI] [PubMed] [Google Scholar]
- 59.Hercus TR, et al. The granulocyte-macrophage colony-stimulating factor receptor: linking its structure to cell signaling and its role in disease. Blood. 2009;114:1289–1298. doi: 10.1182/blood-2008-12-164004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lieschke GJ, et al. Mice lacking granulocyte colony-stimulating factor have chronic neutropenia, granulocyte and macrophage progenitor cell deficiency, and impaired neutrophil mobilization. Blood. 1994;84:1737–1746. [PubMed] [Google Scholar]
- 61.Stanley E, et al. Granulocyte-macrophage colony-stimulating factor-deficient mice show no major perturbation of hematopoiesis but develop a characteristic pulmonary pathology. Proceedings of the National Academy of Sciences, U.S.A. 1994;91:5592–5596. doi: 10.1073/pnas.91.12.5592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dranoff G, et al. Involvement of granulocyte-macrophage colony-stimulating factor in pulmonary homeostasis. Science (Washington) 1994;264:713–716. doi: 10.1126/science.8171324. [DOI] [PubMed] [Google Scholar]
- 63.Bonfield TL, et al. autoantibodies against granulocyte macrophage colony-stimulating factor are diagnostic for pulmonary alveolar proteinosis. Am. J. Respir. Cell Mol. Biol. 2002;27:481–486. doi: 10.1165/rcmb.2002-0023OC. [DOI] [PubMed] [Google Scholar]
- 64.Wiktor-Jedrzejczak W, et al. CSF-1 deficiency in the op/op mouse has differential effects on macrophage populations and differentiation stages. Experimental Hematology. 1992;20:1004–1010. [PubMed] [Google Scholar]
- 65.Lieschke GJ, et al. Mice lacking both macrophage- and granulocyte-macrophage colony-stimulating factor have macrophages and co-existent osteopetrosis and severe lung disease. Blood. 1994;84:27–35. [PubMed] [Google Scholar]
- 66.Pollard JW, Hunt JS, Wiktor-Jedrzejczak W, Stanley ER. A pregnancy defect in the osteopetrotic (op/op) mouse demonstrates the requirement for CSF-1 in female fertility. Developmental Biology. 1991;148:273–283. doi: 10.1016/0012-1606(91)90336-2. [DOI] [PubMed] [Google Scholar]
- 67.Nicola NA, et al. Functional inactivation in mice of the gene for the interleukin-3 (IL-3) – specific receptor β-chain: implications for IL-3 function and the mechanism of receptor transmodulation in hematopoietic cells. Blood. 1996;87:2665–2674. [PubMed] [Google Scholar]
- 68.Nishinakamura R, et al. Hematopoiesis in mice lacking the entire granulocyte-macrophage colony-stimulating factor/interleukin-3/interleukin-5 functions. Blood. 1996;88:2458–2464. [PubMed] [Google Scholar]
- 69.Lantz CS, et al. Role for interleukin-3 in mast-cell and basophil development and in immunity to parasites. Nature. 1998;392:90–93. doi: 10.1038/32190. [DOI] [PubMed] [Google Scholar]
- 70.Mach N, et al. Involvement of interleukin-3 in delayed-type hypersensitivity. Blood. 1998;91:778–783. [PubMed] [Google Scholar]
- 71.Cynshi O, et al. Reduced response to granulocyte colony-stimulating factor in W/Wv and S1/S1d mice. Leukemia. 1991;5:75–77. [PubMed] [Google Scholar]
- 72.Metcalf D, Nicola NA. The clonal proliferation of normal mouse hematopoietic cells: Enhancement and suppression by CSF combinations. Blood. 1992;79:2861–2866. [PubMed] [Google Scholar]
- 73.Metcalf D, Mifsud S, Di Rago L. Murine megakaryocyte progenitor cells and their susceptibility to suppression by G-CSF. Stem Cells. 2005;23:55–62. doi: 10.1634/stemcells.2004-0164. [DOI] [PubMed] [Google Scholar]
- 74.Metcalf D, Di Rago L, Mifsud S. Synergistic and inhibitory interactions in the in vitro control of murine megakaryocyte colony formation. Stem Cells. 2002;20:552–560. doi: 10.1002/stem.200552. [DOI] [PubMed] [Google Scholar]
- 75.Metcalf D, et al. Murine hematopoietic blast colony-forming cells and their progeny have distinctive membrane marker profiles. Proc. Natl. Acad. Sci. 2009;106:19102–19107. doi: 10.1073/pnas.0910354106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Molineux G, Pojda Z, Dexter TM. A comparison of hematopoiesis in normal and splenectomized mice treated with granulocyte colony-stimulating factor. Blood. 1990;75:563–569. [PubMed] [Google Scholar]
- 77.Metcalf D, et al. Hemopoietic responses in mice injected with purified recombinant murine GM-CSF. Experimental Hematology. 1987;15:1–9. [PubMed] [Google Scholar]
- 78.Metcalf D, et al. Effects of purified bacterially synthesized murine Multi-CSF (IL-3) on hematopoiesis in normal adult mice. Blood. 1986;68:46–57. [PubMed] [Google Scholar]
- 79.Lord BI, et al. Myeloid cell kinetics in mice treated with recombinant interkeukin-3, granulocyte colony-stimulating (CSF), or granulocyte-macrophage CSF in vivo. Blood. 1991;77:2154–2159. [PubMed] [Google Scholar]
- 80.Cairo MS, et al. Prophylactic or simultaneous administration of recombinant human granulocyte colony stimulating factor in the treatment of group B streptococcal sepsis in neonatal rats. Pediatric Research. 1990;27:612–616. doi: 10.1203/00006450-199006000-00016. [DOI] [PubMed] [Google Scholar]
- 81.Herbert JC, O’Reilly M, Gamelli RL. Protective effect of recombinant human granulocyte colony-stimulating factor against pneumonococcal infections in splenectomized mice. Archives of Surgery. 1990;125:1075–1078. doi: 10.1001/archsurg.1990.01410200141022. [DOI] [PubMed] [Google Scholar]
- 82.Matsumoto M, et al. Protective effect of human granulocyte colony-stimulating factor on microbial infection in neutropenic mice. Infection and Immunity. 1987;55:2715–2720. doi: 10.1128/iai.55.11.2715-2720.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wakiyama H, et al. Therapeutic effect of granulocyte colony-stimulating factor and cephem antibiotics against experimental infections in neutropenic mice induced by cyclophosphamide. Clinical and Experimental Immunology. 1993;92:218–224. doi: 10.1111/j.1365-2249.1993.tb03383.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yasuda H, et al. Therapeutic efficacy of granulocyte colony-stimulating factor alone and in combination with antibiotics against Pseudomonas aeruginosa infections in mice. Infection and Immunity. 1990;58:2502–2509. doi: 10.1128/iai.58.8.2502-2509.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lang RA, et al. Transgenic mice expressing a hemopoietic growth factor gene (GM-CSF) develop accumulations of macrophages, blindness and a fatal syndrome of tissue damage. Cell. 1987;51:675–686. doi: 10.1016/0092-8674(87)90136-x. [DOI] [PubMed] [Google Scholar]
- 86.Johnson GR, Gonda TJ, Metcalf D, Hariharan IK, Cory S. A lethal myeloproliferative syndrome in mice transplanted with bone marrow cells infected with a retrovirus expressing granulocyte-macrophage colony-stimulating factor. EMBO Journal. 1989;8:441–448. doi: 10.1002/j.1460-2075.1989.tb03396.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chang JM, Metcalf D, Lang RA, Gonda TJ, Johnson GR. Non-neoplastic hematopoietic myeloproliferative syndrome induced by dysregulated Multi-CSF (IL-3) expression. Blood. 1989;73:1487–1497. [PubMed] [Google Scholar]
- 88.Chang JM, Metcalf D, Gonda TJ, Johnson GR. Long-term exposure to retrovirally-expressed G-CSF induces a non-neoplastic granulocytic and progenitor cell hyperplasia without tissue damage in mice. Journal of Laboratory Clinical Investigation. 1989;84:1488–1496. doi: 10.1172/JCI114324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hortner M, Nielsch U, Mayr LM, Johnston JA, Heinrich PC, Haan S. Suppressor of cytokine signaling-3 is recruited to the activated granulocyte-colony stimulating factor receptor and modulates its signal transduction. J. Immunol. 2002;169:1219–1227. doi: 10.4049/jimmunol.169.3.1219. [DOI] [PubMed] [Google Scholar]
- 90.Croker BA, et al. SOCS3 is a critical physiological negative regulator of G-CSF signaling and emergency granulopoiesis. Immunity. 2004;20:153–165. doi: 10.1016/s1074-7613(04)00022-6. [DOI] [PubMed] [Google Scholar]
- 91.Moore MAS, Williams N, Metcalf D, et al. In vitro colony formation by normal and leukemic human hematopoietic cells: Interaction between colony-forming and colony-stimulating cells. Journal of the National Cancer Institute. 1973;50:591–602. doi: 10.1093/jnci/50.3.591. [DOI] [PubMed] [Google Scholar]
- 92.Moore MAS, Spitzer G, Williams N, Metcalf D, Buckley J. Agar culture studies in 127 cases of untreated acute leukemia: The prognostic value of reclassification of leukemia according to in vitro growth characteristics. Blood. 1974;44:1–18. [PubMed] [Google Scholar]
- 93.Miyauchi J, et al. The effects of combinations of the recombinant growth factors GM-CSF, G-CSF, IL-3 and CSF-1 on leukemic blast cells in suspension culture. Leukemia. 1988;2:382–387. [PubMed] [Google Scholar]
- 94.Metcalf D, Moore JG. Divergent disease patterns in GM-CSF transgenic mice associated with differing transgene insertion sites. Proc. Natl. Acad. Sci. USA. 1988;85:7767–7771. doi: 10.1073/pnas.85.20.7767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rasko JEJ, Metcalf D, Alexander B, Strasser A, Begley CG. Establishment of multipotential and antigen presenting cell lines derived from myeloid leukemias in GM-CSF transgenic mice. Leukemia. 1997;11:732–742. doi: 10.1038/sj.leu.2400614. [DOI] [PubMed] [Google Scholar]
- 96.Lang RA, Metcalf D, Gough NM, Dunn AR, Gonda TJ. Expression of a hemopoietic growth factor cDNA in a factor-dependent cell line results in autonomous growth and tumorigenicity. Cell. 1985;43:531–542. doi: 10.1016/0092-8674(85)90182-5. [DOI] [PubMed] [Google Scholar]
- 97.Dührsen U, Metcalf D. A model system for leukemic transformation of immortalized hemopoietic cells in irradiated recipient mice. Leukemia. 1988;2:329–333. [PubMed] [Google Scholar]
- 98.Dührsen U, Stahl J, Gough NM. In vivo transformation of factor-dependent hemopoietic cells: Role of intracisternal A-particle transposition for growth factor gene activation. EMBO Journal. 1990;9:1087–1096. doi: 10.1002/j.1460-2075.1990.tb08214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Perkins A, Kongsuwan K, Visvader J, Adams JM, Cory S. Homeobox gene expression plus autocrine growth factor production elicits myeloid leukemia. Proceedings of the National Academy of Sciences, U.S.A. 1990;87:8398–8402. doi: 10.1073/pnas.87.21.8398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Moore MAS. Converging pathways in leukemogenesis and stem cell self-renewal. Exp. Hematol. 2005;33:719–737. doi: 10.1016/j.exphem.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 101.Young DC, Wagner K, Griffin JD. Constitutive expression of the granulocyte-macrophage colony-stimulating factor gene in acute myeloblastic leukemia. J. Clin. Invest. 1987;79:100–106. doi: 10.1172/JCI112769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Jiang X, Lopez A, Holyoake T, Eaves A, Eaves C. Autocrine production and action of IL-3 and granulocyte colony-stimulating factor in chronic myeloid leukemia. Proc. Natl. Acad.Sci. U.S.A. 1999;96:12804–12809. doi: 10.1073/pnas.96.22.12804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gonda TJ, D’Andrea RJ. Activating mutations in cytokine receptors: implications for receptor function and role in disease. Blood. 1997;89:355–369. [PubMed] [Google Scholar]
- 104.Gabrilove JL, et al. Phase I study of granulocyte colony-stimulating factor in patients with transitional cell carcinoma of the urothelium. Journal of Clinical Investigation. 1988;82:1454–1461. doi: 10.1172/JCI113751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Morstyn G, et al. Effect of granulocyte colony-stimulating factor on neutropenia induced by cytotoxic chemotherapy. Lancet. 1988;i:667–672. doi: 10.1016/s0140-6736(88)91475-4. [DOI] [PubMed] [Google Scholar]
- 106.Bonilla MA, et al. Effects of recombinant human granulocyte colony stimulating factor on neutropenia in patients with congenital agranulocytosis. New England Journal of Medicine. 1989;320:1574–1580. doi: 10.1056/NEJM198906153202402. [DOI] [PubMed] [Google Scholar]
- 107.Lieschke GJ, et al. Effects of bacterially, synthesized recombinant human granulocyte-macrophage colony-stimulating factor in patients with advanced malignancy. Annals of Internal Medicine. 1989;110:357–364. doi: 10.7326/0003-4819-110-5-357. [DOI] [PubMed] [Google Scholar]
- 108.Hammond WP, Price TH, Souza LM, Dale DC. Treatment of cyclic neutropenia with granulocyte colony-stimulating factor. New England Journal of Medicine. 1989;320:1306–1311. doi: 10.1056/NEJM198905183202003. [DOI] [PubMed] [Google Scholar]
- 109.Dale DC, et al. Randomized controlled Phase III trial of recombinant human granulocyte colony-stimulating factor (Filgrastim) for treatment of severe chronic neutropenia. Blood. 1993;81:2496–2502. [PMC free article] [PubMed] [Google Scholar]
- 110.Cole DJ, et al. Phase I trial of recombinant human macrophage colony-stimulating factor administered by continuous intravenous infusion in patients with metastatic cancer. Journal of the National Cancer Institute. 1994;86:39–45. doi: 10.1093/jnci/86.1.39. [DOI] [PubMed] [Google Scholar]
- 111.Postmus RE, et al. Effects of recombinant interleukin-3 in patients with relapsed small-cell lung cancer treated with chemotherapy: A dose-finding study. Journal of Clinical Oncology. 1992;10:1131–1140. doi: 10.1200/JCO.1992.10.7.1131. [DOI] [PubMed] [Google Scholar]
- 112.Bodey GP, Buckley M, Sathe YS, Freireich EJ. Quantitative relationships between circulating leukocytes and infections in patients with acute leukemia. Annals of Internal Medicine. 1966;64:328–340. doi: 10.7326/0003-4819-64-2-328. [DOI] [PubMed] [Google Scholar]
- 113.Bronchud MH, et al. Phase I/II study of recombinant human granulocyte colony-stimulating factor in patients receiving intensive chemotherapy for small cell lung cancer. British Journal of Cancer. 1987;56:809–813. doi: 10.1038/bjc.1987.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Crawford J, et al. Reduction by granulocyte colony-stimulating factor of fever and neutropenia induced by chemotherapy in patients with small-cell lung cancer. N. Engl. J. Med. 1991;325:164–170. doi: 10.1056/NEJM199107183250305. [DOI] [PubMed] [Google Scholar]
- 115.Trillet-Lenoir V, et al. Recombinant granulocyte colony stimulating factor reduces the infectious complications of cytotoxic chemotherapy. Eur. J. Cancer. 1993;29A:319–324. doi: 10.1016/0959-8049(93)90376-q. [DOI] [PubMed] [Google Scholar]
- 116.Gianni AM, et al. Recombinant human granulocyte-macrophage colony-stimulating factor reduces hematologic toxicity and widens clinical applicability of high-dose cyclophosphamide treatment in breast cancer and non-Hodgkin’s lymphoma. J. Clin. Oncol. 1990;8:768–778. doi: 10.1200/JCO.1990.8.5.768. [DOI] [PubMed] [Google Scholar]
- 117.Gerhartz HH, et al. Randomized, double-blind, placebo-controlled, phase III study of recombinant human granulocyte-macrophage colony-stimulating factor as adjunct to induction treatment of high-grade malignant non-Hodgkin’s lymphomas. Blood. 1993;82:2329–2339. [PubMed] [Google Scholar]
- 118.Renwick W, Pettengell R, Green M. Use of filgrastim and pegfilgrastim to support delivery of chemotherapy: Twenty years of clinical experience. BioDrugs. 2009;23:175–186. doi: 10.2165/00063030-200923030-00004. [DOI] [PubMed] [Google Scholar]
- 119.Nemunaitis J, Singer JW, Buckner CD, Durnam D, Epstein C, Hill R, Storb R, Thomas ED, Appelbaum FR. Use of recombinant human granulocyte-macrophage colony-stimulating factor in graft failure after bone marrow transplantation. Blood. 1990;76:245–253. [PubMed] [Google Scholar]
- 120.Nemunaitis J, Singer JW, Buckner CD, Hill R, Storb R, Thomas ED, Appelbaum FR. Use of recombinant human granulocyte-macrophage colony-stimulating factor in autologous marrow transplantation for lymphoid malignancies. Blood. 1988;72:834–836. [PubMed] [Google Scholar]
- 121.Schweizerhof M, et al. Hematopoietic colony-stimulating factors mediate tumor-nerve interactions and bone cancer pain. Nat. Med. 2009;15:802–807. doi: 10.1038/nm.1976. [DOI] [PubMed] [Google Scholar]
- 122.Holmes FA, et al. Blinded, randomized multicenter study to evaluate single administration pegfilgrastim once per cycle versus daily filgrastim as an adjunct to chemotherapy in patients with high-risk stage II or stage III/IV breast cancer. J. Clin. Oncol. 2002;20:729–731. doi: 10.1200/JCO.2002.20.3.727. [DOI] [PubMed] [Google Scholar]
- 123.Green MD, et al. A randomized double-blind multicenter phase III study of fixed-dose single-administration pegfilgrastim versus daily filgrastim in patients receiving myelosuppressive chemotherapy. Ann. Oncol. 2003;14:29–35. doi: 10.1093/annonc/mdg019. [DOI] [PubMed] [Google Scholar]
- 124.Klastersky J, Awada A, Aoun M, Paesmans M. Should the indications for the use of myeloid growth factors for the prevention of febrile neutropenia in cancer patients be extended? Curr. Opin. Oncol. 2009;21:297–302. doi: 10.1097/CCO.0b013e32832c9651. [DOI] [PubMed] [Google Scholar]
- 125.Dale DC. Hematopoietic growth factors for the treatment of severe chronic neutropenia. Stem Cells. 1995;13:94–100. doi: 10.1002/stem.5530130201. [DOI] [PubMed] [Google Scholar]
- 126.D’Souza A, Jaiyesimi I, Trainor L, Venuturumili P. Granulocyte colony-stimulating factor administration: Adverse Events. Transfusion Med. Rev. 2008;22:280–290. doi: 10.1016/j.tmrv.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 127.Miyake T, Kung CK-H, Goldwasser E. Purification of human erythropoietin. J. Biol. Chem. 1977;252:5558–5564. [PubMed] [Google Scholar]
- 128.Jacobs K, et al. Isolation and characterization of genomic and cDNA clones of human erythropoietin. Nature (Lond) 1985;313:806–810. doi: 10.1038/313806a0. [DOI] [PubMed] [Google Scholar]
- 129.Phrommintikul A, Hass SJ, Elsik M, Krum H. Mortality and target haemoglobin concentrations in anaemic patients with chronic kidney disease treated with erythropoietin: a meta-analysis. Lancet. 2007;369:381–388. doi: 10.1016/S0140-6736(07)60194-9. [DOI] [PubMed] [Google Scholar]
- 130.Spivak JL, Gascón P, Ludwig H. Anemia management in oncology and hematology. The Oncologist. 2009;14:43–56. doi: 10.1634/theoncologist.2009-S1-43. [DOI] [PubMed] [Google Scholar]
- 131.Dührsen U, Villeval J-L, Boyd J, Kannourakis G, Morstyn G, Metcalf D. Effects of recombinant human granulocyte-colony stimulating factor on hemopoietic progenitor cells in cancer patients. Blood. 1988;72:2074–2081. [PubMed] [Google Scholar]
- 132.Gianni AM, Siena S, Bregni M, Tarella C, Stern AC, Pileri A, Bonadonna G. Granulocyte-macrophage colony-stimulating factor to harvest circulating haemopoietic stem cells for autotransplantation. Lancet. 1989;2:580–585. doi: 10.1016/s0140-6736(89)90711-3. [DOI] [PubMed] [Google Scholar]
- 133.Socinski MA, Cannistra SA, Elias A, Antman KH, Schnipper L, Griffin JD. Granulocyte-macrophage colony-stimulating factor expands the circulating haemopoietic progenitor cell compartment in man. Lancet. 1988;I:1194–1198. doi: 10.1016/s0140-6736(88)92012-0. [DOI] [PubMed] [Google Scholar]
- 134.Molineux G, Podja Z, Hampson IN, Lord BI, Dexter TM. Transplantation potential of peripheral blood stem cells induced by granulocyte colony-stimulating factor. Blood. 1990;76:2153. [PubMed] [Google Scholar]
- 135.Haas R, et al. Successful autologous transplantation of blood stem cells mobilized with recombinant human granulocyte-macrophage colony-stimulating factor. Exp. Hematol. 1990;18:94–98. [PubMed] [Google Scholar]
- 136.Sheridan WP, et al. Effect of peripheral-blood progenitor cells mobilized by Filgrastim (GCSF) on platelet recovery after high-dose chemotherapy. Lancet. 1992;339:640–644. doi: 10.1016/0140-6736(92)90795-5. [DOI] [PubMed] [Google Scholar]
- 137.Van Hoef ME. Haematological recovery after high-dose consolidation chemotherapy with peripheral blood progenitor cell rescue: the effects of the mobilization regimen and post-transplant growth factors. Neph. J. Med. 1998;52:30–39. doi: 10.1016/s0300-2977(97)00077-6. [DOI] [PubMed] [Google Scholar]
- 138.Chao NJ, et al. Granulocyte colony-stimulating factor “mobilized” peripheral blood progenitor cells accelerate granulocyte and platelet recovery after high-dose chemotherapy. Blood. 1993;81:2031–2035. [PubMed] [Google Scholar]
- 139.Richman CM, Weiner RS, Yankee RA. Increase in circulating stem cells following chemotherapy in man. Blood. 1976;47:1031–1039. [PubMed] [Google Scholar]
- 140.Quesenberry PJ, et al. Stem cell engraftment strategies. Annals. N.Y. Acad. Sci. 2001;938:54–61. doi: 10.1111/j.1749-6632.2001.tb03574.x. [DOI] [PubMed] [Google Scholar]
- 141.Hölig K, et al. Safety and efficacy of hematopoietic stem cell collection from mobilized peripheral blood in unrelated volunteers: 12 years of single-center experience in 3928 donors. Blood. 2009;114:3757–3763. doi: 10.1182/blood-2009-04-218651. [DOI] [PubMed] [Google Scholar]
- 142.Caux C, Dezutter-Dambuyant C, Schmitt D, Banchereau J. GM-CSF and TNF-alpha cooperate in the generation of dendritic Langerhans cells. Nature. 1992;360:258–261. doi: 10.1038/360258a0. [DOI] [PubMed] [Google Scholar]
- 143.Inaba K, et al. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J. Exp. Med. 1992;176:1693–1702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J. Exp. Med. 1994;179:1109–1118. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Vremec D, et al. The influence of granulocyte/macrophage colony-stimulating factor on dendritic cell levels in mouse lymphoid organs. Eur. J. Immunol. 1997;27:40–44. doi: 10.1002/eji.1830270107. [DOI] [PubMed] [Google Scholar]
- 146.Jinushi M, Tahara H. Cytokine gene-mediated immunotherapy: current status and future perspectives. Cancer Sci. 2009;100:1389–1396. doi: 10.1111/j.1349-7006.2009.01202.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Dranoff G, et al. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proc. Natl. Acad. Sci. USA. 1993;90:3539–3543. doi: 10.1073/pnas.90.8.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Soiffer R, et al. Vaccination with irradiated, autologous melanoma cells engineered to secrete granulocyte-macrophage colony-stimulating factor by adenoviral-mediated gene transfer augments antitumor immunity in patients with metastatic melanoma. J. Clin. Oncol. 2003;21:3343–3350. doi: 10.1200/JCO.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 149.Soiffer R, et al. Vaccination with irradiated, autologous melanoma cells engineered to secrete human granulocyte-macrophage colony stimulating factor generates potent anti-tumor immunity in patients with metastatic melanoma. Proc. Natl. Acad. Sci. USA. 1998;95:13141–13146. doi: 10.1073/pnas.95.22.13141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Salgia R, et al. Vaccination with irradiated autologous tumor cells engineered to secrete granulocyte-macrophage colony-stimulating factor augments antitumor immunity in some patients with metastatic non-small-cell lung carcinoma. J. Clin. Oncol. 2003;21:624–630. doi: 10.1200/JCO.2003.03.091. [DOI] [PubMed] [Google Scholar]
- 151.Nemunaitis J, et al. Granulocyte-macrophage colony-stimulating factor gene-modified autologous tumor vaccines in non-small-cell lung carcinoma. J. Natl. Cancer Inst. 2004;96:326–331. doi: 10.1093/jnci/djh028. [DOI] [PubMed] [Google Scholar]
- 152.Simons J, et al. Induction of immunity to prostate cancer antigens: results of a clinical trial of vaccination with irradiated autologous prostate tumor cells engineered to secrete granulocyte-macrophage colony-stimulating factor using ex vivo gene transfer. Cancer Res. 1999;59:5160–5168. [PubMed] [Google Scholar]
- 153.Tani K, et al. Phase I study of autologous tumor vaccines transduced with the GM-CSF gene in four patients with stage IV renal cell cancer in Japan: clinical and immunological findings. Mol. Ther. 2004;10:799–816. doi: 10.1016/j.ymthe.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 154.Small EJ, et al. Granulocyte macrophage colony-stimulating factor - secreting allogeneic cellular immunotherapy for hormone-refractory prostate cancer. Clin. Cancer Res. 2007;13:3883–3891. doi: 10.1158/1078-0432.CCR-06-2937. [DOI] [PubMed] [Google Scholar]
- 155.Kirkwood JM, et al. Immunogenicity and antitumor effects of vaccination with peptide vaccine +/− granulocyte-monocyte colony-stimulating factor and/or IFN-α2b in advanced metastatic melanoma: Eastern Cooperative Oncology Group Phase II Trial E1696. Clin. Cancer Res. 2009;15:1443–1451. doi: 10.1158/1078-0432.CCR-08-1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Grigg AP, et al. Optimizing dose and scheduling of filgrastim (Granulocyte Colony-Stimulating Factor) for mobilization and collection of peripheral blood progenitor cells in normal volunteers. Blood. 1995;86:4437–4445. [PubMed] [Google Scholar]
- 157.Merad M, Manz MG. Dendritic cell homeostasis. Blood. 2009;113:3418–3427. doi: 10.1182/blood-2008-12-180646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Graf T, Enver T. Forcing cells to change lineages. Nature. 2009;462:587–594. doi: 10.1038/nature08533. [DOI] [PubMed] [Google Scholar]
- 159.Sheridan WP, et al. Granulocyte-colony-stimulating factor and neutrophil recovery after high-dose chemotherapy and autologous bone marrow transplantation. Lancet. 1989;ii:891–895. doi: 10.1016/s0140-6736(89)91552-3. [DOI] [PubMed] [Google Scholar]