Abstract
Nucleofection permits efficient transfection even with difficult cell types such as primary and non-dividing cells, and is used to deliver various nucleic acids including DNA, mRNA, and siRNA. Unlike DNA and siRNA, mRNA is subject to rapid degradation, which necessitates instant early translation following mRNA delivery. We examined factors important in translation following nucleofection and observed rapid phosphorylation of eukaryotic initiation factor 2 alpha (eIF2α) following nucleofection, which occurred in the absence of delivered nucleic acid. We studied the involvement of 3 ubiquitous kinases capable of phosphorylating eIF2α in mammalian cells and identified that nucleofection-mediated phosphorylation of eIF2α was dependent on general control non-derepressible 2 (GCN2) and protein kinase RNA-activated (PKR)-like ER kinase (PERK) but not PKR. A reduction in translation due to eIF2α phosphorylation was observed post nucleofection demonstrating functional significance. Understanding the impact of nucleofection on translational machinery has important implications for therapeutics currently under development based on the delivery of mRNA, DNA, and siRNA. Strategies to circumvent eIF2α phosphorylation and other downstream effects of activating GCN2 and PERK will facilitate further advancement of nucleic acid-based therapies.
Keywords: Nucleofection, mRNA therapy, eIF2α, PERK, GCN2
Introduction
Nucleofection is an advanced electroporation technique that varies electrical parameters and buffers to optimize delivery for specific cell types with high efficiency and reproducibility.1 A major advantage of nucleofection is its versatility in transfecting a wide variety of primary dividing and non-dividing cell types.1-3 Nucleofection can be used to deliver a variety of nucleic acids including mRNA,4, 5 siRNA,1, 6 miRNA,7, 8 and DNA.2, 9, 10
An increasingly common use of nucleofection is delivery of mRNA. Gene transfer based on mRNA is safe, because unlike DNA-based and viral vector approaches, mRNA-based gene transfer does not bear the risks of chromosomal integration.11 Protein expression is rapid, beginning almost immediately upon mRNA reaching the cytoplasm. High transfection efficiency is obtained, in part because there is no requirement for mRNA to reach the nucleus.12-14 Unlike other gene delivery strategies, no additional RNA transcripts are made following transfection, but mRNA degrades rapidly, thus translation rates immediately following delivery are a key consideration for mRNA-based gene delivery applications.
A convergent response to cellular stress induced by a variety of insults is the phosphorylation of eukaryotic initiation factor 2 alpha (eIF2α), which results in a general decrease in translation initiation events and a global decrease in translation (see 15 for review). There are four known eIF2α kinases in mammalian cells, each responding to different forms of cellular stress: RNA-dependent protein kinase (PKR), PKR-like ER kinase (PERK), general control non-derepressible-2 (GCN2), and heme-regulated inhibitor (HRI). Activation of PKR occurs principally with binding to structured RNAs,16 but can also be activated through cellular protein binding partners under stress conditions17 and is primarily characterized as an antiviral sensor, although it functions in multiple pathways.18 HRI is active primarily in erythroid cells during heme deprivation.19 PERK is activated under conditions of ER stress as part of the unfolded protein response.20 Lastly, GCN2 is stimulated by a variety of stresses, including amino acid starvation,21 uncharged tRNAs,22 proteosome inhibition,23 and UV irradiation.24
Here, we show that nucleofection induces phosphorylation of eIF2α in the absence of a delivered nucleic acid, and GCN2 and PERK are the eIF2α kinases responsible for this phosphorylation. Furthermore, we show that translation is inhibited following nucleofection. The inhibition of translation resulting from this phosphorylation is potentially clinically relevant as it was found to occur in primary non-dividing human cells that are current targets of therapies involving nucleofection. The identification and understanding of non-specific effects of nucleofection are important for understanding the results obtained with its use. In addition, developing approaches to overcome nucleofection-induced eIF2α phosphorylation will enhance the use of nucleofection in clinical therapeutics.
Results
Nucleofection induces eIF2α phosphorylation in WT MEF cells
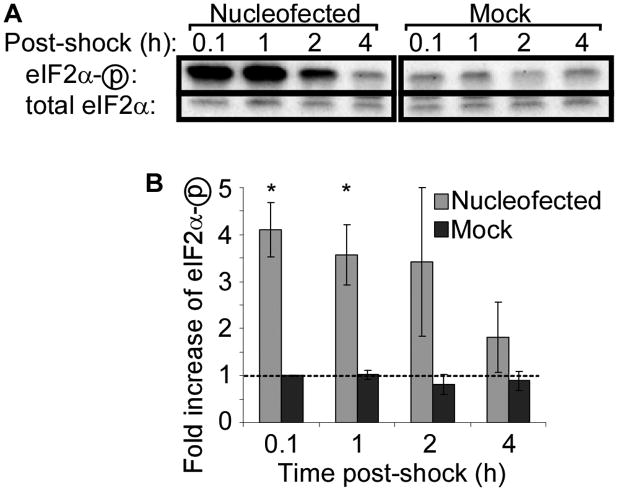
The impact of nucleofection on translation was first studied in a mouse embryonic fibroblast (MEF) cell line derived from wild-type (WT) C57Bl/6 mice. Nucleofection conditions were optimized according to manufacturer's guidelines, and it was determined that program T-020 provided the best cell survival with high transfection efficiency. WT MEFs were then nucleofected without including any nucleic acid in the transfection mix. As a negative control, cells were mock treated by subjecting them to the same manipulation and buffers but without electric shock. Following nucleofection, eIF2α phosphorylation was assessed by western blotting using an antibody specific for eIF2α phosphorylated at serine 51. As shown in Figure 1, nucleofection induced phosphorylation of eIF2α four-fold over the baseline level present in mock-treated cells.
Figure 1. Nucleofection causes phosphorylation of eIF2α in wild-type cells.
Wild-type MEF cells were nucleofected or mock-treated and lysed at the indicated times. (A) Phosphorylation of eIF2α was assessed by western blotting with an antibody specific for phosphorylated eIF2α (eIF2αP), and then re-probed for total eIF2α. Representative data from one of three independent experiments is shown. (B) Quantitation of western blot band densities. Values were calculated as the ratio of phosphorylated to total eIF2α and normalized to the values obtained in mock-treated cells at 0.1 hours post-shock. Data displayed is mean ± SEM of n = 3 experiments. Asterisks indicate P-value <0.05 compared to mock treatment.
Lipid and polymer transfection reagents do not induce eIF2α phosphorylation in WT MEF cells
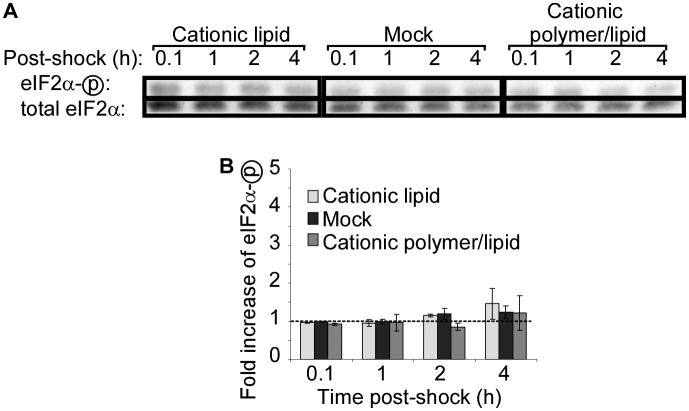
To determine if eIF2α phosphorylation is a common feature of transfection, we treated the cells with Lipofectin, a commonly used cationic lipid, consisting of N-[1-(2,3-dioleyloxy)propyl]-n,n,n-trimethylammonium chloride (DOTMA) and dioleoyl phophotidylethanolamine (DOPE), and TransIT, a cationic polymer/lipid transfection reagent. Reagents were prepared and delivered according to manufacturer instructions, but without including nucleic acid in the transfection mixes. Neither lipid-based nor polymer/lipid-based transfection protocols induced phosphorylation of eIF2α (Figure 2).
Figure 2. Cationic polymer and lipid transfection reagents do not induce phosphorylation of eIF2α in wild-type cells.
Wild-type MEF cells were treated with the indicated transfection reagent or mock-treated and lysed at indicated time points. (A) Phosphorylation of eIF2α was assessed by western blotting. Representative data from one of two independent experiments is shown. (B) Quantitation of western blot band densities. Values were calculated as the ratio of phosphorylated to total eIF2α and normalized to the values obtained in mock-treated cells at 0.1 hours post-shock. Data displayed is mean ± SEM of n = 2 experiments.
GCN2 knockout reduces nucleofection-induced eIF2α phosphorylation
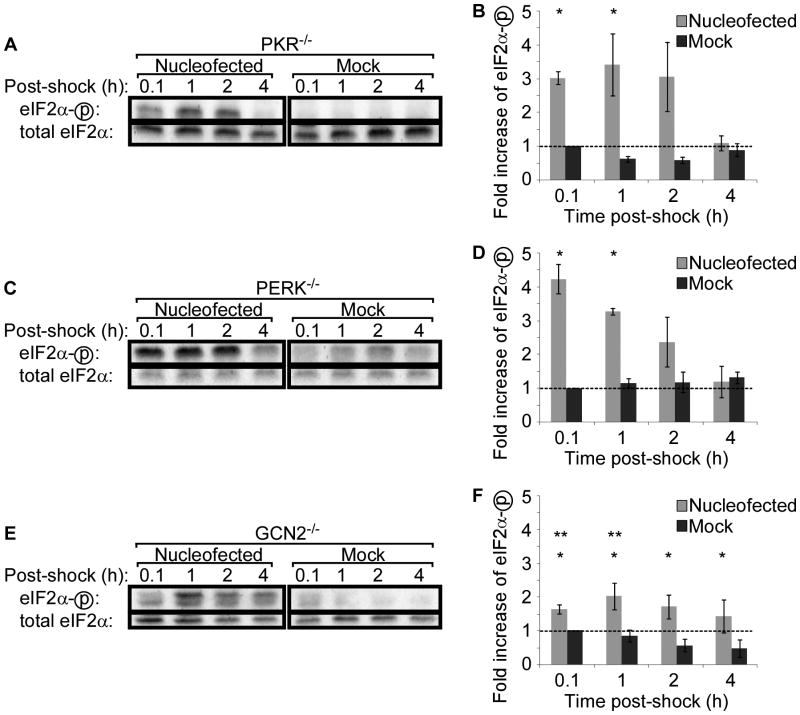
Of the four mammalian eIF2α kinases, three, PKR, PERK, and GCN2, are widely distributed in all cell types, whereas HRI is reported to function primarily in erythroid cells.19 Therefore, to identify the eIF2α kinase responsible for nucleofection-induced eIF2α phosphorylation, we took advantage of MEF cell lines created from mice deficient in PKR, PERK, and GCN2. As was done with WT MEFs, cells were nucleofected and eIF2α phosphorylation was assessed by western blotting. Nucleofection of PKR−/− MEF cells resulted in eIF2α phosphorylation comparable to that induced in WT MEF cells (Figure 3A&B), as did nucleofection of PERK−/− MEF cells (Figure 3C&D). Nucleofection also induced eIF2α phosphorylation in GCN2−/− MEF cells, but the peak level of phosphorylation was reduced (Figure 3E&F).
Figure 3. The absence of GCN2 reduces phosphorylation of eIF2α following nucleofection.
MEF cells deficient in the eIF2α kinases, PKR (A-B), PERK (C-D), or GCN2 (E-F), were nucleofected or mock-treated and lysed at indicated times. Phosphorylation of eIF2α was assessed by western blotting (A, C, E). Representative data of 2–4 independent experiments is shown. For quantitation of western blot band densities, values were calculated as the ratio of phosphorylated to total eIF2α and normalized to the values obtained in mock-treated cells at 0.1 hours post-shock (B, D, E). Data displayed is mean ± SEM of n = 2 to 4 experiments. Asterisks indicate P-value <0.05 compared to mock treatment. Double asterisks indicate P-value <0.05 compared to nucleofected WT MEFs.
GCN2 phosphorylation is induced by nucleofection
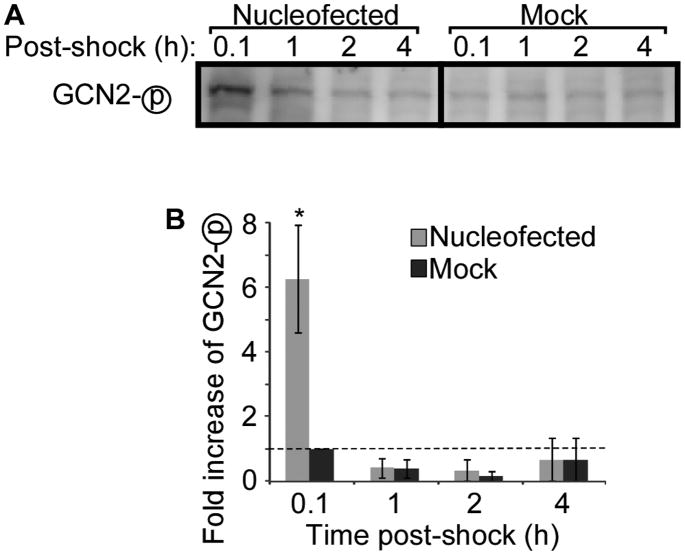
A partial but incomplete reduction in eIF2α phosphorylation was observed only in GCN2−/− MEF cells; therefore, to confirm the role of GCN2, we examined GCN2 activation in WT cells following nucleofection. Activation of GCN2 was evaluated by assessing the level of phosphorylated GCN2 by western blotting. Nucleofection induced phosphorylation of GCN2, which was not observed in mock treated cells, suggesting at least a partial role for GCN2 in nucleofection induced phosphorylation of eIF2α (Figure 4).
Figure 4. Nucleofection induces GCN2 phosphorylation in WT cells.
WT MEF cells were nucleofected or mock-treated and then lysed at the indicated time points. (A) Phosphorylation of GCN2 was assessed by western blotting. Representative data of three independent experiments is shown. (B) Quantitation of western blot band densities. Values were normalized to the values obtained in mock-treated cells at 0.1 hours post-shock. Data displayed is mean ± SEM of n = 3 experiments. Asterisk indicates P-value <0.05 compared to mock treatment.
GCN2 and PERK are responsible for nucleofection-induced eIF2α phosphorylation
The removal of a single eIF2α kinase did not fully prevent nucleofection-induced eIF2α phosphorylation, suggesting the possibility that multiple kinases are involved. Therefore, we assessed phosphorylation of eIF2α following nucleofection of MEF cells derived from a dual-knockout GCN2−/−/PERK−/− mouse on a C57Bl/6 background. No eIF2α phosphorylation was observed in nucleofected GCN2−/−/PERK−/− MEF cells (Figure 5). An apparent lack of any baseline eIF2α phosphorylation that was seen in WT or single knockout MEFs was observed in these cells. The dual knockout cells responded to poly(I:C) demonstrating functional PKR and the ability of their eIF2α to be phosphorylated (Figure 5). These data demonstrated that both GCN2 and PERK were required for nucleofection-induced phosphorylation of eIF2α.
Figure 5. Phosphorylation of eIF2α is mediated by both GCN2 and PERK following nucleofection.
GCN2−/−/PERK−/− MEF cells were nucleofected without nucleic acid (Nucleofected), mock-treated, or nucleofected with poly(I:C), and then lysed at the indicated times. Phosphorylation of eIF2α was assessed by western blotting. Representative data from one of three independent experiments is shown.
Nucleofection induces eIF2α phosphorylation in human dendritic cells
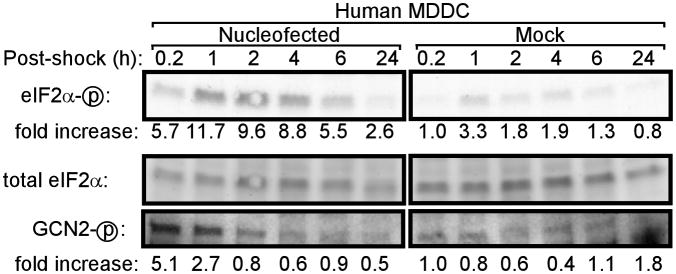
Nucleofection is increasingly used to deliver mRNA to human dendritic cells (DCs) and T cells and has entered clinical trials. Therefore, we assessed the influence of nucleofection on eIF2α phosphorylation in primary human monocyte-derived dendritic cells (hMDDCs). As seen in MEF cell lines, nucleofection induced phosphorylation of eIF2α in hMDDCs (Figure 6). Furthermore, phosphorylation of GCN2 was also induced in hMDDC following nucleofection but not mock treatment (Figure 6).
Figure 6. Nucleofection of primary human dendritic cells induces phosphorylation of eIF2α and GCN2.
Primary human MDDC were nucleofected or mock-treated and lysed at the indicated times. Phosphorylation of eIF2α and GCN2 was assessed by western blotting. For quantitation of western blot band densities, values were calculated as the ratio of phosphorylated eIF2α or GCN2 to total eIF2α and normalized to the values obtained in mock-treated cells at 0.2 hours post-shock.
Nucleofection reduces translation in a GCN2- and PERK-dependent manner
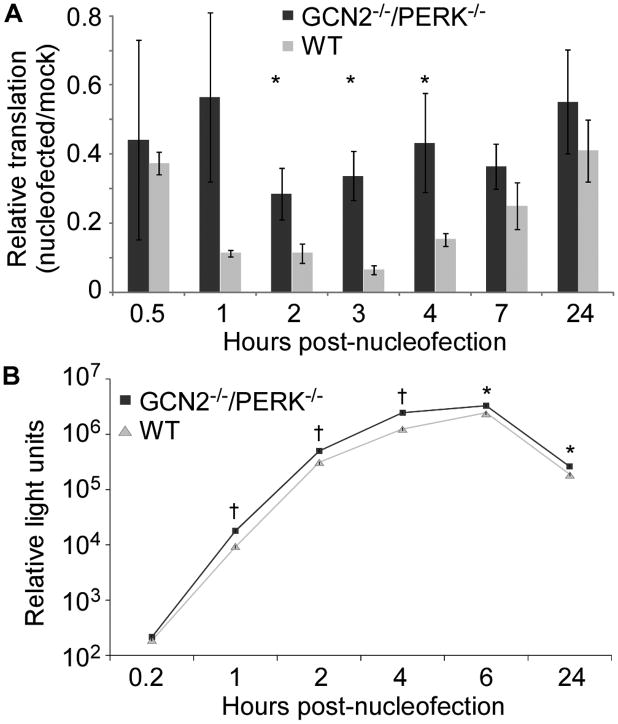
The functional relevance of nucleofection-induced eIF2α phosphorylation was assessed by measuring translation following nucleofection. One day prior to nucleofection, WT and GCN2−/−/PERK−/− MEF cells were transfected with a firefly luciferase expression plasmid. Cells were nucleofected in the absence of a nucleic acid and translation was measured by quantitation of luciferase enzyme activity. In WT cells, translation was decreased following nucleofection corresponding to the timeframe of eIF2α phosphorylation. However, nucleofected GCN2−/−/PERK−/− MEFs had a significantly smaller decrease in translation post nucleofection (Figure 7A). Nucleofection, in general, leads to toxicity and cell death, which was apparent in both cell lines as a reduction in translation that continued at 24 hr and beyond.
Figure 7. Nucleofection reduces translation in a GCN2 and PERK dependent manner.
WT or GCN2−/−/PERK−/− MEF cells were nucleofected and then lysed at the indicated time. Luciferase enzymatic activity was measured as relative light units (RLU). Asterisks indicate P-value <0.05 and daggers indicate P-value <0.001 comparing GCN2−/−/PERK−/− to WT MEFs. Data is representative of 3 independent experiments. (A) Cells were transfected with pCMV-luciferase plasmid 24 hours prior to nucleofection. Luciferase activity was normalized to RLU present in non-nucleofected cells (mock) at the same timepoint. Data displayed is mean ± SEM of four replicate wells. (B) Luciferase mRNA was delivered by nucleofection. Data is mean ± SEM of three replicate wells.
Translation of a transfected nucleic acid was compared after directly delivering luciferase-encoding mRNA by nucleofection to WT and GCN2−/−/PERK−/− MEF cells. To circumvent RNA-induced activation of PKR, the in vitro transcribed mRNA contained pseuoduridines instead of uridines25 and was HPLC purified.26 Translation of nucleofected mRNA was significantly higher in GCN2−/−/PERK−/− MEFs than in WT MEFs by 1 hr and continuing through 24 hr following nucleofection (Figure 7B). To control for the effect of cell line derivation and clonality, the WT and GCN2−/−/PERK−/− MEF cells were transfected with the same luciferase-encoding mRNA using TransIT that we demonstrated did not result in phosphorylation of eIF2α (Figure 2). The WT MEF cells had slightly higher levels of translation (0-10%) throughout the time course, demonstrating that the reduction in translation of delivered mRNA was due to nucleofection.
Discussion
Nucleofection-mediated delivery of nucleic acids to cells in vitro, ex vivo, and in vivo is an established approach that is now being utilized in clinical trials for the development of therapeutics. The advantage of this technique is that the efficiency of transfection of non-dividing cells is greatly increased.2 We demonstrate that nucleofection induces phosphorylation of eIF2α. Using knockout cell lines, we identify that the eIF2α kinases GCN2 and PERK are responsible for nucleofection-induced phosphorylation. Furthermore, nucleofection induced phosphorylation of eIF2α in primary human DCs, indicating that this effect is relevant to the primary cell types currently under investigation for clinical therapeutics. Global and nucleofected mRNA translation was inhibited in WT MEFs following nucleofection, which was mitigated by absence of GCN2 and PERK, confirming the functional impact of nucleofection-induced eIF2α phosphorylation.
We identify that the electrical shock component of nucleofection leads to the activation of GCN2 and PERK and subsequent phosphorylation of eIF2α. This effect of electrical shock has not been previously observed and could be a common feature of electroporation or may be a specific effect of nucleofection. Underhill et al.27 examined eIF2α phosphorylation following electroporation, but did not observe an increase unless DNA was also included in the electroporation. These studies utilized the transformed CHO cell line and the EasyJect Plus (Equibio) electroporation device and only analyzed phosphorylation of eIF2α 24 hrs later, a time where we typically do not see nucleoporation-induced phosphorylation of eIF2α. Tesfay et al studied PKR−/− MEFs electroporated by a Bio-Rad GenePulser. Examination of the eIF2α western blots suggested an increase in the amount of phosphorylated eIF2α two hours after electroporation, but quantitation is not provided to allow accurate determination.28
In wild-type cells and hMDDC, phosphorylation of GCN2 was induced by nucleofection. In single-knockout cell lines, the absence of GCN2 resulted in a pronounced but not complete reduction in nucleofection-induced eIF2α phosphorylation, while no effect was observed with the absence of PERK alone. Elimination of nucleofection-induced eIF2α phosphorylation required the absence of both GCN2 and PERK. The observation that GCN2 phosphorylation coincides with eIF2α phosphorylation and then wanes while eIF2α phosphorylation remains elevated suggests a model where eIF2α is phosphorylated at early time points by GCN2 and at later time points by PERK. This is reminiscent of eIF2α phosphorylation in response to UVC light, which is mediated by GCN2 at 1 hour24 and by PERK at 4 hours29 following UV exposure.
GCN2 is activated by a range of stresses, including nutrient limitation, proteosome inhibition, oxidizing conditions, high salinity, and UV irradiation. In all cases, it is thought that GCN2 activation requires the binding of uncharged tRNA (see 30, 31). PERK is an ER-associated transmembrane protein that normally exists in an inactive form as a heterodimer with the chaperone BiP. ER stresses, such as excess misfolded protein, cause dissociation of BiP, allowing PERK homodimerization and activation.20 In contrast to WT cells, in GCN2−/− cells, we observed low level nucleofection-induced eIF2α phosphorylation, suggesting that GCN2 is the major kinase responsible for phosphorylating eIF2α and that nucleofection has an immediate impact on the availability of charged tRNAs. The absence of nucleofection-induced eIF2α phosphorylation in GCN2−/−/PERK−/− cells suggests that the phosphorylation of eIF2α seen in GCN2−/− cells results from PERK activation and occurs later than GCN2 activation following nucleofection. Nucleofection may directly cause ER stress leading to PERK activation. Alternatively, nucleofection-induced PERK activation may occur as a consequence of the absence of GCN2. In this scenario, GCN2 activation following nucleofection results in translational repression and thereby prevents secondary ER stress. However, in the absence of GCN2, unrepressed translation then leads to ER stress and PERK activation. Thus, while the data suggests that both GCN2 and PERK mediate nucleofection-induced eIF2α phosphorylation, we cannot rule out whether GCN2 is, in fact, the only kinase. Attempts to measure phosphorylation of PERK were unsuccessful.
Nucleofection-induced eIF2α phosphorylation typically returned to baseline levels by four to six hours following nucleofection, although in some cases extended through 24 hours (data not shown). Given this timeframe, transgene expression following delivery of mRNA is likely to be affected most dramatically, although expression following plasmid transfection would be impacted as well. In addition to reducing translation of the transfected gene, eIF2α phosphorylation has a general impact by repressing global translation in cells. This is of particular concern for nucleofection of primary cells, which are often more sensitive and where a minimal disturbance can be detrimental. Numerous therapeutic approaches using mRNA delivery are under exploration, including transfection of DCs with mRNA encoding tumor antigens,32-36 delivery of mRNA encoding vaccine antigens,37 cancer immunotherapy through transfection of T cells with mRNA encoding chimeric antigen receptors,14, 38 stem cell research,39 and induced pluripotent stem (iPS) cell generation.40-44 Importantly, clinical trials utilizing nucleofection delivery are ongoing and trials with related technologies that deliver electrical stimulation applied to cells ex vivo45 or after nucleic acid delivery in vivo are under intense study.46 Whether such related technologies also lead to phosphorylation of eIF2α will need to be determined.
To achieve maximum benefit from nucleofection, it will be valuable to design methods to obviate nucleofection-induced eIF2α phosphorylation. When serine 51 of eIF2α is mutated to alanine (eIF2α-S51A), eIF2α cannot be phosphorylated, and, therefore, is unaffected by eIF2α kinase activity, allowing ongoing translation despite eIF2α kinase activation.47, 48 Co-delivery of mRNA encoding eIF2α-S51A with an mRNA encoding a protein of interest would generate an increasing pool of non-phosphorylated eIF2α, which could offset nucleofection-induced eIF2α phosphorylation. A similar approach was seen to enhance translation following delivery of plasmid DNA.27 Due to the long half-life of eIF2α, estimated as 10 days,49 this approach would be expected to have a long-lasting impact in transfected cells, with potentially deleterious effects.50 Similarly, mRNA encoding inhibitors of eIF2α kinases, such as the vaccinia K3L protein,51 could be co-transfected with an mRNA of interest to limit eIF2α phosphorylation. Alternatively, mRNA transcripts could be designed to take advantage of eIF2α phosphorylation rather than attempting to prevent it. In contrast to the majority of transcripts, translation of select cellular mRNAs are upregulated following eIF2α phosphorylation, including GCN4 (ref. 50) and ATF4 (ref. 21), and this property is dependent on the 5′ UTR of these transcripts. Producing in vitro transcribed mRNAs containing the GCN4 5′UTR might allow selective translation through the duration of eIF2α phosphorylation following nucleofection.
We demonstrate that nucleofection of cells stimulates phosphorylation of the translation initiation factor eIF2α in immortalized cell lines and primary cells. This phosphorylation is mediated by the kinases GCN2 and PERK and results in global and delivered mRNA translational repression. In general, the primary consequence of eIF2α phosphorylation is inhibition of translation, thus, for nucleofection-based gene delivery, the immediate impact is reduced translation of the nucleofected nucleic acid. In addition to decreased translation, phosphorylation of eIF2α also leads to secondary consequences, including inhibited translation of most cellular transcripts, enhanced translation of stress genes, and increased levels of apoptosis. Therefore, in addition to translational repression, other effects involving eIF2α phosphorylation need to be considered. The efficacy of treatments could be limited both by translational repression and by cell stress sequelae, and, therefore, eIF2α phosphorylation must be considered in the design of nucleofection-based delivery approaches.
Materials and Methods
Cells and reagents
Immortalized mouse embryonic fibroblasts (MEFs) were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 2 mM L-glutamine (Life Technologies, Carlsbad, CA, USA), 100 U/ml penicillin and 100 μg/ml streptomycin (Invitrogen, Carlsbad, CA, USA), 10% fetal calf serum (HyClone, Logan, UT, USA), MEM non-essential amino acids (Invitrogen), and 55 μM β-mercaptoethanol (Bio-Rad, Hercules, CA, USA). Human monocyte derived dendritic cells (hMDDCs) were prepared as described12 in serum-free AIM-V media supplemented with 50 ng/mL recombinant human (rhu)GM-CSF and 100 ng/mL rhuIL-4. Polyinosinic:polycytidylic acid (poly(I:C)) was purchased from Sigma (St Louis, MO, USA) and used at a concentration of 5 μg per 100 μl nucleofection.
Nucleofection
MEF cells were nucleofected using program T-020 and nucleofector V kit (Lonza, Basel, Switzerland). Human MDDCs were nucleofected using program U-002 and human dendritic cell nucleofector kit (Lonza). After 15 minutes recovery in RPMI, cells were plated in complete media (supplemented DMEM/10% FBS and RPMI/10% FBS for MEFs and hMDDCs, respectively) and incubated at 37° C with 5% CO2. At the indicated time following nucleofection, cells were lysed in RIPA lysis buffer supplemented with protease inhibitor cocktail (Sigma) and Halt phosphatase inhibitor (Pierce, Rockford, IL, USA) for western blotting.
Lipid and polymer transfections
Cells were seeded into 48-well plates at a density of 1.0 × 105 cells/well one day prior to transfection. Cells were exposed to 50 μl DMEM containing lipid-based lipofectin (Invitrogen), 50 μl DMEM medium alone, or 200 μl complete DMEM medium containing polymer/lipid-based TransIT-mRNA (Mirus, Madison, WI, USA) for 1 hour, which was then replaced with complete medium and further cultured. At the indicated time following exposure to lipid and polymer transfection reagents, cells were lysed as described for nucleofected cells.
Western blotting
Equal mass of protein (10–30 μg) for each sample was separated by 12% SDS-PAGE, transferred to Hybond-P PVDF membranes (GE Amersham, Piscataway, NJ, USA), blocked with 2.5% non-fat milk in TBS containing 0.05% Tween 20, and probed with antibodies for GCN2-pT899 (Epitomics, Burlingame, CA, USA) and eIF2α-pS51 (Cell Signaling Technology, Danvers, MA, USA), followed by HRP-conjugated anti-rabbit antibody (GE Amersham), and Pico or Femto chemiluminescent substrates (Pierce). Membranes were stripped by agitating gently in a buffer of 2% SDS, 100 mM β-mercaptoethanol, 62.5 mM Tris, pH 6.7 for 30 minutes at 50° C, then subsequently re-blocked and re-probed for total eIF2α (Cell Signaling Technology). Images were captured using the LAS1000 digital imaging system (FujiFilm, Valhalla, NY, USA) and densitometry was performed using MultiGauge v2.2 software.
Detection of reporter proteins in nucleofected cells
For plasmid-based reporter expression, one day prior to nucleofection WT and PERK−/−/GCN2−/− MEFs were transfected with pCMV-luciferase plasmid using Fugene-6 as described by the manufacturer (Roche, Basel, Switzerland).
For mRNA-based reporter expression, in vitro transcribed firefly luciferase mRNA containing cap1, pseudouridine modifications, and a 101-nt poly-A tail was generated, as previously described.52, 53 Briefly, Megascript T7 RNA polymerase (Ambion, Austin, TX) was used to transcribe luciferase mRNA from linearized plasmid pTEVlucA101, replacing uridine triphosphate with pseudouridine triphosphate (TriLink, San Diego, CA) in the transcription mix. The mRNA was capped using the m7G capping kit with 2′-O-methyltransferase (CellScript, Madison, WI) to obtain cap1. The mRNA was then HPLC purified as described.26 Nucleofection of WT and PERK−/−/GCN2−/− MEFs was performed with 2.0 × 106 cells and 5 μg of luciferase mRNA per 100 μl nucleofection. Cell number and viability were monitored and were equal between treatment conditions at each time point.
Cells were trypsinized (0.05%, Life Technologies), nucleofected, seeded onto plates coated with collagen (0.01 mg/ml) (Invitrogen), and lysed at the indicated time points in 25 μl cell lysis buffer (Promega, Madison, WI, USA). Two μl aliquots were assayed with the luciferase assay system (Promega) and a LUMAT LB 950 luminometer (Berthold, Oak Ridge, TN, USA) at a 10-second measuring time.
Statistical analysis
All data are reported as mean ± standard error of the mean (SEM). Statistical differences between treatment groups were calculated by the Student's t-test using Microsoft Excel. For all statistical testing, a P-value <0.05 was considered significant.
Acknowledgments
MEF cell lines were generously provided by David Ron (WT, GCN2−/−, and PERK−/−), Robert Silverman (PKR−/−), and Alan Diehl [with permission from Douglas Cavener] (PERK−/−/GCN2−/−). This work was supported by the National Institutes of Health [R01AI50484 and R21DE019059 to D.W., T32DK07748 to B.R.A., R42HL87688 to K.K.].
Funding: This work was supported by the National Institutes of Health [R01AI50484 and R21DE019059 to D.W., T32DK07748 to B.R.A., R42HL87688 to K.K.].
Footnotes
Conflict of Interest: The authors report no conflicts of interest.
References
- 1.Gresch O, Engel FB, Nesic D, Tran TT, England HM, Hickman ES, et al. New non-viral method for gene transfer into primary cells. Methods. 2004;33:151–63. doi: 10.1016/j.ymeth.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 2.Hamm A, Krott N, Breibach I, Blindt R, Bosserhoff AK. Efficient transfection method for primary cells. Tissue Eng. 2002;8:235–45. doi: 10.1089/107632702753725003. [DOI] [PubMed] [Google Scholar]
- 3.Harriague J, Bismuth G. Imaging antigen-induced PI3K activation in T cells. Nat Immunol. 2002;3:1090–6. doi: 10.1038/ni847. [DOI] [PubMed] [Google Scholar]
- 4.Coughlin CM, Vance BA, Grupp SA, Vonderheide RH. RNA-transfected CD40-activated B cells induce functional T-cell responses against viral and tumor antigen targets: implications for pediatric immunotherapy. Blood. 2004;103:2046–54. doi: 10.1182/blood-2003-07-2379. [DOI] [PubMed] [Google Scholar]
- 5.Van De Parre TJ, Martinet W, Schrijvers DM, Herman AG, De Meyer GR. mRNA but not plasmid DNA is efficiently transfected in murine J774A.1 macrophages. Biochem Biophys Res Commun. 2005;327:356–60. doi: 10.1016/j.bbrc.2004.12.027. [DOI] [PubMed] [Google Scholar]
- 6.Schacht V, Ramirez MI, Hong YK, Hirakawa S, Feng D, Harvey N, et al. T1alpha/podoplanin deficiency disrupts normal lymphatic vasculature formation and causes lymphedema. EMBO J. 2003;22:3546–56. doi: 10.1093/emboj/cdg342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Poy MN, Eliasson L, Krutzfeldt J, Kuwajima S, Ma X, Macdonald PE, et al. A pancreatic islet-specific microRNA regulates insulin secretion. Nature. 2004;432:226–30. doi: 10.1038/nature03076. [DOI] [PubMed] [Google Scholar]
- 8.Vo N, Klein ME, Varlamova O, Keller DM, Yamamoto T, Goodman RH, et al. A cAMP-response element binding protein-induced microRNA regulates neuronal morphogenesis. Proc Natl Acad Sci U S A. 2005;102:16426–31. doi: 10.1073/pnas.0508448102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roy J, Paquette JS, Fortin JF, Tremblay MJ. The immunosuppressant rapamycin represses human immunodeficiency virus type 1 replication. Antimicrob Agents Chemother. 2002;46:3447–55. doi: 10.1128/AAC.46.11.3447-3455.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blindt R, Zeiffer U, Krott N, Filzmaier K, Voss M, Hanrath P, et al. Upregulation of the cytoskeletal-associated protein Moesin in the neointima of coronary arteries after balloon angioplasty: a new marker of smooth muscle cell migration? Cardiovasc Res. 2002;54:630–9. doi: 10.1016/s0008-6363(02)00252-3. [DOI] [PubMed] [Google Scholar]
- 11.Hacein-Bey-Abina S, Von Kalle C, Schmidt M, McCormack MP, Wulffraat N, Leboulch P, et al. LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science. 2003;302:415–9. doi: 10.1126/science.1088547. [DOI] [PubMed] [Google Scholar]
- 12.Weissman D, Ni H, Scales D, Dude A, Capodici J, McGibney K, et al. HIV gag mRNA transfection of dendritic cells (DC) delivers encoded antigen to MHC class I and II molecules, causes DC maturation, and induces a potent human in vitro primary immune response. J Immunol. 2000;165:4710–7. doi: 10.4049/jimmunol.165.8.4710. [DOI] [PubMed] [Google Scholar]
- 13.Cannon G, Weissman D. RNA based vaccines. DNA Cell Biol. 2002;21:953–61. doi: 10.1089/104454902762053882. [DOI] [PubMed] [Google Scholar]
- 14.Zhao Y, Moon E, Carpenito C, Paulos CM, Liu X, Brennan AL, et al. Multiple injections of electroporated autologous T cells expressing a chimeric antigen receptor mediate regression of human disseminated tumor. Cancer Res. 2010;70:9053–61. doi: 10.1158/0008-5472.CAN-10-2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holcik M, Sonenberg N. Translational control in stress and apoptosis. Nat Rev Mol Cell Biol. 2005;6:318–27. doi: 10.1038/nrm1618. [DOI] [PubMed] [Google Scholar]
- 16.Nallagatla SR, Toroney R, Bevilacqua PC. Regulation of innate immunity through RNA structure and the protein kinase PKR. Curr Opin Struct Biol. 2011;21:119–27. doi: 10.1016/j.sbi.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Daher A, Laraki G, Singh M, Melendez-Pena CE, Bannwarth S, Peters AH, et al. TRBP control of PACT-induced phosphorylation of protein kinase R is reversed by stress. Mol Cell Biol. 2009;29:254–65. doi: 10.1128/MCB.01030-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pindel A, Sadler A. The role of protein kinase R in the interferon response. J Interferon Cytokine Res. 2011;31:59–70. doi: 10.1089/jir.2010.0099. [DOI] [PubMed] [Google Scholar]
- 19.Lu L, Han AP, Chen JJ. Translation initiation control by heme-regulated eukaryotic initiation factor 2alpha kinase in erythroid cells under cytoplasmic stresses. Mol Cell Biol. 2001;21:7971–80. doi: 10.1128/MCB.21.23.7971-7980.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bertolotti A, Zhang Y, Hendershot LM, Harding HP, Ron D. Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat Cell Biol. 2000;2:326–32. doi: 10.1038/35014014. [DOI] [PubMed] [Google Scholar]
- 21.Harding HP, Novoa I, Zhang Y, Zeng H, Wek R, Schapira M, et al. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol Cell. 2000;6:1099–108. doi: 10.1016/s1097-2765(00)00108-8. [DOI] [PubMed] [Google Scholar]
- 22.Wek SA, Zhu S, Wek RC. The histidyl-tRNA synthetase-related sequence in the eIF-2 alpha protein kinase GCN2 interacts with tRNA and is required for activation in response to starvation for different amino acids. Mol Cell Biol. 1995;15:4497–506. doi: 10.1128/mcb.15.8.4497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiang HY, Wek RC. Phosphorylation of the alpha-subunit of the eukaryotic initiation factor-2 (eIF2alpha) reduces protein synthesis and enhances apoptosis in response to proteasome inhibition. J Biol Chem. 2005;280:14189–202. doi: 10.1074/jbc.M413660200. [DOI] [PubMed] [Google Scholar]
- 24.Deng J, Harding HP, Raught B, Gingras AC, Berlanga JJ, Scheuner D, et al. Activation of GCN2 in UV-irradiated cells inhibits translation. Curr Biol. 2002;12:1279–86. doi: 10.1016/s0960-9822(02)01037-0. [DOI] [PubMed] [Google Scholar]
- 25.Anderson BR, Muramatsu H, Nallagatla SR, Bevilacqua PC, Sansing LH, Weissman D, et al. Incorporation of pseudouridine into mRNA enhances translation by diminishing PKR activation. Nucleic Acids Res. 2010;38:5884–92. doi: 10.1093/nar/gkq347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kariko K, Muramatsu H, Ludwig J, Weissman D. Generating the optimal mRNA for therapy: HPLC purification eliminates immune activation and improves translation of nucleoside-modified, protein-encoding mRNA. Nucleic Acids Res. 2011 doi: 10.1093/nar/gkr695. Epub ahead of print: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Underhill MF, Coley C, Birch JR, Findlay A, Kallmeier R, Proud CG, et al. Engineering mRNA translation initiation to enhance transient gene expression in chinese hamster ovary cells. Biotechnol Prog. 2003;19:121–9. doi: 10.1021/bp025560b. [DOI] [PubMed] [Google Scholar]
- 28.Tesfay MZ, Yin J, Gardner CL, Khoretonenko MV, Korneeva NL, Rhoads RE, et al. Alpha/beta interferon inhibits cap-dependent translation of viral but not cellular mRNA by a PKR-independent mechanism. J Virol. 2008;82:2620–30. doi: 10.1128/JVI.01784-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu S, Hu Y, Wang JL, Chatterjee M, Shi Y, Kaufman RJ. Ultraviolet light inhibits translation through activation of the unfolded protein response kinase PERK in the lumen of the endoplasmic reticulum. J Biol Chem. 2002;277:18077–83. doi: 10.1074/jbc.M110164200. [DOI] [PubMed] [Google Scholar]
- 30.Dever TE, Dar AC, Sicheri F. The eIF2alpha kinases. In: Mathews MB, Sonenberg N, Hershey JWB, editors. Translational Control in Biology and Medicine. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 2007. pp. 319–344. [Google Scholar]
- 31.Hinnebusch AG. Translational regulation of GCN4 and the general amino acid control of yeast. Annu Rev Microbiol. 2005;59:407–50. doi: 10.1146/annurev.micro.59.031805.133833. [DOI] [PubMed] [Google Scholar]
- 32.Shurin MR, Gregory M, Morris JC, Malyguine AM. Genetically modified dendritic cells in cancer immunotherapy: a better tomorrow? Expert Opin Biol Ther. 2010;10:1539–53. doi: 10.1517/14712598.2010.526105. [DOI] [PubMed] [Google Scholar]
- 33.Gilboa E, Vieweg J. Cancer immunotherapy with mRNA-transfected dendritic cells. Immunol Rev. 2004;199:251–263. doi: 10.1111/j.0105-2896.2004.00139.x. [DOI] [PubMed] [Google Scholar]
- 34.Lesterhuis WJ, De Vries IJ, Schreibelt G, Schuurhuis DH, Aarntzen EH, De Boer A, et al. Immunogenicity of dendritic cells pulsed with CEA peptide or transfected with CEA mRNA for vaccination of colorectal cancer patients. Anticancer Res. 2010;30:5091–7. [PubMed] [Google Scholar]
- 35.Perche F, Benvegnu T, Berchel M, Lebegue L, Pichon C, Jaffres PA, et al. Enhancement of dendritic cells transfection in vivo and of vaccination against B16F10 melanoma with mannosylated histidylated lipopolyplexes loaded with tumor antigen messenger RNA. Nanomedicine. 2011 doi: 10.1016/j.nano.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 36.Van Tendeloo VF, Van de Velde A, Van Driessche A, Cools N, Anguille S, Ladell K, et al. Induction of complete and molecular remissions in acute myeloid leukemia by Wilms' tumor 1 antigen-targeted dendritic cell vaccination. Proc Nat Acad Sci. 2010;107:13824–9. doi: 10.1073/pnas.1008051107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pascolo S. Vaccination with messenger RNA (mRNA) Handb Exp Pharmacol. 2008:221–35. doi: 10.1007/978-3-540-72167-3_11. [DOI] [PubMed] [Google Scholar]
- 38.Birkholz K, Hombach A, Krug C, Reuter S, Kershaw M, Kampgen E, et al. Transfer of mRNA encoding recombinant immunoreceptors reprograms CD4+ and CD8+ T cells for use in the adoptive immunotherapy of cancer. Gene Ther. 2009;16:596–604. doi: 10.1038/gt.2008.189. [DOI] [PubMed] [Google Scholar]
- 39.Ponsaerts P, van der Sar S, Van Tendeloo VF, Jorens PG, Berneman ZN, Singh PB. Highly efficient mRNA-based gene transfer in feeder-free cultured H9 human embryonic stem cells. Cloning Stem Cells. 2004;6:211–6. doi: 10.1089/clo.2004.6.211. [DOI] [PubMed] [Google Scholar]
- 40.Angel M, Yanik MF. Innate immune suppression enables frequent transfection with RNA encoding reprogramming proteins. PLoS One. 2010;5:e11756. doi: 10.1371/journal.pone.0011756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kaji K, Norrby K, Paca A, Mileikovsky M, Mohseni P, Woltjen K. Virus-free induction of pluripotency and subsequent excision of reprogramming factors. Nature. 2009;458:771–5. doi: 10.1038/nature07864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sul JY, Wu CW, Zeng F, Jochems J, Lee MT, Kim TK, et al. Transcriptome transfer produces a predictable cellular phenotype. Proc Natl Acad Sci U S A. 2009;106:7624–9. doi: 10.1073/pnas.0902161106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Warren L, Manos PD, Ahfeldt T, Loh YH, Li H, Lau F, et al. Highly Efficient Reprogramming to Pluripotency and Directed Differentiation of Human Cells with Synthetic Modified mRNA. Cell Stem Cell. 2010;7:618–630. doi: 10.1016/j.stem.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yakubov E, Rechavi G, Rozenblatt S, Givol D. Reprogramming of human fibroblasts to pluripotent stem cells using mRNA of four transcription factors. Biochem Biophys Res Commun. 2010;394:189–93. doi: 10.1016/j.bbrc.2010.02.150. [DOI] [PubMed] [Google Scholar]
- 45.Im GI, Kim HJ. Electroporation-mediated gene transfer of SOX trio to enhance chondrogenesis in adipose stem cells. Osteoarthritis Cartilage. 2011;19:449–57. doi: 10.1016/j.joca.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 46.Mallilankaraman K, Shedlock DJ, Bao H, Kawalekar OU, Fagone P, Ramanathan AA, et al. A DNA vaccine against chikungunya virus is protective in mice and induces neutralizing antibodies in mice and nonhuman primates. PLoS Negl Trop Dis. 2011;5:e928. doi: 10.1371/journal.pntd.0000928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Scheuner D, Song B, McEwen E, Liu C, Laybutt R, Gillespie P, et al. Translational control is required for the unfolded protein response and in vivo glucose homeostasis. Mol Cell. 2001;7:1165–76. doi: 10.1016/s1097-2765(01)00265-9. [DOI] [PubMed] [Google Scholar]
- 48.Zeenko VV, Wang C, Majumder M, Komar AA, Snider MD, Merrick WC, et al. An efficient in vitro translation system from mammalian cells lacking the translational inhibition caused by eIF2 phosphorylation. RNA. 2008;14:593–602. doi: 10.1261/rna.825008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Duncan R, Hershey JW. Regulation of initiation factors during translational repression caused by serum depletion. Abundance, synthesis, and turnover rates. J Biol Chem. 1985;260:5486–92. [PubMed] [Google Scholar]
- 50.Jousse C, Oyadomari S, Novoa I, Lu P, Zhang Y, Harding HP, et al. Inhibition of a constitutive translation initiation factor 2alpha phosphatase, CReP, promotes survival of stressed cells. J Cell Biol. 2003;163:767–75. doi: 10.1083/jcb.200308075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kawagishi-Kobayashi M, Silverman JB, Ung TL, Dever TE. Regulation of the protein kinase PKR by the vaccinia virus pseudosubstrate inhibitor K3L is dependent on residues conserved between the K3L protein and the PKR substrate eIF2alpha. Mol Cell Biol. 1997;17:4146–4158. doi: 10.1128/mcb.17.7.4146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kariko K, Buckstein M, Ni H, Weissman D. Suppression of RNA recognition by Toll-like receptors: the impact of nucleoside modification and the evolutionary origin of RNA. Immunity. 2005;23:165–75. doi: 10.1016/j.immuni.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 53.Kariko K, Muramatsu H, Welsh FA, Ludwig J, Kato H, Akira S, et al. Incorporation of pseudouridine into mRNA yields superior nonimmunogenic vector with increased translational capacity and biological stability. Mol Ther. 2008;16:1833–40. doi: 10.1038/mt.2008.200. [DOI] [PMC free article] [PubMed] [Google Scholar]