Abstract
Background
Psilocybin is a well-characterized classic hallucinogen (psychedelic) with a long history of religious use by indigenous cultures, and nonmedical use in modern societies. Although psilocybin is structurally related to migraine medications, and case studies suggest that psilocybin may be efficacious in treatment of cluster headache, little is known about the relationship between psilocybin and headache.
Methods
This double-blind study examined a broad range of psilocybin doses (0, 5, 10, 20, and 30 mg/70 kg) on headache in 18 healthy participants.
Results
Psilocybin frequently caused headache, the incidence, duration, and severity of which increased in a dose-dependent manner. All headaches had delayed onset, were transient, and lasted no more than a day after psilocybin administration.
Conclusions
Possible mechanisms for these observations are discussed, and include induction of delayed headache through nitric oxide release. These data suggest that headache is an adverse event to be expected with the nonmedical use of psilocybin-containing mushrooms as well as the administration of psilocybin in human research. Headaches were neither severe nor disabling, and should not present a barrier to future psilocybin research.
Keywords: psilocybin, headache, cluster headache, migraine, classic hallucinogen, LSD, psychedelic
1. Introduction
Psilocybin is a relatively well-characterized hallucinogen that has a long history of religious use by indigenous cultures of Mesoamerica and South America in the form of Psilocybe mushrooms. These mushrooms have also been used for recreational and spiritual purposes in industrialized societies. Psilocybin exerts its effects via its active metabolite, psilocin. Psilocybin, like mescaline from the peyote cactus and lysergic acid diethylamide (LSD), is considered a “classic” hallucinogen, producing psychoactive effects that are similar and primarily mediated by 5-HT2A receptor agonism. Psilocybin and LSD belong to a subclass of classic hallucinogens that, like the neurotransmitter serotonin, are structurally based on a tryptamine chemical backbone (Nichols, 2004). Other tryptamine-based compounds such as sumatriptan and ergotamine constitute the primary and most effective class of acute migraine treatment for migraine and related primary headaches (Brandes et al., 2010); in fact, chemist Albert Hofmann discovered LSD while searching for novel treatments for migraine, among other disorders (Hofmann, 1980). A recent case series also provided preliminary evidence that psilocybin and LSD may treat cluster headache (Sewell et al., 2006), and evidence suggests that the non-hallucinogenic LSD analog 2-bromo-LSD (BOL-148) may share similar efficacy (Sicuteri, 1963; Karst et al., 2010).
In a previous study, 36 hallucinogen-naïve volunteers received 30 mg/70 kg psilocybin and a comparison drug (40 mg/70 kg methylphenidate; a psychoactive “active placebo” used to maintain study blinding) in different sessions (Griffiths et al., 2006; Griffiths et al., 2008). Although reduction or induction of cephalic pain was not specifically assessed, it was our impression that more participants spontaneously reported headache after psilocybin study days than after methylphenidate days. Although reports of headache following psilocybin use were few, our reliance on spontaneous self-report likely underestimated the true incidence. A subsequent literature search uncovered several reports of headache following classic hallucinogen use.
The first such report came nearly a hundred and twenty years ago, when Prentiss and Morgan reported that one of their experimental subjects given mescaline experienced a three-day headache severe enough to be debilitating on the second day (Prentiss and Morgan, 1895). Other healthy volunteers reported “persistent ache and feeling of exhaustion in the occipital region, that persisted for several days” (Prentiss and Morgan, 1896). The pharmacologist Arthur Heffter’s account of his personal experience with mescaline on June 5, 1887 reads: “Nausea, occipital headache, intense dizziness, and clumsiness in moving began about half an hour after the last dose” (Heffter, 1898). The American neurologist Weir Mitchell described his own experience with mescaline in an 1896 talk to the American Neurological Society as characterized by “left frontal pain (not severe) and soon after a dull occipital ache felt on both sides and at or about the occipital bosses” (Mitchell, 1896). The headache persisted for two days, prompting Mitchell to editorialize that the mescaline experience was “worth one such headache… but not worth a second.” The psychologist Havelock Ellis also reported a “slight headache which passed off in the course of the morning” in his detailed description of the effects he experienced after taking mescaline. He also reported that a poet friend who also took it complained of “a very slight headache, which came and went” as the only negative side effect (Ellis, 1902).
The next report of headache from classic hallucinogen use came sixty years later, in a report of 16 individual administrations of psilocybin (across 13 participants in doses ranging from 5 to 14 mg). 50% resulted in reports of headache, but further details were not provided (Delay et al., 1958). Two years later a study in which psilocybin was given in doses ranging from 8 to 36 mg reported headache in five of 14 volunteers (Malitz et al., 1960). However, Malitz did not characterize the headaches further, or state at what dose they occurred. Another description of psilocybin published the same year listed “headache” under “later effects” (i.e., more than 12 hours post drug administration) but gave no further details (Hollister et al., 1960). The same author measured the after effects of 37 to 209 μg/kg psilocybin in 17 subjects, and reported that the most frequent complaints were occasional headaches and fatigue “likened to a mild hangover” (Hollister, 1961). Another study reported headache as an adverse event following psilocybin administration, but did not quantify this (Rümmele, 1958). A more recent summary of psilocybin experiments conducted in Switzerland reported that “headaches, head pressure or face pain” were reported by 12.5% to 37.5% of participants in a dose-related manner (Studerus et al., 2010). Other studies suggest effects of classic hallucinogen administration on cephalic pain, although the implications are unclear. For example, one study found that participants who regularly suffered from migraine or other “essential” (idiopathic or primary) headaches showed increased psychoactive effects from very low doses of psilocybin and LSD compared to age-matched controls (Fanciullacci et al., 1974).
In order to more rigorously characterize the headaches that had been spontaneously reported in the first study, our second psilocybin administration study specifically examined this potential side effect. Our informed consent process described headache on or after the session day as a possible adverse effect of psilocybin administration. We explicitly asked participants about headaches, and carefully characterized those reported. This study is therefore the first to prospectively assess the dose-dependent incidence of headache following psilocybin administration.
2. Methods
2.1 Participants
Participants were 10 female and 8 male medically and psychiatrically healthy volunteers (Griffiths et al., 2011). Only one had previously used hallucinogens; he had taken psilocybin on two occasions more than 20 years before study screening. Demographics, including self-reported headache history, are presented in Table 1. Participants were not paid for participation, and generally reported being motivated by curiosity about psilocybin’s effects, altered states of consciousness, and the opportunity for self-reflection. Study conduct, including participant selection, followed the safety guidelines for human hallucinogen research recommended by our laboratory (Johnson et al., 2008).
Table 1.
Volunteer demographics.
| Vol. ID | Sex | Race | Age (years) | Education (years) | Self-reported history of headache at screening |
|---|---|---|---|---|---|
| 201 | M | Caucasian | 34 | 16 | none |
| 202 | F | Caucasian | 29 | 16 | none |
| 205 | M | Caucasian | 30 | 16 | none |
| 206 | F | Caucasian | 46 | 18 | Had 3–4 headaches in previous year, but virtually never had headaches before that. Participant believes they are related to perimenopause |
| 207 | M | Caucasian | 44 | 18 | none |
| 210 | M | Caucasian | 42 | 16 | Has 1–2 migraines/month; prescribed sumatriptan |
| 211 | F | Caucasian | 34 | 18 | none |
| 213 | F | Caucasian | 52 | 18 | none |
| 214 | M | African American | 38 | 16 | At ages 22–23 had 1 headache/month; from ages 24–38 had 1–5 headaches/year |
| 215 | F | Caucasian | 41 | 18 | none |
| 217 | F | Caucasian | 49 | 18 | Since age 40 (during perimenopause) has had 10 headaches, 5 of which were debilitating & required a day in bed |
| 218 | M | African American | 55 | 20 | none |
| 219 | M | Caucasian | 51 | 20 | none |
| 222 | F | Caucasian | 62 | 16 | none |
| 223 | F | Caucasian | 53 | 16 | none |
| 226 | M | Caucasian | 56 | 18 | 8 years before study had persistent severe headache for 6 month duration. CT scan did not identify a problem |
| 228 | M | Caucasian | 55 | 20 | none |
| 230 | F | Caucasian | 49 | 18 | none |
2.2 Procedure
Experimental methods, inclusion and exclusion criteria are described in detail elsewhere (Griffiths et al., 2011). Psilocybin was administered in doses of 0, 5, 10, 20, and 30 mg/70 kg in five eight-hour drug sessions conducted at approximately one-month intervals in a double-blind, crossover design. Psilocybin doses increased sequentially in nine randomly selected participants and decreased sequentially in the other nine. The single placebo session was intermixed among the four active doses as detailed elsewhere (Griffiths et al., 2011). These two sequences were included in order to examine whether ascending vs. descending dose sequence changed the psychological effects of psilocybin.
Study monitors met individually with each participant for a total of eight hours before the first session and for two hours between sessions to develop rapport and trust, which can reduce adverse reactions. The eight-hour drug sessions were conducted in a comfortable living-room-like environment designed for the study. Two monitors were present throughout the session. Participants were encouraged to lie on a couch, wear an eye mask, listen to music through headphones, and focus attention on their inner experiences. At the end of the session volunteers rated drug effects on several questionnaires, and then were released into the care of a friend or family member, who took them home. Although pain medication was not allowed during sessions, participants were told they could use over-the-counter headache medicine after session completion.
2.3 Measures
A variety of outcome measures included observer-rated drug effects during the psilocybin session, participant-rated drug effects, and assessments of persistent effects at follow-up visits three weeks after each session and 14 months after the last session. These results are reported elsewhere (Griffiths et al., 2011). Relevant to the present analysis, at a follow up session participants were specifically asked whether they had had a headache during or after the session. This follow up occurred one or two days after the session for 82% of sessions (SD 2.1; maximum 14). Participants reporting headache were asked the onset and ending time of the headache (used to calculate headache duration), whether the headache was a single episode or intermittent, whether the headache had ended already, the severity of the headache (1=“mild”, 2=“moderate”, 3=“severe”), and whether medication was taken to treat the headache. Although participants were not explicitly asked about headaches at three-week and fourteen-month follow-up visits, they were provided an opportunity to report persisting headaches or any other negative effects resulting from study participation.
2.4 Analyses
Headache incidence was analyzed with repeated measures regression with effects of sequence (ascending, descending) and dose (0, 5, 10, 20, 30 mg/70kg) (SAS PROC MIXED, α=0.05). Tukey post-hoc tests were used to compare each psilocybin dose to placebo. Several repeated-measures multiple regression analyses also examined the effect of dose on other variables, including headache incidence as a dichotomous covariate to examine the relationship of headache with other measurements. These included the Hood Mysticism Scale total score (Hood et al., 2001; Spilka et al., 2003) and Mystical Experience Questionnaire total score (Griffiths et al., 2006) as measures of mystical quality of subjective psilocybin effects; the intensity subscale of the Hallucinogen Rating Scale (HRS) (Strassman et al., 1994) as a measure of drug effect strength; and peak and area under the curve (AUC) values for systolic and diastolic blood pressure and heart rate throughout each session.
3. Results
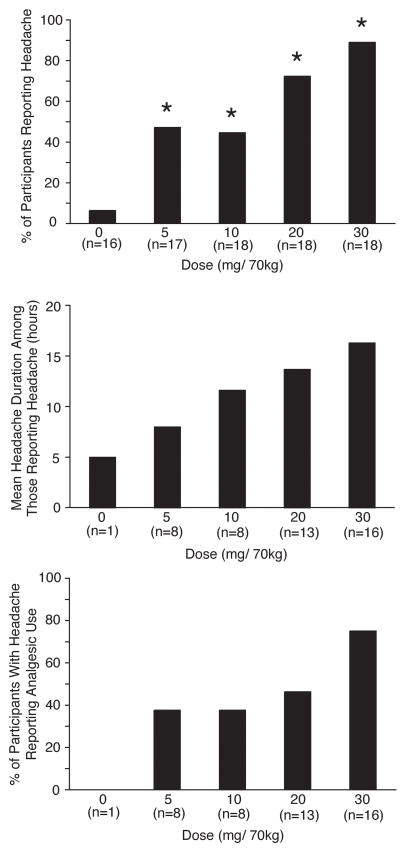
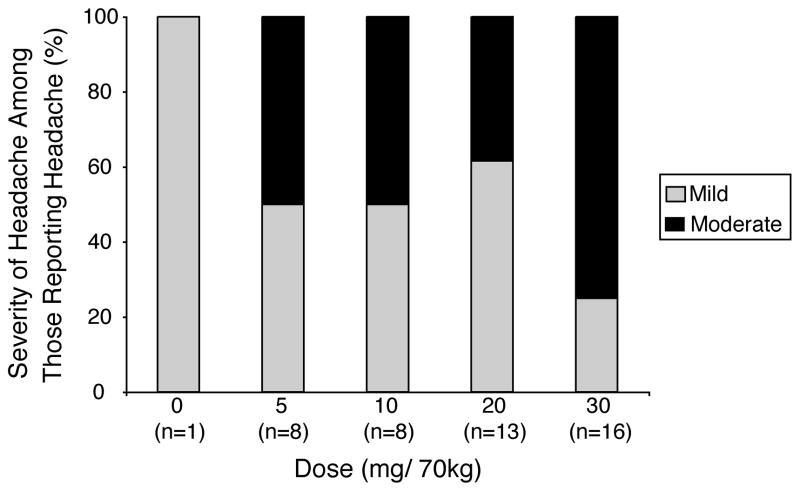
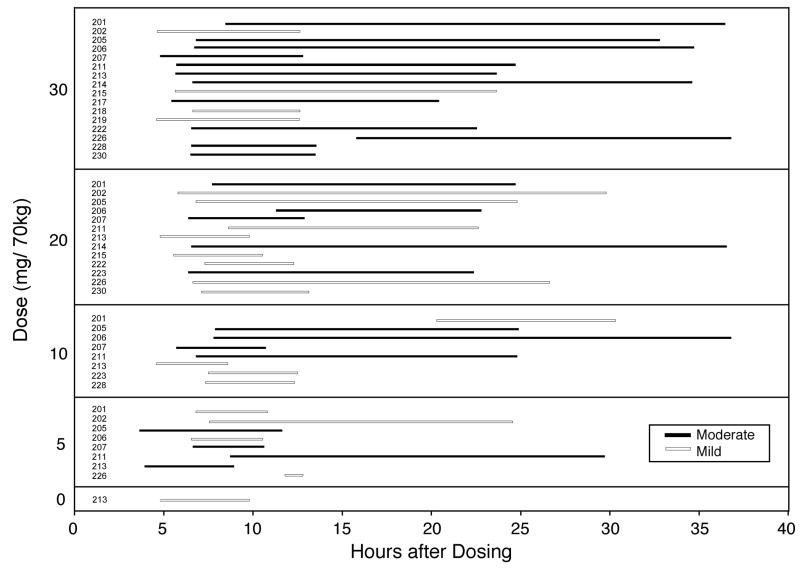
Table 1 shows headache-relevant individual participant demographics. Four participants reported history of headache at screening. In three sessions participants reported headache at the time of drug administration. In these cases the onset of headache occurred at a mean of 13.3 hours before drug administration, and total headache duration was a mean of 28.5 hours. These sessions were excluded from further analysis. Headache incidence increased in a dose-related manner (Figure 1 top panel). Repeated-measures regression found a significant effect of Dose (F4,64=12.74, p<.0001), but not Sequence or Dose × Sequence. Tukey post-hoc tests verified that headache occurred in a significantly greater number of participants at each dose of psilocybin compared to placebo (all p<0.03). Headache duration among those who reported headache was dose-related (Figure 1 middle panel). All headaches had resolved before headache data collection. There was a dose-dependent increase in the percentage of participants who took headache medicine (in all cases OTC acetaminophen, ibuprofen, or aspirin) for headache among those who reported headache (Figure 1 bottom panel). Headache severity among those who reported headache was dose related (Figure 2). All headaches were rated mild or moderate; no participant rated their headache as severe at any dose. Figure 3 presents individual participant data showing the incidence, timing (including onset time and duration), and severity of headaches for each dose. Headaches started a mean of 7.0 hours after psilocybin administration, with little difference in onset time between doses, although a few headaches started substantially later. There was no systematic relationship between individual participant demographics and Figure 3 data. The effect of the headache covariate was not significant in any repeated-measures multiple-regression analyses, suggesting no relationship between headache and mystical experience, hallucinogenic drug effects, drug strength, blood pressure, or heart rate.
Fig. 1.
Top panel: Incidence of headache across dose conditions. Bars show percent of participants reporting headache after each dose condition. Asterisks indicate a significant difference between that condition and the placebo (0 mg/70 kg) condition (Tukey post-hoc tests). Middle panel: Mean headache duration among those reporting headache. Bottom panel: Percent of participants with headache reporting analgesic use. Number of volunteers reporting headache (indicated by n) increased across dose conditions for all panels.
Fig. 2.
Ratings of headache severity among those reporting headache for each dose condition. Percents of participants reporting “mild” and “moderate” severity are shown for each dose condition. No participant rated any headache as “severe.” The number of volunteers reporting headache (indicated by n) increased across dose conditions.
Fig. 3.
Individual participant data showing the incidence, timing (including onset time and duration), and severity of headaches for each dose condition. The y-axis shows the different dose conditions categorically. Each bar horizontal bar shows data for the participant number listed to right of the y-axis. Data are not shown for sessions without reported headache and sessions with missing data.
4. Discussion
For most volunteers, psilocybin caused a delayed headache, the incidence and severity of which was dose-related. Although the headaches observed in this study were likely to have been migraines, the diagnosis of migraine requires determination of factors in addition to severity (nausea, vomiting, photophobia or phonophobia, unilaterality, pulsatile quality, and worsening with physical activity) that were not assessed in this study, precluding accurate determination of headache type. To explore these results, we will first consider the role of serotonin in headache, then compare psilocybin with other drugs that cause headache, then speculate on mechanisms, including a possible relationship between psilocybin headache induction and the putative ability of psilocybin to treat cluster headache.
4.1 Serotonin and headache
Serotonin plays a pivotal role in many aspects of migraine pathogenesis. Cranial, meningeal, and cerebral blood vessels are sensitive to serotonin and can either contract or relax via activation of 5-HT2A, 5-HT1B, or 5-HT7 receptors, with serotonin exhibiting its lowest affinity at 5-HT2A receptors (Martin, 1994; Saxena, 1995; De Vries et al., 1999; Bouchelet et al., 2000). Serotonin constricts large arteries and arteriovenous anastomoses mainly via 5-HT1B receptors, and dilates arterioles via 5-HT7 receptors (De Vries et al., 1998; De Vries et al., 1999).
There is considerable evidence for tonically low serotonin levels in migraine patients. During migraine attacks, plasma levels of serotonin are increased; between attacks, serotonin is decreased (Humphrey, 1991; Ferrari and Saxena, 1993). Cortical auditory evoked potentials are also increased in migraine patients between attacks, consistent with low serotonin levels (Wang et al., 1996). Tryptophan depletion, which lowers brain serotonin levels, induces nausea, dizziness, and motion sickness in healthy controls but not in migraineurs, suggesting that migraineurs are tolerant to low serotonin levels (Drummond, 2005). Patients who carry the short allele of the serotonin transporter (which has been associated with reduced serotonin neurotransmission) have an increased risk for migraine, and increased migraine frequency (Lesch et al., 1996; Kotani et al., 2002).
Several drugs that release serotonin from neurons and blood platelets (fenfluramine and reserpine) and some serotonin reuptake inhibitors (zimeldine and femoxetine) are able to provoke migraine attacks more frequently in migraineurs than in controls (Humphrey, 1991; Ferrari and Saxena, 1993; Panconesi and Sicuteri, 1997). Effective antimigraine drugs target specific populations of serotonin receptors. Paradoxically, fenfluramine and reserpine, when used for an extended period, reduce serotonin and can confer resistance to migraine headache (Hamel, 2007), and serotonin given during an attack will relieve headache, indicating that the role of serotonin in headache is complex. These observations suggest that migraine is a disorder of tonically low central serotonin associated with an increase in serotonin release during attacks (Ferrari et al., 1989). In a low-serotonin state, the cortex is more vulnerable both to cortical spreading depression (CSD) and CSD-induced trigeminal nociception (Supornsilpchai et al., 2006).
Receptors that trigger migraine attack (possibly 5-HT2B) appear to be different from those that relieve migraine headache (5-HT1 family). The ability of the 5-HT2B/C receptor agonist m-chlorophenylpiperazine (mCPP) to induce attacks in migraine sufferers that are indistinguishable from spontaneous headaches (Brewerton et al., 1988), together with the 5-HT2B affinity of prophylactic antimigraine 5-HT2 receptor antagonists such as methysergide, cyproheptadine, and mianserin, support the involvement of 5-HT2B supersensitivity in migraine attacks (Fozard and Kalkman, 1994; Kalkman, 1994; Schmuck et al., 1996).
Triptans are a novel class of selective 5-HT1B/1D antagonists that are the mainstay of current acute migraine treatment. Some triptans also interact with 5-HT1A and 5-HT1F receptors, but rizatriptan is almost completely selective for 5-HT1B/1D (Tfelt-Hansen et al., 2000), and alniditan, a non-triptan 5-HT1B/1D antagonist that is no longer in clinical development, is effective for migraine (Goldstein et al., 1996) but has no affinity for 5-HT1F (Leysen et al., 1996). Sumatriptan, zolmitriptan, eletriptan, and frovatripan show micromolar affinity at the 5-HT7 receptor, which mediates smooth muscle relaxation in cerebral arteries (Terron and Falcon-Neri, 1999).
The 5-HT2A receptor, which mediates psilocybin’s psychoactive effects, also mediates several important cardiovascular effects in both the peripheral nervous system and CNS, including vascular smooth muscle contraction, platelet aggregation, thrombus formation, and coronary artery spasm. The selective 5-HT2A antagonists ketanserin, AT-1015, and sarpogrelate all have vasodilatative properties (Kyriakides et al., 1999; Kihara et al., 2000; Miyata et al., 2000). Methysergide, a 5-HT2A/2C receptor antagonist (and migraine prophylactic agent), inhibits the vasoconstrictive and pressor effects of serotonin as well as the serotonin actions on extravascular smooth muscle. Conversely, the 5-HT2A agonist DOI constricts rat veins, an effect that is blocked by the 5-HT2A antagonists ketanserin (Cohen et al., 1993).
4.2 Drug-induced headache
A number of different drugs have been used experimentally to induce headache. Foremost among these is nitroglycerin. Nitric oxide (NO) is a free-radical gas with vasorelaxant effects (Silberstein, 1994). Nitroglycerin serves as an NO donor, causing a nonspecific headache within five to six hours in healthy individuals, presumably from cGMP activation (Miki et al., 1977). Migraine patients are more sensitive to the immediate effects of nitroglycerin than healthy subjects (Schnitker and Schnitker, 1947; Peters, 1953; Dalsgaard-Nielsen, 1955), as are cluster headache patients during their cluster periods (Horton, 1956; Ekbom, 1968). First-degree relatives of migraine patients also develop migraine after nitroglycerin administration, suggesting a genetic predisposition (Sicuteri, 1963). In migraineurs, an immediate headache of mild-to-moderate intensity begins during nitroglycerin infusion; five to six hours later a characteristic migraine attack reproducibly occurs in 75% of migraine patients (Thomsen et al., 1994; Thomsen et al., 1996; Christiansen et al., 2000; Afridi et al., 2004). A similar response is observed after sublingual administration of the long-acting isosorbide dinitrate (Bellantonio et al., 1997). The surprisingly long latency period between nitroglycerin exposure and migraine suggests that release of NO initiates a slow cortical spreading depression that results in an attack. In rats, NO has been shown to mediate both the cortical hyperexcitability and the facilitation of trigeminal nociception characteristic of serotonin depletion, increasing both cortical spreading depression and the resultant trigeminal nociception in decreased serotonin states (le Grand et al., 2011). NO donors also can induce a bilateral, pulsating frontotemporal headache as they clear from the blood. Because 5-HT2B/2C receptor stimulation liberates NO (Glusa and Richter, 1993; Fozard, 1995), 5-HT2B/2C antagonists such as the anti-migraine medications methysergide and pizotifen may well exert their action by reducing NO production, and psilocin may directly trigger migraine through 5-HT2B-mediated NO release (Schmuck et al., 1996).
4.3 Psilocybin induction of headache
Psilocybin is 50% absorbed following oral administration (Brown, 1972; Hopf and Eckert, 1974) and appears in the plasma 20 to 40 minutes after oral administration (Hasler et al., 1997; Passie et al., 2002). First-pass hepatic metabolism converts psilocybin into psilocin (4-hydroxy-N,N-dimethyltryptamine, the pharmacologically active metabolite of psilocybin), which is detectable in plasma 30 to 50 minutes after administration (Hasler et al., 1997; Lindenblatt et al., 1998). Psilocin plasma levels increase rapidly, plateau for about 50 minutes, then slowly decline, with approximately two-thirds excreted after three hours (Holzmann, 1995).
The time-course of psilocybin-triggered headache matches closely that observed from nitroglycerin, and is consistent with a possible role for psilocybin as an NO releaser. Psilocybin increases expression of a number of genes, including Iκβ-α, the main inhibitor of NFκβ, an activating component for immune response (Nichols and Sanders-Bush, 2002; Gonzalez-Maeso et al., 2003). Expression of inducible nitric oxide synthase (iNOS), which enhances NO release, is dependent on NFκβ (Reuter et al., 2002), which is directly suppressed by LSD and probably also by psilocybin (Nichols, 2004). Nitroglycerin causes a delayed expression of iNOS (Reuter et al., 2001), and psilocybin may exert effects through this mechanism also. Direct evidence for or against psilocybin as an NO releaser is currently lacking, however.
Alternately, psilocybin may trigger headache through an increase in brainstem serotonergic activity. Psilocin affects primarily serotonergic receptors (5-HT1A,D, 5-HT2A, C, 5-HT5, 5-HT6, and 5-HT7 receptor subtypes; NIMH Psychoactive Drug Screening Program: http://pdsp.med.unc.edu). It binds with high affinity at 5-HT2A and to a lesser extent at 5-HT1A receptors, but has no affinity for dopamine receptors, α- or β-adrenergic receptors, SERT, or 5-HT3 receptors. Psilocin’s psychoactive effects are primarily mediated through 5-HT2A receptors (Nichols, 2004), which are located predominantly on cortical pyramidal cell apical dendrites (Jakab and Goldman-Rakic, 1998). When activated, these receptors lead to increased cortical activity driven by glutamatergic excitatory postsynaptic potentials, largely in the frontal cortex (Vollenweider et al., 1997), although 5-HT2A receptors are also localized on GABA interneurons and thus have the potential to also decrease cortical activation (Nichols, 2004).
The pain from headache is transmitted through a plexus of largely unmyelinated fibers that surround the large cerebral vessels, pial vessels, large venous sinuses and dura mater to the ophthalmic division of the trigeminal ganglion (Liu-Chen et al., 1984) and upper cervical dorsal roots (Arbab et al., 1986). When stimulated, these trigeminal nociceptive fibers release substance P, calcitonin gene-related peptide (CGRP; Uddman et al., 1985), and glutamate (Carlton, 2001), promoting leakage of plasma from the capillaries into the surrounding tissue and structural changes in the dura, including mast cell degranulation and platelet aggregation in postcapillary venules (Dimitriadou et al., 1991; Dimitriadou et al., 1992). This extravasation can be blocked by ergot alkaloids and sumatriptan (Moskowitz and Cutrer, 1993). Drugs such as psilocin that also act on serotonergic receptors (5-HT1B, 5-HT1D, and 5-HT1F) that are located on second-order peptidergic nociceptors here will reduce cell activity (Potrebic et al., 2003) and block headache effects; simultaneously, drugs that facilitate cortical glutamatergic activity will promote headache. The laterality, or lack thereof, of psilocybin-induced headaches in our experiment was not recorded, but those accounts of psychedelic-induced headache with localization generally indicate a bilateral, occipital location, which is consistent with referred pain from the trigeminal complex (Mitchell, 1896; Prentiss and Morgan, 1896). As AMPA-kainate glutamate receptor antagonists have been found effective in terminating migraine in small studies (Merritt and Williams, 1990) it is possible that glutamate receptor activation such as produced by psilocybin administration directly induces migraine.
Psilocybin headache induction also may be mediated through 5-HT1A receptors. The 5-HT1A partial agonist buspirone has been shown effective against migraine in patients with anxiety (Lee et al., 2005), although a role for 5-HT1A in migraine pathogenesis has not been established. In contrast to 5-HT2A receptors, inhibitory 5-HT1A receptors are highly and presynaptically expressed in the dorsal raphe nucleus of the brainstem, which does not manifest other serotonin receptor subtypes (Sotelo et al., 1990). Because 5-HT1A autoreceptors are more numerous and sensitive to serotonin than their postsynaptic counterparts (Barnes and Sharp, 1999), the primary effect of 5-HT1A receptor activation by psilocin is to reduce dorsal raphe cell firing rate, resulting in decreased serotonin release in terminal projection fields (Aghajanian and Hailgler, 1975). Dense concentrations of postsynaptic 5-HT1A receptors also have been identified in the hippocampus (Hamon et al., 1990), and in axon hillocks of pyramidal cells in the prefrontal cortex (PFC; Pazos and Palacios, 1985; DeFelipe et al., 2001; Czyrak et al., 2003) where they co-localize with 5-HT2A receptors (Santana et al., 2004) and inhibit pyramidal cell activity in a manner proportional to dorsal raphe serotonin release (Puig et al., 2005). Intraparenchymal microcirculation (supplying neurons and glia) in the cerebral cortex is also regulated by serotonergic dorsal raphe neurons (Cohen et al., 1996).
Dorsal raphe inhibition activates noradrenergic neurons in the nearby locus ceruleus (LC), a major pontine center for sensory integration, making them more sensitive to sensory input (Aghajanian, 1980; Rasmussen and Aghajanian, 1986; Aghajanian, 1994). LC neurons also control cerebral circulation and may induce vascular changes similar to those found in migraine (Agnoli and De Marinis, 1985). Stimulation of the LC in animals can cause a frequency-dependent reduction in cerebral blood flow, maximal (up to 25%) in the occipital cortex, through an α2 adrenoreceptor-linked mechanism (Goadsby and Duckworth, 1989) as well as a strong antinociception that is mediated by spinal α2-adrenoreceptors. Inhibition of serotonin release from the dorsal raphe nucleus, in combination with an increase in cortical activation induced by psilocybin, likely reduces the relative influence of the brainstem nuclei on cortical response. By selectively depressing the activity of neurons in the dorsal raphe and thereby decreasing serotonin release, psilocybin removes the tonic inhibition of downstream neurons mediated by serotonin. This ability to evoke a cessation of serotonergic cell firing appears unrelated to the production of hallucinogenic activity (Aghajanian and Marek, 2000).
Although there is no direct anatomical connection between dorsal raphe neurons and the meningeal/cerebral blood vessels (Cohen et al., 1992; Cohen et al., 1996; Mathiau et al., 1993), these vessels can nevertheless respond to changes in central serotonin neurotransmission. Lesions of the dorsal raphe nucleus induce supersensitivity to serotonin in isolated cerebral arteries (Moreno et al., 1991). An abrupt increase in brainstem serotonergic neuron activity or platelet discharge of serotonin following a stressful stimulus results in the activation of sensitized neuronal/vascular serotonin receptors, and possibly of the pain-generating process also. Psilocin’s suppression of dorsal raphe nucleus activity, with subsequent LC overactivity, suggests that “rebound” dorsal raphe overactivity and LC suppression may generate headache from supersensitivity to serotonin once the direct pharmacological effects of psilocin wear off. That being said, direct evidence that activation of trigeminovascular fibers can be achieved by a change in the firing rate of dorsal raphe neurons is currently lacking, and although psilocybin’s ability to do so has been demonstrated in vitro, evidence that it does so in vivo is also lacking.
Some investigators have combined psilocybin with other drugs with specific receptor effects and measured incidence of headache, providing possible clues as to psilocybin’s mechanism. One study in which the 5-HT2A receptor blocker ketanserin 50 mg was administered 90 min before psilocybin 215 μg/kg in ten subjects found equally high rates of headache in psilocybin and psilocybin + ketanserin groups, but none in placebo or ketanserin alone groups, suggesting that psilocybin headache is not mediated via effects at 5-HT2A (Carter et al., 2005). This is consistent with genetic association studies excluding alleles of 5-HT2A and 5-HT2C as candidate genes for migraine susceptibility (Buchwalder et al., 1996). Other evidence against a role for 5-HT2A is that the 5-HT2A agonist LSD does not appear to cause headache, as discussed in the introduction. Another study combining haloperidol 1.5 mg/70 kg with psilocybin 260 μg/kg in five subjects found a 60% incidence of headache in the psilocybin-alone group 10 hours after drug administration, but none when psilocybin was combined with haloperidol, or in the placebo or haloperidol-alone groups (Vollenweider et al., 1998), consistent with haloperidol’s migraine-blocking effects (Honkaniemi et al., 2006). Drug interaction studies thus far have suffered from small sample sizes and inconsistent measurement of headache, but provide intriguing clues as the mechanism of psilocybin’s cephalalgic effects, suggesting that it is unrelated to 5-HT2A and related psychedelic effects. Anecdotal reports that mescaline, which does not bind to 5-HT1 receptors, also induces headache suggests that post-psilocybin headache also may be unrelated to 5-HT1. Coadministration of psilocybin with a 5-HT1 receptor antagonist could determine this. Studerus and colleagues, in their review of 110 healthy subjects given psilocybin in various studies at doses ranging from 45 to 315 mg/kg, also reported dose-dependent headaches, head pressure, or head pain following psilocybin administration (Studerus et al., 2010). Their laboratory found an incidence of headache half of that observed in our sample. The reason for this is unclear. There may have been a lower proportion of subjects with a genetic predisposition to headache, or there may have been cultural differences in what subjects considered to be a reportable side effect.
Several lines of evidence suggest that the cause of post-psilocybin headache may not be 5-HT2A-mediated vasoconstriction followed by rebound vasodilatation. Although 5-HT2A receptors are thought to have pro-nociceptive effects in the peripheral nervous system (Sokolov et al., 2011). 5-HT2A antagonists such mianserin, sergolexole, ketanserin, and ICI169369 are not effective preventative drugs for migraine headache (Silberstein, 1994). Although antipsychotics with antagonist effects at 5-HT2A have been reported effective in treating migraines (Dusitanond and Young, 2009), all have effects at multiple receptors other than 5-HT2A, precluding conclusions about the role of 5-HT2A. Also, because rebound vasodilatation would likely be indicated by frontal hypermetabolism, and because PET shows frontal hypermetabolism early during acute psilocybin effects (Vollenweider et al., 1997), if vasodilatation was responsible for headache one would expect headaches to occur similarly early during psilocybin effects, rather than showing the delayed onset observed in the present study. Other evidence also argues against a role for psilocybin as a vasoconstrictor. Although high doses of psilocybin have been demonstrated to constrict sheep umbilical veins in vitro (Dyer and Gant, 1973), this observation may have limited relevance to clinical doses, at which psilocybin causes only mildly elevated pulse and blood pressure (Hasler et al., 2004; Griffiths et al., 2006; Griffiths et al., 2008; Griffiths et al., 2011). Moreover, epidemiological data do not suggest that stroke or myocardial infarction results from psilocybin as would be expected if vasoconstrictive effects were prominent (e.g., psilocybin is mentioned in 0.1% of drug-related emergency department visits, likely providing sufficient opportunity to detect an association with such cardiovascular adverse effects; SAMHSA, 2003). Although the related class of ergot alkaloids contains many drugs with severe direct vasoconstrictive effects, psilocybin does not appear to share these effects.
Other arguments have been made for 5-HT2C mediation of migraine pathophysiology (Fozard and Kalkman, 1994). 5-HT2C is expressed throughout the CNS, particularly in the choroid plexus (Hartig et al., 1990) and the suprasegmental parts that transmit nociceptive information. mCPP can trigger migraine attacks at doses that yield relevant agonism at 5-HT2C only (Brewerton et al., 1988), and a number of drugs that have prophylactic effects against migraine share high affinity for 5-HT2C as their only common pharmacological property (Fozard and Kalkman, 1992). Although the role of 5-HT2C in the pathogenesis of headache is not yet clear, psilocybin’s strong agonism at 5-HT2C may be related to its effects on headache.
4.4 Psilocybin treatment of headache
The first scientific mention of psilocybin’s possible therapeutic effects on headache came in the form of a 2005 case report (Matharu et al., 2005); prior to that, there had been no reports of effects of psilocybin on any sorts of pain. This was followed by other reports (Sempere et al., 2006; Sewell et al., 2006) and a movement by patients to advocate for research into psilocybin and related drugs as headache therapeutics (www.clusterbusters.com). If psilocybin is indeed effective for cluster headache, the mechanism remains unclear. Psilocybin is structurally similar to triptans, which are also indole-based. Many nonpsychedelic indole alkaloids that are structurally related to psilocybin, (e.g., methysergide, ergotamine, dihydroergotamine, and methylergonovine) are used as treatments for cluster headache, although they do not abort cluster periods or extend remission period as psilocybin is noted to do. Recent reports that the non-psychoactive LSD analog BOL-148 is also effective in treating cluster headache (Karst et al., 2010) suggest that the therapeutic effects of these drugs are not mediated by 5-HT2A; in fact, a dissociation between the autonomic and the psychoactive properties of psilocybin has already been described (Fischer, 1968).
Prolonged efficacy in terminating cluster periods and extending remission periods is difficult to explain as a consequence of receptor stimulation or antagonism, which should persist only for the duration of acute pharmacologic effect, or from receptor up- or down-regulation, which generally resolves over two weeks to two months. A number of other possibilities have been suggested, including disruption of the circadian rhythm through 5-HT1A, 5-HT2C, or 5-HT7 modulation, or through 5-HT2A-mediated gene induction (Sewell et al., 2006). It would not be surprising if psilocybin both induced and treated headache by a related mechanism; however, this mechanism is as yet unclear.
4.5 Conclusion
Psilocybin’s inductive effects on headache may be due to a number of different mechanisms: 1) induction of delayed headache through NO release, 2) facilitation of glutamate release in the cortex, with headache generation in those susceptible; 3) suppression of dorsal raphe nucleus inhibitory activity on the LC with α2 adrenoreceptor-linked vasoconstriction during the period of acute drug effects, followed by vasodilatation and headache, 4) direct triggering of migraine attack through 5-HT2B agonism in migraine-susceptible research subjects, 5) induction of Iκβ-α, inhibiting NFκβ, TNF-α, and iNOS, with subsequent “rebound” headache; or a combination of the above. The relative contributions of these various mechanisms to psilocybin cephalalgia remain to be explored. The most likely explanation is that of delayed NO release; more evidence exists, however, for dorsal raphe nucleus activity, rebound meningeal vasodilatation, and headache in the susceptible.
In order to differentiate between the potential mechanistic explanations, it will be important in future studies to characterize the nature of psilocybin-induced headache more carefully by using more detailed headache scales. Also, participants in psilocybin experiments should be screened for past or family history of migraine, and genetic analyses for mitochondrial DNA polymorphisms implicated in migraine pathogenesis might also be instructive. Because the headaches that resulted from psilocybin in these experiments were neither severe nor disabling, predisposition to such headache should not be considered an exclusion criterion in future psilocybin experiments. Similarly, the data do not suggest that administering psilocybin during an ongoing headache is a safety risk. In fact, future studies may require this in order to answer mechanistic and therapeutic questions related to psilocybin’s relationship with headache. Some circumstances may lead to a relative contraindication, however; for example, if a headache might detract from experimentally induced mystical-type effects intended to mediate a desired therapeutic outcome (e.g., anxiolytic, antidepressant, or anti-addiction), researchers may choose to postpone drug administration if a headache is present.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Afridi SK, Kaube H, Goadsby PJ. Glyceryl trinitrate triggers premonitory symptoms in migraineurs. Pain. 2004;110:675–680. doi: 10.1016/j.pain.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Aghajanian GK. Mescaline and LSD facilitate the activation of locus coeruleus neurons by peripheral stimuli. Brain Res. 1980;186:492–498. doi: 10.1016/0006-8993(80)90997-x. [DOI] [PubMed] [Google Scholar]
- Aghajanian GK, Hailgler HJ. Hallucinogenic indoleamines: preferential action upon presynaptic serotonin receptors. Psychopharmacol Commun. 1975;1:619–629. [PubMed] [Google Scholar]
- Aghajanian GK, Marek GJ. Serotonin model of schizophrenia: emerging role of glutamate mechanisms. Brain Res. 2000;31:302–312. doi: 10.1016/s0165-0173(99)00046-6. [DOI] [PubMed] [Google Scholar]
- Aghajanian G. LSD and phenethylamine hallucinogens: common sites of neuronal action. In: Pletscher A, Ladewig D, editors. 50 Years of LSD. Current Status and Perspectives of Hallucinogens. Parthenon; New York: 1994. pp. 27–42. [Google Scholar]
- Agnoli A, De Marinis M. Vascular headaches and cerebral circulation: an overview. Cephalalgia. 1985;5(Suppl 2):9–15. doi: 10.1177/03331024850050S202. [DOI] [PubMed] [Google Scholar]
- Arbab MA, Wiklund L, Svendgaard NA. Origin and distribution of cerebral vascular innervation from superior cervical, trigeminal and spinal ganglia investigated with retrograde and anterograde WGA-HRP tracing in the rat. Neuroscience. 1986;19:695–708. doi: 10.1016/0306-4522(86)90293-9. [DOI] [PubMed] [Google Scholar]
- Barnes NM, Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology. 1999;38:1083–1152. doi: 10.1016/s0028-3908(99)00010-6. [DOI] [PubMed] [Google Scholar]
- Bellantonio P, Micieli G, Buzzi MG, Marcheselli S, Castellano AE, Rossi F, Nappi G. Haemodynamic correlates of early and delayed responses to sublingual administration of isosorbide dinitrate in migraine patients: a transcranial Doppler study. Cephalalgia. 1997;17:183–187. doi: 10.1046/j.1468-2982.1997.1703183.x. [DOI] [PubMed] [Google Scholar]
- Bouchelet I, Case B, Olivier A, Hamel E. No contractile effect for 5-HT1D and 5-HT1F receptor agonists in human and bovine cerebral arteries: similarity with human coronary artery. Br J Pharmacol. 2000;129:501–508. doi: 10.1038/sj.bjp.0703081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandes JL, Buchanan TM, Welch KM. Acute treatment of migraine. In: Vinken PJ, Bruyn GW, editors. Handbook of Clinical Neurology. Elsevier; Amsterdam: 2010. pp. 323–336. [DOI] [PubMed] [Google Scholar]
- Brewerton TD, Murphy DL, Mueller EA, Jimerson DC. Induction of migrainelike headaches by the serotonin agonist m-chlorophenylpiperazine. Clin Pharmacol Thera. 1988;43:605–609. doi: 10.1038/clpt.1988.83. [DOI] [PubMed] [Google Scholar]
- Brown FC. Hallucinogenic Drugs. Charles C Thomas Pub Ltd; Springfield, IL: 1972. [Google Scholar]
- Buchwalder A, Welch SK, Peroutka SJ. Exclusion of 5-HT2A and 5-HT2C receptor genes as candidate genes for migraine. Headache. 1996;36:254–258. doi: 10.1046/j.1526-4610.1996.3604254.x. [DOI] [PubMed] [Google Scholar]
- Carlton SM. Peripheral excitatory amino acids. Curr Opin Pharmacol. 2001;1:52–56. doi: 10.1016/s1471-4892(01)00002-9. [DOI] [PubMed] [Google Scholar]
- Carter OL, Burr DC, Pettigrew JD, Wallis GM, Hasler F, Vollenweider FX. Using psilocybin to investigate the relationship between attention, working memory, and the serotonin 1A and 2A receptors. J Cogn Neurosci. 2005;17:1497–1508. doi: 10.1162/089892905774597191. [DOI] [PubMed] [Google Scholar]
- Christiansen I, Daugaard D, Lykke Thomsen L, Olesen J. Glyceryl trinitrate induced headache in migraineurs - relation to attack frequency. Eur J Neurol. 2000;7:405–411. doi: 10.1046/j.1468-1331.2000.00094.x. [DOI] [PubMed] [Google Scholar]
- Cohen ML, Johnson MP, Schenck KW, Susemichel A, Wainscott DB, Robertson DW, Nelson DL. DOI and alpha-methylserotonin: comparative vascular and nonvascular smooth muscle effects and central 5-hydroxytryptamine2 receptor affinities. J Pharmacol Exp Ther. 1993;266:943–949. [PubMed] [Google Scholar]
- Cohen Z, Bonvento G, Lacombe P, Hamel E. Serotonin in the regulation of brain microcirculation. Prog Neurobiol. 1996;50:335–362. doi: 10.1016/s0301-0082(96)00033-0. [DOI] [PubMed] [Google Scholar]
- Cohen Z, Bovento G, Lacombe P, Seylaz J, MacKenzie ET, Hamel E. Cerebrovascular nerve fibers immunoreactive for tryptophan-5-hydroxylase in the rat: distribution, putative origin and comparison with sympathetic noradrenergic nerves. Brain Res. 1992;598:203–214. doi: 10.1016/0006-8993(92)90184-b. [DOI] [PubMed] [Google Scholar]
- Czyrak A, Czepiel K, Mackowiak M, Chocyk A, Wedzony K. Serotonin 5-HT1A receptors might control the output of cortical glutamatergic neurons in rat cingulate cortex. Brain Res. 2003;989:42–51. doi: 10.1016/s0006-8993(03)03352-3. [DOI] [PubMed] [Google Scholar]
- Dalsgaard-Nielsen T. Migraine diagnostics with special reference to pharmacological tests. Int Arch Allerg. 1955;7:312–322. doi: 10.1159/000228235. [DOI] [PubMed] [Google Scholar]
- De Vries P, Villalon CM, Heiligers JP, Saxena PR. Characterization of 5-HT receptors mediating constriction of porcine carotid arteriovenous anastomoses; involvement of 5-HT1B/1D and novel receptors. Br J Pharmacol. 1998;123:1561–1570. doi: 10.1038/sj.bjp.0701770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vries P, Villalon CM, Saxena PR. Pharmacological aspects of experimental headache models in relation to acute antimigraine therapy. Eur J Pharmacol. 1999;375:61–74. doi: 10.1016/s0014-2999(99)00197-1. [DOI] [PubMed] [Google Scholar]
- DeFelipe J, Arellano JI, Gomez A, Azmitia EC, Munoz A. Pyramidal cell axons show a local specialization for GABA and 5-HT inputs in monkey and human cerebral cortex. J Comp Neuro. 2001;433:148–155. doi: 10.1002/cne.1132. [DOI] [PubMed] [Google Scholar]
- Delay J, Pichot P, Lemperiere T, Nicolas-Charles P. The psychophysiological effects of psilocybine. CR Hebd Seances Acad Sci. 1958;247:1235–1238. [PubMed] [Google Scholar]
- Dimitriadou V, Buzzi MG, Moskowitz MA, Theoharides TC. Trigeminal sensory fiber stimulation induces morphological changes reflecting secretion in rat dura mater mast cells. Neuroscience. 1991;44:97–112. doi: 10.1016/0306-4522(91)90253-k. [DOI] [PubMed] [Google Scholar]
- Dimitriadou V, Buzzi MG, Theoharides TC, Moskowitz MA. Ultrastructural evidence for neurogenically mediated changes in blood vessels of the rat dura mater and tongue following antidromic trigeminal stimulation. Neuroscience. 1992;48:187–203. doi: 10.1016/0306-4522(92)90348-6. [DOI] [PubMed] [Google Scholar]
- Drummond PD. Effect of tryptophan depletion on symptoms of motion sickness in migraineurs. Neurology. 2005;65:620–622. doi: 10.1212/01.wnl.0000172339.15577.a6. [DOI] [PubMed] [Google Scholar]
- Dusitanond P, Young WB. Neuroleptics and migraine. Cent Nerv Syst Agents Med Chem. 2009;9:63–70. doi: 10.2174/187152409787601888. [DOI] [PubMed] [Google Scholar]
- Dyer DC, Gant DW. Vasoconstriction produced by hallucinogens on isolated human and sheep umbilical vasculature. J Pharmacol Exp Ther. 1973;184:366–375. [PubMed] [Google Scholar]
- Ekbom K. Nitroglycerin as a provocative agent in cluster headache. Arch Neurology. 1968;19:487–493. doi: 10.1001/archneur.1968.00480050057005. [DOI] [PubMed] [Google Scholar]
- Ellis H. Mescal--the divine plant. Pop Sci Monthly. 1902;51:52–71. [Google Scholar]
- Fanciullacci M, Franchi G, Sicuteri F. Hypersensitivity to lysergic acid diethylamide (LSD-25) and psilocybin in essential headache. Experientia. 1974;30:1441–1443. doi: 10.1007/BF01919685. [DOI] [PubMed] [Google Scholar]
- Ferrari MD, Odink J, Tapparelli C, Van Kempen GM, Pennings EJ, Bruyn GW. Serotonin metabolism in migraine. Neurology. 1989;39:1239–1242. doi: 10.1212/wnl.39.9.1239. [DOI] [PubMed] [Google Scholar]
- Ferrari MD, Saxena PR. On serotonin and migraine: a clinical and pharmacological review. Cephalalgia. 1993;13:151–165. doi: 10.1046/j.1468-2982.1993.1303151.x. [DOI] [PubMed] [Google Scholar]
- Fischer R. Psilocybin-induced autonomic, perceptual and behavioral change. Psychopharmkologie. 1968;1:291–302. [Google Scholar]
- Fozard JR. The 5-hydroxytryptamine-nitric oxide connection: the key link in the initiation of migraine? Arch Int Pharmacodyn Ther. 1995;329:111–119. [PubMed] [Google Scholar]
- Fozard JR, Kalkman HO. 5-HT in nervous system disease and migraine. Curr Opin Neurol Neurosurg. 1992;5:496–502. [PubMed] [Google Scholar]
- Fozard JR, Kalkman HO. 5-Hydroxytryptamine (5-HT) and the initiation of migraine: new perspectives. Naunyn-Schmiedebergs Archives Pharmacol. 1994;350:225–229. doi: 10.1007/BF00175026. [DOI] [PubMed] [Google Scholar]
- Glusa E, Richter M. Endothelium-dependent relaxation of porcine pulmonary arteries via 5-HT1C-like receptors. Naunyn-Schmiedebergs Arch Pharmacol. 1993;347:471–477. doi: 10.1007/BF00166737. [DOI] [PubMed] [Google Scholar]
- Goadsby PJ, Duckworth JW. Low frequency stimulation of the locus coeruleus reduces regional cerebral blood flow in the spinalized cat. Brain Res. 1989;476:71–77. doi: 10.1016/0006-8993(89)91537-0. [DOI] [PubMed] [Google Scholar]
- Goldstein J, Dahlof CG, Diener HC, Olesen J, Schellens R, Senard JM, Simard D, Steiner TJ. Alniditan in the acute treatment of migraine attacks: a subcutaneous dose-finding study. Subcutaneous Alniditan Study. Group Cephalalgia. 1996;16:497–502. doi: 10.1046/j.1468-2982.1996.1607497.x. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Maeso J, Yuen T, Ebersole BJ, Wurmbach E, Lira A, Zhou M, Weisstaub N, Hen R, Gingrich JA, Sealfon SC. Transcriptome fingerprints distinguish hallucinogenic and nonhallucinogenic 5-hydroxytryptamine 2A receptor agonist effects in mouse somatosensory cortex. J Neurosci. 2003;23:8836–8843. doi: 10.1523/JNEUROSCI.23-26-08836.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths R, Richards W, Johnson M, McCann U, Jesse R. Mystical-type experiences occasioned by psilocybin mediate the attribution of personal meaning and spiritual significance 14 months later. J Psychopharmacol. 2008;22:621–632. doi: 10.1177/0269881108094300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths RR, Johnson MW, Richards WA, Richards BD, McCann U, Jesse R. Psilocybin occasioned mystical-type experiences: immediate and persisting dose-related effects. Psychopharmacology (Berl) 2011 doi: 10.1007/s00213-011-2358-5. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths RR, Richards WA, McCann U, Jesse R. Psilocybin can occasion mystical-type experiences having substantial and sustained personal meaning and spiritual significance. Psychopharmacology (Berl) 2006;187:268–283. doi: 10.1007/s00213-006-0457-5. discussion 284–292. [DOI] [PubMed] [Google Scholar]
- Hamel E. Serotonin and migraine: biology and clinical implications. Cephalalgia. 2007;27:1293–1300. doi: 10.1111/j.1468-2982.2007.01476.x. [DOI] [PubMed] [Google Scholar]
- Hamon M, Gozlan H, el Mestikawy S, Emerit MB, Bolanos F, Schechter L. The central 5-HT1A receptors: pharmacological, biochemical, functional, and regulatory properties. Ann NY Acad Sci. 1990;600:114–129. doi: 10.1111/j.1749-6632.1990.tb16877.x. discussion 129–131. [DOI] [PubMed] [Google Scholar]
- Hartig PR, Hoffman BJ, Kaufman MJ, Hirata F. The 5-HT1C receptor. Ann NY Acad Sci. 1990;600:149–166. doi: 10.1111/j.1749-6632.1990.tb16879.x. discussion 166–167. [DOI] [PubMed] [Google Scholar]
- Hasler F, Bourquin D, Brenneisen R, Bar T, Vollenweider FX. Determination of psilocin and 4-hydroxyindole-3-acetic acid in plasma by HPLC-ECD and pharmacokinetic profiles of oral and intravenous psilocybin in man. Pharm Acta Helv. 1997;72:175–184. doi: 10.1016/s0031-6865(97)00014-9. [DOI] [PubMed] [Google Scholar]
- Hasler F, Grimberg U, Benz MA, Huber T, Vollenweider FX. Acute psychological and physiological effects of psilocybin in healthy humans: a double-blind, placebo-controlled dose-effect study. Psychopharmacology (Berl) 2004;172:145–156. doi: 10.1007/s00213-003-1640-6. [DOI] [PubMed] [Google Scholar]
- Heffter A. Ueber Pellote. Beitrag zur chemischen und pharmakologischen Kenntnis der Cacteen Naunyn-Schmiedebergs. Arch Exp Path Pharmacol. 1898;40:385–429. [Google Scholar]
- Hofmann A. LSD, My Problem Child. McGraw-Hill; New York: 1980. [Google Scholar]
- Hollister LE. Clinical, biochemical and psychologic effects of psilocybin. Arch Int Pharmacodyn Ther. 1961;130:42–52. [PubMed] [Google Scholar]
- Hollister LE, Prusmack JJ, Paulsen A, Rosenquist N. Comparison of three psychotropic drugs (psilocybin, JB-329, and IT-290) in volunteer subjects. J Nerv Mental Dis. 1960;131:428–434. doi: 10.1097/00005053-196011000-00007. [DOI] [PubMed] [Google Scholar]
- Holzmann P. Bestimmung Von Psilocybin-Metaboliten Im Humanplasma Und Urin. University of Tuebingen; Tuebingen, Germany: 1995. [Google Scholar]
- Honkaniemi J, Liimatainen S, Rainesalo S, Sulavuori S. Haloperidol in the acute treatment of migraine: a randomized, double-blind, placebo-controlled study. Headache. 2006;46:781–787. doi: 10.1111/j.1526-4610.2006.00438.x. [DOI] [PubMed] [Google Scholar]
- Hood RW, Jr, Ghorbani N, Watson PJ, Ghramaleki AF, Bing MN, Davison HK, Morris RJ, Williamson WP. Dimensions of the mysticism scale: confirming the three-factor structure in the United States and Iran. J Sci Study Relig. 2001;40:691–705. [Google Scholar]
- Hopf A, Eckert H. Distribution patterns of 14-C-psilocin in the brains of various animals. Activitas Nervosa Superior. 1974;16:64–66. [PubMed] [Google Scholar]
- Horton B. Histamine cephalalgia: differential diagnosis and treatment. Proc Staff Meet Mayo Clin. 1956;31:525–533. [PubMed] [Google Scholar]
- Humphrey PP. 5-Hydroxytryptamine and the pathophysiology of migraine. J Neurology. 1991;238(Suppl 1):S38–S44. doi: 10.1007/BF01642905. [DOI] [PubMed] [Google Scholar]
- Jakab RL, Goldman-Rakic PS. 5-Hydroxytryptamine2A serotonin receptors in the primate cerebral cortex: possible site of action of hallucinogenic and antipsychotic drugs in pyramidal cell apical dendrites. Proc Natl Acad Sci USA. 1998;95:735–740. doi: 10.1073/pnas.95.2.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson M, Richards W, Griffiths R. Human hallucinogen research: guidelines for safety. J Psychopharmacol. 2008;22:603–620. doi: 10.1177/0269881108093587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalkman HO. Is migraine prophylactic activity caused by 5-HT2B or 5-HT2C receptor blockade? Life Sci. 1994;54:641–644. doi: 10.1016/0024-3205(94)00546-x. [DOI] [PubMed] [Google Scholar]
- Karst M, Halpern JH, Bernateck M, Passie T. The non-hallucinogen 2-bromo-lysergic acid diethylamide as preventative treatment for cluster headache: an open, non-randomized case series. Cephalalgia. 2010;30:1140–1144. doi: 10.1177/0333102410363490. [DOI] [PubMed] [Google Scholar]
- Kihara H, Hirose K, Koganei H, Sasaki N, Yamamoto H, Kimura A, Nishimori T, Shoji M, Yoshimoto R. AT-1015, a novel serotonin (5-HT)2 receptor antagonist, blocks vascular and platelet 5-HT2A receptors and prevents the laurate-induced peripheral vascular lesion in rats. J Cardiovasc Pharmacol. 2000;35:523–530. doi: 10.1097/00005344-200004000-00002. [DOI] [PubMed] [Google Scholar]
- Kotani K, Shimomura T, Shimomura F, Ikawa S, Nanba E. A polymorphism in the serotonin transporter gene regulatory region and frequency of migraine attacks. Headache. 2002;42:893–895. doi: 10.1046/j.1526-4610.2002.02209.x. [DOI] [PubMed] [Google Scholar]
- Kyriakides ZS, Sbarouni E, Nikolaou N, Antoniadis A, Kremastinos DT. Intracoronary ketanserin augments coronary collateral blood flow and decreases myocardial ischemia during balloon angioplasty Cardiovasc. Drugs Ther. 1999;13:415–422. doi: 10.1023/a:1007851906207. [DOI] [PubMed] [Google Scholar]
- le Grand SM, Supornsilpchai W, Saengjaroentham C, Srikiatkhachorn A. Serotonin depletion leads to cortical hyperexcitability and trigeminal nociceptive facilitation via the nitric oxide pathway. Headache. 2011;51:1152–1160. doi: 10.1111/j.1526-4610.2011.01931.x. [DOI] [PubMed] [Google Scholar]
- Lee ST, Park JH, Kim M. Efficacy of the 5-HT1A agonist, buspirone hydrochloride, in migraineurs with anxiety: a randomized, prospective, parallel group, double-blind, placebo-controlled study. Headache. 2005;45:1004–1011. doi: 10.1111/j.1526-4610.2005.05181.x. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, Benjamin J, Muller CR, Hamer DH, Murphy DL. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274:1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- Leysen JE, Gommeren W, Heylen L, Luyten WH, Van de Weyer I, Vanhoenacker P, Haegeman G, Schotte A, Van Gompel P, Wouters R, Lesage AS. Alniditan, a new 5-hydroxytryptamine1D agonist and migraine-abortive agent: ligand-binding properties of human 5-hydroxytryptamine1D alpha, human 5-hydroxytryptamine1D beta, and calf 5-hydroxytryptamine1D receptors investigated with [3H]5-hydroxytryptamine and [3H]alniditan. Mol Pharmacol. 1996;50:1567–1580. [PubMed] [Google Scholar]
- Lindenblatt H, Kraemer E, Holzmann-Erens P, Gouzoulis-Mayfrank E, Kovar KA. Quantitation of psilocin in human plasma by high-performance liquid chromatography and electrochemical detection: comparison of liquid-liquid extraction with automated on-line solid-phase extraction. J Chromatogr B Biomed Sci Appl. 1998;709:255–263. doi: 10.1016/s0378-4347(98)00067-x. [DOI] [PubMed] [Google Scholar]
- Liu-Chen LY, Gillespie SA, Norregaard TV, Moskowitz MA. Co-localization of retrogradely transported wheat germ agglutinin and the putative neurotransmitter substance P within trigeminal ganglion cells projecting to cat middle cerebral artery. J Comp Neurol. 1984;225:187–192. doi: 10.1002/cne.902250204. [DOI] [PubMed] [Google Scholar]
- Malitz S, Esecover H, Wilkens B, Hoch PH. Some observations on psilocybin, a new hallucinogen, in volunteer subjects. Compr Psychiatry. 1960;1:8–17. doi: 10.1016/s0010-440x(60)80045-4. [DOI] [PubMed] [Google Scholar]
- Martin GR. Vascular receptors for 5-hydroxytryptamine: distribution, function and classification. Pharmacol Ther. 1994;62:283–324. doi: 10.1016/0163-7258(94)90048-5. [DOI] [PubMed] [Google Scholar]
- Matharu MS, van Vliet JA, Ferrari MD, Goadsby PJ. Verapamil induced gingival enlargement in cluster headache. J Neurol Neurosurg Psychiatry. 2005;76:124–127. doi: 10.1136/jnnp.2003.024240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathiau P, Riche D, Behzadi G, Dimitriadou V, Aubineau P. Absence of serotonergic innervation from raphe nuclei in rat cerebral blood vessels--I. Histological evidence. Neuroscience. 1993;52:645–655. doi: 10.1016/0306-4522(93)90413-a. [DOI] [PubMed] [Google Scholar]
- Merritt JE, Williams PB. Vasospasm contributes to monosodium glutamate-induced headache. Headache. 1990;30:575–580. doi: 10.1111/j.1526-4610.1990.hed3009575.x. [DOI] [PubMed] [Google Scholar]
- Miki N, Kawabe Y, Kuriyama K. Activation of cerebral guanylate cyclase by nitric oxide. Biochem Biophys Res Commun. 1977;75:851–856. doi: 10.1016/0006-291x(77)91460-7. [DOI] [PubMed] [Google Scholar]
- Mitchell SW. Remarks on the effects of Anhelonium [sic] lewinii (the mescal button) BMJ. 1896;2:1625–1629. doi: 10.1136/bmj.2.1875.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata K, Shimokawa H, Higo T, Yamawaki T, Katsumata N, Kandabashi T, Tanaka E, Takamura Y, Yogo K, Egashira K, Takeshita A. Sarpogrelate, a selective 5-HT2A serotonergic receptor antagonist, inhibits serotonin-induced coronary artery spasm in a porcine model. J Cardiovasc Pharmacol. 2000;35:294–301. doi: 10.1097/00005344-200002000-00018. [DOI] [PubMed] [Google Scholar]
- Moreno MJ, Conde MV, de la Luz Fraile M, Fernandez-Lomana H, Lopez de Pablo AL, Marco EJ. Lesion of the dorsal raphe nucleus induces supersensitivity to serotonin in isolated cat middle cerebral artery. Brain Res. 1991;538:324–328. doi: 10.1016/0006-8993(91)90448-5. [DOI] [PubMed] [Google Scholar]
- Moskowitz MA, Cutrer FM. SUMATRIPTAN: a receptor-targeted treatment for migraine. Ann Rev Med. 1993;44:145–154. doi: 10.1146/annurev.me.44.020193.001045. [DOI] [PubMed] [Google Scholar]
- Nichols CD, Sanders-Bush E. A single dose of lysergic acid diethylamide influences gene expression patterns within the mammalian brain. Neuropsychopharmacology. 2002;26:634–639. doi: 10.1016/S0893-133X(01)00405-5. [DOI] [PubMed] [Google Scholar]
- Nichols DE. Hallucinogens. Pharmacol Ther. 2004;101:131–181. doi: 10.1016/j.pharmthera.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Panconesi A, Sicuteri R. Headache induced by serotonergic agonists--a key to the interpretation of migraine pathogenesis? Cephalalgia. 1997;17:3–14. doi: 10.1046/j.1468-2982.1997.1701003.x. [DOI] [PubMed] [Google Scholar]
- Passie T, Seifert J, Schneider U, Emrich HM. The pharmacology of psilocybin. Addict Biol. 2002;7:357–364. doi: 10.1080/1355621021000005937. [DOI] [PubMed] [Google Scholar]
- Pazos A, Palacios JM. Quantitative autoradiographic mapping of serotonin receptors in the rat brain. I Serotonin-1 receptors. Brain Res. 1985;346:205–230. doi: 10.1016/0006-8993(85)90856-x. [DOI] [PubMed] [Google Scholar]
- Peters GA. Migraine: diagnosis and treatment with emphasis on the migraine-tension head-ache, provocative tests and use of rectal suppositories. Proc Staff Meet Mayo Clin. 1953;28:673–686. [PubMed] [Google Scholar]
- Potrebic S, Ahn AH, Skinner K, Fields HL, Basbaum AI. Peptidergic nociceptors of both trigeminal and dorsal root ganglia express serotonin 1D receptors: implications for the selective antimigraine action of triptans. J Neurosci. 2003;23:10988–10997. doi: 10.1523/JNEUROSCI.23-34-10988.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prentiss D, Morgan F. Anhalonium lewinii (mescal buttons) a study of a drug with special reference to its hysiological action upon man. Thera Gazette. 1895;19:577–585. [Google Scholar]
- Prentiss D, Morgan F. Mescal Buttons: Anahalonium Lewinii--Hennings (Lophophora Williamsii Lewin-Ii--Coulter) The Publisher’s Printing Co; New York: 1896. [Google Scholar]
- Puig MV, Artigas F, Celada P. Modulation of the activity of pyramidal neurons in rat prefrontal cortex by raphe stimulation in vivo: involvement of serotonin and GABA. Cereb Cortex. 2005;15:1–14. doi: 10.1093/cercor/bhh104. [DOI] [PubMed] [Google Scholar]
- Rasmussen K, Aghajanian GK. Effect of hallucinogens on spontaneous and sensory-evoked locus coeruleus unit activity in the rat: reversal by selective 5-HT2 antagonists. Brain Res. 1986;385:395–400. doi: 10.1016/0006-8993(86)91090-5. [DOI] [PubMed] [Google Scholar]
- Reuter U, Bolay H, Jansen-Olesen I, Chiarugi A, Sanchez del Rio M, Letourneau R, Theoharides TC, Waeber C, Moskowitz MA. Delayed inflammation in rat meninges: implications for migraine pathophysiology. Brain. 2001;124:2490–2502. doi: 10.1093/brain/124.12.2490. [DOI] [PubMed] [Google Scholar]
- Reuter U, Chiarugi A, Bolay H, Moskowitz MA. Nuclear factor-kappaB as a molecular target for migraine therapy. Ann Neurology. 2002;51:507–516. doi: 10.1002/ana.10159. [DOI] [PubMed] [Google Scholar]
- Rümmele V. Die Stellung des Psilocybins unter anderen psychotropen Substanzn. Schweiz Arch Neuro. 1958;84:348–352. [Google Scholar]
- SAMHSA. Emergency Department Trends from the Drug Abuse Warning Network, Final Estimates 1995–2002 (DHHS Publication no. (SMA) 03–3780) Substance Abuse and Mental Health Services Administration, Office of Applied Studies; Rockville, Maryland: 2003. [Google Scholar]
- Santana N, Bortolozzi A, Serrats J, Mengod G, Artigas F. Expression of serotonin1A and serotonin2A receptors in pyramidal and GABAergic neurons of the rat prefrontal cortex. Cereb Cortex. 2004;14:1100–1109. doi: 10.1093/cercor/bhh070. [DOI] [PubMed] [Google Scholar]
- Saxena PR. Serotonin receptors: subtypes, functional responses and therapeutic relevance. Pharmacol Therapeutics. 1995;66:339–368. doi: 10.1016/0163-7258(94)00005-n. [DOI] [PubMed] [Google Scholar]
- Schmuck K, Ullmer C, Kalkman HO, Probst A, Lubbert H. Activation of meningeal 5-HT2B receptors: an early step in the generation of migraine headache? Eur J Neuroscience. 1996;8:959–967. doi: 10.1111/j.1460-9568.1996.tb01583.x. [DOI] [PubMed] [Google Scholar]
- Schnitker MT, Schnitker MA. A clinical test for migraine. JAMA. 1947;135:89. doi: 10.1001/jama.1947.62890020001007. [DOI] [PubMed] [Google Scholar]
- Sempere AP, Berenguer-Ruiz L, Almazan F. Chronic cluster headache: response to psilocybin Rev. Neurol. 2006;43:571–572. [PubMed] [Google Scholar]
- Sewell RA, Halpern JHGPH., Jr Response of cluster headache to psilocybin and LSD. Neurology. 2006;66:1920–1922. doi: 10.1212/01.wnl.0000219761.05466.43. [DOI] [PubMed] [Google Scholar]
- Sicuteri F. Prophylactic treatment of migraine by means of lysergic acid derivatives. Triangle. 1963;67:116–125. [PubMed] [Google Scholar]
- Silberstein SD. Serotonin (5-HT) and migraine. Headache. 1994;34:408–417. doi: 10.1111/j.1526-4610.1994.hed3407408.x. [DOI] [PubMed] [Google Scholar]
- Sokolov AY, Lyubashina OA, Panteleev SS. The role of serotonin receptors in migraine headaches. Neurochem J. 2011;5:92–99. [Google Scholar]
- Sotelo C, Cholley B, El Mestikawy S, Gozlan H, Hamon M. Direct immunohistochemical evidence of the existence of 5-HT1A autoreceptors on serotoninergic neurons in the midbrain raphe nuclei. Eur J Neurosci. 1990;2:1144–1154. doi: 10.1111/j.1460-9568.1990.tb00026.x. [DOI] [PubMed] [Google Scholar]
- Spilka B, Hood RW, Hunsberger B, Gorsuch R. The Psychology of Religion: An Empirical Approach. The Guilford Press; New York: 2003. [Google Scholar]
- Strassman RJ, Qualls CR, Uhlenhuth EH, Kellner R. Dose-response study of N,N-dimethyltryptamine in humans. II Subjective effects and preliminary results of a new rating scale. Arch Gen Psychiatry. 1994;51:98–108. doi: 10.1001/archpsyc.1994.03950020022002. [DOI] [PubMed] [Google Scholar]
- Studerus E, Kometer M, Hasler F, Vollenweider FX. Acute, subacute and long-term subjective effects of psilocybin in healthy humans: a pooled analysis of experimental studies. J Psychopharmacol. 2010 doi: 10.1177/0269881110382466. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Supornsilpchai W, Sanguanrangsirikul S, Maneesri S, Srikiatkhachorn A. Serotonin depletion, cortical spreading depression, and trigeminal nociception. Headache. 2006;46:34–39. doi: 10.1111/j.1526-4610.2006.00310.x. [DOI] [PubMed] [Google Scholar]
- Terron JA, Falcon-Neri A. Pharmacological evidence for the 5-HT7 receptor mediating smooth muscle relaxation in canine cerebral arteries Br. J Pharmacol. 1999;127:609–616. doi: 10.1038/sj.bjp.0702580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tfelt-Hansen P, De Vries P, Saxena PR. Triptans in migraine: a comparative review of pharmacology, pharmacokinetics and efficacy. Drugs. 2000;60:1259–1287. doi: 10.2165/00003495-200060060-00003. [DOI] [PubMed] [Google Scholar]
- Thomsen LL, Lawton FG, Knowles RG, Beesley JE, Riveros-Moreno V, Moncada S. Nitric oxide synthase activity in human gynecological cancer. Cancer Res. 1994;54:1352–1354. [PubMed] [Google Scholar]
- Thomsen L, Daugaard D, Iversen HK, Olesen J. Normal radial artery dilatation during reactive hyperemia in migraine without aura. Endothelium. 1996;4:199–206. [Google Scholar]
- Uddman R, Edvinsson L, Ekman R, Kingman T, McCulloch J. Innervation of the feline cerebral vasculature by nerve fibers containing calcitonin gene-related peptide: trigeminal origin and coexistence with substance P. Neurosci Lett. 1985;62:131–136. doi: 10.1016/0304-3940(85)90296-4. [DOI] [PubMed] [Google Scholar]
- Vollenweider FX, Leenders KL, Scharfetter C, Maguire P, Stadelmann O, Angst J. Positron emission tomography and fluorodeoxyglucose studies of metabolic hyperfrontality and psychopathology in the psilocybin model of psychosis. Neuropsychopharmacol. 1997;16:357–372. doi: 10.1016/S0893-133X(96)00246-1. [DOI] [PubMed] [Google Scholar]
- Vollenweider FX, Vollenweider-Scherpenhuyzen MF, Babler A, Vogel H, Hell D. Psilocybin induces schizophrenia-like psychosis in humans via a serotonin-2 agonist action. Neuroreport. 1998;9:3897–3902. doi: 10.1097/00001756-199812010-00024. [DOI] [PubMed] [Google Scholar]
- Wang W, Timsit-Berthier M, Schoenen J. Intensity dependence of auditory evoked potentials is pronounced in migraine: an indication of cortical potentiation and low serotonergic neurotransmission? Neurology. 1996;46:1404–1409. doi: 10.1212/wnl.46.5.1404. [DOI] [PubMed] [Google Scholar]