Abstract
Background
Cutaneous squamous cell carcinoma (cSCC) is the most common malignancy after solid organ transplantation, with an increased risk of recurrence and metastasis over the general population. The newly updated 7th edition American Joint Committee on Cancer (AJCC) staging system for cSCC is based on consensus expert opinion and requires validation in large cohort studies and in specific patient subpopulations.
Objective
Our objective was to evaluate the risk of cSCC recurrence in a high-risk population of heart and lung transplant recipients, based on the 7th edition AJCC staging system.
Methods
We performed a 10-year retrospective cohort study of all primary cSCC diagnosed in heart and lung transplant recipients at a tertiary care academic dermatology center.
Results
The cumulative incidence of local recurrence was 4% for cSCC in situ and 19% for Stage I cSCC at 5 years, and 54% for Stage II cSCC at 3 years. Stage II tumors had a 10-fold greater risk of recurrence than stage I, and a 43-fold greater risk of recurrence than in situ tumors.
Limitations
This study is limited to a specific patient subgroup at a tertiary care center, and may not be generalizable to all populations.
Conclusions
Heart and lung transplant recipients are at high risk for local recurrence of cSCC. These data substantiate the prognostic accuracy of the newly updated 7th edition AJCC staging system for stage 0, I and II cSCC in this population and demonstrate the aggressive behavior of this cancer in immunosuppressed patients.
Keywords: Squamous Cell Carcinoma, Lung Transplantation, Heart Transplantation, Staging
Introduction and Background
Cutaneous squamous cell carcinoma (cSCC) is the most common malignancy after solid organ transplantation, with a five-year incidence of 30% in lung transplant recipients1 and up to 26% in heart transplant recipients2,3. The aggressive nature of cSCC in immunosuppressed patients is manifested by an increased risk of local recurrence, nodal and distant metastasis (NM, DM), and disease-specific death (DSD)4–10.
There is currently no single system for defining high-risk cSCC. Case series and retrospective cohort studies have identified tumor and patient characteristics associated with a high risk of tumor recurrence and metastasis11–14. These characteristics include clinical features such as location on the ear or lip, histologic features such as perineural invasion, and host factors such as immunosuppression or radiation exposure15. Once unresectable disease or distant metastasis occurs, treatment options are limited and prognosis is poor16–18. However, cSCC without high-risk features have a very low risk of NM, DM, and DSD12,13,19,20.
In the past, the American Joint Committee on Cancer (AJCC) TNM staging system for non-melanoma skin cancer (NMSC) was primarily based on tumor diameter, and a single staging system was used for all types of NMSC. A previous study assessing the 6th edition staging system identified key flaws in the earlier system21. Indeed, based on the 6th edition staging system, Stage I and II cSCC had overlapping Kaplan-Meier curves for recurrence; Stage II tumors had a lower incidence of recurrence at 5-years than Stage I.
Recognizing these flaws, in 2010 the AJCC revised the staging system for cSCC. In this 7th edition of the staging system, high-risk features are now defined as: tumor diameter ≥ 2 cm; invasion into cranial bone; anatomic location on the ear or lip; tumor thickness > 2mm or Clark's level ≥ 4; poor differentiation; and perineural invasion22.
The new staging system has notable advantages over the 6th edition staging system. It is specific to cSCC and recognizes the high-risk tumor features of location, tumor thickness and level, differentiation, and perineural invasion. However, population-based studies for recurrence and metastasis were not available for committee review, so the new system was developed by consensus opinion of a panel of experts based on the available literature. Therefore, this new staging system requires validation of prognostic accuracy in various patient populations.
The goal of this study is to determine the risk of cSCC recurrence based on the 7th edition AJCC staging system for cSCC in a specific high-risk patient subgroup of heart and lung transplant recipients. To do so, we performed a 10-year retrospective cohort study of primary cSCC in this patient population at a single academic center. We report here that heart and lung transplant recipients are at high risk of tumor recurrence, and that the new AJCC staging system accurately predicts risk of tumor recurrence for stage 0, I and II cSCC tumors in this high-risk population.
Methods
Study Design
This is a ten-year retrospective cohort study of all heart and lung transplant recipients with primary cSCC seen in the Department of Dermatology at the University of California, San Francisco Medical Center between 2001 and 2010. This is a tertiary care academic setting at a hospital with an active organ transplantation program. This study was performed with approval of the UCSF Committee on Human Research in accordance with the Helsinki principles.
Subjects
All heart and lung transplant recipients seen in dermatology between January 1, 2001 and December 31, 2010 were identified through billing records by International Classification of Disease, 9th edition (ICD-9) codes V42.1 (heart transplant) or V42.6 (lung transplant). Complete medical records were reviewed to collect details of transplantation and to identify any primary cSCC diagnosed during this time period. Data collected for each tumor included size, location, depth of invasion, perineural involvement, level of differentiation, desmoplasia, treatment modality, and outcome. To validate the accuracy of the pathology reports for the high-risk features of interest, a random set of 10% of tumor slides were selected for review by a dermatopathologist and Mohs micrographic surgeon (T.M. and S.T.A.). No discrepancies were found.
The primary outcome of interest was the time to development of local recurrence (LR). Local recurrence was defined as a biopsy-proven cSCC at the site of the primary, if also noted as recurrent by the treating physician. There were no nodal or distant metastases in this dataset.
Tumor Staging
Tumors were staged according to the 2010 American Joint Committee on Cancer (AJCC) 7th edition tumor staging system23 (Table 1). A tumor was defined as T1 if the maximum pre-biopsy diameter was ≤ 2 cm and had at most one of the following high-risk features: poorly differentiated histology, location on the mucosal (non-hair bearing) lip or ear, presence of perineural invasion, or depth of invasion to Clark's level ≥ IV or > 2 mm. A tumor was classified as T2 if it was > 2 cm in maximum pre-biopsy diameter or had ≥ 2 of the 7th edition AJCC high-risk features. A tumor was classified as T3 if it invaded the one or more of the facial bones (including the maxilla, mandible, orbit, or temporal bones). A tumor was classified as T4 if it invaded other bone or had perineural invasion of the skull base. None of the subjects in the cohort had nodal or metastatic staging workups at presentation. Therefore, tumor stage is equivalent to TNM stage: (Tis = Stage 0, T1 = Stage I, and T2 = Stage II).
Table 1.
AJCC 7th edition cSCC tumor staging system1.
| TX | Primary tumor cannot be assessed |
| T0 | No evidence of primary tumor |
| Tis | Carcinoma in situ |
| T1 | Tumor ≤ 2 cm in greatest dimension with < 2 high-risk features2 |
| T2 | Tumor > 2 cm in greatest dimension with or without one additional high-risk feature, or any size with ≥ 2 high-risk features2 |
| T3 | Tumor with invasion of maxilla, mandible, orbit, or temporal bone |
| T4 | Tumor with invasion of skeleton (axial or appendicular) or perineural invasion of skull base. |
In the absence of nodal or distant metastasis, Tis = Stage 0, T1 = Stage I, and T2 = Stage II.
High-risk features incude depth >2mm or Clark level ≥ 4, perineural invasion, location on the ear or lip, and poor differentiation.
Statistical Analysis
Variables were analyzed with the Student's t-test or two-sided Fisher's exact test, as appropriate. We assessed pairwise correlations between predictors including male sex, time since transplantation at primary cSCC diagnosis, tumor stage, and treatment type (local destruction vs. surgical excision). Correlation coefficients were below 0.3 in all cases with the exception of treatment type and tumor stage, which had a correlation coefficient of 0.41. In situ tumors were far more likely to be treated with local destruction than Stage I/II tumors, which were primarily treated with surgical excision. This moderate correlation was considered analytically by stratifying time-to-event models by treatment modality.
Time to recurrence was graphically assessed using standard Kaplan-Meier methods. Subjects were right-censored at the date of last follow-up if the tumor had not recurred. Parametric estimates of the instantaneous risk of recurrence were estimated using hazard models. Preliminary univariate hazard model analyses tested each variable against the outcome of time-to-recurrence. Variables associated with local recurrence on univariate analysis at a p ≤0.05 level were sex, duration of immunosuppression, and tumor stage. These variables were included in the final multivariate model. To evaluate the proportionality of hazards, we plotted scaled Schoenfeld residuals with respect to time and assessed log-negative-log plots. We further tested the proportional hazards assumption with the Schoenfeld test. We used Cox proportional hazard models to assess the instantaneous risk of developing a recurrence, clustering for tumors within patient. We next performed binary tests of interaction between all predictors, which revealed an interaction between sex and both duration of immunosuppression and tumor stage. To address this interaction, we stratified the survival model by sex. The final model had stage as the primary predictor, was adjusted for duration of immunosuppression at the primary diagnosis, and was stratified by sex and treatment type. Statistical analysis was performed using STATA v11.2 (STATA Corp, College Station, TX).
Results
41 subjects were identified, including 17 heart (41%), 22 lung (54%), and 2 combined heart-lung transplant recipients (5%) (Table 2). The mean age at transplant was 53.3 ± 10.9 years. 71% of the patients were male. All patients were Caucasian, one self-identified as being Hispanic. 33 patients (81%) presented with more than one primary cSCC (range 2-20). 10 patients (24.4%) had a recurrence of their primary cSCC. There was no difference in the frequency of recurrence based on sex, organ transplanted, or age at transplant.
Table 2. Patient Demographics.
Data presented as n (%) or mean ± SD. Local recurrence was defined as a biopsy-proven cSCC at the site of the primary, confirmed as recurrent in medical records by the treating physician.
| Local Recurrence | No Recurrence | P-value | |
|---|---|---|---|
| N | 10 (24.4) | 31 (75.6) | |
| Sex | |||
| Female | 5 (41.7) | 7 (58.3) | 0.124 |
| Male | 5 (17.2) | 24 (82.8) | |
| Race/Ethnicity | |||
| White, Non-Hispanic | 10 (25.0) | 30 (75.0) | 0.756 |
| White, Hispanic | 1 (100) | 0 (0) | |
| Age at Transplant | 49.1 ± 11.1 | 54.1 ± 10.6 | 0.181 |
| Organ Transplanted | |||
| Heart | 4 (23.5) | 13 (76.5) | |
| Lung | 5 (22.7) | 17 (77.3) | 0.696 |
| Heart/Lung | 1 (50) | 1 (50) |
A total of 225 primary cSCCs were diagnosed and followed for a median follow-up time of 15.2 months (range 6 days-9 years) (Table 3). 19 tumors had at least one recurrence (8.4%). The median time to recurrence was 6.3 months (range 3.1- 48.1), which was significantly shorter than the median follow-up time for tumors that did not recur before censoring (median 22.7 months, range 5.0-15.0, p=0.014). No primary tumors in this cohort presented with, or subsequently developed nodal or distant metastasis without first recurring locally.
Table 3. Primary Tumor Characteristics.
Data presented as n (%) or mean ± SD. C&E: curettage and electrodesiccation. MMS: Mohs micrographic surgery. WLE: wide local excision.
| Local Recurrence | No Recurrence | P-value | |
|---|---|---|---|
|
|
|||
| N | 19 (8.4) | 206 (91.6) | |
| Perineural Invasion | |||
| Absent | 19 (8.5) | 205 (91.5) | 0.916 |
| Present | 0 | 1 (100) | |
| Desmoplasia | |||
| Absent | 18 (8.2) | 202 (91.8) | 0.359 |
| Present | 1 (20.0) | 4 (80.0) | |
| Differentiation | |||
| Moderate-Well | 17 (7.9) | 199 (92.1) | 0.17 |
| Poor | 2 (22.2) | 7 (77.8) | |
| Anatomic Location | |||
| Not on Ear or Lip | 16 (7.8) | 189 (92.2) | 0.231 |
| On Ear or Lip | 3 (15.0) | 17 (85.0) | |
| Mean Diameter (mm) | 15.8 ± 15.3 | 10.2 ± 7.1 | 0.007 |
| < 20mm | 11 (5.9) | 177 (94.2) | |
| ≥ 20mm | 5 (26.3) | 14 (73.7) | |
| Not Noted | 3 (16.7) | 15 (83.3) | 0.009 |
| Treatment Modality | |||
| C&E / Local Destruction | 2 (3.5) | 55 (96.5) | |
| Surgical (MMS, WLE) | 15 (8.7) | 147 (91.3) | |
| None/Not Noted | 3 (42.9) | 4 (57.1) | 0.16 |
| AJCC Stage | |||
| 0 | 2 (2.6) | 75 (97.4) | |
| I | 9 (7.6) | 109 (92.4) | |
| II | 5 (3.33) | 10 (66.7) | 0.002 |
| Could not be staged | 3 (20.0) | 12 (80.0) | |
| Patient Age at Diagnosis (years) | 61.8 ± 10.7 | 60.7 ± 10.1 | 0.641 |
| Duration of Immunosuppression at Diagnosis (years) | 10.9 ± 6.5 | 8.2 ± 5.6 | 0.049 |
Eighteen of the 225 primary cSCC tumors (8%) did not have a pre-biopsy size noted. Of the remaining 207 primary tumors, the mean size was 10.6 ± 7.1 mm. Tumors that recurred had a larger pre-biopsy diameter than those that did not recur (15.8 vs.10.2 mm, p=0.007). Primary tumors that were ≥ 20 mm, the size cutoff for upstaging to T2 in the AJCC staging system, were significantly more likely to recur (p=0.009). No primary tumor invaded beyond the dermis. No association was found between risk of recurrence and poor degree of histologic differentiation (p=0.17), presence of perineural invasion (p=0.92), desmoplasia (p=0.36), or anatomic location on the ear or lip (p=0.23) (Table 3).
Tumors were staged according to the 7th edition AJCC staging system for cSCC. Staging criteria are detailed in Table 1. Of the 225 primary tumors, 210 were staged as follows: 77 (37%) Stage 0 (cSCC in situ), 118 (56%) Stage I, and 15 (7%) Stage II. Fifteen primary tumors had no pre-biopsy size noted and fewer than 2 high-risk features; therefore these could not be staged according to the AJCC guidelines. No primary tumors in this study had nodal or distant metastasis at the time of diagnosis. Therefore, the primary T stage was equivalent to the overall TNM stage. Of the 19 tumors that recurred, 2 (11%) were Stage 0, 9 (47%) were Stage I, 5 (26%) were Stage II, and 3 (16%) were unstaged. There were no T3 or T4 primary tumors in this cohort.
Fifty seven (26%) of the tumors in the cohort were treated with local tumor destruction: 56 by electrodesiccation and curettage (C&E) and 1 by Erbium:YAG laser ablative therapy. 161 (74%) primary cSCC tumors were treated with surgical excision: 147 by Mohs micrographic surgery and 14 by wide local excision. 7 primary tumors (3%) had no information on treatment modality available. As expected, there was a significant correlation (r = 0.41) between tumor stage and treatment modality, with in situ tumors more likely to be treated with local destruction and Stage I/II tumors more likely to be treated with surgical excision. For this reason, our analysis was stratified by treatment type.
A Cox regression model was fit for AJCC stage adjusted for duration of immunosuppression at the primary diagnosis, and was stratified by sex and treatment modality. Overall, higher stage was associated with an increased relative hazard of local recurrence. Stage II tumors had a 10-fold increased risk of recurrence compared to Stage I (HR 9.9, 95%CI 3.0-32.7, p <0.0001) and a 43-fold risk of recurrence compared to cSCC in situ (Stage 0) (HR 43.5, 95% CI 5.6-337.5, p < 0.0001). Stage I tumors had a 4.4-fold increased risk of recurrence compared to cSCC in situ, but this did not achieve statistical significance (HR 4.4, 95% CI 0.8-25.2, p=0.1). Recurrence-free survival is shown in Figure 1.
Figure 1.
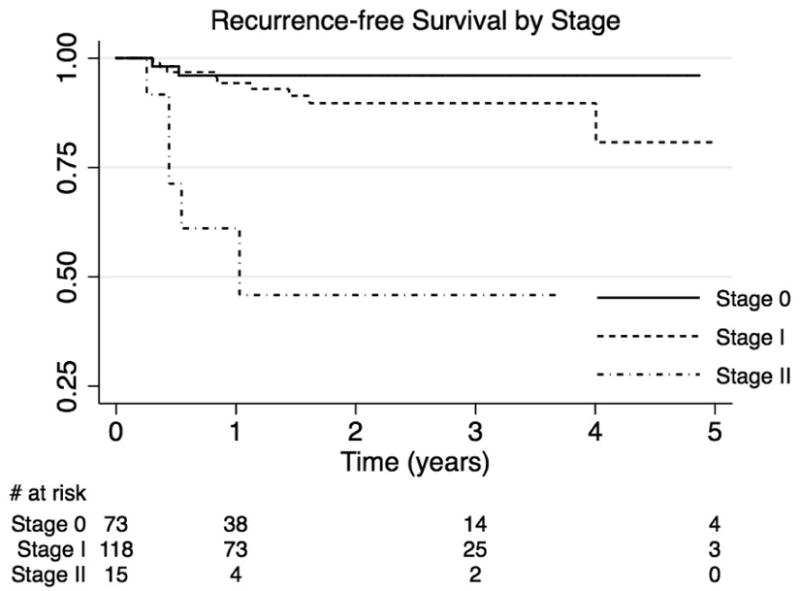
Cutaneous squamous cell carcinoma. Recurrence-free survival by stage.
Higher tumor stage was associated with an increased incidence of recurrence (p <0.0001, Table 4). Stage II cSCC had 39% incidence of recurrence at 1 year and 54% incidence of recurrence at 3 years. No data was available for the 5-year time point. The incidence of recurrence for Stage I cSCC was 6%, 10%, and 19% at 1, 3, and 5 years, respectively. 4% of cSCC in situ recurred by 1 year, with no further recurrences during the follow up period.
Table 4.
Cumulative Incidence of Recurrence by Stage. N.D.: no data.
| Cumulative Incidence | Std. Error | 95% Conf. Int. | ||
|---|---|---|---|---|
| Stage 0 | ||||
| 1 year | 0.04 | 0.03 | 0.01-0.15 | |
| 3 years | 0.04 | 0.03 | 0.01-0.15 | |
| 5 years | 0.04 | 0.03 | 0.01-0.15 | |
| Stage I | ||||
| 1 year | 0.06 | 0.02 | 0.02- 0.13 | |
| 3 years | 0.10 | 0.04 | 0.05- 0.20 | |
| 5 years | 0.19 | 0.09 | 0.07- 0.45 | |
| Stage II | ||||
| 1 year | 0.39 | 0.15 | 0.17-0.74 | |
| 3 years | 0.54 | 0.18 | 0.26-0.87 | |
| 5 years | N.D. | N.D. | N.D. |
Discussion
This study examined 224 primary cutaneous squamous cell carcinoma (cSCC) from 41 heart and lung transplant recipients and found a high incidence of local recurrence. The 5-year cumulative incidence of local recurrence was 4% for cSCC in situ, 19% for Stage I cSCC, and 54% for Stage II cSCC. The overall 5-year incidence of recurrence in this cohort was 16%, consistent with prior studies estimating the risk of recurrence of cSCC in solid organ transplant recipients (sOTR) between 7% and 13%24–26.
The median time to recurrence in our population was 6 months, ranging from 3 months to four years. This is significantly shorter than the 4-year median time to recurrence previously reported in immunocompetent patients27, and suggests that sOTR should have close follow-up in the immediate post-treatment period, potentially at 3 and 6 months post-treatment.
The incidence of recurrence was predicted by stage based on the newly revised 7th edition AJCC staging system, validating the prognostic accuracy of this system for stage 0, I and II cSCC tumors in this high-risk patient population.
We included cSCC in situ (Stage 0) in our study to evaluate the risk of recurrence in this high-risk population. 2 of 77 (2.6%) Stage 0 tumors recurred within the six months after treatment, with no additional recurrences beyond that point. As most in situ tumors in our study were treated with locally destructive methods, this rapid time to recurrence may represent persistence after incomplete destruction instead of recurrence. Nevertheless, these findings suggest that close follow-up in the first year post treatment and consideration for definitive surgical treatment in high-risk patients diagnosed with cSCC in situ may be indicated.
Our study faces certain limitations. It was limited to a retrospective analysis of primary cSCC in heart and lung transplant recipients at a single, tertiary care academic center and, thus, may not be generalizable to all organ transplant recipients or transplant recipients in a community setting.
In the current AJCC T-staging system, in order to remain similar to the head and neck cSCC staging system, T3 and T4 designations were reserved for primary tumors with bony invasion. There were no primary T3 or T4 tumors found in our cohort. This is consistent with previous findings that the incidence of primary cSCC presenting with bony invasion is rare22. Given the infrequency of these events, a larger cohort will be required to assess recurrence in higher stage primary tumors.
To validate the 7th edition AJCC prognostic staging system, larger collaborative studies are needed to capture tumors of all stages and to measure the outcomes of of local recurrence, nodal metastasis, distant metastasis, and disease-specific death. There were no nodal or distant metastases in our cohort, except in cases with prior local recurrence. However, first recurrences have previously been shown to put patients at increased risk for subsequent nodal and or distant metastasis11,12,21, and as such first recurrence is an outcome clinicians should aim to prevent.
Recently, the ICD-9 coding designations for non-melanoma skin cancer have changed. Previously, all non-melanoma skin cancers were grouped together under the ICD-9 code 173.X. Now squamous cell carcinoma has been given the unique designation of 173.X2, which should make the implementation of large scale study more feasible in the future.
A practical limitation of the new staging system is that it can be difficult to determine depth of invasion or the presence of perineural invasion on a superficial shave biopsy, which is the standard diagnostic procedure for NMSC. Staging could be more accurately performed after excision or Mohs micrographic surgery, during which the above features can be completely assessed. As cSCC are currently not routinely staged in clinical practice or captured in tumor registries, population-based studies will be difficult to perform but will significantly add to our understanding of this cancer.
In 2011, the National Comprehensive Cancer Network (NCCN) released their treatment guidelines for cSCC, which are based on slightly different high-risk tumor features including large size, depth of invasion ≥ 4mm or Clark's level IV, poorly defined borders, rapid growth, poor differentiation, acantholytic, desmoplastic, or adenosquamous histology, neurologic symptoms, perineural invasion, recurrence after treatment, and those occurring in the setting of immunosuppression or at a site of prior radiation or chronic inflammation11. The AJCC prognostic staging system and the NCCN treatment guidelines now have considerably more overlap, but are still not ideally aligned. The lack of a consensus on clinically relevant high risk features illustrates the need for additional studies. This would allow for more accurate predictions of prognosis and stage-specific treatment guidelines.
cSCC is more aggressive in immunocompromised patients, but immunosuppression is currently not a factor in AJCC staging. This study was not designed to evaluate whether immunosuppression is an independent risk factor for recurrence. We attempted to construct and alternate staging model that incorporated immunosuppression into the AJCC system, but in this limited cohort only 16 tumors were upstaged from T1 to T2. Therefore, we were unable to evaluate whether this alternate model provided a better fit to the data. A future case-control study would be better suited for comparison of recurrence rates between immunosuppressed and immunocompetent patient populations.
In conclusion, the newly revised 7th edition AJCC staging system for cutaneous squamous cell carcinoma is a useful tool for predicting risk of local recurrence in heart and lung transplant recipients, a phenomenon that is significantly associated with poor outcomes including subsequent nodal and distant metastasis and death11,12,21. Future studies will be necessary to validate the new staging system in the general population and for higher stage tumors.
In current clinical practice, most dermatologists do not stage primary cSCC. Our study provides support that staging provides important prognostic implications in known high-risk patient populations. Given the aggressive nature of this cancer in organ transplant recipients, we advocate for staging of primary cSCC and close follow-up in this high-risk population.
Acknowledgments
S.T.A. is supported by an NIH/NCRR/OD UCSF-CTSI grant number KL2 RR024130. The funding agency played no role in the collection, analysis, interpretation, or publication of this data. The authors wish to thank the UCSF Heart and Lung Transplant teams and the Nina Ireland Lung Disease Program for their support in this project.
Abbreviations
- AJCC
American Joint Committee on Cancer
- C&E
Curettage and electrodesiccation
- CI
Confidence interval
- cSCC
cutaneous squamous cell carcinoma
- DM
distant metastasis
- DSD
disease-specific death
- HR
Hazard ratio
- ICD-9
International Classification of Disease, 9th edition
- LR
local recurrence
- MMS
Mohs micrographic surgery
- NCCN
National Comprehensive Cancer Network
- NM
nodal metastasis
- NMSC
Non-melanoma skin cancer
- SD
Standard Deviation
- sOTR
Solid organ transplant recipients
- WLE
Wide local excision
Footnotes
Financial Disclosures: The authors have no relevant financial interests to disclose.
This work has not been presented previously.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Boker A, Singer JP, Metchnikoff C, et al. High Dose Exposure to Voriconazole is Associated with Cutaneous Squamous Cell Carcinoma in Lung Transplant Recipients. J Heart Lung Transplant. 2011 doi: 10.1016/j.healun.2012.02.033. In submission. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alam M, Brown RN, Silber DH, et al. Increased incidence and mortality associated with skin cancers after cardiac transplant. Am J Transplant. 2011;11(7):1488–1497. doi: 10.1111/j.1600-6143.2011.03598.x. [DOI] [PubMed] [Google Scholar]
- 3.Brewer JD, Colegio OR, Phillips PK, et al. Incidence of and risk factors for skin cancer after heart transplant. Arch Dermatol. 2009;145(12):1391–1396. doi: 10.1001/archdermatol.2009.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Veness MJ, Quinn DI, Ong CS, et al. Aggressive cutaneous malignancies following cardiothoracic transplantation: the Australian experience. Cancer. 1999;85(8):1758–1764. [PubMed] [Google Scholar]
- 5.Penn I. Post-transplant malignancy: the role of immunosuppression. Drug Saf. 2000;23(2):101–113. doi: 10.2165/00002018-200023020-00002. [DOI] [PubMed] [Google Scholar]
- 6.Ong CS, Keogh AM, Kossard S, Macdonald PS, Spratt PM. Skin cancer in Australian heart transplant recipients. J Am Acad Dermatol. 1999;40(1):27–34. doi: 10.1016/s0190-9622(99)70525-6. [DOI] [PubMed] [Google Scholar]
- 7.Euvrard S, Kanitakis J, Pouteil-Noble C, et al. Aggressive squamous cell carcinomas in organ transplant recipients. Transplant Proc. 1995;27(2):1767–1768. [PubMed] [Google Scholar]
- 8.Adamson R, Obispo E, Dychter S, et al. High incidence and clinical course of aggressive skin cancer in heart transplant patients: a single-center study. Transplant Proc. 1998;30(4):1124–1126. doi: 10.1016/s0041-1345(98)00178-x. [DOI] [PubMed] [Google Scholar]
- 9.Pollard JD, Hanasono MM, Mikulec AA, Le QT, Terris DJ. Head and neck cancer in cardiothoracic transplant recipients. Laryngoscope. 2000;110(8):1257–1261. doi: 10.1097/00005537-200008000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Glover MT, Niranjan N, Kwan JT, Leigh IM. Non-melanoma skin cancer in renal transplant recipients: the extent of the problem and a strategy for management. Br J Plast Surg. 1994;47(2):86–89. doi: 10.1016/0007-1226(94)90164-3. [DOI] [PubMed] [Google Scholar]
- 11.Miller SJ, Alam M, Andersen J, et al. Basal cell and squamous cell skin cancers. J Natl Compr Canc Netw. 2010;8(8):836–864. doi: 10.6004/jnccn.2010.0062. [DOI] [PubMed] [Google Scholar]
- 12.Rowe DE, Carroll RJ, Day CL., Jr Prognostic factors for local recurrence, metastasis, and survival rates in squamous cell carcinoma of the skin, ear, and lip. Implications for treatment modality selection. J Am Acad Dermatol. 1992;26(6):976–990. doi: 10.1016/0190-9622(92)70144-5. [DOI] [PubMed] [Google Scholar]
- 13.Clayman GL, Lee JJ, Holsinger FC, et al. Mortality risk from squamous cell skin cancer. J Clin Oncol. 2005;23(4):759–765. doi: 10.1200/JCO.2005.02.155. [DOI] [PubMed] [Google Scholar]
- 14.Mullen JT, Feng L, Xing Y, et al. Invasive squamous cell carcinoma of the skin: defining a high-risk group. Ann Surg Oncol. 2006;13(7):902–909. doi: 10.1245/ASO.2006.07.022. [DOI] [PubMed] [Google Scholar]
- 15.Jennings L, Schmults CD. Management of high-risk cutaneous squamous cell carcinoma. J Clin Aesthet Dermatol. 2010;3(4):39–48. [PMC free article] [PubMed] [Google Scholar]
- 16.Goepfert H, Dichtel WJ, Medina JE, Lindberg RD, Luna MD. Perineural invasion in squamous cell skin carcinoma of the head and neck. Am J Surg. 1984;148(4):542–547. doi: 10.1016/0002-9610(84)90385-4. [DOI] [PubMed] [Google Scholar]
- 17.Veness MJ, Morgan GJ, Palme CE, Gebski V. Surgery and adjuvant radiotherapy in patients with cutaneous head and neck squamous cell carcinoma metastatic to lymph nodes: combined treatment should be considered best practice. Laryngoscope. 2005;115(5):870–875. doi: 10.1097/01.MLG.0000158349.64337.ED. [DOI] [PubMed] [Google Scholar]
- 18.Weinberg AS, Ogle CA, Shim EK. Metastatic cutaneous squamous cell carcinoma: an update. Dermatol Surg. 2007;33(8):885–899. doi: 10.1111/j.1524-4725.2007.33190.x. [DOI] [PubMed] [Google Scholar]
- 19.Brantsch KD, Meisner C, Schönfisch B, et al. Analysis of risk factors determining prognosis of cutaneous squamous-cell carcinoma: a prospective study. Lancet Oncol. 2008;9(8):713–720. doi: 10.1016/S1470-2045(08)70178-5. [DOI] [PubMed] [Google Scholar]
- 20.Cherpelis BS, Marcusen C, Lang PG. Prognostic factors for metastasis in squamous cell carcinoma of the skin. Dermatol Surg. 2002;28(3):268–273. doi: 10.1046/j.1524-4725.2002.01169.x. [DOI] [PubMed] [Google Scholar]
- 21.Kyrgidis A, Tzellos TG, Kechagias N, et al. Cutaneous squamous cell carcinoma (SCC) of the head and neck: risk factors of overall and recurrence-free survival. Eur J Cancer. 2010;46(9):1563–1572. doi: 10.1016/j.ejca.2010.02.046. [DOI] [PubMed] [Google Scholar]
- 22.Farasat S, Yu SS, Neel VA, et al. A new American Joint Committee on Cancer staging system for cutaneous squamous cell carcinoma: creation and rationale for inclusion of tumor (T) characteristics. J Am Acad Dermatol. 2011;64(6):1051–1059. doi: 10.1016/j.jaad.2010.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Edge SB, Byrd DR, Compton CC, et al. AJCC Cancer Staging Manual. Seventh. NY, NY: Springer; 2010. Chapter 29. [Google Scholar]
- 24.Lewis KG, Weinstock MA. Nonmelanoma skin cancer mortality (1988-2000): the Rhode Island follow-back study. Arch Dermatol. 2004;140(7):837–842. doi: 10.1001/archderm.140.7.837. [DOI] [PubMed] [Google Scholar]
- 25.Buell JF, Hanaway MJ, Thomas M, Alloway RR, Woodle ES. Skin cancer following transplantation: the Israel Penn International Transplant Tumor Registry experience. Transplant Proc. 2005;37(2):962–963. doi: 10.1016/j.transproceed.2004.12.062. [DOI] [PubMed] [Google Scholar]
- 26.Lott DG, Manz R, Koch C, Lorenz RR. Aggressive behavior of nonmelanotic skin cancers in solid organ transplant recipients. Transplantation. 2010;90(6):683–687. doi: 10.1097/TP.0b013e3181ec7228. [DOI] [PubMed] [Google Scholar]
- 27.Chren MM, Torres JS, Stuart SE, et al. Recurrence after treatment of nonmelanoma skin cancer: a prospective cohort study. Arch Dermatol. 2011;147(5):540–546. doi: 10.1001/archdermatol.2011.109. [DOI] [PMC free article] [PubMed] [Google Scholar]


