Abstract
Over the course of natural history, countless animal species have evolved adaptive behavioral systems to cope with dangerous situations and promote survival. Emotional memories are central to these defense systems because they are rapidly acquired and prepare organisms for future threat. Unfortunately, the persistence and intrusion of memories of fearful experiences are quite common and can lead to pathogenic conditions, such as anxiety and phobias. Over the course of the last thirty years, neuroscientists and psychologists alike have attempted to understand the mechanisms by which the brain encodes and maintains these aversive memories. Of equal interest, though, is the neurobiology of extinction memory formation as this may shape current therapeutic techniques. Here we review the extant literature on the neurobiology of fear and extinction memory formation, with a strong focus on the cellular and molecular mechanisms underlying these processes.
Keywords: fear conditioning, extinction, memory, amygdala, prefrontal cortex, hippocampus, long-term potentiation, synaptic plasticity, rat
1. Introduction
Each day, animals are faced with a multitude of situations that require the assessment of risk and organization of defensive behavior to contend with imminent or future threats. Failure to do so might result in harm or even death. Fear is an emotion that is central to the organization of defensive behaviors to threat, and therefore has an essential role in survival. Indeed, both innate and learned fears are central for motivating defensive behaviors that allow for successful coping in risky situations (Mineka and Ohman, 2002; Ohman and Mineka, 2001). For example, in a natural setting, when animals encounter a predator, they will flee, freeze or attempt to threaten their opponent (Bolles, 1970). Additionally, organisms use subtle predictive cues, such as noises or odors, to determine if danger is imminent and respond preemptively, which may enhance their chances of survival.
In the laboratory setting, Pavlovian fear conditioning has become the quintessential method of investigating aversive learning and memory (Maren, 2001). In this paradigm, an innocuous stimulus (conditioned stimulus, CS) is repeatedly presented with a noxious stimulus, such as a footshock (unconditioned stimulus, US). After several pairings, the CS alone predicts the US and engenders a state of fear, indicated by freezing and increased heart rate, among other reactions. Though psychologists historically thought of fear conditioning in terms of conditioned reflexes, it is now regarded as a compilation of hierarchical associations that informs the organism about its world (Rescorla, 1988). That is, not only is the CS-US association learned, but also the relation of this aversive event to other stimuli and their structure in the environment. Thus, when an individual undergoes a traumatic event, a complex representation of the experience is formed that may persist indefinitely. These memories are typically adaptive, allowing individuals to cope with future threats. Unfortunately, in some cases, dysfunction in the fear system produces inappropriate and exaggerated fears that lead to psychopathology.
Indeed, disorders of fear and anxiety, including specific phobias and post-traumatic stress-disorder (PTSD), are largely due to and maintained by pathological fear memories. Based on recent statistics, nearly 82% of Americans will encounter a traumatic event in their life (Sledjeski et al., 2008). The National Institute of Mental Health reports that 3.5% of American adults are diagnosed with PTSD and of those, only 49% receive treatment (National Institute of Mental Health, 2011). Due to the prevalence of the disorder, research in the last several decades has focused on understanding the neural bases of fear memory formation with the aim of developing appropriate clinical interventions. One challenge that clinicians face is that fear memories endure over long periods of time and can generalize across contexts (Bouton, 1988; Rasmusson and Charney, 1997). Moreover, common behavioral therapies, including exposure therapy, tend to produce transient fear reduction that is often bound to the context in which the therapy was administered. This is also the case for extinction, a commonly used laboratory procedure in which a CS is repeatedly presented in the absence of the US, resulting in a decrease in fear. Therefore, the resilience of fear memories and the fragility of extinction memories make successful treatment of disorders such as PTSD a challenge.
In recent years, there have been great strides in understanding the neurobiological underpinnings of both fear and extinction memory formation. This offers a foundation upon which novel therapeutic intereventions for fear and anxiwty might bebuilt. The purpose of the current paper is to review both the history and recent advances in this field, with a specific focus on cellular and molecular findings. Evidence will mostly come from investigations using rodent models; this does not preclude the importance of research with human subjects. For an overview of the human fear and extinction literature, we refer you to several exhaustive reviews (Hartley and Phelps, 2010; Milad et al., 2006; Phelps and LeDoux, 2005).
2. Formation of Aversive Memories
2.1. Where do fear memories live?
2.1.1. The amygdala
The search for the locus of emotion began in the 1920’s with Walter Cannon and Phillip Bard implicating the hypothalamus and its projections in the mediation of emotional behavior. Later in 1937, James Papez extended this emotional circuit to include more medial temporal lobe structures. Specifically, he injected rabies virus into the hippocampus of a cat and observed its course throughout the brain. Based on these results, he described the emotional circuit as emanating from the hippocampus, traveling through the mammillary bodies, anterior thalamus, and anterior cingulate cortex (Papez, 1937). Paul MacLean revised this circuit to include the prefrontal cortex and amygdala and labeled it the “visceral brain” or more commonly, the “limbic system” (MacLean, 1949). The most convincing evidence for the amygdala’s role in emotion, particularly fear, came from the seminal work by Kluver and Bucy (1937). They found that the bilateral removal of the medial temporal lobes in rhesus monkeys resulted in abnormal emotional behavior (Kluver and Bucy, 1937). Before the temporal lobectomy, the monkeys were fearful and withdrew from their human handlers; after the surgical procedure, however, the monkeys no longer feared human beings and did not display anger or aggression. Importantly, they also showed avid interest in exploring objects in the environment, regardless if they posed a threat. Because Kluver and Bucy’s lesions included many brain structures such as the hippocampus, amygdala, and temporal neocortex, Weiskrantz (1956) reexamined lesions restricted to the amygdala and observed the same pattern of behavior, especially the loss of fear. These behavioral phenomena would later be replicated many times in various species (Fonberg, 1972; Goddard, 1964) and would come to be known as “Kluver-Bucy” syndrome. Along with reports that amygdaloid seizures result in fear-like behavior (Depaulis et al., 1997), these early studies provide irrefutable evidence that the amygdala is crucial for attributing emotional significance to situations and regulating fear behavior.
The role of the amygdala in fear learning and memory was first demonstrated in the laboratory using instrumental conditioning paradigms, such as avoidance learning. For example, bilateral lesions of the amygdala decrease an animal’s preference of a nonshocked chamber over one in which a shock was delivered (Brady et al., 1954; Robinson, 1963). Similarly, the amygdala has been implicated in mediating conditioned emotional responses, including conditioned suppression. For instance, Kellicutt and Schwartzbaum (1963) trained rats with amygdala lesions to bar-press for food and then fear conditioned them. They found that rats with amygdala lesions took longer to suppress bar-pressing in response to the CS (Kellicutt and Schwartzbaum, 1963). Several years later, Goldstein (1965) reported that amygdala lesions resulted in deficits in the acquisition and retention of fear responses, as measured by the latency to jump out of a compartment in which a tone and shock were paired. Blanchard and Blanchard (1972) followed up these studies by showing that restricted amygdala lesions impaired the acquisition of contextual fear conditioning, in which animals learn to associate the shock with the surrounding environment. These seminal studies provided the foundation from which the neural circuit underlying fear learning and memory formation has been built.
With regard to fear conditioning, the amygdala is typically described as having two different functional subdivisions: the basolateral complex of the amygdala (BLA) and the central nucleus of the amygdala (CeA; Maren, 2003; Pitkanen et al., 1997). The BLA itself contains the lateral nucleus (LA), the basolateral nucleus (BA) and the basomedial nucleus (BM; Davis et al., 1994; Krettek and Price, 1978). These nuclei themselves can be further divided into separate regions. The LA, located in the dorsal most part of the amygdala between the external capsule and CeA, is divided into the dorsolateral, mediolateral and ventrolateral regions (Pitkanen et al., 1997). The BA is situated below the LA and is made up of the magnocellular, intermediate and parvicellular regions. Lastly, the BM, also known as the accessory basal nucleus, lies ventral to the BA and also consists of the magnocellular, intermediate and parvicellular regions. The LA is the largest nucleus within the BLA, yet contains small tightly packed neurons with average soma diameters of approximately 10–15 µm. In comparison, the BA contains the largest neurons of the BLA: the average soma diameter of BA neurons is approximately 15–20 µm. The size of the neurons in the BA ranges from large in the anterior most part of the BA to small in the more posterior section. The BM, like the LA, also consists of smaller neurons (Davis et al., 1994; Krettek and Price, 1978; Sah et al., 2003).
As a whole, the morphology of the BLA is similar to that of the cortex with the exception that BLA neurons are organized randomly rather than in layers. The population of neurons is within the BLA is heterogeneous as there are two different types of neurons: pyramidal neurons and interneurons. Pyramidal neurons (class I) make up approximately 80% of the BLA and are large, spine-dense, and contain glutamate. These neurons form synapses on many other BLA neurons, in addition to forming most of the extrinsic connections to areas outside the BLA (i.e. CeA, hippocampus). The remaining 20% of neurons within the BLA consist of GABAergic interneurons (class II), which mostly form local circuits within the BLA. In comparison to pyramidal neurons, interneurons are small, stellate and spine-sparse. There are many different types of interneurons in the BLA which are differentiated by unique protein expression signatures, similar to interneurons in the cortex. (Davis et al., 1994; McDonald, 1982b; Pape and Pare, 2010; Pitkanen et al., 1997; Sah et al., 2003; Swanson and Petrovich, 1998).
The CeA consists of the lateral central amygdala (CeL), the medial central amygdala (CeM) and the capsular region of the central amygdala (CeLc). In general, CeA is primarily made up of GABAergic interneurons, which have been likened to neurons in the dorsal and ventral striatopallidal region of the brain. However, there are slight differences between the cells in the CeL and the CeM. The CeL contains medium-sized spine-dense neurons that branch prolifically. Neurons in the CeM have larger soma than the CeL, yet do not contain many dendritic spines and branch sparsely. As a whole, CeA neurons express a variety of peptides, such as enkephalin, neurotensin and corticotropin-releasing hormone. CeA neurons project extensively to extrinsic structures, such as the hypothalamus and periacqueductal gray (Davis et al., 1994; McDonald, 1982a, 1985; Pape and Pare, 2010; Pitkanen et al., 1997; Sah et al., 2003; Swanson and Petrovich, 1998).
In addition, recent work reveals that local inhibitory networks exist within the Ce that regulate the overall activity in this region (Ciocchi et al., 2010; Haubensak et al., 2010). For example, Haubensak et al. (2011) have provided evidence that there are two distinct populations of neurons within the CeL based on the presence or absence of protein kinase C-δ (PKC-δ). Not only did they find that PKC-δ-positive and PKC-δ-negative cells make inhibitory connections with one another, they also observed that PKC-δ-positive cells had monosynaptic connections with CeM neurons. Interestingly, Haubensak et al. (2010) found that these separate populations of CeL cells map onto behaviorally responsive cells in vivo. For example, when PKC-δ-positive cells in the CeL were silenced, firing activity in “CeL-off cells”, or neurons that exhibit a strong inhibitory response to a CS, was suppressed. However, CeL neurons that typically display an excitatory response to a CS (“CeL-on cells”) were not affected by the inhibition of PKC-δ-positive cells in the CeL, suggesting that CeL-off neurons may be PKC-δ-positive cells. Together, these studies are the first to demonstrate that although the CeA does have important extrinsic projections, inhibitory local circuits also exist both within the CeL and between the CeL and CeM that may regulate its overall activity.
In addition to instrinsic interneurons within the BLA and CeA, there are also clusters of GABAergic neurons located at the interface of the BLA and CeA. These so-called intercalated cell masses (ITC) have recently garnered interest as a cellular substrate for gating information flow between the BLA and CeA. There are three main groups of ITC clusters located in fiber bundles in and around the amygdala (Millhouse, 1986; Sah et al., 2003). The lateral cluster is situated within the external capsule on the outside of the BLA (ITC-L). The more medial, or intermediate, cluster sits amidst the fibers between the BLA and CeA (ITC-M). Lastly, there is a large ITC cluster located medially to the BA and ventral to the CeA (ITC-V). There are two types of neurons found within the ITC clusters, both of which have been compared to striatal neurons. The first kind consists of medium spiny neurons that synapse on neurons within the lateral, basal and central nuclei (Millhouse, 1986). The second group has a large soma and a mixture of spiny and aspiny dendrites, which travel in parallel to the BLA and CeA. This latter group, though, may not be GABAergic as it stains positive for acetylcholine rather than GABA. As a whole, the ITC local network is oriented in a dorsal to ventral direction in the rat (Amir et al., 2011). That is, GABAergic inhibition is always directed ventrally, which allows for an ideal mechanism for the BLA to control activity in the CeA.
In the last three decades, investigators have made great strides in uncovering the extrinsic connections of the amygdala underlying fear conditioning. Considerable work has demonstrated that the lateral nucleus of the amygdala (LA) is the primary sensory interface of the amygdala (Figure 1). Work by LeDoux and colleagues has established that the medial geniculate nucleus (MGN) directly relays auditory information to the LA during fear conditioning (Doron and Ledoux, 1999; LeDoux et al., 1990; LeDoux et al., 1986; LeDoux et al., 1985; LeDoux et al., 1984). As such, disrupting communication between the MGN and the amygdala results in deficits in the acquisition of fear (Iwata et al., 1986). Importantly, it has also been shown that after stimulation of the MGN, LA neurons are highly responsive to auditory stimuli (Bordi and LeDoux, 1992) and display increases in neuronal firing (Clugnet et al., 1990) as well as long-term potentiation (LTP; discussed in detail below), a mechanism thought to mediate synaptic plasticity (Clugnet and LeDoux, 1990). Auditory information is also transmitted in parallel indirectly from the MGN to the LA via the auditory cortex (Brunzell and Kim, 2001; Romanski and LeDoux, 1992). Contextual stimuli, which themselves predict aversive USs, are processed by the hippocampus (Fanselow and Poulos, 2005) and sent from the ventral subiculum and ventral CA1 to the basal nuclei of the amygdala (BL and BM; Canteras and Swanson, 1992; Maren and Fanselow, 1995; Pitkanen et al., 2000).
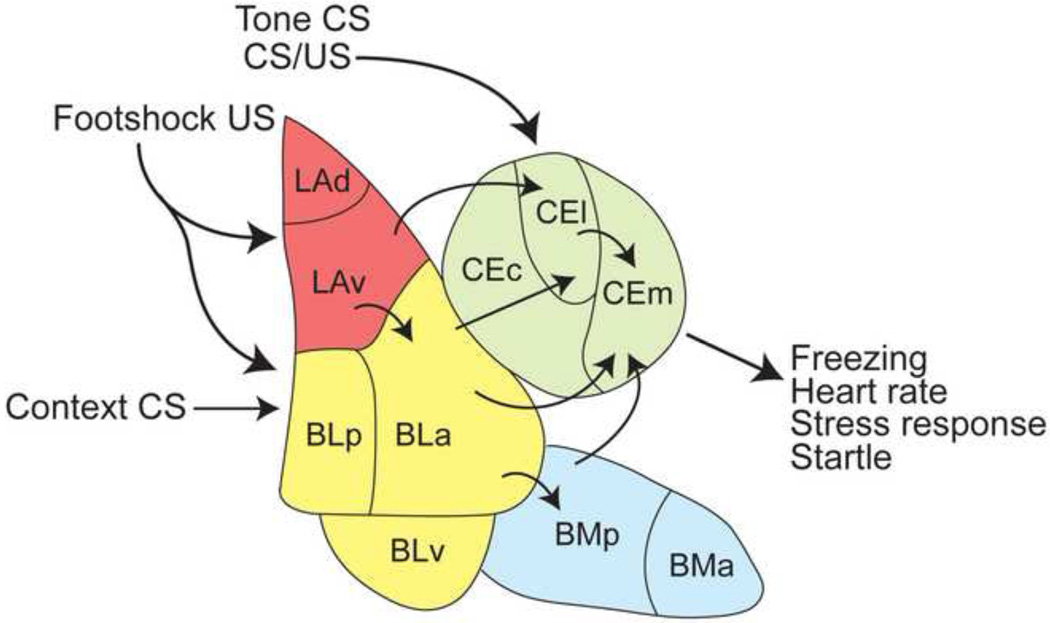
Figure 1. Amygdala anatomy and connectivity during fear conditioning in rats.
The amygdala consists of several nuclei that are integral to acquisition and retention of fear memories. Tone conditioned stimulus (CS) and footshock unconditioned stimulus (US) information converges within the lateral amygdala [LA; dorsal lateral amygdala (LAd) and ventral lateral amygdala (LAv)], as well as the basolateral amygdala [BL; posterior basolateral amygdala (BLp), anterior basolateral amygdala (BLa) and ventral basolateral amygdala (BLv)]. Additionally, CS and US information are processed in parallel within the central amygdala [CeA; centrolateral amygdala (CEl) and centromedial amygdala (CEm). Lastly, contextual conditioned stimulus information is transmitted to the basal amygdala. From the BL, information is relayed to the CeM, thought to be the output of the amygdala, either through the CeL or by coursing through the basomedial amygdala [BM; posterior basomedial amgydala (BMp) and anterior basomedial amygdala (BMa)]. The CeM projects to downstream structures, such as the periaqueductal gray, that produce various fear responses, including freezing and increases in heart rate among others.
Information about footshock unconditioned stimuli (USs) is relayed to the LA from thalamic and cortical regions. However, the exact pathway(s) that convey US information are still not clear. Some have suggested that the posterior intralaminar nucleus of the thalamus (PIN) and the insular cortex are responsible for relaying US information to the amygdala (Shi and Davis, 1999; Shi and Cassell, 1998). Indeed, combined lesions of the PIN and the insular cortex prevent the acquisition of fear-potentiated startle, another aversive learning paradigm (Shi and Davis, 1999). Additionally, pairing a CS with stimulation of the PIN as the US resulted in reliable conditioned responses (Cruikshank et al., 1992). However, others have found that combined lesions of the PIN and the insular cortex have no effect on fear conditioning (Brunzell and Kim, 2001; Lanuza et al., 2004). Rather, it is suggested that the PIN and insular cortex are part of a larger network of structures that process and convey US information to the amygdala (Brunzell and Kim, 2001; Lanuza et al., 2004). For example, nociceptive information can also be transmitted to the amygdala from the parabrachial nucleus and the spinal cord (Bernard et al., 1993; Lanuza et al., 2008).
As expected, LA neurons respond to both auditory and somatic stimuli (Romanski et al., 1993), suggesting a convergence of CS and US information on the same neurons within the LA. Indeed, a recent cellular imaging technique has allowed for the visualization of converging CS and US inputs onto the amygdala. Cellular compartmental analysis of temporal activity by fluorescence in situ hybridization (catFISH) capitalizes on the expression profile of the immediate early gene activity-regulated cytoskeletal-associated protein (Arc/also termed Arg3.1; Guzowski and Worley, 2001). Immediate early genes are activated rapidly in response to cellular stimuli and can result in the transcription and translation of proteins that may contribute to synaptic plasticity (Chaudhuri, 1997). Arc is observed within the nucleus up to 5 minutes after an animal has undergone a behavioral task and within the cytoplasm up to 25–30 minutes after the experience (Guzowski et al., 2005). Using this technique, Barot et al. (2008) showed that amygdala neurons that respond to the CS are also activated by the US in a conditioned taste aversion paradigm. Similar results were obtained using a contextual fear conditioning paradigm (Barot et al., 2009). Rats were placed into a conditioning chamber and received one footshock 26 minutes later. This paradigm allowed for the presentation of the CS (context) and US (footshock) to be divided into 2 different experiences that could be visualized separately. Indeed, during contextual fear conditioning, there were neurons within the BLA that exhibited both cytoplasmic (due to the CS) and nuclear (due to the US) staining. This was not observed in any of the control groups (immediate shock, latent inhibition and no shock). This strongly suggests that afferents to the amygdala that carry CS and US information converge on the same population of neurons.
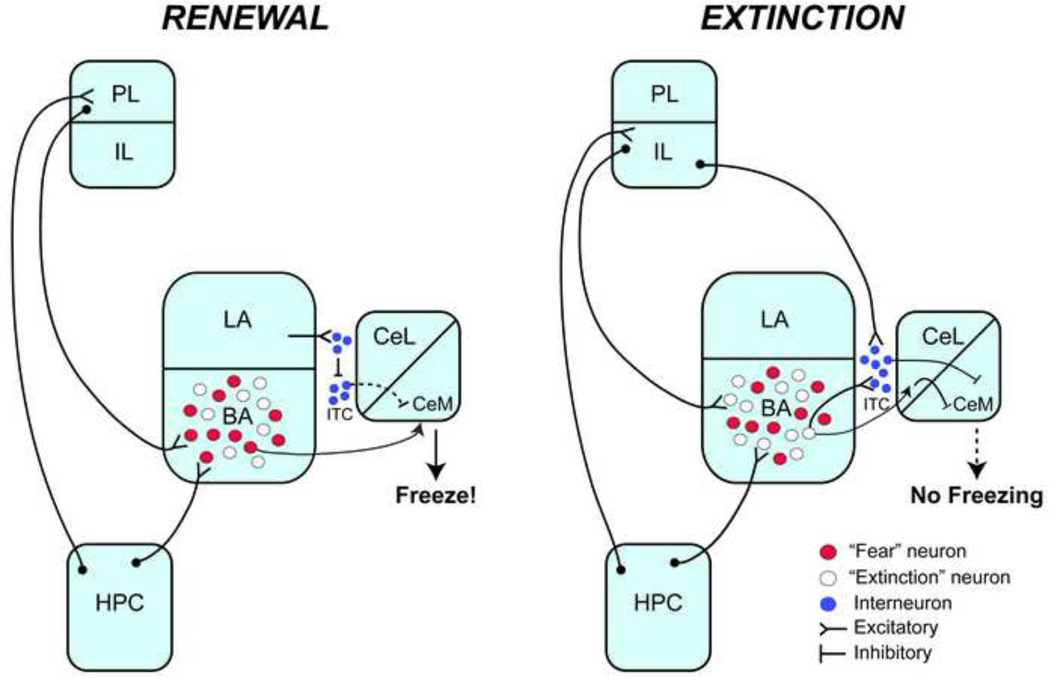
To generate a fear response, information must flow from the BLA to the CeA, which is thought to be the output center of the amygdala (Figure 1). The LA transmits CS-US information to the CeA via two routes (Krettek and Price, 1978; Pape and Pare, 2010; Pare and Smith, 1998). In a direct connection with the CeA, the LA sends unidirectional excitatory projections to the CeL. The CeL then synapses on the CeM, which has many projections to regions involved in fear responses, such as the periacqueductal gray (freezing), the paraventricular nucleus of the hypothalamus (glucocorticoid release), the parabrachial nucleus (respiration) and the lateral hypothalamus (increased heart rate and respiration; Davis and Whalen, 2001; Fendt and Fanselow, 1999; Maren, 2001). The LA also projects to the basal nuclei (BM and BL); these nuclei innervate neurons with the CeM. dditionally, BLA neurons also make connections with ITC cells before synapsing on CeA neurons (Pare and Smith, 1993, 1998; Royer et al., 1999). As these GABAergic ITC cells generate feed-forward inhibition in the CeA, it is thought that they gate the information flow between the BLA and CeA (Royer et al., 1999, 2000). Interestingly, ITC cells also receive robust excitatory input from the prefrontal cortex (Amir et al., 2011), which has been suggested to play a role in the expression of fear (Corcoran and Quirk, 2007; Sierra-Mercado et al., 2011). Thus, it is conceivable that excitation of ITC cells via prefrontal input could also result in the feedforward inhibition of the CeM. As a result of multiple inhibitory inputs onto the CeM, conditioned fear responses are thus generated by the disinhibition of CeM neurons via the ITC and CeL neurons (Ehrlich et al., 2009). Evidence for the latter comes from recent work indicating that CeL-off neurons can project to and inhibit CeM inhibitory output neurons, resulting in their net disinhibition (Ciocchi et al., 2010; Haubensak, et al., 2010).
It has been established that the BLA is the site of CS-US association insofar as lesions or reversible inactivation disrupt the acquisition of conditioned fear (Goosens and Maren, 2001; Koo et al., 2004; LeDoux et al., 1990; Maren et al., 1996a), as well as other indices of conditional fear behavior, such as fear-potentiated startle (Campeau and Davis, 1995). In fact, these deficits can be seen up to from one month (Maren et al., 1996a; Lee et al., 1996) to one year (Gale et al., 2004) after training. Support from electrophysiological studies confirms the BLA’s role in associative plasticity in fear memory formation. During aversive learning, neurons within the BLA exhibit enhanced responding to the CS (Maren et al., 1991; Quirk et al., 1997; Quirk et al., 1995; Repa et al., 2001; Rogan et al., 1997b). These changes are associative in nature, and can be dissociated from fear states and behavioral fear responses that are consequences of fear conditioning (Goosens et al., 2003). Importantly, the synaptic plasticity underlying aversive learning also occurs in the LA (Blair et al., 2001), as it receives converging inputs from the cortex and thalamus. Consistent with this notion, changes in the LA precede the actual behavioral changes in the animal (Repa et al., 2001) as well as tone-induced firing in other brain areas, such as the auditory cortex (Quirk et al., 1997). Disruption of LA activity does not, however, affect plasticity observed in the thalamus during fear conditioning, ruling out the possibility that plasticity in the LA is a reflection of changes in afferent structures (Schafe et al., 2005).
Evidence has also emerged that suggests that the CeA, rather than being a passive relay station to fear generating structures, is involved in fear memory formation. For example, temporary inactivation of the CeA (Wilensky et al., 2006; Ciocchi et al., 2010) or CeL alone (Ciocchi et al., 2010) prior to fear conditioning disrupts the acquisition of fear learning. Additionally, rats with BLA lesions undergoing overtraining are able to acquire conditional freezing (Ponnusamy et al., 2007; Maren, 1999a), although this is short-lived (Poulos et al., 2008). Both the bed nucleus of the stria terminalis (BNST; Poulos et al., 2010) and the CEA (Zimmerman et al. 2007) have been suggested to mediate overtrained fear in rats with BLA lesions. However, unlike the BNST, the CEA is required for both contextual and auditory CS memories, suggesting that it is ultimately responsible for mediating fear in the absence of the BLA (Zimmerman and Maren, 2010). Thus, it is conceivable that CS-US information is processed in the CeA in parallel to the LA, or that BLA-CeA projections themselves are the essential site of plasticity in fear conditioning (Maren, 2008). Indeed, auditory information can reach the CeA from the posterior thalamic nucleus (LeDoux et al., 1985; Linke et al., 2000; Turner and Herkenham, 1991). This nucleus receives auditory input from areas of the inferior colliculus and the dorsal nucleus of the lateral lemniscus. Consistent with this, thalamic stimulation results in changes in synaptic efficacy in the CeM that are independent of LA input to the CeA (Samson and Pare, 2005). In addition, US information is relayed to the CeA via the spinal cord and the parabrachial complex of the pons (Lanuza et al., 2004). This evidence suggests that amygdala is endowed with multiple routes by which fearful information can be processed and retained indefinitely.
2.1.2 The hippocampus
It is well known that during fear conditioning, contextual cues become associated with the aversive stimulus. The hippocampus is responsible for assembling a contextual representation of the conditioning environment and transmitting it to the amygdala (Fanselow and Poulos, 2005; Maren, 2001). Some of the earliest investigations of the role of the hippocampus in aversive learning began by assessing the effects of electrolytic lesions of the dorsal hippocampus (DH) on contextual fear conditioning. Electrolytic lesions yielded major deficits in contextual fear (Kim and Fanselow, 1992; Phillips and LeDoux, 1992), resembling the amnesic effects seen in humans with damage to the medial temporal lobe (Scoville and Milner, 1957). The effects of DH lesions are also time-dependent whereby the observed deficit diminishes across time (Anagnostaras et al., 1999; Kim and Fanselow, 1992). For example, Kim and Fanselow (1992) only observed contextual fear deficits in rats that received DH lesions one day after training. However, rats retained the fear memories if they had had surgery 7–28 days after training. Similarly, in a within-subjects study, rats trained 50 days prior to surgery displayed intact remote context fear memory, but impaired memory for training that occurred 1 day prior to surgery (Anagnostaras et al., 1999). This effect has also been replicated with neurotoxic lesions, which spare fibers of passage (Maren et al., 1997). This accumulated evidence suggests that the hippocampus is important for the initial acquisition and storage of the contextual memory, but over time, the memory is transferred elsewhere and rendered hippocampus-independent (Frankland et al., 2004; but see Sutherland and Lehmann, 2011; Sutherland et al., 2010). However, in contrast, other groups have proposed that the hippocampus may in fact have a more permanent role in the storage of contextual fear memories (Lehmann et al., 2007; Sutherland et al., 2008; Sutherland and Lehmann, 2011; Sutherland et al., 2010; Goshen et al., 2011). For example, Goshen et al. (2011) have recently used optogenetics to demonstrate that inhibition hippocampal area CA1 disrupts the retrieval of fear memories when assessed 9 or 12 weeks after training. Importantly, they showed that this was effect was only obtained when optogenetic induced-inhibition was limited to the duration of the test; when it was extended to include the 30 minutes prior to the test session, Goshen et al. (2011) did not observe a deficit in memory recall. This indicates that the hippocampus may have an enduring role in remote context memory, and other brain structures can rapidly compensate for its loss under some conditions.
Interestingly, many groups have reported that when neurotoxic lesions of the DH are made prior to training, there are no observable deficits in contextual fear (Cho et al., 1999; Gisquet-Verrier et al., 1999; Maren et al., 1997; Richmond et al., 1999, but see Selden et al., 1991). Based on both the pre- and post-training lesion data, it has been theorized that an organism can acquire fear using a hippocampal-dependent configural strategy or a hippocampal-independent elemental strategy (Maren et al., 1997; Rudy and O’Reilly, 1999; Rudy et al., 2002; Maren and Holt, 2004; Biedenkapp and Rudy, 2009; Zelikowsky et al, 2011; Fanselow, 2010). For the configural strategy, an organism assembles the various elements of the context, such as odors, tactile information, and visual stimuli, into one configuration that is represented as the context and subsequently associated with the aversive US. Conversely, elemental learning consists of associating one specific salient feature of the environment with the US. With an intact hippocampus, organisms use a configural strategy in which they assemble the many sensory elements of the conditioning situation into a coherent contextual representation. Evidence for this comes from the observation that if an animal is placed into a chamber and immediately shocked, the rats do not learn the association between the context and shock (Fanselow, 1986; Fanselow, 1990). If the rats undergo pre-exposure to the context prior to the shock, this deficit is alleviated, suggesting that the hippocampus requires a certain amount of time to form a representation of the environment. Thus, rats with post-training lesions are still able to use a configural strategy during contextual fear learning; however, after the hippocampus is ablated, they are no longer able to retrieve that memory. However, when the DH is lesioned prior to training, rats are unable to use a configural stratey and thus must employ another strategy in which to learn about the aversive situation. It has been suggested that rats use an elemental strategy in the absence of a hippocampus, resulting in the successful acquisition and retention of contextual fear memories. These findings imply that the hippocampus can interfere with or inhibit other non-hippocampal systems that typically employ an elementral strategy during fear acquisition. A recent study by Sparks et al. (2011) has provided evidence for this notion of hippocampal overshadowing of non-hippocampal systems. They demonstrated that while rats were able to successfully acquire contextual fear memories with an inactivated hippocampus, they were impaired during the test session when hippocampal activity was restored. This suggests that under normal conditions, the hippocampus interferes with other systems during learning and retrieval; when offline, non-hippocampal systems are released from this control and can mediate contextual learning.
Though many groups have demonstrated that pre-training lesions or inactivation of the hippocampus have no deleterious effect on subsequent learning, Wiltgen et al., (2006) have found deficits under some conditions. This group found that rats with pre-training lesions of the DH show impairments in contextual fear conditioning after one training trial. Increasing the number of training trials alleviated this impairment. Importantly, it was also shown that increasing the time between placement in the training chamber and the delivery of the shock strengthened contextual conditioning in lesioned animals. Because this is characteristic of a configural strategy, it may be that organisms without a hippocampus are still able to form configural representations of the environment and thus learn about the context, albeit at a slower rate. Taken together, accumulated data over the last decade demonstrate that the hippocampus is important for context fear learning, but in its absence, it is possible for other neural structures to compensate for this loss.
Importantly, many laboratories have also shown that the ventral hippocampus is necessary for the acquisition of both auditory and contextual fear. The VH has reciprocal connections to the amygdala and robust projections to the NAcc (Canteras and Swanson, 1992). Thus, it is perfectly situated to regulate activity in both structures during aversive learning. Many have reported that both electrolytic (Biedenkapp and Rudy, 2009; Maren, 1999b; Trivedi and Coover, 2004) and neurotoxic (Bannerman et al., 2003; Maren, 1999b; Richmond et al., 1999) lesions impair conditioning to both contextual and auditory cues. Infusions of glutamate receptor antagonists (Zhang et al., 2001), GABA agonists (Bast et al., 2001; Esclassan et al., 2009; Maren and Holt, 2004) or sodium channel blockers (Bast et al., 2001) also prevent the acquisition of context or auditory fear. Because lesions or inactivation of the DH typically do not affect fear to explicit CSs, some believe that the dorsal and ventral hippocampus mediate different aspects of learning and more generally, cognition. Specifically, the DH is involved in the spatial and contextual aspects of learning and may transmit this information through the VH to the amygdala, where the CS-US association occurs. The VH, on the other hand, is particularly important for processing and transmitting discrete emotional stimuli to the amygdala. Consistent with the notion that the DH and VH may subserve different aspects of learning and cognition, Dong et al. (2009) found that the dorsal CA1 area of the hippocampus (CA1d) and ventral CA1 area of the hippocampus (CA1v) display clear regional-specificity with regard to the expression of certain genes. For example, gene markers in the CA1d correlate highly with those found in the cortical and subcortical structures innervated by the CA1d that are involved in spatial processing and navigation. On the other hand, the CA1v shares gene expression patterns with other areas of the brain that receive projections from the CA1v and that have been shown to mediate endocrine and emotional responses. Thus, it appears that rather than being a homogenous structure, the hippocampus can in fact be parceled into distinct subregions, each with their own gene expression patterns. As a result, each hippocampal subfield mediates different aspects of behavior (for an comprehensive review on this subject, please refer to Fanselow and Dong, 2010).
2.2. Synaptic plasticity underlying memory formation
2.2.1. Long-term potentiation
One cellular mechanism that may underlie synaptic plasticity in the amygdala and the hippocampus during fear learning is long-term potentiation (LTP; Bliss and Collingridge, 1993). Several important properties of LTP make it a leading candidate for subserving fear memory formation. For example, the property of associativity refers to the fact that the pairing of a weak stimulus (such as the CS) with a strong stimulus (such as the US) will result in an overall strengthening of both pathways (McNaughton et al., 1978). Synapse-specificity, another characteristic of LTP, refers to the observation that LTP occurs only at synapses that share coincident activity with a postsynaptic neuron (for example, thalamo-LA or cortico-LA monosynaptic connections). Lastly, the property of cooperativity refers to the fact that a stimulated neuron needs to reach a certain depolarization threshold before LTP will be induced (McNaughton et al., 1978).
First studied in the hippocampus (Bliss and Gardner-Medwin, 1973; Bliss and Lomo, 1973), LTP has been described in many brain areas including the amygdala (Clugnet and LeDoux, 1990; Maren and Fanselow, 1995; Racine et al., 1983). In the laboratory, LTP can be induced in excitatory synaptic pathways by a high-frequency stimulus, or tetanus. This pattern of stimulation results in the presynaptic release of glutamate, an excitatory neurotransmitter, which binds to α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) and N-methyl-D-aspartate (NMDA) receptors on the post-synaptic membrane (Bliss and Collingridge, 1993; Goosens and Maren, 2002). However, until the depolarization threshold is reached, only the AMPA receptors are active as the NMDA channel is blocked by magnesium. When the depolarization threshold is reached, the magnesium leaves the NMDA channel and allows calcium to flow into the neuron. The influx of calcium results in a variety of changes that as a whole increase synaptic efficacy (Figure 2; Huang and Kandel, 1998). For example, additional AMPA receptors are trafficked into the postsynaptic membrane (Kessels and Malinow, 2009; Makino and Malinow, 2009; Maren et al., 1993; Tocco et al., 1992). Calcium also binds to proteins already found in the neuron, which initiates a variety of molecular cascades resulting post-synaptic modifications. Importantly, it has been reported that there are two phases of LTP: the early and late phase (Schafe et al., 2001; Kandel, 2001). The early phase of LTP (E-LTP) is independent of RNA and protein synthesis whereas the late phase of LTP (L-LTP) can last several hours to several days and depends upon de novo RNA and protein synthesis.
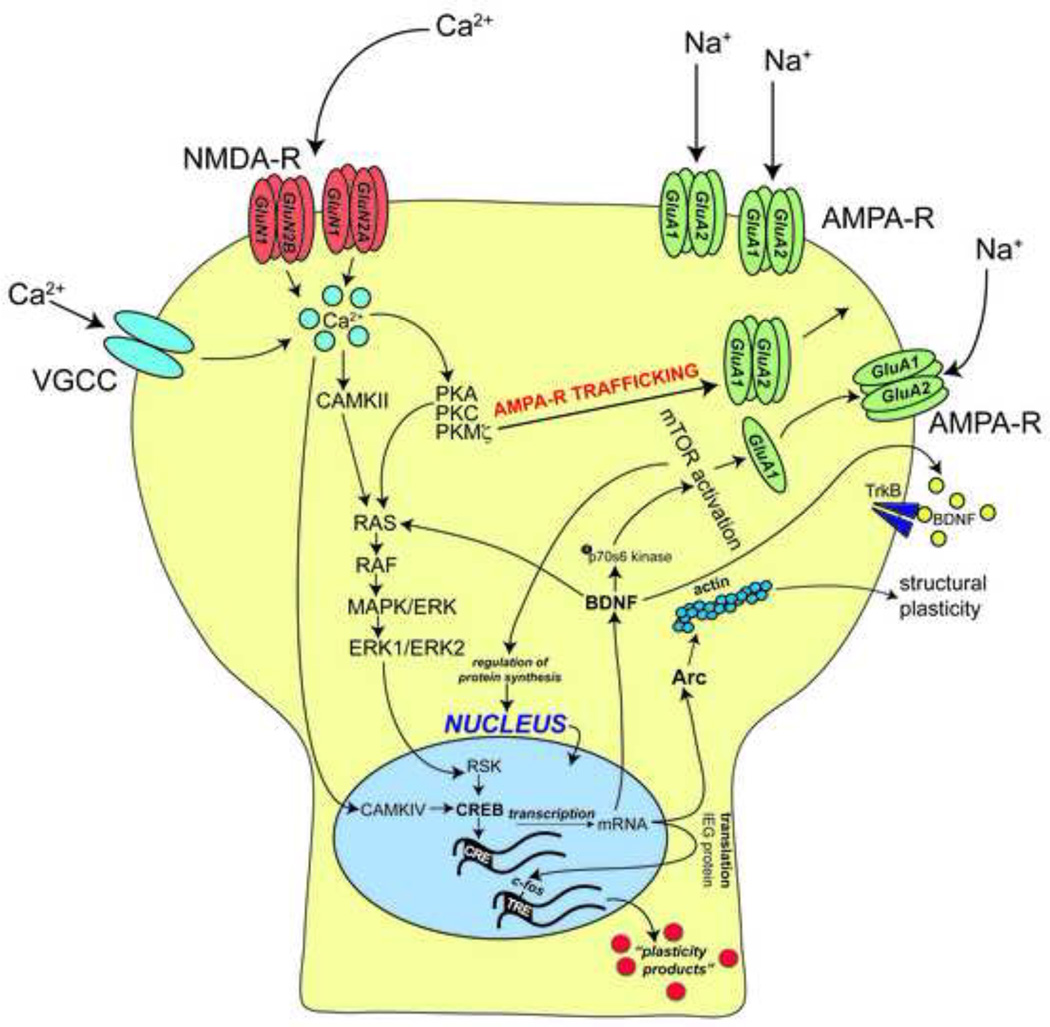
Figure 2. Signaling cascades underlying synaptic plasticity thought to mediate fear learning.
During strong postysnaptic depolarization, which is mediated by AMPA receptors (AMPA-R), calcium (Ca2+) entry through NMDA receptors (NMDA-R) and voltage-gated calcium channels (VGCC) initiates synaptic plasticity. Calcium-dependent protein kinases (e.g. protein kinase A, protein kinase C and protein kinase M ζ, and Ca2+/calmodulin protein kinase II) regulate the trafficking of AMPA-Rs into the synapse as well as the activation of the ERK/MAPK pathway, which can directly interact with transcription factors, such as CREB, within the nucleus. Calcium ions can also travel directly to the nucleus and interact with Ca2+/calmodulin kinase IV, also leading to the activation of CREB. Gene transcription within the nucleus results in a plethora of newly synthesized proteins, such as brain-derived neurotrophic factor (BDNF), activity-regulated cytoskeleton-associated protein (Arc) and c-fos. Importantly, BDNF regulates the ERK/MAPK pathway (Ou and Gean, 2006), in addition to activating mammalian target of rapamycin (mTOR; Slipczuk et al., 2009). mTOR activation results in the insertion of AMPA-R subunits into the membrane as well as the regulation of protein synthesis. In addition, BDNF is secreted from the neuron and binds to TrkB receptors, which are thought to be important for the late phase of long-term potentiation (Korte et al., 1995; Korte et al., 1998). Arc protein, in contrast, interacts with actin filaments of the cytoskeleton; this interaction has been shown to be crucial for changes in structural plasticity, such as dendritic spine enlargement in neurons (Matsuzaki et al, 2004).
2.2.2. Neurotransmission
Researchers have shown that fear conditioning, like LTP induction by stimulation, can result in synaptic changes in LA neurons. Rogan & LeDoux (1997) were one of the first to demonstrate that changes in LA neurons after fear conditioning display changes that are typically seen after LTP induction. Extending this, others have shown that these synaptic changes in the amygdala require NMDA and AMPA glutamate receptors (Maren, 2005; Walker and Davis, 2002). Indeed, inputs from both the cortex and thalamus to the LA are glutamatergic and synapse on neurons that have both types of receptors (Mahanty and Sah, 1999). Moreover, LTP in the amygdala has been found to be NMDA-receptor dependent (Bauer et al., 2002; Maren and Fanselow, 1995). As with LTP in the hippocampus (Collingridge et al., 1983), infusions of d,l-2-amino-5-phosphonovaerate (APV), a NMDA receptor antagonist, into the amygdala block the acquisition of aversive memories (Campeau et al., 1992; Fanselow and Kim, 1994; Goosens and Maren, 2003; Maren et al., 1996b; Miserendino et al., 1990). In addition to preventing learning, NMDA receptor antagonism also blocks conditioning-related firing changes in LA neurons as well as amygdala LTP (Goosens and Maren, 2004; Maren and Fanselow, 1995).
Endogenous NMDA receptors consist of a combination of several subunits: GluN1, and several different GluN2s. Of particular interest is the GluN2B subunit as it has been famously shown in the Doogie mice that overexpression of this subunit results in enhanced activation of NMDA receptors and superior learning on several behavioral tasks (Tang et al., 1999). Importantly, GluN2B subunits are found on dendritic spines of neurons that receive synapses from the MGN and PIN (Radley et al., 2007). The blockade of this subunit with ifenprodil, a GluN2B antagonist, blocks the acquisition of fear conditioning (Rodrigues et al., 2001) as well as LTP at thalamo-LA synapses (Bauer et al., 2002). Lastly, interruption of phosphorylation of GluN2B subunits disrupts conditioned freezing and impairs LTP at thalamo-LA synapses (Nakazawa et al., 2006). Together with the fact that NMDA receptors with GluN1-GluN2B compositions show slower decay after an excitatory action potential, it is clear that GluN2B subunits are important components of NMDA receptors in synaptic plasticity. However, these findings with GluN2B subunits do not preclude the importance of GluN2A subunits in aversive learning. Walker and Davis (2008) infused a selective GluN2A antagonist into the BLA and found that it blocks the acquisition and expression of fear-potentiated startle. Because they saw effects on both acquisition and expression, they concluded that rather than being essential for fear learning like the GluN2B subunits are, the GluN2A subunits may have a more general role in synaptic transmission.
Though the above studies clearly demonstrate a role for NMDA receptors in fear learning and amygdala LTP, there have been several other studies that suggest non-NMDA receptor-dependent plasticity is important for fear learning. For instance, voltage-gated calcium channels (VGCCs) have been implicated in some forms of long-lasting synaptic plasticity. Consistent with this, Weisskopf and LeDoux (1999) used an in vitro preparation to pair presynaptic thalamic inputs with postsynaptic LA depolarization in order to assess whether the resulting LTP was NMDA-dependent. They found that blockade of NMDA receptors had no effect on LTP whereas chelating calcium or applying a VGCC antagonist prevented thalamo-amygdala LTP (Weisskopf et al., 1999). Similarly, in in vivo preparations, the blockade of L-type VGCCs also impairs long-term fear memory formation (Bauer et al., 2002). Moreover, others have shown that both cortical and thalamic synapses on large LA dendritic spines require L-type VGCCs (Humeau et al., 2005). However, it has also been reported that L-type VGCCs are not important for fear acquisition at all (Cain et al., 2002). Currently, it is believed that LTP induction protocols using weak stimuli are NMDA dependent whereas those that involve strong pre- and postsynaptic depolarization also depend upon VGCCs (Bauer et al., 2002; Pape and Pare, 2010).
Similar to NMDA receptor antagonists, AMPA receptor antagonism in the BLA also prevents both the acquisition and expression of aversive fear (Walker and Davis, 2002). Conversely, the facilitation of AMPA receptors increases the rate of fear conditioning (Rogan et al., 1997a). Others have shown that the stimulation of afferents to the LA results in excitatory synaptic currents mediated by both AMPA and NMDA receptors localized on the same pyramidal dendritic spines (Mahanty and Sah, 1999). Rumpel et al. (2005) have now shown that the encoding of fear memories requires functional AMPA receptors. They infected LA neurons with a virus containing a vector that had the AMPA subunit GluA1 fused to a green fluorescent protein (GFP; “plasticity-tag”; Rumpel et al., 2005). Upon recombination, AMPA receptors would show greater inward rectification than endogenous AMPA receptors. In another group of animals, LA neurons were infected with a variant of the virus that contained a construct of GluA1 that would prevent incorporation of endogenous GluA1-containing receptors into the synaptic membrane (“plasticity-block”). Mice infected with the former virus were then fear conditioned with either paired or unpaired tones and shocks. Mice in the paired group displayed higher levels of freezing during the test session relative to those in the unpaired condition. More importantly, they found that mice infected with the “plasticity-tag” had AMPA receptors that showed greater inward rectification during paired fear conditioning as compared to control groups. In contrast, mice infected with the latter construct prior to conditioning displayed short-term and long-term impairments in fear memory. This sophisticated experiment suggests that during fear conditioning, AMPA receptors are trafficked into the postsynaptic membrane and this delivery is crucial to the formation of long-term fear memories.
Although substantial evidence indicates that LTP is expressed by postsynaptic modifications, there is also evidence that suggests that presynaptic changes accompany LTP (Huang and Kandel, 1998). Indeed, Maren and Fanselow (1995) reported that LTP in the BLA resulted in a decrease in the expression of paired-pulse facilitation (PPF; Maren and Fanselow, 1995), a phenomenon in which a second stimulation of equal magnitude to the first evokes a larger response; this was later replicated in the LA (Huang and Kandel, 1998). There are also data indicating that marked decreases in PPF (induced by increasing the probability of neurotransmitter release) at thalamo-LA synapses in fear-conditioned animals are mediated by AMPA receptors (McKernan and Shinnick-Gallagher, 1997). Because changes in PPF typically reflect presynaptic changes, these results suggest that amygdala LTP leads to presynaptic modifications, such as an increase in neurotransmitter release (Tsvetkov et al., 2002). Interestingly, it has also been shown that LTP can induce presynaptic modifications at cortico-LA synapses (Humeau et al., 2003). Moreover, this effect only occurs when both cortico-LA and thalamo-LA pathways are stimulated, demonstrating the associative nature of LTP. Together, these findings reveal a more complicated model of synaptic plasticity underlying fear learning whereby LTP induces both pre- and postsynaptic modifications through the activation of cortico- and thalamo-LA synapses.
Though excitatory transmission is crucial for LTP and aversive learning, it is also clear that inhibitory synaptic transmission regulates amygdala activity. Indeed, 20% of the neurons in the amygdala are GABAergic inhibitory interneurons. In addition, both GABAA and GABAB receptors are found widely within this limbic structure. In an attempt to characterize GABA receptors within the LA, Lang and Pare (1997) recorded from LA projection neurons and observed consistent hyperpolarizing potentials, which they determined were mediated partially by GABA interneurons synapsing onto projection neurons. Physiological data indicate that LA interneurons receive direct excitatory input from both cortical (Lang and Pare, 1997, 1998) and thalamic areas (Li et al., 1996) as well as indirect input through projection neurons (Szinyei et al., 2000). Though calcium-permeable AMPA receptors on interneurons have been shown to be involved in amygdala LTP (Mahanty and Sah, 1998), there is also evidence that NMDA receptors specifically containing GluN2B subunits are present on LA interneurons receiving both thalamic and cortical input (Szinyei et al., 2003). Behaviorally, it has shown that after fear conditioning, extracellular GABA levels are reduced (Stork et al., 2002), the availability of the GABA synthesizing enzyme is reduced (Heldt and Ressler, 2007), and plasticity at interneurons is impaired (Szinyei et al., 2007). Additionally, the genetic deletion or antagonism of the alpha 1 unit of GABAA receptors enhances fear conditioning and plasticity within the LA (Wiltgen et al., 2009). This suggests that during aversive learning, changes in inhibition may shift the balance of activity towards excitation of glutamatergic neurons, allowing for LTP to occur. Indeed, it has been shown that dopamine suppresses feed-forward inhibition from amygdala interneurons during LTP (Bissiere et al., 2003). Thus, in order for aversive memories to be properly acquired, the regulatory role of GABA is reduced so that there is enough excitation to allow for synaptic plasticity to occur.
More recently, it has been shown that electrical junctions between GABA interneurons within the dorsal hippocampus are important for encoding contextual information during fear conditioning. Bissiere et al. (2011) have shown that systemically blocking connexin 36, which joins GABA interneurons, with either carbenoxolone or mefloquine resulted in a deficit in context fear, but not tone fear. Additionally, this manipulation rendered extinction context-independent. Locally injecting either antagonist into the dorsal hippocampus similarly impaired context learning. The authors also observed that if an animal that had received pre-training infusions of the antagonist was placed back into the training context, there was an increase in c-fos expression in the CA1 and CA3 subfields of the hippocampus. These results indicate that the connexin antagonist blocked the encoding of the context during training; when placed back into the training context, they did not recognize it as familiar and treated it as a novel environment. Together, results from these pharmacological manipulations suggest that gap junctions between interneurons in the hippocampus are required for encoding contextual information during learning. Finally, Bissiere et al. (2011) demonstrated that blocking these gap junctions interfered with hippocampal theta oscillations, which have been previously shown to be important for exploratory behavior. This suggests that GABA communication via electrical synapses in the dorsal hippocampus promotes theta activity during contextual encoding, which may become synchronized with the amygdala during fear memory learning and consolidation.
2.3. Stabilization of fear memories
2.3.1. Signaling cascades
In order to maintain newly acquired fear memories over long periods of time, new information needs to be stabilized or consolidated into a more permanent form. Ultimately, the consolidation of fear memories requires new protein synthesis (Figure 2). Indeed, infusions of protein synthesis inhibitors into the BLA prevent the retention of auditory and contextual fear memories (Maren et al., 2003; Schafe and LeDoux, 2000; Schafe et al., 1999; Schafe et al., 2001). However, there are various upstream molecular signaling cascades within the amygdala and hippocampus that are crucial to the synthesis of new macromolecules. Many of these cascades are initiated by the influx of calcium through NMDA and AMPA receptors as well as VGCCs. One such cascade is that of Ca2+/calmodulin-dependent protein kinase II (CAMKII). Importantly, CAMKII’s activation stimulates autophosphorylation of its own subunits at a threonine (Thr286) residue that slows calcium/calmodulin dissociation rate and slows the rate of inactivation when they do eventually dissociate (Colbran and Brown, 2004). Once fully bound, CAMKII translocates to the post-synaptic density (PSD) where it acts through various means to enhance synaptic efficacy. For example, CAMKII binds to both NMDA and AMPA receptors in the postsynaptic membrane (Lisman et al., 2002). Interactions with the former receptor, specifically with the GluN2B subunit, promote longer action of the kinase as it prevents CAMKII’s inactivation when calcium/calmodulin dissociates (Bayer et al., 2001). CAMKII can also phosphorylate the GluA1 subunit of AMPA receptors, which enhances the channel conductance of the receptor (Barria et al., 1997; Derkach et al., 1999). Lastly, CAMKII is directly involved in trafficking and anchoring AMPA receptors into the postsynaptic membrane (Hayashi et al., 2000; Lisman et al., 2002), which as described above, has been shown to be necessary for the formation of fear memories. Accordingly, Mayford and colleagues (1996) showed that specifically turning off CAMKII in the amygdala prevents the consolidation and retention of auditory and contextual fear memories. Blocking calcium/calmodulin yields similar results (Rodrigues et al., 2004). Additionally, Rodrigues et al. (2004) have shown that that within the LA, αCAMKII, one of several isoforms of CAMKII, co-localizes with GluN2B subunits at postsynaptic targets of the auditory thalamus and that fear conditioning specifically results in the autophosphorylation of Thr286 residue in αCAMKII in LA dendritic spines.
CAMKII is also important for LTP and fear memory consolidation within the hippocampus. Consistent with this, tissue extracted from fear-conditioned rats has increased levels of αCAMKII up to two hours after the training experience. Much of the evidence for CAMKII mediating memory consolidation in the hippocampus comes from transgenic mouse experiments. For example, in an early study by Silva et al. (1992), mice deficient in αCAMKII do not display hippocampal LTP. Additionally, mice have also been generated that have a mutation of the αCAMKII gene that prevents autophosphorylation at the Thr286 residue. When mice are homozygous for this point mutation, contextual fear conditioning and hippocampal NMDA-dependent LTP are disrupted (Giese et al., 1998). Mice that are heterozygous for this mutation, however, do not exhibit these deficits. However, when a NMDA antagonist was systemically administered to heterozygous mice, contextual fear conditioning was impaired (Ohno et al., 2001) and hippocampal LTP was prevented (Ohno et al., 2002). As the dose given had no effect on wild-type mice, this suggests that NMDA receptor-dependent autophosphorylation of αCAMKII is necessary for contextual fear conditioning. Given what is known about CAMKII’s necessity for the induction of LTP in the amygdala and hippocampus and the successful acquisition of auditory and contextual fear, it is no wonder why people have thought of it as the molecular substrate for memory.
During memory stabilization, calcium can also interact with other protein kinases, such as Ca2+/phospholipid-dependent protein kinase (PKC), and cAMP-dependent protein kinase (PKA). Once activated, both kinases are involved in a variety of actions that mediate LTP maintenance and synaptic plasticity. Specifically, the activation of these kinases is thought to alter transcriptional activity within the nucleus. Evidence for their involvement in memory formation comes from studies using pharmacological or genetic manipulations. An early study demonstrated that PKA is essential for the presynaptic expression of LTP in the LA as inhibitors of this kinase prevented LTP induced by tetanus in amygdala slices (Huang and Kandel, 1998). Similarly, when PKA inhibitors are applied to hippocampal slices, L-LTP is prevented with little effect on E-LTP. Lastly, the transcription of cAMP response element (CRE)-mediated genes, typically induced by L-LTP, is blocked by PKA inhibitors (Impey et al., 1996). These in vitro experiments suggest a role for the kinases in promoting lasting neuronal changes due to LTP. In vivo, when H-7, an inhibitor of both PKC and PKA, is infused into the BLA prior to fear conditioning, short-term fear memory remains intact whereas long-term fear memory is impaired (Goosens et al., 2000). Schafe and colleagues (2000) also reported impairments in long-term fear memory with a more specific PKA inhibitor, Rp-cAMPS (Schafe and LeDoux, 2000; Schafe et al., 1999). Other laboratories have generated transgenic mice to assess the role of these kinases in fear memory. Abel et al. (1997) created transgenic mice that contained an inhibitory form of one of the regulatory subunits of PKA. This variation in PKA was mostly restricted to the hippocampus and resulted in impairments in both L-LTP and long-term contextual fear memories (Abel et al., 1997). Similarly, mice with knockouts of the β isoform of PKC suffer learning deficits in both auditory and contextual fear conditioning (Weeber et al., 2000). These deficits were attributed to effects within the BLA as these mutant mice displayed normal hippocampal electrophysiological properties. More recently, researchers developed a mouse with a knockout (KO) of β-arrestin-2, a molecule that regulates receptor signaling (Li et al., 2009). Importantly, β-arrestin-2 interacts with another compound that normally inhibits PKA. These KO mice displayed impairments in the acquisition and consolidation of fear memories. Moreover, LTP in both thalamo-LA and cortico-LA synapses was depressed. Broadly, these findings suggest that, in addition to PKA activation, the regulation of its activity within the amygdala is just as important for the stabilization of fear memories.
Recently, a specific isoform of PKC, PKMζ, has been identified as the molecular substrate for long-term memory storage. Most other PKC isoforms typically contain a catalytic domain and a regulatory domain that consists of a binding site for second messengers as well as an autoinhibitory pseudosubstrate sequence (Sacktor, 2008; Serrano et al., 2008). This sequence of the regulatory domain interacts with the catalytic domain to result in the inhibition of the kinase. However, when a second messenger binds to the regulatory domain, PKC undergoes a conformational change and is released from its autoinhibition. Once the second messengers are metabolized, PKC returns to its inactive form. PKMζ is unique in that it only contains the catalytic domain. Therefore, there is no inhibition of the catalytic domain, rendering PKMζ, constitutively active. The persistent activation of PKMζ makes it a good candidate for maintaining LTP and presumably long-term memory storage.
Indeed, in an early in vitro study, Sacktor et al. (1993) demonstrated that PKMζ increases during the induction and maintenance of hippocampal LTP. In fact, of all the protein kinases, PKMζ is the only that has been shown to be necessary and sufficient for L-LTP (Ling et al., 2002). Moreover, application of PKMζ inhibitors will completely reverse established L-LTP (Ling et al., 2002; Serrano et al., 2005). Interestingly, both the maintenance of L-LTP and the increase in PKMζ requires new protein synthesis (Osten et al., 1996) of PKMζ that derives from its own unique PKMζ mRNA (Hernandez et al., 2003). Instead of PKMζ mRNA being translated in the cytoplasm of the neuron, PKMζ mRNA is actually transported to dendritic spines for translation. This allows for a more integrated mechanism of signal transduction and synaptic strengthening. One way in which PKMζ can strengthen synapses during L-LTP is by aiding in the trafficking of AMPA receptors into the postsynaptic membrane (Ling et al., 2006). Indeed, addition of PKMζ to hippocampal slices results in a potentiation of AMPA receptors and the twofold addition of AMPA receptors. When a PKMζ inhibitor was applied to the slices, this reversed the observed enhancement. In behaving animals, it has been shown that PKMζ within the amygdala is necessary for the maintenance of long-term fear memories. Intracranial infusions of zeta inhibitory peptide (ZIP), which mimics the missing autoinhibitory pseudosubstrate sequence, into the BLA disrupts both auditory and contextual fear conditioning (Serrano et al., 2008). It is important to note that these infusions took place 22 hours after training occurred, indicating that PKMζ may be important for the permanent storage of fear memories within the amygdala. In contrast, however, a more recent study has shown that the effects of ZIP in the amygdala are transient (Parsons and Davis, 2011). That is, rats displayed deficits in fear expression when tested two days after ZIP adminstration, but exhibited intact fear expression when tested ten or fifteen days after ZIP infusions. Curiously, Parsons and Davis (2011) also demonstrated that if rats are tested shortly after ZIP infusions and then given a second test ten days later, fear expression is disrupted, suggesting that the short-term retrieval process predisposes the memory to long-term disruption. Together, this recent evidence indicates that permanent maintenance of fear memories may require synaptic activity in structures outside the amygdala, such as the cortex and hippocampus. Interestingly, PKMζ inhibitors in the DH do not affect the storage of contextual fear memories, but do affect other spatial tasks, such as the Morris water maze and radial arm maze (Kwapis et al., 2009; Serrano et al., 2008). This suggests that PKMζ, though important in other hippocampal-dependent learning tasks, specifically mediates the storage of associative fear memories within the amygdala shortly after learning.
Protein kinases can in fact regulate and act upon other kinase signaling pathways. Indeed, CAMKII, PKC and PKA converge upon mitogen-activated protein kinase (MAPK) signaling pathway, which is intimately involved in regulating transcription of plasticity-related genes. In vertebrates, there are actually seven different MAPK pathways, each of which is characterized by a core cascade of three sequential kinases, a MAP kinase kinase kinase (MAPKKK), a MAP kinase kinase (MAPKK) and a MAP kinase (Adams and Sweatt, 2002). Of these different pathways, the extracellular-regulated kinase (ERK)/MAPK pathway has been implicated in synaptic plasticity and fear memory consolidation (Thomas and Huganir, 2004). When CAMKII, PKC and PKA are activated, they interact with the G-protein Ras, which then activates another protein kinase, RAF (Adams and Sweatt, 2002). RAF, serving as the MAPKKK, phosphorylates the MAPK/ERK kinase (MEK); MEK will finally phosphorylate ERK1 and ERK 2 on their tyrosine and threonine residues. ERK1 and ERK 2, also known as p44 MAPK and p42 MAPK respectively, will translocate to the nucleus and act upon transcription factors, other kinases and cytoskeletal proteins. This results in a variety of changes that lead to strengthening of the synapse. For example, Wu et al. (2001) found that repeated membrane depolarization increases pERK in hippocampal neuron dendrites. Moreover, the presence of activated ERK in the dendrites resulted in the formation of new spines as well as the alteration of existing ones (Wu et al., 2001). Others have demonstrated that the MAPK cascade promotes the delivery of GluA1-containing AMPA receptors during LTP (Zhu et al., 2002).
The first evidence for MAPK in LTP came from English and Sweatt (1996 and 1997) who demonstrated that inhibition of the MAPK cascade with PD098059 attenuated LTP. Additionally, it was shown that levels of ERK2 are elevated following NMDA receptor stimulation (English and Sweatt, 1996) and during an influx of calcium (Impey et al., 1999). These early studies suggested that NMDA activation during LTP promotes synaptic changes via the MAPK signaling pathway. Shortly after these discoveries, Brambila et al. (1997) created a mouse that lacked RasGRF, which normally activates Ras. They found that these knockout mice are impaired in the consolidation of both auditory and contextual fear conditioning (Brambilla et al., 1997). This effect was attributed to abnormal plasticity within the BLA as region-specific stimulation resulted in hippocampal LTP, but not BLA LTP in mutant mice. Similarly, it has been shown that infusions of a Ras inhibitor into the BLA impair the acquisition of auditory and contextual fear conditioning (Merino and Maren, 2006). Consistent with this, the blockade of MEK systemically (Atkins et al., 1998) or locally within the LA (Schafe et al., 2000) impairs the long-term retention of fear memories and blocks LTP in the LA in vitro. Moreover, stimulation of the auditory thalamus results in phosphorylated ERK (pERK) within the LA that is necessary for LTP in this pathway (Schafe et al., 2008). Importantly, the phosphorylation of ERK due to stimulation was restricted to regions of the LA that also show an increase in pERK during fear memory consolidation (Schafe et al., 2000). Interestingly, it has also been shown that infusions of a MEK inhibitor into the auditory thalamus occlude LTP in the thalamo-LA pathway (Apergis-Schoute et al., 2005), which suggests that MAPK signaling with the auditory thalamus contributes to LA plasticity during fear memory formation. Notably, mice lacking ERK1 demonstrate no impairments in fear conditioning (Selcher et al., 2001), suggesting that ERK2 may mediate learning-related changes. Just as the activation of ERK is important for fear memory, it has also been shown that the regulation of its phosphorylation is also crucial to memory consolidation. Striatal-enriched protein tyrosine phosphatase (STEP) co-localizes with ERK in the LA and regulates its activity (Paul et al., 2007). During fear conditioning, there is an increase in de novo protein synthesis of STEP in the LA. Accordingly, mutations of STEP prevent nuclear translocation of ERK, block amygdala LTP and disrupt fear conditioning. Thus, it seems that this protein has an important role in not only regulating ERK activity, but also in modulating synaptic plasticity within the amygdala.
2.3.2. Transcription and gene expression
The various activated signaling cascades converge upon transcription factors within the nucleus. cAMP responsive element binding protein (CREB) is one transcription factor in particular that is responsible for regulating protein synthesis. Phosphorylation of CREB at Ser133 occurs when upstream signaling cascades, such as the MAPK pathway, are activated by cellular stimulation (Hernandez and Abel, 2008). Within the MAPK pathway, ERK does not actually phosphorylate Ser133 directly, but instead, activates another kinase, RSK, that acts directly on CREB (Impey et al., 1998a; Thomas and Huganir, 2004). Interestingly, PKA is required for the translocation of ERK to the nucleus and the subsequent activation of RSK (Impey et al., 1998a). Calcium can also activate CREB through its interactions with the nuclear calcium/calmodulin-dependent kinase IV (CAMKIV). Importantly, it has been shown that CAMKIV is more important for the rapid activity-dependent phosphorylation of CREB, whereas prolonged CREB phosphorylation requires RSK/MSK (Thomas and Huganir, 2004). Upon phosphorylation, CREB binds to the CRE promoter and transcribes a variety of genes, resulting in the production of growth factors, other transcription factors, immediate early gene products and retrograde messengers (Hernandez and Abel, 2008).
Early in vitro experiments first demonstrated both CRE-mediated gene expression and the phosphorylation of CREB were increased during L-LTP in hippocampal neurons (Impey et al., 1996). To assess whether CRE-dependent expression is involved during conditioning, Impey et al. (1998) developed a mouse that contained a CRE-driven β-galactosidase gene, which allows for the visualization of the gene products. Using this mouse, they found that during auditory and contextual fear conditioning, there was an increase in CRE-mediated gene expression in the amygdala and hippocampus, respectively (Impey et al., 1998b). Though these seminal studies were useful in identifying CREB’s role in LTP and learning, the most fruitful experiments came from work done with mice with null mutations of CREB’s various isoforms. For example, one of the first experiments of this nature demonstrated that mice deficient in either the α or δ isoforms exhibit intact short-term auditory fear memory, but impaired long-term fear memory (Bourtchuladze et al., 1994). This has been since replicated in other laboratories using various aversive learning paradigms (Gass et al., 1998; Josselyn et al., 2004; Kogan et al., 1997).
However, the most illuminating work about CREB’s role in memory formation has come from studies by Sheena Josselyn and colleagues. She was the first to show that overexpressing CREB specifically within the lateral amygdala enhances long-term fear memory (Josselyn et al., 2001). Using a herpes simplex virus type 1 (HSV) vector-mediated gene transfer, CREB was delivered to the amygdala prior to training. To specifically assess whether CREB would enhance memory formation, they used a training protocol that typically does not result in long-term memory formation. They found that the overexpression of CREB resulted in a striking enhancement of long-term memory and more importantly, this facilitation occurred despite the fact that only 15% of LA neurons overexpressed CREB. As this effect has been replicated in other laboratories with various fear and anxiety paradigms (Jasnow et al., 2005; Wallace et al., 2004), it seemed to suggest that the small proportion of LA neurons that overexpressed CREB might be outcompeting their neighboring neurons in the attempt to be incorporated into the long-term memory trace. In support of this hypothesis, infusions of this viral vector into the LA of CREB deficient mice (lacking both the α and δ isoforms) completely rescued their impairment in auditory fear conditioning (Han et al., 2007). Moreover, the detection of Arc-positive neurons shortly after conditioning was restricted to CREB-deficient mice that received the viral vector, indicating that CREB-expressing neurons were selectively activated during learning and presumably incorporated into the memory trace. Using an elegant molecular design, Han et al. (2009) then went onto to show that selective ablation of these CREB-overexpressing neurons in the LA after conditioning permanently erased the fear memory. What, then, makes these overexpressing CREB neurons so special? It has been shown that neurons with increased levels of CREB are more excitable than their neighbors and display enhanced synaptic transmission (Zhou et al., 2009). For example, Zhou et al. (2009) demonstrated that in neurons that overexpress CREB, the action potential threshold was lowered and there were more action potentials elicited by stimulation in transfected CREB neurons than nontransfected neurons. As such, there does not seem to be an even distribution of memory storage within the LA; CREB involvement determines which specific neurons are incorporated into the memory trace (Josselyn, 2010). The increased excitability of these neurons in the LA may prime them to be preferentially included in the memory trace. Whether these CREB-expressing neurons are randomly dispersed throughout the LA or are the specific targets of extrinsic structures, such as the auditory thalamus, is currently not known. The latter possibility is very attractive given that it has been shown that discrete CS and US information converge within the LA. The use of anatomical tracing of these selectively incorporated neurons would clarify how this integration occurs.
Along with other transcription factors, CREB transcribes immediate early genes (IEG) during fear conditioning (Miyashita et al., 2008). IEGs can be divided into two different categories: activity-induced regulatory transcription factors (RTF) and effector IEGs (Chaudhuri, 1997). The former group consists of IEGs such as c-fos and zif268; they are devoted to regulating the transcription of other proteins. Effector IEGs, such as Arc, BDNF and Homer 1a, are involved in cellular growth, synaptic modifications, and neuronal homeostasis. Importantly, the expression of these IEGs has been correlated with memory formation and memory strength. For example, numerous studies have shown that during contextual and auditory fear conditioning, there is an increase in c-fos expression in the hippocampus and amygdala, respectively (Ploski et al., 2010; Radulovic et al., 1998; Radwanska et al., 2002; Ressler et al., 2002; Stanciu et al., 2001; Wilson and Murphy, 2009). Interestingly, the increase in c-fos expression during fear conditioning seems to be restricted to the ventral portions of the dorsal LA (Radwanska et al., 2002; Wilson and Murphy, 2009), which is consistent with the finding that neurons within this area exhibit enhanced neuronal firing during training and through extinction (Repa et al., 2001). A similar increase in zinc-finger transcription factor (zif268/EGR-1/knox24) is observed in the hippocampus (Hall et al., 2001) and amygdala during fear memory consolidation (Ploski et al., 2010; Ressler et al., 2002) and retrieval. Moreover, the disruption of zif268 in the amygdala with an antisense oligodeoxynucleotide impairs long-term contextual fear memory (Malkani et al., 2004).
Another IEG that is activated downstream of CREB is activity-regulated cytoskeletal-associated protein (Arc/Arg3.1). Interestingly, Arc mRNA is able to leave the nucleus and move to the dendrites where it selectively accumulates at sites of synaptic activity (Rodriguez et al., 2005) via actin polymerization and ERK activation (Huang et al., 2007). Once incorporated into the dendrites, it is then locally translated into its protein form. Arc has been implicated in L-LTP in the hippocampus and spatial learning tasks (Guzowski et al., 2000; Plath et al., 2006). Additionally, Ploski (2008) provided the first direct evidence that Arc in the LA is required for the consolidation of fear memory. Specifically, they infused Arc antisense oligodeoxynucleotide into the LA and found that rats exhibited impaired long-term, but not short-term, auditory fear conditioning (Ploski et al., 2008). Additionally, as mentioned above, Arc is selectively activated in LA neurons that overexpress CREB during fear conditioning (Han et al., 2007). Given the importance of Arc in fear learning, what does Arc do to promote memory consolidation? Evidence suggests that in addition to co-localizing with cytoskeletal elements, such as actin, during its delivery to dendrites, Arc regulates actin polymerization after LTP (Messaoudi et al., 2007). It has been shown that LTP induction is associated with increases in actin polymerization in dendritic spines (Fukazawa et al., 2003), which is necessary for dendritic spine enlargement (Matsuzaki et al., 2004). Thus, through stabilizing actin at locally active synapses, Arc aids in the structural synaptic changes that promote the maintenance of LTP and presumably, long-term fear memory.
2.3.3. Neurotrophic factors
CREB also regulates the transcription of various neurotrophic factors, which play a role in the regulation of neuronal structure and growth. Though the neurotrophin family is large and diverse, the most widely distributed neurotrophic factor in the brain is brain-derived neurotrophic factor (BDNF). BDNF primarily has its effects through its interactions with its receptor, tropomyosin-related kinase B (TrkB), which is expressed on the surface membrane of neurons throughout the brain. To interact with TrkB, BDNF must be secreted out of the neuron (pre- or postsynaptically) where it can exogenously bind to the receptor. Unique to this specific neurotrophin, BDNF’s secretion is entirely activity-dependent (Lessmann et al., 2003; Schinder and Poo, 2000). It is no surprise, then, that BDNF has been found to be involved in L-LTP, presumably through its interactions with TrkB. Indeed, exogenously applied BDNF to hippocampal slices increases synaptic strength (Kang and Schuman, 1995; Messaoudi et al., 2002) whereas mice lacking BDNF do not exhibit L-LTP (Korte et al., 1995; Korte et al., 1998). Furthermore, blockade of TrkB in hippocampal slices also blocks L-LTP (Korte et al., 1995; Korte et al., 1998). Interestingly, it has also been shown that BDNF-induced LTP requires the upregulation of Arc in the hippocampus (Messaoudi et al., 2002) and influences dendritic spine growth (Alonso et al., 2004; Tyler and Pozzo-Miller, 2003). Together, these findings indicate an important role for BDNF in maintaining L-LTP through synaptic (Bamji et al., 2006) and structural changes.
Rattiner et al. (2004) were the first to connect amygdala-dependent learning with BDNF’s purported role in neural plasticity. They reported an increase in BDNF mRNA and TrkB phosphorylation in the BLA only after a CS-US pairing that promoted aversive learning, but not after exposure to the individual stimuli alone (Rattiner et al., 2004). Additionally, infusions of a general Trk antagonist impaired the acquisition of fear. To functionally demonstrate that TrkB receptors are specifically involved in amygdala-dependent learning, they constructed a lentiviral vector containing a truncated isoform of TrkB (TrkB.T1), which was injected bilaterally into the BLA. They found that expression of TrkB.T1 within the BLA impaired the acquisition of fear conditioning, indicating that BDNF signaling via these receptors in the amygdala is necessary for aversive learning. One way in which BDNF may mediate learning is by affecting the MAPK signaling cascade (Ou and Gean, 2006). Indeed, application of BDNF to amygdala slices increases Ras and MAPK. MEK inhibitors both impaired aversive learning in vivo and prevented BDNF-induced MAPK phosphorylation in vitro. In a more recent study, these same authors set out to characterize how fear learning modulates the signaling cascade that regulates BDNF expression (Ou and Gean, 2007). They found that during fear conditioning, there is an increase in amygdalar BDNF exon I- and exon III-containing mRNA and that protein synthesis inhibitors, transcription inhibitors, NMDA and VGCC antagonists, and PKA and CAMKII blockers significantly attenuate this increase. Lastly, they specifically demonstrated that after fear conditioning, phosphorylated CREB binds to the proximal region of the BNDF promoters I and III in the amygdala, which results in de novo BDNF protein synthesis. These findings provide a comprehensive understanding of the signaling cascade that underlies fear learning induced-BDNF expression.
2.3.4. Post-translation regulation
One important BDNF effect has on synaptic plasticity is its activation of proteins that regulate protein translation. The mammalian target of rapamycin (mTOR) kinase is indeed activated by BDNF (Slipczuk et al., 2009; Takei et al., 2004) and can initiate downstream protein synthesis by directly or indirectly phosphorylating eIF4E-binding protein 1 (4E-BP1) and p70s6 kinase (Raught et al., 2001). Interestingly, previous work has shown that mTOR-dependent translation activation by BDNF is required for protein synthesis specifically within cortical dendrites (Takei et al., 2004). For example, upregulation of Arc and CAMKII in dendrites after BDNF application is prevented by rapamycin, a selective inhibitor of mTOR. As rapamycin has also been to shown to block L-LTP (Tang et al., 2002), it follows that mTOR signaling would be crucial for fear memory formation. Indeed, Parsons et al. (2006) were the first to demonstrate the involvement of mTOR proteins in the amygdala during fear conditioning. They first showed that fear conditioning results in an increase in the phosphorylation of p70s6 kinase (Parsons et al., 2006), which decreases after intra-amygdala infusions of rapamycin. Furthermore, post-training and post-retrieval infusions of rapamycin into the amygdala prevent the formation of fear memories and disrupt the retention of the memory, respectively. In a more recent study, it was shown that one effect that BDNF induced-mTOR activation may have is the increase in the expression of the GluA1 AMPA subunit in the hippocampus (Slipczuk et al., 2009). This indicates at least one mechanism by which both BDNF and translation regulators directly contribute to synaptic changes underlying memory stabilization.
2.4 Reconsolidation of fear memories
After several days, memories will become resistant to amnesic agents that typically impair consolidation. However, if these stabilized memories are retrieved, they can in fact become labile again. In this state, they are susceptible to transcription and protein synthesis inhibitors, indicating that they must re-stabilize (Alberini, 2005). This process of re-stabilization, or reconsolidation, first came to light when Nader and colleagues (2000) demonstrated that upon retrieval, auditory fear memories were again sensitive to protein synthesis inhibitors 24 hours or 2 weeks after training. This retrieval-dependent fragility of fear memories has since been replicated many times (Debiec et al., 2002; Sara, 2000; Tronson and Taylor, 2007). In the last several years, the focus has been on understanding the molecular cascades underlying reconsolidation. It seems as though reconsolidation shares many similar cellular and molecular characteristics with consolidation (Alberini, 2005; Tronson and Taylor, 2007). For example, as Nader et al. (2000) showed, reconsolidation, like consolidation, of fear memories requires protein synthesis in the basolateral amygdala (Duvarci and Nader, 2004; Mamiya et al., 2009; Nader et al., 2000). Similarly, protein synthesis in the hippocampus is required for both the consolidation and reconsolidation of contextual fear memories (Debiec et al., 2002; Mamiya et al., 2009). Other research has also indicated that the MAPK signaling cascade within the LA is required for reconsolidation of auditory fear memories as infusions of U0126, a MEK inhibitor, impair long-term memory retention after retrieval (Doyere et al., 2007; Duvarci et al., 2005). As with consolidation, previous work also shows that CRE-mediated transcription in the amygdala is required for the re-stabilization of fear memories (Kida et al., 2002; Mamiya et al., 2009). Lastly, translational regulation is also important for reconsolidation as post-retrieval delivery of rapamycin into the amygdala disrupts this process (Parsons et al., 2006).
Though there seems to many similarities between consolidation and reconsolidation, it is important to note that there are in fact some differences. Lee et al. (2004) reported a double dissociation between molecular requirements for the two processes in the hippocampus. Specifically, BDNF was found to be important for the consolidation, but not reconsolidation, of contextual fear memories (Lee et al., 2004). Conversely, zif268 expression within the hippocampus was found to only be upregulated during reconsolidation. There also seem to be discrepancies between consolidation and reconsolidation depending on the species, behavioral task and brain region (for a review, see Alberini, 2005; Tronson and Taylor, 2007). For example, hippocampal protein synthesis is necessary for the consolidation, but not reconsolidation, of inhibitory avoidance (Taubenfeld et al., 2001). Similar results are found with protein synthesis inhibitors in the nucleus accumbens with instrumental learning tasks (Hernandez et al., 2002). Taken together, it seems that, although consolidation and reconsolidation may share several common molecular pathways, they may in fact be disparate processes as their requirements vary across brain region and learning task.
Though some believe that reconsolidation is a process by which the memory is updated with new information, others suggest that reconsolidation allows for the initial memory to be strengthened, with no changes to the underlying associative nature of the memory. That is, no new information becomes linked or associated with the original memory during reconsolidation. Recent work provides strong evidence for the latter. To ask whether new information can be incorporated into a reactivated memory, researchers have used aversive learning protocols that were modified for a second-order conditioning paradigm (Debiec et al., 2006; Tronel et al., 2005). That is, animals learn to associate a previously conditioned stimulus (CS1; e.g. tone) with another conditioned stimulus (CS2; e.g. light) during the reactivation session. Interestingly, selective disruption of reconsolidation of the CS1-US memory impaired memory for CS1, but did not have any effect on the association of CS2-CS1-US. Thus, it appears that although reactivation renders a memory labile, it does not allow new information to become linked to it. To provide direct evidence that reconsolidation serves to strengthen memories, Inda et al. (2011) demonstrated that multiple brief reactivation sessions of a young memory enhance memory retention. Additionally, Lee (2008) showed that contextual memory is strengthened with a second training session. Furthermore, he showed that the blockade of BDNF with an antisense oligodeoxynucleotide (ODN), which disrupts consolidation and not reconsolidation in the hippocampus, did not disrupt memory retention when given prior to the second learning session (Lee, 2008). However, the blockade of zif268 with an antisense ODN, which disrupts reconsolidation but not consolidation in the hippocampus, impaired contextual fear memory retention. Thus, reconsolidation does not involve the stabilization of newly acquired information (which occurs during consolidation), but merely serves to strengthen the original memory. The notion of memory strengthening during reconsolidation is also supported by studies that show that pharmacological agents can enhance memory when administered after reactivation (Lee et al., 2006; Tronson and Taylor, 2007; Tronson et al., 2006). What is still a matter of debate is how memories are actually strengthened during reconsolidation. For example, it is possible that the same population of neurons that encode the original fear memory is re-engaged during reconsolidation and through distinct molecular cascades, strengthens the memory at the synaptic level. Alternatively, it may be that a different population of cells is recruited during reconsolidation, which serves to strengthen the memory by synapsing upon those neurons that store the original memory trace. Additionally, memory strengthening during reconsolidation could be mediated not only at the cellular level, but at the structural level as well. For example, during reconsolidation, the memory trace may become distributed across several different structures, such as the prefrontal cortex, creating a stronger and more resilient long-term memory. However, whether any of these theories hold true empirically is not known but are worthwhile avenues of further research.
3. Extinction Memory Formation
Though a great deal of information has been learned about the neural bases of fear conditioning, the mechanisms underlying fear extinction are not as well characterized. Given that extinction has significant implications for clinical interventions in treating anxiety disorders, phobias and PTSD, recent research has been devoted to developing a framework for understanding extinction processes. Indeed, extinction has strong parallels to exposure therapy, a common cognitive behavioral therapeutic technique, in which the patient receives extensive exposure to the fear-evoking stimulus. Repeated exposure of the aversive stimulus results in a gradual decrease in fear behavior. Because of the similarity between extinction and exposure therapy, it seems prudent to investigate the mechanisms underlying extinction so as to improve methods used to treat patients with pathogenic fear disorders.
3.1. Behavioral features and theories of extinction
Ivan Pavlov was the first to document the extinction process with appetitive conditioned stimuli (Pavlov, 1927). In his hallmark experiment, Pavlov paired the sound of a metronome (CS) with the delivery of meat powder (US). Initially, the meat powder alone elicited a salivary response (unconditioned response, UR), but upon several CS-US pairings, the metronome alone resulted in a salivary response (CR). Importantly, the repeated presentation of the CS in the absence of the US led to a gradual decrement in the magnitude of the CR. Similarly, repeated CS presentations without an aversive US result in the extinction of the fear response. Though seemingly simple, extinction is actually a complicated phenomenon. In this section, several core behavioral properties of extinction will be described, followed by some of the more prominent theories of extinction learning.
To begin, it is important to appreciate that extinction is not the same as forgetting (Myers and Davis, 2007). Forgetting implies that there is a decrease in the fear response due to the passage of time. In fact, fear memories are resistant to forgetting as they can persist for over a year (Gale et al., 2004). However, extinction specifically refers to a decrement in behavior due to the presentation of the CS without the US.
An important property of extinction is its context-dependence. An illustrative example of this is the renewal effect, which refers to the return of conditioned fear when the CS is presented in a context different from that in which extinction occurred (Bouton, 2004; Bouton and Bolles, 1985). This effect has been observed even after massive extinction training consisting of up to 160 CS-alone presentations (Bouton and Swartzentruber, 1989; Denniston and Miller, 2003; Gunther et al., 1998). Importantly, renewal is not due to excitatory or inhibitory contextual conditioning occurring during fear conditioning and extinction, respectively. Rather, the context comes to modulate or “set the occasion” for CS-US and CS-‘no US’ associations. As such, after extinction, the CS has two possible meanings: 1) the CS predicts the US and 2) the CS predicts the absence of the US. The context in which the CS is presented ultimately determines which association is retrieved and therby determines whether fear is expressed or not. For example, if an extinguished CS is presented in the context in which extinction occurred, fear is suppressed. Conversely, if the CS it presented in an ambiguous or novel context, fear to the CS will return or renew. Thus, fear behavior after extinction depends specifically on the environment in which the cue is presented.
Extinction is not permanent loss of conditional responding. That is, learned fear responses are quick to return if there is a delay between extinction and retention testing. This property of extinction is termed spontaneous recovery (Rescorla, 2004). It is typically reported that the longer the extinction-to-test interval, the more robust the recovery is (Quirk, 2002). Similar to renewal, spontaneous recovery can also be explained by contextual modulation. Rather than a change in spatial cues, the passage of time creates a change in “temporal context” (Bouton, 1993). Interestingly, if there is both a physical and temporal change, there is even more of an enhanced recovery than that observed with each context shift alone (Rosas, 1998).
Lastly, extinguished fear responses can be reinstated with unsignaled presentations of the US. Specifically, if the US is delivered after extinction, there will be a recovery of conditioned responding when the CS is subsequently presented. There are two important points to demonstrate that reinstatement is a context-specific. First, reinstatement will only occur if the CS test after extinction occurs in the context in which the US was presented. Second, reinstatement does not occur if an unsignaled US is presented after fear conditioning. This suggests that reinstatement of fear is due to contextual conditioning between the US and the context in which it is presented; this contextual conditioning triggers a fear response upon the presentation of the extinguished CS. Thus, the return of fear during reinstatement is the result of the summation of a weak context-US association and the residual excitatory associative properties of the extinguished CS.
Given the various behavioral properties of extinction, several different theories of what is learned during extinction have been posited. Robert Rescorla and Alan Wagner’s model of associative learning describes extinction as a loss of associative strength to a CS that had accrued during conditioning; that is, extinction is a form of unlearning (Delamater, 2004; Miller et al., 1995; Rescorla and Wagner, 1972). Key to their associative learning model, Rescorla and Wagner claim that extinction learning is determined by US expectancy. By this view, CS-alone presentations lead to a surprising absense of the US, resulting in a decrease in the associative strength of the CS. There are various empirical reports that support the idea that extinction occurs because of a violation of US expectancy (Holtzman-Assif et al.; Huh et al., 2009; McNally and Westbrook, 2003). For example, Huh et al. (2009) reported that there was increased pERK in the hippocampus when an expected footshock was not delivered. Furthermore, increasing US expectancy was associated with faster extinction and increases in pERK in the hippocampus. Huh et al. (2009) claimed that ERK signaling during extinction was specific to coding prediction error, as there was no ERK activation during habituation, or continual reinforcement. Others have also reported that opioid signaling within the periacqueductal gray (PAG) also mediates negative prediction errors during extinction (McNally et al., 2004; McNally and Westbrook, 2003; Quirk and Mueller, 2008) whereas dopamine in the nucleus accumbens is important for regulating prediction error during extinction (Holtzman-Assif et al., 2010)
However, the Rescorla-Wagner model cannot account for the recovery of conditional responding observed during renewal, reinstatement and spontaneous recovery. As such, others have proposed that extinction is actually a form of new learning, a notion that is supported by the observed properties of extinction (Bouton, 2004; Pearce and Hall, 1980; Konorski, 1967) as well as the fact that fear can be rapidly re-acquired after extinction (Bouton, 2004). By this view, a new inhibitory association between the CS and US is formed (a CS-“no US” association) during extinction that co-exists and competes with the original CS-US memory (Konorski, 1967; Bouton, 1993; Myers and Davis, 2007). After extinction, the net sum of each association is zero; subsequent contextual cues, whether temporal, interoceptive or spatial in nature, gate which association is retrieved and ultimately expressed.
Other theorists have suggested that extinction is a form of non-associative learning. For example, extinction has been likened to the process of habituation, in which there is a decrease in responding to a stimulus when the stimulus is repeatedly presented over a long period of time (McSweeney and Swindell, 2002; Storsve et al., 2010). By this view, an organism may initially attend to the fact that the US no longer follows the CS, but once it is familiar with CS-alone presentations, it will ignore the CS (Kamprath and Wotjak, 2004; McSweeney and Swindell, 2002; Pearce and Hall, 1980). Interestingly, Rescorla and Heth (1975) argued that during CS extinction, habituation to the US also occurs. In other words, with initial CS presentations, the US representation is reactivated as it is a fundamental part of the CS-US association, but over the course of extinction, the US representation is devalued (Rescorla and Heth, 1975; Storsve et al., 2010). Though habituation and extinction have some features in common (McSweeney and Swindell, 2002), habituation does not account for the context-dependence of extinction.
Ultimately, it seems that multiple processes contribute to the acquisition of extinction. Both new inhibitory learning and loss of associative strength together might explain extinction learning. For example, it has been shown in numerous studies that the age at which extinction occurs critically determines whether new learning occurs during extinction (Kim and Richardson, 2007b, 2010). Kim et al. (2007b) extinguished rats on either postnatal day 17 (P17) or postnatal day 24 (P24). Both age groups demonstrated low levels of fear at the end of extinction, but, remarkably, only the P24 rats renewed their fear the following day (Kim and Richardson, 2007b). In addition to renewal, it was also shown that P17 rats do not exhibit reinstatement and spontaneous recovery (Gogolla et al., 2009; Kim and Richardson, 2007a). This suggests that extinction erases the original CS-US association in preweanling rats.
What mediates the transition from “unlearning” to “new learning” during extinction? It has been suggested that the participation of several neural structures during extinction is limited in P17 rats. In adult rats, extinction is mediated by a distributed network of neural structures that includes the hippocampus, amygdala and prefrontal cortex (PFC). In P17 rats, however, only the amygdala seems to be required for the extinction of conditioned fear (Kim et al., 2009; Kim and Richardson, 2008). This is consistent with developmental literature showing that the hippocampus and the PFC are delayed in their full maturation (Van Eden and Uylings, 1985a, b; Wilson, 1984). As such, the unlearning or erasure that occurs during extinction in the P17 rats may be due to their relatively immature neural organization. Another contributing factor to the development from “unlearning” to “new learning” may be the involvement of perineuronal nets (PNNs), an extracellular matrix consisting of chondroitin sulfate proteoglycans (CSPGs). PNNs have been found to be involved in the induction of ocular dominance in the visual cortex during a critical period of development. Along these lines, Gogolla et al. (2009) hypothesized that PNNs in the amygdala may enable plasticity during development that ultimately prevents the erasure of the original fear memory typically observed in young animals. They found that the time course for the development of PNNs and the persistence of fear memories after extinction were positively correlated (Gogolla et al., 2009). More importantly, intracranial infusions of a compound that disrupts CSPGs into the BLA in adult mice prevented renewal and spontaneous recovery after extinction. This evidence, along with other developmental literature, suggests that the dissociable extinction mechanisms in young and adult rats are due to distinct neural and cellular developmental processes.
Others have also shown that the interval between fear conditioning and extinction is a critical determinant of extinction learning. Maren and Chang (2006) demonstrated that if extinction occurs 15 minutes after fear conditioning (immediate extinction), there is no long-term retention of the extinction memory. That is, there is a within session decrease in fear during extinction, but when tested 48 hours later, there is recovery of the fear response (Maren and Chang, 2006, but see Myers et al., 2006). In a follow-up study, Chang and Maren (2009) showed that if rats were tested 15 minutes after immediate extinction, rats do show suppression of fear behavior, but it does not last (Chang and Maren, 2009). This short-term fear suppression, however, is context-independent insofar as rats that received immediate extinction did not renew their fear when tested 15 minutes after extinction in a different context. Based on their findings, they concluded that rather than learning a CS-“no US” association during immediate extinction, rats were using nonassociative mechanisms during extinction. Specifically, animals were habituating to the CS, independent of the context in which it was presented. Like extinction, habitation can display spontaneous recovery (McSweeney and Swindell, 2002), which is consistent with the fact that rats exhibited high levels of fear during the retention test. Unlike extinction, however, short-term habitation does not seem to be context-dependent. These results are especially informative when considering therapeutic treatments for anxiety disorders as it suggests that early interventions may actually exacerbate the recovery of traumatic memories.
3.2. Neurobiology
Similar to fear conditioning, extinction is not mediated by one specific brain region. Rather, extinction depends on plasticity within a distributed neural network. The amygdala, prefrontal cortex (PFC) and hippocampus have all been implicated in the acquisition, consolidation and retrieval of extinction of conditioned fear. Specifically, the amygdala is thought to be the site of acquisition and storage of the extinction memory; the prefrontal cortex, specifically the infralimbic area (IL) of the PFC, is thought to mediate the consolidation of extinction. Finally, the hippocampus plays a role in the context-dependent expression of extinction. In this section, the focus will be on how the circuit-level interactions between these brain regions mediate extinction. This will be followed by a review of some of the cellular mechanisms thought to underlie plasticity during extinction learning.
3.2.1. Brain region interactions
There are robust reciprocal connections between the amygdala and the hippocampus (Canteras and Swanson, 1992; Pitkanen et al., 2000). Specifically, projections from the hippocampus to the amygdala arise in the ventral subiculum/ventral CA1 region of the hippocampus and traverse through the ventral angular bundle (VAB). Projections from the ventral subiculum terminate heavily in the LA, BM, BA and CeM of the amygdala. The ventral CA1 however primarily projects only to the BA. Projections from the amygdala to the hippocampus mostly arise in the BA of the amygdala and terminate in the ventral subiculum, CA1, CA2 and CA3 subfields of the hippocampus. Given the heavy connections between these two areas, it is possible for both the amygdala and hippocampus to communicate with one another. This communication seems to be especially important for the context-specific retrieval of extinction. Indeed, it has been shown that the hippocampus regulates context-specific firing within the amygdala after extinction (Maren and Hobin, 2007). That is, infusions of muscimol into the dorsal hippocampus block the increase in LA firing typically observed during the renewal of fear. Furthermore, it has been shown that the ventral hippocampus projects onto neurons within the amygdala that are selectively active during renewal (Herry et al., 2008). It is also possible that communication between these two structures also is involved in extinction learning, given that hippocampal inactivation impairs the acquisition of extinction (Corocoran et al., 2005). It is important to point out that these effects are different than those observed with pre-training hippocampal lesions (Frohardt et al, 2000; Zelikowsky et al., 2011) as they do not interfere with the acquisition of extinction. This is because other neural structures are able to compensate for the loss of the hippocampus during conditioning and thus encode the memory using an elemental strategy (but see Wiltgen et al., 2006). If the hippocampus is intact during conditioning, however, any manipulation thereafter will cause impairments in fear expression and extinction, as the source of the configural representation of the context is gone.
Like the amygdala, the prefrontal cortex is a major target of hippocampal projections (Jay and Witter, 1991; Vertes, 2004; Cenquizca and Swanson, 2007; but see Swanson, 1981). The PFC is comprised of various subregions, but the ones that have been specifically implicated in extinction are the prelimbic (PL) and infralimbic areas located in the ventromedial PFC. Both the PL and IL receive input from the ventral CA1 and ventral subiculum (Hoover and Vertes, 2007). Low-frequency electrical stimulation (LFS) of the dorsal hippocampus attenuates extinction-related LTP within the PFC (Farinelli et al., 2006) and impairs extinction recall. In contrast, high-frequency stimulation of the hippocampus restores extinction-related potentiation within the PFC and, importantly, facilitates the recall of extinction. Similarly, others have shown that the stimulation of the ventral hippocampus results in similar LTP-like changes as those observed after extinction (Hugues et al., 2006). Moreover, this prefrontal plasticity can be blocked by infusions of a MAPK inhibitor. Interestingly, a more recent study suggests that hippocampal input to the IL is a major source of BDNF that is necessary for successful suppression of conditioned fear (Peters et al., 2010). Taken together, this evidence suggests that hippocampal projections to the PFC elicit synaptic changes that may be responsible for the consolidation of extinction.
Additionally, there are strong reciprocal connections between the PFC and the amygdala. Anatomical studies show that IL projects to the BM, CeA and ITC, whereas the PL sends robust projections to the BLA and CeA (McDonald et al., 1996; Vertes, 2004). Interactions between the prefrontal cortex and amygdala have received the most attention in regards to extinction. Specifically, it is thought that the mPFC influences CeA activity, which can result in the suppression of fear. However, there are various theories as to how this actually occurs. In vivo work in anesthetized rats has shown that the stimulation of either the IL or PL results in the inhibition of BLA through the activation of BLA inhibitory interneurons (Grace and Rosenkranz, 2002; Rosenkranz and Grace, 2001). In fact, it has been demonstrated that PFC stimulation in anesthetized rats suppressed LA activity in response to the presentation of a previous conditioned stimulus (Rosenkranz et al., 2003). This suggests that during extinction, the excitability of BLA projections neurons is reduced via local inhibitory circuits. This subsequently results in a decrease in CeA activity and fear behavior.
It has also been posited that the mPFC, specifically the IL, regulates amygdala activity through its projections to the ITC cells. For example, Quirk et al. (2003) were the first to show that stimulation of the PFC reduced CeA responsiveness to BLA excitatory input. However, this decrease in CeA activity is not thought to be due to PFC synapses on BLA interneurons, as Rosenkranz and Grace proposed. Specifically, others have shown that PFC stimulation excites BLA neurons (Likhtik et al., 2005) and that the PFC projects onto BA neurons selectively active during extinction (Herry et al., 2008). This indicates that, downstream of the BLA, there is an active gating mechanism that inhibits CeA activity during extinction (Likhtik et al., 2005; Quirk et al., 2003). The ITC cells have emerged as likely candidates because they are GABAergic, project to the CeA and receive glutamatergic input from both the IL and BLA (Royer et al., 1999; Royer and Pare, 2002). Consistent with this, chemical stimulation of the IL with picrotoxin results in an increase in c-fos in the ITC cells (Berretta et al., 2005) while selective lesions of ITC cells impair the expression of extinction (Likhtik et al., 2008). In a recent study, Amano et al. (2010) examined how the IL modulates BLA input onto ITC cells during extinction. Twenty-four hours after extinction, rats were sacrificed and coronal slices of their amygdalae were prepared (Amano et al., 2010). Whole-cell recordings were made from CeM neurons during BLA stimulation. They found that in rats that were extinguished, there was greater synaptic inhibition in the CeM. In addition, they reported that ITC cells of extinguished rats were significantly more responsive than ITC cells of control groups (animals that were conditioned or received unpaired CS and US presentations). This enhancement of ITC responsiveness was due to an increase in BLA neurotransmitter release and an alteration in the phosphorylation level of ionotropic glutamate receptors on ITC cells. Lastly, they demonstrated that the increased BLA-ITC efficacy is dependent upon the IL. This provides strong evidence that suppression of fear during extinction is mediated to a large extent by the regulation of ITC cells by IL glutamatergic activity.
3.3. Synaptic plasticity underlying extinction memory formation and stabilization
3.3.1. Neurotransmission
Similar to fear memories, the formation of extinction memories is NMDA-dependent. Indeed, systemic infusions of NMDA antagonists block extinction of conditioned fear (Baker and Azorlosa, 1996; Cox and Westbrook, 1994; Langton et al., 2007; Liu et al., 2009; Santini et al., 2001). Furthermore, pre-extinction infusions of NMDA antagonists directly into the BLA produce the same deficits in extinction (Falls et al., 1992; Laurent et al., 2008; Lin et al., 2003c; Walker and Davis, 2002; Zimmerman and Maren, 2010). Several lines of evidence show that GluN2B subunits of NMDA in the BLA are specifically involved in the acquisition of extinction. For example, the overexpression of the GluN2B subunit results in faster extinction (Tang et al., 1999). Additionally, pre-extinction infusions of ifenprodil, a GluN2B antagonist, into the LA impair extinction (Sotres-Bayon et al., 2007) whereas post-extinction infusions do not (Sotres-Bayon et al., 2009). Interestingly, post-extinction infusions of ifenprodil into the PFC prevent the retrieval of extinction memories whereas pre-extinction infusions have no effect; this indicates that these subunits in the PFC mediate the consolidation of extinction (Sotres-Bayon et al., 2009). Consistent with this, post-extinction infusions of the NMDA antagonist CPP [3-((+/−)2-carboxypiperazin-4yl)propel-1-phosphate] into the PFC not only eliminate the retention of extinction, but also reduce burst firing in the IL, which, under drug-free conditions, strongly predicts extinction recall (Burgos-Robles et al., 2007). These data indicate that NMDA-dependent plasticity in the BLA is necessary for encoding extinction memories, but that NMDA receptors in the PFC are required for the consolidation of extinction.
Although NMDA antagonists have been found to impair extinction learning, NMDA agonists have the opposite effect. That is, it has been found that the NMDA partial agonist, D-cycloserine (DCS), enhances extinction of conditioned fear by acting on the glycine binding site of NMDA receptors (Walker et al., 2002). Using a fear-conditioned startle paradigm, Walker et al. (2002) were the first to demonstrate that pre-extinction systemic or intra-BLA infusions of DCS facilitated extinction, and many others have reported similar results (Bouton et al., 2008; Langton and Richardson, 2008; Ledgerwood et al., 2003; Woods and Bouton, 2006). Post-extinction injections of DCS also faciliate extinction, suggesting that DCS enhances the consolidation of extinction (Langton and Richardson, 2010; Ledgerwood et al., 2003). Interestingly, DCS has been reported to prevent some of the recovery phenomena associated with extinction, such as reinstatement (Ledgerwood et al., 2004, but see Yamada et al., 2009), but has no effect on others, such as renewal (Woods and Bouton, 2006). One possible explanation for the effect on reinstatement, but not renewal, is that DCS promotes inhibitory conditioning of the context, which interferes with the excitatory conditioning between the US and the context that normally allows reinstatement to occur. This would have no effect on the renewal of fear, though, because the presentation of the extinguished CS occurs outside an inhibitory context. In addition, DCS also promotes the generalization of extinction to other non-extinguished CSs (Ledgerwood et al., 2005), which provides more evidence that during extinction, DCS explicitly enhances inhibitory conditioning to the context (Woods and Bouton, 2006). Chang and Maren (2011) have also reported that infusions of DCS into the infralimbic cortex facilitate extinction under conditions in which it normally does not occur (Chang and Maren, 2011). These results suggest that augmenting NMDA receptor function facilitates extinction learning under some conditions.
Though voltage-gated calcium channels (VGCC) have an unequivocal role in the acquisition of fear memories, there is less of a consensus regarding their participation in the extinction of conditioned fear. Initially, it was shown that systemic injections of two different L-type VGCC antagonists, nifedipine and nimodipine, impair the extinction of auditory and contextual fear in mice (Cain et al., 2002; Cain et al., 2005; Suzuki et al., 2004). However, later studies report data that are in stark contrast to these initial results. For example, it has been shown that mice with a knockout of CaV1.3, a specific subunit of L-type VGCCs found in the brain, displayed normal within-session extinction and intact retention of extinction 24 hours later (McKinney and Murphy, 2006). More recently, McKinney et al. (2008) replicated this finding with mice lacking the CaV1.2 subunit. However, in this same study, they found that systemic injections of nifedipine impaired acquisition and retention of extinction (McKinney et al., 2008). Along with this impairment, though, they observed that the drug affected locomotor activity in an open-field test, suggesting that the impairments observed with pharmacological manipulations may be due to aversive qualities of the drug itself. Specifically, it is thought that the VGCC antagonist causes a stress response by interacting with peripheral VGCCs (Busquet et al., 2008; Waltereit et al., 2008). In support of this notion, intracerebroventricular infusions of nifedipine have no effect on extinction (Busquet et al., 2008). Additionally, Waltereit et al. (2008) showed that if nifedipine is delivered subcutaneously 4 hours prior to extinction, extinction is impaired even though nifedipine is no longer present in the bloodstream. While these studies suggest that VGCCs do not participate in extinction, further investigation is warranted.
As extinction is thought to be a form of inhibitory learning, it is no surprise that GABA neurotransmission is involved. In one of the first studies addressing GABA’s role in extinction, Harris and Westbrook (1998) reported that systemic injections of GABA antagonists impaired the acquisition and context-dependent expression of extinction. Conversely, infusions of GABA agonists into either the IL or BLA facilitate extinction (Akirav et al., 2006). This suggests that GABA-mediated inhibition is required for suppression of conditioned fear. Consistent with this, the acquisition of extinction is associated with an upregulation of a variety of GABA-related genes, mRNA and proteins in the amygdala (Chhatwal et al., 2005b; Heldt and Ressler, 2007). For example, mRNA and protein levels of gephyrin, the GABAA receptor clustering protein, are increased in the amygdala after extinction (Chhatwal et al., 2005b; Heldt and Ressler, 2007; Lin et al., 2009a). In a comprehensive investigation of changes in GABA-associated genes in the amygdala during fear and extinction acquisition, Heldt and Ressler (2007) reported that during extinction, there were increases in the mRNA coding for gephryin, GABA α2 and β2 receptor subunits and GAD67, a GABA synthesizing enzyme. They also observed a decrease in the mRNA of GABA transporter-1, which mediates presynaptic uptake of GABA, during extinction. Importantly, it was also found that during extinction, there is increased binding of H3-flunitrazepam, a GABAA agonist, suggesting that extinction favors the trafficking of GABA receptors into the synapse. Consistent with this, it was recently shown that blocking insertion of GABAA receptors impairs extinction (Lin et al., 2009a). Together, these results strongly suggest that during extinction, GABA neurotransmission is especially important for inhibitory learning.
More recently, Jungling et al. (2008) have revealed that a newly discovered transmitter, neuropeptide S (NPS), is important in modulating the release of glutamate from LA principal neurons onto the GABAergic cells among the ITC islands between the BLA and CeA (medial paracapsular neurons; mpara neurons). Consistent with its known anyxiolytic properties (Xu et al., 2004), administration of exogenous NPS reduces anxious behavior as assessed by the open field and elevated plus maze. Further, the antagonism of endogenous NPS results in anxiogenic behavior in these behavioral tasks. As would then be expected, local application of exogenous NPS in the BLA facilitates extinction learning, whereas the infusion of a NPS antagonist impairs extinction learning and extinction recall. Given that NPS is highly expressed in the ITC clusters of the amygdala (Xu et al, 2007), Jungling et al. (2008) hypothesized that their behavioral effects were due to NPS modulating ITC synapses. To investigate this, they made whole cell recordings from amygdala slices from mice that were created to tag GAD67-expressing neurons with EGFP. Recordings were taken from LA principal neurons as well as from the EGFP-labeled GABAergic cells of the mpara ITC clusters. They found that bath application of NPS resulted in an enhancement of the evoked excitatory response in mpara cells due to stimulation of the LA principal neurons. This effect of NPS on glutamatergic synaptic responses was specific to mpara cells as it was not observed in local LA interneurons, other principal neurons or the lateral paracapsular ITC neurons that are located along the external capsule. They further showed that NPS specifically modulates the actual release of glutamate, as the NPS receptors are located presynaptically on LA principal neurons that send monosynaptic connections to mpara cells. Taken together, this evidence suggests that NPS is essential for the communication between LA principal neurons and ITC mpara neurons in order to reduce anxiety, and more specifically, facilitate extinction of conditioned fear.
In the last decade, research has also focused on endocannabinoid (EBC) neurotransmission in extinction. Within this system, there are two main endogenous cannabinoid receptors (CB): CB1 and CB2. The former is found primarily throughout the peripheral and central nervous system whereas the latter is highly expressed in the immune system (Chhatwal and Ressler, 2007). As all the literature regarding cannabinoids and extinction involve the CB1 receptor, this review will focus on this specific receptor rather than CB2 receptors. The CB1 receptor is a G-protein coupled receptor that is expressed presynaptically; they are activated by retrograde transmission of endogenous cannabinoids, such as anandamide. Importantly, activation of the CB1 receptor causes a decrease in the excitability of the presynaptic neuron, which subsequently results in a decrease in neurotransmitter release. CB1 receptors are expressed at high levels in the amygdala, hippocampus and cortex (Chhatwal and Ressler, 2007; Herkenham et al., 1990; Katona et al., 2001). Specifically, these receptors have been found on both glutamatergic neurons and GABAergic neurons, especially those expressing the neuropeptide cholecystokinin (CKK) (Azad et al., 2008; Katona et al., 2001; Marsicano and Lutz, 1999).
Marsicano et al. (2002) were the first to report a definitive role for the EBC system in extinction. In an elegant study, they used both CB1 receptor knockout mice as well as pharmacological manipulations to demonstrate that CB1 receptors are essential for successful extinction (Marsicano et al., 2002). Furthermore, they showed that extinction training elevated levels of anandamide and 2-arachidonoyglycerol (2-AG), another EBC, in the BLA. It is important to note, however, that their use of multiple extinction sessions in their behavioral paradigm does not allow for a conclusive examination of CB1 receptors in different phases of memory (i.e. acquisition versus consolidation). Nevertheless, others have also demonstrated a role for CB1 receptors in fear extinction. For instance, it has been shown that systemic injections (Chhatwal et al., 2005a; Pamplona et al., 2006; Suzuki et al., 2004), intra-IL (Lin et al., 2009b) or intra-BLA infusions (Roche et al., 2007) of CB1 receptor antagonists impair extinction whereas systemic (Pamplona et al., 2006) or intra-IL (Lin et al., 2009b) delivery of CB1 agonists facilitate extinction. Additionally, others have shown that acutely blocking EBC degradation accelerates extinction of conditioned fear (Chhatwal et al., 2005a). In a more recent study, Chhatwal and colleagues (2009) discovered that the EBC system interacts with the CKK neuropeptide system during extinction. As mentioned above, CB1 receptors are highly expressed on the presynaptic terminals of GABA interneurons, especially those that express CKK (Katona et al., 2001; Marsicano and Lutz, 1999). Chhatwal (2009) reported that a CCK agonist or CB1 receptor antagonist impaired extinction; however, the impairment in extinction by the CB1 receptor antagonist was ameliorated when a CCK antagonist, but not an agonist, was infused into the BLA. Based on their data, they suggest that CB1 receptor/CKK-expressing interneurons that synapse onto BLA neurons may be important for the synaptic plasticity underlying extinction learning (Chhatwal et al., 2009).
Based on these data, it appears that CB1 receptors have a critical role in the extinction of fear. However, several studies suggest the contrary. First, Lin et al. (2008) have reported that chronic administration of CB1 agonists retards extinction training-induced reduction of fear and results in a decrease in the levels of CB1 receptors in the IL. This suggests that although acute treatment with CB1 agonists may facilitate extinction, long-term exposure may have compromising effects. Secondly, it has been posited that rather that contributing to inhibitory associative learning during extinction, CB1 receptors mediate extinction through a habituation-like process (Kamprath et al., 2006). Specifically, Kamprath et al. (2006) argue that EBC involvement is not exclusive to the process of extinction. Rather, they suggest that this system is recruited in situations in which habituation occurs, such as extinction (Myers and Davis, 2007). The idea that extinction can be learned through non-associative processes was initially introduced by Kamprath and Wotjak (2004). They purported that associative learning and sensitization (non-associative learning) occur simultaneously during fear learning and that a habituation-like process during extinction counteracts the influence of the non-associative component of the fear memory acquired through sensitization. To demonstrate that CB1 receptors are important for the non-associative component of extinction, they first showed that CB1 receptor knockout mice were able to acquire conditioned and sensitized fear; in contrast to their wild-type counterparts, though, knockout mice displayed impairments in long-term habituation that closely resembled the deficits in extinction previously reported in CB1 receptor knockout mice (Marsicano et al., 2002). However, it is important to consider that their sensitization procedures are akin to generalized contextual fear conditioning: administering an inescapable footshock in one context and then presenting a novel nonreinforced tone in another context, which results in an increase in fear to the tone. By this view, the decline in fear during the extinction of “sensitized fear” would conceivably be more similar to that acquired through associative processes thought to underlie extinction of fear in more standard paradigms. If this is the case, CB1 receptors are indeed important for learning the CS-“no US” association during extinction, as previously reported (Marsciano et al., 2002; Chhatwal et al., 2005a; Pamplona et al., 2006; Suzuki et al., 2004).
Another neurotransmitter that has received considerable attention within the context of extinction learning and consolidation is dopamine. Early studies have shown that cocaine (Willick and Kokkinidis, 1995; Borowski and Kokkinidis, 1998) and amphetamine (Borowski and Kokkinidis, 1998), which cause the release of dopamine, attenuate the retention of extinction of fear-potentiated startle. Moreover, the specific D1 receptor agonist, SKF 38393, resulted in similar deficits in extinction recall (Borowski and Kokkinidis, 1998). In contrast, however, others have reported that either genetic deletion of D1 receptors (Eh-Ghundi et al., 2001) or dopamine loss within the prefrontal cortex (Fernandez Espejo, 2003) resulted in the perseveration of contextual fear and a major delay in its extinction. Though it is not clear what accounts for these contradictory findings, the latter results are consistent with more recent evidence showing that infusions of a D1 receptor antagonist into the IL impair the consolidation of extinction of cued fear (Hikind and Maroun, 2008). Interestingly, the infusion of the same D1 antagonist into the BLA impaired the acquisition, but not the consolidation, of extinction, demonstrating a functional dissociation between the role of these receptors in the prefrontal cortex and amygdala.
In addition to D1 receptors, D2 receptors have also been implicated in modulating extinction learning. An early investigation by Nader and LeDoux (1999) suggested that D2 receptors are required for the recall of learned fear as the systemic administration of quinpirole, a D2 agonist, blocked the extinction of cued fear. Later findings, however, showed that quinpirole only partially blocks extinction, whereas the systemic injection of sulpiride, a D2 antagonist, facilitates extinction learning in mice (Ponnusamy et al., 2005). Interestingly, it has also been shown that massed extinction has a facilitatory effect on extinction, regardless of drug treatment. Based on this evidence, Ponnusamy et al. (2005) suggested that D2 receptors constrain extinction in an inhibitory manner such that only the blockade of these receptors or extensive CS presentations can alleviate this constraint. However, these findings have also not been replicated; Mueller et al. (2010) recently demonstrated that either systemic or intra-IL infusions of raclopride, a D2 antagonist, impairs extinction consolidation. In addition, this manipulation attenuated extinction-evoked firing in IL neurons. One reason for the difference between this more recent study and that of Ponnsumay et al. (2005) is that raclopride is a more selective D2 antagonist than sulpride, which has been shown to bind to non-D2 receptor sites. Rather than constraining extinction, Mueller et al. (2010) proposed that D2 receptor signaling in the IL is required for extinction. Given that both D1 and D2 receptors in the IL are required for extinction consolidation and that projections from the IL to the amygdala seem to be important for the reduction of fear after extinction, it is conceivable that dopaminergic signaling modulates the IL’s influence on the amygdala. However, it is less clear the role that dopamine plays in other neural structures involved in extinction as the findings from systemic studies are quite contradictory and do not point to a specific locus of action.
Lastly, Gavin McNally and colleagues have convincingly demonstrated that endogenous opiods, specifically within the ventrolateral column of the periaqueductal gray (vlPAG), play an important role in extinction learning. For example, McNally and Westbrook (2003) showed that pre-extinction systemic injections of naloxone, an opiod antagonist, impair the development of extinction. Direct infusions of naloxone into the vlPAG, but not dorsal PAG, had similar effects (McNally et al., 2004). Importantly, these effects were not due to either consolidation or expression deficits insofar as naloxone treatment after extinction did not impair freezing as assessed during a drug-free test. Additionally, it was shown that the administration of RB101(S), an inhibitor of catabolizing enzymes that degrade enkephalin (a precursor of opiod peptides), facilitates the extinction of auditory fear (McNally et al., 2005a). Together, this evidence points to an essential role of opiods in fear extinction. Given that previous literature indicates that the opiod system is important for error prediction in fear conditioning (McNally and Cole, 2006; McNally et al., 2004), McNally and colleagues (2004) suggest that endogenous opiods in the vlPAG signal negative prediction errors that encode the discrepancy between what is predicted (the US) and the actual outcome (no US). Fear extinction normally occurs when the expected outcome exceeds the actual outcome, and this negative discrepancy drives inhibitory learning. Given that opiod receptor antagonism impairs the development of extinction, it is conceivable that opiod receptors within the vlPAG provide the error signal that induces extinction memory formation in structures such as the amygdala.
McNally et al. (2005b) then went on to explore the molecular requirements involved in opiod signaling during fear extinction. First, McNally et al. (2005b) infused µ-, δ- or κ-opiod receptor antagonists into the vlPAG prior to extinction. They found that only µ-opiod receptor antagonism impaired extinction in a regionally-specific manner. They further demonstrated that reductions in intracellular cyclic-AMP (cAMP) in the vlPAG are required for the extinction of conditioned fear whereas both PKA and ERK/MAPK in the vlPAG are not involved. This finding is in line with the fact that activation of µ-opiod receptors results in the inhibition of adenyl cyclase, which under normal conditions promotes the increase in cAMP. This µ-opiod receptor-induced decrease in cAMP causes an overall reduction in neuronal excitability. Interestingly, it has been shown that opiod receptor activation also results in an increase in potassium conductance and a decrease in calcium conductance and neurotransmitter release (Williams et al., 2001), though the connection between these cellular changes and extinction has not been explicitly shown. Taken together, it appears that error signals during extinction are mediated by µ-opiod receptor activation in the vlPAG, which results in the inhibition and reduction of adenyl cyclase and cAMP, respectively. How these changes affect other neural structures involved in extinction, such as the prefrontal cortex and amygdala, remains unclear and warrants further investigation.
3.3.2. Signaling cascades
Similar to the formation of fear memories, molecular cascades involving protein kinases, such as PKA, CAMK and phosphatidylinositol-3 (PI3k), are involved in the stabilization of extinction memories (Bevilaqua et al., 2006; Mueller et al., 2008; Szapiro et al., 2003; Yang and Lu, 2005). Of the many kinases, MAPK signaling cascades in the amygdala, prefrontal cortex and hippocampus have been found to play a major role in extinction of conditioned fear. Indeed, infusions of MAPK antagonists into the BLA reduce extinction of fear-potentiated startle (Lin et al., 2003c; Lu et al., 2001) and conditioned freezing (Herry et al., 2006). Consistent with this, extinction of auditory fear results in an increase in pERK in the BLA, indicating that this signaling pathway is specifically activated during extinction (Herry et al., 2006). Interestingly, previous work has shown that the facilitating effects of DCS are mediated partially by the MAPK signaling cascade in the BLA (Yang and Lu, 2005). Similarly, the infusion of a MAPK antagonist into the IL immediately after extinction impaired the retention of the extinction memory as well as the phosphorylation of ERK (Hugues et al., 2006; Hugues et al., 2004). Importantly, activation of this signaling cascade in the PFC during extinction is specific to adult organisms, as rats that underwent extinction in postnatal day 17 do not display increase in pERK in the PFC (Kim et al., 2009). Other work has similarly shown that MAPK/ERK is necessary in the hippocampus for the extinction of contextual fear (Fischer et al., 2007; Szapiro et al., 2003). Interestingly, CB1 receptors regulate the activity of the MAPK signaling cascade in the BLA, prefrontal cortex and hippocampus during extinction (Cannich et al., 2004; Lin et al., 2009b). For example, CB1 receptor knockout mice had reduced levels of pERK after extinction in all of these brain regions, as compared to their wild-type counterparts (Cannich et al., 2004). Conversely, CB1 receptor agonists significantly enhance levels of pERK during extinction (Lin et al., 2009b). As such, it seems that the MAPK signaling pathway is not only activated by the influx of calcium, but also is induced by the endocannabinoid system during extinction.
3.3.3. Gene expression
As with fear memory formation, signaling cascades engaged by extinction learning ultimately converge upon transcription factors through which protein synthesis is modulated. For example, previous work has shown that activation of the transcription factor CREB is increased within the amygdala and prefrontal cortex and that its blockade impairs fear suppression (Mamiya et al., 2009). Furthermore, transcription inhibitors within the BLA attenuate the facilitating effects of DCS during extinction (Yang and Lu, 2005). Others have demonstrated that extinction is protein synthesis dependent (Berman and Dudai, 2001; Mamiya et al., 2009; Pedreira and Maldonado, 2003; Santini et al., 2004; Suzuki et al., 2004; Vianna et al., 2001). Importantly, it has been shown that genes that regulate and code for protein synthesis are required for extinction of conditioned fear. For example, Herry and Mons (2004) demonstrated that extinction is accompanied by an increase in c-fos and zif268 in the prefrontal cortex and c-fos in the BLA. However, incomplete extinction, as evidenced by a high rate of spontaneous recovery after extinction, was associated with impairments in IEG expression in these brain regions (Herry and Mons, 2004). Consistent with these results, others have shown that rats bred for high anxiety exhibit impaired extinction of auditory fear as well as low levels of c-fos expression in the IL and LA and high levels in the CeA (Muigg et al., 2008). Along similar lines, Hefner et al. (2008) showed that a specific strain of mice with characteristic impairments in extinction also have attenuated IEG expression in the IL and BLA, but increased expression within the CeA. Together, these studies demonstrate that successful consolidation of extinction requires gene expression within the prefrontal cortex and amygdala. Impairments in extinction, however, reflect abnormalities within the cortico-amygdala circuit.
3.3.4. Neurotrophic factors
Similar to fear memory formation, previous work has shown that BDNF has an important role in the extinction of conditioned fear. For example, mice with a knock-in of a variant BDNF gene displayed an inability to extinguish cued fear (Soliman et al., 2010). Additionally, Chhatwal and colleagues (2006) have demonstrated that two hours, but not 30 minutes or 4 hours, after extinction, there is a marked increase in BDNF mRNA in the BLA, suggesting a role for this neurotrophic factor in the stabilization of the extinction memory. Using a viral gene-delivery system, they also showed that the expression of a truncated TrKB receptor resulted in impairments in extinction (Chhatwal et al., 2006). Similarly, within the hippocampus, there is an increase in BDNF protein after successful extinction (Peters et al., 2010). If the hippocampus is depleted of BDNF, extinction of contextual fear is impaired as assessed by both freezing and fear-potentiated startle (Heldt et al., 2007). Interestingly, it has recently been shown that the hippocampus provides BDNF to the infralimbic cortex during the consolidation of extinction (Peters et al., 2010). In this investigation, Peters et al. (2010) first showed that infusions of BDNF into the IL enhanced extinction when administered prior to extinction training. More importantly, infusions of BDNF into the IL without extinction training resulted in a decrease in fear, indicating that BDNF alone could substitute for extinction. Importantly, it was shown that this was not due to the erasure of the original fear memory. They then either infused BDNF into the hippocampus and saline into the IL or infused BDNF into the hippocampus after already having infused a BDNF-inactivating agent into this area. They found that the former treatment reduced conditioned fear as compared to controls (saline infusions into both the IL and hippocampus) whereas the latter treatment eliminated this effect. Consistent with the notion that BDNF mediates consolidation of extinction in the IL, it has also been found that epigenetic modulation of BDNF genes in the IL are associated with fear extinction (Bredy et al., 2007).
3.3.5. Proteolytic activity
Very recently, research has emerged demonstrating a role for serine protease inhibitors in extinction of conditioned fear (Meins et al., 2010). In general, serine proteases are normally involved in digesting proteins; broadly, they play a role in neural plasticity, neural development, neural degeneration and neural inflammation Interestingly, certain serine proteases have been shown to be involved in long-term memory formation and hippocampal LTP (Wang et al., 2008). Finally, they have been implicated in modulating phosphorylation of receptors, such as AMPA receptors. Within the brain, serine proteases are regulated by serine protease inhibitors, or serpins. One specific serpin, protein nexin-1 (PN-1), has been shown to be important for NMDAR-dependent LTP within the hippocampus (Luthi et al., 1997). Given that NMDA receptors are required for extinction and that PN-1s are found in the amygdala and hippocampus, Meins et al. (2010) investigated whether these protease inhibitors are involved in extinction of fear. They first characterized the distribution of PN-1s within the nuclei of the amygdala. They found that PN-1s are highly expressed within GABAergic neurons in the CeA and ITC cells and within glial cells in the BLA (Meins et al., 2010). To assess whether PN-1 is involved in extinction, they created PN-1 knock out (PN-1KO) mice. During extinction, these PN-1KO mice exhibited high levels of freezing throughout the extinction session, indicating a deficit in extinction learning. Furthermore, Meins and colleagues found that impaired extinction in the PN-1KO was associated with abnormal activity-dependent markers within the BA, ITC and CeL. That is, compared to the wild-type controls, there was a decrease in c-fos levels with the BA and a reduction in phosphorylated αCAMKII in the ITC and CeL in the PN-1KO mice. Together, these results suggest that PN-1s, specifically within GABAergic neurons in the CeA and ITC, are important for extinction learning. Whether PN-1s are modulating NMDA receptors on these inhibitory neurons is not clear from this investigation. Additionally, these authors only focused on nuclei within the amygdala. It would be interesting to see whether similar results were obtained with direct manipulations of PN-1s in other areas, such as the PFC and hippocampus, which are known to be involved in fear extinction.
3.3.6. Depotentiation during extinction
Thus far, the research reviewed has supported the notion that extinction is indeed new learning. That is, the observed molecular changes during extinction suggest an increase in synaptic efficacy. However, observations from electrophysiological studies show that during extinction, there is a decrease in neuronal firing in the LA (Quirk et al., 1995). Furthermore, the presentation of an extinguished CS causes long-term depression in firing of LA neurons (Rogan et al., 2005). Others have directly shown that extinction of fear reverses the fear conditioning-induced LTP in thalamo-LA pathways (Kim et al., 2007). This reversal, or depotentiation, has been observed in the amygdala both in vitro and in vivo by administering low-frequency stimulation and is accompanied by a reduction of fear (Kim et al., 2007; Lin et al., 2003a). Interestingly, the depotentiation of LA synapses has been shown to be dependent upon both NMDA receptors and VGCCs (Lin et al., 2003a; Lin et al., 2003b) and results in the dephosphorylation of certain protein kinases, such as MAPK and phosphatidylinositol 3-kinase (PI-3)(Lin et al., 2003b). Curiously, protein kinase dephosphorylation resulting from depotentiation is associated with an increase in calcineurin, a protein phosphatase; application of calcineurin inhibitors in the amygdala eliminates depotentiation in vitro (Kim et al., 2007) and blocks extinction in rats (Lin et al., 2003b). Thus, it seems that during extinction, certain molecular changes occur that actually reverse conditioning-induced synaptic alterations.
In addition to changes in phosphorylation patterns, both depotentiation and extinction have been associated with a decrease in the surface expression of AMPA receptor subunits. For example, Kim et al. (2007) demonstrated that during conditioning, there was an enhancement in GluA1 and GluA2 subunits within the LA; however, extinction completely reversed this increase in subunit levels. Blockade of AMPA receptor endocytosis also impaired depotentiation within the LA (Kim et al., 2007). Consistent with this, the disruption of AMPA receptor endocytosis impairs long-term depression as well as the acquisition and retention of extinction of conditioned fear (Dalton et al., 2008). In a more recent study, it was found that delivery of DCS both facilitated extinction as well as reduced the conditioning-induced increase in AMPA/NMDA receptor ratio in the LA (Lin et al., 2010). Furthermore, blockade of AMPA receptor endocytosis impaired the enhancing effects of DCS.
AMPA receptor endocytosis has also been found to occur during an extinction protocol that has been shown to completely erase the original fear memory (Clem and Huganir, 2010; Monfils et al., 2009, but see Chan et al., 2010). Monfils et al. (2009) reported that if a CS is presented shortly before extinction, the fear memory is completely destabilized and permanently erased. Indeed, they showed that renewal, reinstatement and spontaneous recovery did not occur when a retrieval trial preceded extinction. They reasoned that the CS-alone retrieval test brought about a wave of reconsolidation and that if extinction occurs within the reconsolidation window, the original memory is updated with the information that the CS no longer predicts an aversive event. Using this protocol, Clem and Huganir (2010) investigated the means by which this retrieval trial, or “reconsolidation update”, erased the original fear memory. By recording at thalamo-amygdala synapses, they found that the reconsolidation update reverses the increase in calcium-permeable AMPA (CP-AMPA) receptor rectification seen during fear conditioning and that this is dependent upon the activation of the metabotropic glutamate receptor 1 (Clem and Huganir, 2010). Importantly, there was actually a marked decrease in CP-AMPA receptor rectification, indicating that these receptors were being trafficked out of the membrane. They extended these findings by demonstrating that phosphorylation at the serine-845 site on the GluA1 subunits of CP-AMPA receptors is a prerequisite for the memory erasure evoked by the reconsolidation update.
Taken together, this evidence suggests that extinction of conditioned fear induces synaptic weakening that is dependent upon NMDA and metabotropic glutamate receptors, as well as VGCCs. This depotentiation causes an increase in calcineurin, which works to weaken the original fear memory by dephosphorylating protein kinases that were involved in original fear memory consolidation. In addition, calcineurin may interact with AMPA receptors to traffic them out of the surface membrane. Ultimately, this would result in the weakening or complete erasure of the original fear memory. However, this is in complete contrast with the behavioral phenomena that demonstrate that after extinction, fear can remerge (e.g. renewal, spontaneous recovery and reinstatement). Thus, how can these two theories be reconciled? The most parsimonious explanation to account for the various mechanisms underlying extinction is that extinction causes a redistribution of the fear memory both within the amygdala and the circuit itself. For example, it might be that the changes that are thought to weaken the original memory only occur in a certain portion of the amygdala. For example, Repa et al. (2001) have shown that there are two segregated populations of neurons that behave differently during fear conditioning and extinction. Furthermore, it has been reported that within the BA, there are neurons that selectively respond during fear conditioning and renewal (“fear” neurons) or during extinction (“extinction” neurons) (Herry et al., 2008). Thus, it is conceivable that within the amygdala, there are neurons that undergo depotentiation during extinction, but there are also cells that mediate the “CS-noUS” associative learning.
Additionally, it may be that interactions between the prefrontal cortex and the amygdala mediate the inhibitory learning during extinction. Evidence suggests that the prefrontal cortex, especially the IL, is required for the consolidation of extinction (Herry et al., 2010; Quirk and Mueller, 2008). As the IL sends glutamatergic projections to both the ITC clusters and local inhibitory interneurons in the BLA, synaptic plasticity at these synapses could be responsible for new learning during extinction, even though depotentiation may occur elsewhere within the LA.
Lastly, it may be that changes at the level of the circuit (amygdala, hippocampus, PFC) undergo synaptic plasticity that mediate inhibitory learning, even though at the cellular level, alterations such as dephosphorylation are occurring. For example, it has been suggested that after extinction, sensory information about the CS may be primarily relayed to the amygdala via the auditory cortex (Pape and Pare, 2010) as depotentiation has occurred at thalamo-LA synapses. Consistent with this idea, previous work has shown that after fear conditioning, a population of neurons, located in a region of the LA without thalamic input, continue to fire throughout extinction (Repa et al., 2001). Thus, it is conceivable that the auditory cortex could still provide CS information during extinction. Interestingly, Quirk et al. (1995) have shown that there are cells within the auditory cortex that are resistant to extinction. Thus, it has been suggested that transmission of CS information shifts from being relayed by the thalamus to being transferred by the auditory cortex (Pape and Pare, 2010). Even though this model has not been explicitly tested, it speaks to the notion that after extinction, CS information is redistributed throughout the fear circuit. This reorganization of the fear memory after extinction would allow for flexible changes in behavior depending on contextual cues, as the memory is stored in multiple brain areas.
4. Contextual modulation of the expression of extinction
As described above, retrieval of an extinction memory is under the control of contextual cues. For example, the expression of extinction will only occur if the extinguished CS is presented in the context in which extinction occurred. However, if the CS is presented in a different context, fear to the CS will renew. Similarly, fear to a CS will become reinstated only if it is presented in the context in which an unsignaled US occurred after extinction. In the last decade, considerable research has focused on the neurobiology underlying the contextual modulation of behavior after extinction (Maren and Quirk, 2004; Maren, 2005, 2011). It is widely accepted that, similar to the acquisition of extinction, the context-specific expression of extinction is mediated by a distributed network, including the hippocampus, prefrontal cortex and amygdala. In this section, we will review the extant literature pertaining to the contextual modulation of fear after extinction and offer a neural model that describes how this modulation may occur.
4.1. Neurobiology
Because the hippocampus has been shown to be important for contextual processing and discrimination, it has been hypothesized that this region may be responsible for contextually modulating fear behavior after extinction. Early studies show that pre-conditioning lesions of the fornix (Wilson et al., 1995) or the hippocampus (Frohardt et al., 2000) only abolish reinstatement (Frohardt et al., 2000) and spontaneous recovery (Wilson et al., 1995), but do not have any disrupting effects on renewal (Frohardt et al., 2000; Wilson et al., 1995). However, the results from these early studies do not preclude the hippocampus from being involved in the renewal of fear: because lesions were performed prior to any behavioral sessions, it is possible that other brain structures compensated for the loss of the hippocampus. To avoid this methodological problem, we have used inactivation techniques in our laboratory to temporarily inhibit the hippocampus only during the test session. With this procedure, we have demonstrated that reversible inactivation of the dorsal (Corcoran et al., 2005; Corcoran and Maren, 2001, 2004) or ventral hippocampus (Hobin et al., 2006) with muscimol, a GABA-A agonist, prior to the retention test eliminated renewal, as evidenced by low levels of fear when the CS presentation occurred outside the extinction context. More recently, Knapska and Maren (2009) have shown that c-fos expression is elevated in the ventral CA1 and dentate gyrus of the hippocampus during both renewal and extinction memory retrieval. Together with the inactivation data, this suggests that the hippocampus is involved in disambiguating the meaning of CS using contextual cues.
In a further attempt to explore the role of the hippocampus in the renewal of fear, Zelikowsky et al. (2011) varied the time in which rats received DH lesions and assessed the effects of the lesions on renewal of fear. Similar to previous work (Frohardt et al., 2000), rats that received pre-training lesions of the DH renewed their fear to the extinguished CS. In contrast, rats that received post-extinction lesions failed to renew their fear. This suggests that if the hippocampus participates in the acquisition and extinction of fear, it is necessary for the renewal of fear. However, if rats are trained and extinguished without a hippocampus, they are still able to display renewal, which is presumably mediated by another compensatory brain structure. This pattern of results is similar to that seen with the effects of pre-training versus post-training lesions on the expression of non-extinguished fear. Interestingly, Zelikowsky et al. (2011) also showed that rats could only renew their fear in the absence of a hippocampus if the tone duration during extinction training and test matched. When lesioned rats were extinguished with a discrete tone, but were tested with a continuous tone, renewal was severely impaired. Based on these findings, Zelikowsky and colleagues (2011) claim that without a hippocampus, rats become more sensitive to temporal changes in the CS. In intact rats, however, the hippocampus allows the animal to generalize across temporal differences in CSs to guide conditional responding.
The prefrontal cortex has also been implicated in regulating fear behavior after extinction. For example, the PL is thought to be involved in renewal of fear. Support for this comes from studies showing the PL is involved in the expression of conditioned fear (Blum et al., 2006; Corcoran and Quirk, 2007). Furthermore, stimulation of the PL results in freezing behavior (Vidal-Gonzalez et al., 2006) and elicits firing within the BA (Likhtik et al., 2005). Burgos-Robles et al. (2009) have recently shown that PL firing patterns parallel freezing behavior during fear conditioning and extinction. That is, there is sustained firing within the PL during fear conditioning, which gradually decreases during extinction (Burgos-Robles et al., 2009). Finally, renewal of fear is associated with elevated levels of c-fos expression within the PL (Knapska and Maren, 2009). In contrast, the IL is thought to mediate the retrieval of extinction. In early studies, it was shown that lesions of the medial prefrontal cortex that specifically included the IL prevented retention of the extinction memory (Lebron et al., 2004; Quirk et al., 2000, but see Garcia et al., 2006). Furthermore, inactivation of the IL prior to an extinction retrieval test results in high levels of freezing, indicating the IL is necessary for the retrieval of the extinction memory (Sierra-Mercado et al., 2006). Using electrophysiological methods, it has been shown that the IL selectively responds to extinguished CSs, which is associated with low levels of fear (Herry and Garcia, 2002; Milad and Quirk, 2002, but see Chang et al., 2010). Interestingly, the recall of extinction is accompanied by an increase in neuronal bursting in the IL, which is thought to increase the chance of the IL activating inhibitory cells within the amygdala to gate fear expression (Chang et al., 2010; Santini et al., 2008). Additionally, if the IL is stimulated in conjunction with a non-extinguished CS, rats display low levels of freezing, analogous to a post-extinction state (Milad and Quirk, 2002; Milad et al., 2004). Finally, Knapska and Maren (2009) observed a significant increase in c-fos expression within the IL during the retrieval of extinction as compared to the renewal of fear. Taken together, it seems that the prelimbic and infralimbic areas working in an opposing manner: activity in the prelimbic results in fear perseveration whereas activity in the IL promotes successful suppression of fear.
Given that the amygdala is important for the acquisition of extinction (Falls et al., 1992), it is no surprise that it has been shown to be important for the context-dependent expression of extinction. In an elegantly designed experiment, Hobin and Maren examined the role of the LA in the context-dependent expression of extinguished fear (2003). Rats were trained with two different CSs (CS1 and CS2), which were then extinguished in different contexts the following day. After implanting a recording electrode in the LA, rats underwent retention tests in which each CS was presented within its extinction context (consistent, CONS) as well as in the context in which the other CS was extinguished (inconsistent, INCONS). During the CONS test, rats exhibited low levels of fear; however, rats displayed significantly more fear during the INCONS test. Remarkably, spike firing within the LA was highest when the CS was presented outside the extinction context. This indicates that after extinction, the LA represents CSs that are ambiguous with respect to their associative meaning. Interestingly, it was further shown that the inactivation of the dorsal hippocampus eliminates this context-dependent firing pattern in the LA (Maren and Hobin, 2007). This finding suggests that contextual information processed by the hippocampus converges on both inhibitory and excitatory associations within the amygdala; this integration results in either the expression of extinction or renewal, respectively.
In a more recent study, Herry et al. (2008) discovered that within the BA, there are two separate populations of neurons that are selectively active during extinction (“extinction” neurons) and renewal (“fear” neurons). To first identify these populations, these authors used a discriminative fear conditioning protocol in which CS+ was paired with a footshock whereas CS− was presented alone. “Fear” neurons in the BA showed an increase in firing during and after conditioning; extinction of the CS+ eliminated firing within these BA neurons. Importantly, “fear” neurons did not respond to the CS−. During extinction of the CS+, however, there was an increase in firing in the “extinction” neurons that was absent during and after fear conditioning. To further demonstrate that “fear” and “extinction” neurons were selectively recruited during fear behavior and extinction, respectively, mice underwent a discriminative extinction paradigm in which two different CSs (CS1 and CS2) were fear-conditioned, but only CS1 was extinguished. “Fear” neurons only responded to the non-extinguished CS2 whereas the “extinction” neurons specifically fired in response to the extinguished CS2. Remarkably, during extinction, “extinction” neurons began to increase their firing pattern before the activity of the “fear” neurons disappeared, clearly demonstrating that within the BA, neuronal activity can be switched between active and inactive states very quickly. Extending these findings, these authors went on to show that activity in “fear” neurons reemerges after extinction if the CS is presented outside the extinction context. Similarly, when mice are tested for extinction memory retrieval, “extinction” neurons are selectively engaged. Interestingly, it was shown that these segregated populations of neurons have distinctly different connections with the hippocampus and prefrontal cortex. Using orthodromic and antidromic stimulation of BA afferents and efferents, respectively, Herry et al. (2008) showed that “fear” neurons receive input from the ventral hippocampus and project to the prefrontal cortex. “Extinction” neurons, on the other hand were reciprocally connected with the prefrontal cortex only. The notion that neurons within the BA are active during both the expression of extinction and renewal aligns well with findings from Knapska and Maren (2009) in which they observe high levels of c-fos expression in the BA when rats are tested within or outside the extinction context. Thus, this evidence suggests that the BA is required for the context-dependent expression of extinction, possibly to allow for discrimination between CSs with different meanings. Interestingly, there is also indirect evidence that indicates that there may also be similar populations of neurons within the LA. For example, Repa et al. (2001) describe LA neurons that are extinction resistant whereas Hobin et al. (2003) report that approximately 25% of LA neurons selectively fire within the extinction context, suggesting the presence of “fear” and “extinction neurons within the LA, respectively. Whether these populations of neurons truly exist within the LA, however, is not clear and thus, requires further study.
To explore how the VH and PFC interact with the BA during renewal, our lab recently used cellular imaging of c-fos in anatomically defined neurons that project to the BA (Orsini et al., 2011). Rats received unilateral infusions of cholera toxin subunit b (CTb), a monosynaptic retrograde tracer, into the BA and underwent fear conditioning and extinction. Twenty-four hours later, rats were tested either within the extinction context (SAME) or outside the extinction context (DIFF). Rats in the DIFF condition renewed their fear to the tone whereas those in the SAME condition exhibited a suppression of fear to the extinguished CS. Interestingly, there was a striking pattern with respect to the proportion of BA-projecting neurons engaged during renewal vs. extinction recall. Specifically, BA-projecting neurons within the PL and VH were selectively engaged during the renewal of fear; in contrast, BA-projecting neurons within the IL were recruited during the recall of extinction. This suggests that during renewal, both the VH and PL actively communicate with the BA. However, this data does not indicate whether the direct (VH to BA) and indirect (VH through PL to BA) pathways to the BA are both required for renewal, or whether one is able to compensate in the absence of the other. As such, we severed communication between the VH and PL or the VH and BA using asymmetric electrolytic lesions immediately after extinction. This manipulation completely disrupted renewal of fear, clearly indicating that both the direct and indirect routes from the hippocampus to the amygdala are required for the regulation of fear behavior after extinction. Together, these data suggest that convergent input from the VH and PL in the BA is necessary for renewal of fear, possibly to overcome extinction-induced inhibition.
4.2. Theoretical circuit model of contextual modulation of fear after extinction
Though there seems to be concrete evidence as to the role of these brain regions in the context-dependent expression of fear, it is less clear how this contextual modulation occurs. It is currently appreciated that the hippocampus is positioned in such a way to influence amygdala activity, as it has connections with both the amygdala and the PFC (Figure 3). By this view, the hippocampus can modulate amygdala activity through its direct connections to the BA and through its indirect pathway via the prefrontal cortex (Orsini et al., 2011). Input from both pathways then converge on neurons within the BA that, based on previous work (Herry et al., 2008), can decipher the associative meaning of the CS (CS-“no US” or CS-US) and, via the CeA, generate the appropriate behavior output. In addition to cells in the BA, the ITC cells receive input from the IL and can generate feed-forward inhibition so as to suppress fear responses during the recall of extinction. However, this theoretical model has not been explicitly tested and thus warrants further investigation.
Figure 3. Contextual modulation of fear after extinction.
After extinction, rats suppress their fear to the conditioned stimulus (CS) within the extinction context, but exhibit high levels of fear to the CS in other contexts. The renewal of fear and the recall of extinction are regulated by hippocampal-prefrontal control of the amygdala. As illustrated in the left panel, during renewal, the hippocampus (HPC) transmits contextual information to the basal amygdala (BA) via a direct projection, as well as through indirect projections via the prelimbic cortex (PL) of the prefrontal cortex (Orsini et al., 2011). These projections may synapse upon “fear” neurons within the BA, which selectively fire to a CS during fear conditioning and renewal (Herry et al., 2008). ”Fear” neurons in the BA may send excitatory projections directly to the centromedial amygdala (CeM) to drive the expression of fear (Tye et al., 2011). CeM excitation might also be mediated by dampening GABergic inhibition from intercalated cells (ITC) and CeL inputs to CEm (Royer et al., 1999; Tye et al., 2011). During extinction (right panel), the infralimbic cortex (IL) of the prefrontal cortex inhibits CEm output by driving inhibitory ITC neurons (Quirk et al., 2003; Berretta et al., 2005). IL inputs might also synapse on “extinction” neurons within the BA, which have been shown to fire selectively to an extinguished CS (Herry et al., 2008). “Extinction” neurons might then influence activity within the CeA through several routes, possibly by driving inhibitory ITC or CeL neurons that limit CeM activity. BA-CeL pathway is anxiolytic and this decrease in anxiety is mediated by the inhibition of the CeM (Tye et al., 2011). Although the hippocampus is not necessary for the expression of extinction, its projections to both IL and BA may be involved in extinction under some conditions.
5. Conclusion
Over the course of the last several decades, neuroscientists have made enormous progress in defining and understanding the neurobiology of fear memory formation. The amygdala and hippocampus have been shown to be especially important for encoding aversive events. Within these structures, various cellular changes occur that allow for the stabilization of these memories. For example, auditory fear memory formation within the amygdala requires NMDA-dependent long-term potentiation, which results in the activation of signaling cascades and subsequent transcription and translation of new proteins. Structural and synaptic changes, such as dendrite outgrowth and AMPA receptor insertion, also accompany fear memory formation. This considerable accumulation of knowledge about aversive memory formation allows researchers to speculate with more confidence about the neural bases for post-traumatic disorder (PTSD) and other anxiety disorders.
Similarly, the surge of research into the neural mechanisms of extinction has enabled scientists to postulate that this process involves inhibitory learning mediated by a distributed neural circuit including the prefrontal cortex, amygdala and hippocampus. Importantly, extinction involves a re-organization of the original fear memory throughout this network that may involve both increases in synaptic strength as well as the depotentiation of certain synapses. The overall result is a suppression of conditioned fear that is context-dependent and not permanent. This is especially relevant to therapeutic interventions for patients with disorders such as PTSD as it suggests that exposure therapy, the clinical equivalent to extinction, is a quite fragile process that is very susceptible to disruption by contextual cues. Fortunately, certain pharmacological treatments have emerged that seem to facilitate the extinction of aversive memories. For example, d-cycloserine (DCS), shown to enhance extinction in rats, has been found to be helpful as an adjunct to exposure therapy in patients with acrophobia (Ressler et al., 2004). By continuing our quest for a fuller understanding of the cellular correlates of extinction, we may discover newer and better interventions to permanently rid individuals of their traumatic memories and reduce their suffering.
Highlights.
Fear memory formation requires synaptic changes within the amygdala and hippocampus.
Synaptic changes corresponding with memory formation are thought to be dependent on long-term potentiation and a variety of intracellular signaling cascades that result in both pre- and postsynaptic modifications.
Extinction memory formation is mediated by a distributed neural circuit, including the amygdala, prefrontal cortex and hippocampus.
The expression of extinction is context-specific, which has important implications for clinical interventions targeting pathogenic conditions, such as post-traumatic disorder.
Acknowledgements
This work was supported by grants from the National Institutes of Health to SM (R01MH065961) and CAO (F31MH091822).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abel T, Nguyen PV, Barad M, Deuel TA, Kandel ER, Bourtchouladze R. Genetic demonstration of a role for PKA in the late phase of LTP and in hippocampus-based long-term memory. Cell. 1997;88:615–626. doi: 10.1016/s0092-8674(00)81904-2. [DOI] [PubMed] [Google Scholar]
- Adams JP, Sweatt JD. Molecular psychology: roles for the ERK MAP kinase cascade in memory. Annu Rev Pharmacol Toxicol. 2002;42:135–163. doi: 10.1146/annurev.pharmtox.42.082701.145401. [DOI] [PubMed] [Google Scholar]
- Akirav I, Raizel H, Maroun M. Enhancement of conditioned fear extinction by infusion of the GABA(A) agonist muscimol into the rat prefrontal cortex and amygdala. Eur J Neurosci. 2006;23:758–764. doi: 10.1111/j.1460-9568.2006.04603.x. [DOI] [PubMed] [Google Scholar]
- Alberini CM. Mechanisms of memory stabilization: are consolidation and reconsolidation similar or distinct processes? Trends Neurosci. 2005;28:51–56. doi: 10.1016/j.tins.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Alonso M, Medina JH, Pozzo-Miller L. ERK1/2 activation is necessary for BDNF to increase dendritic spine density in hippocampal CA1 pyramidal neurons. Learn Mem. 2004;11:172–178. doi: 10.1101/lm.67804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amano T, Unal CT, Pare D. Synaptic correlates of fear extinction in the amygdala. Nat Neurosci. 2010;13:489–494. doi: 10.1038/nn.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amir A, Amano T, Pare D. Physiological identification and infralimbic responsiveness of rat intercalated amygdala neurons. J Neurophysiol. 2011;105:3054–3066. doi: 10.1152/jn.00136.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anagnostaras SG, Maren S, Fanselow MS. Temporally graded retrograde amnesia of contextual fear after hippocampal damage in rats: within-subjects examination. J Neurosci. 1999;19:1106–1114. doi: 10.1523/JNEUROSCI.19-03-01106.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apergis-Schoute AM, Debiec J, Doyere V, LeDoux JE, Schafe GE. Auditory fear conditioning and long-term potentiation in the lateral amygdala require ERK/MAP kinase signaling in the auditory thalamus: a role for presynaptic plasticity in the fear system. J Neurosci. 2005;25:5730–5739. doi: 10.1523/JNEUROSCI.0096-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkins CM, Selcher JC, Petraitis JJ, Trzaskos JM, Sweatt JD. The MAPK cascade is required for mammalian associative learning. Nat Neurosci. 1998;1:602–609. doi: 10.1038/2836. [DOI] [PubMed] [Google Scholar]
- Azad SC, Kurz J, Marsicano G, Lutz B, Zieglgansberger W, Rammes G. Activation of CB1 specifically located on GABAergic interneurons inhibits LTD in the lateral amygdala. Learn Mem. 2008;15:143–152. doi: 10.1101/lm.741908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker JD, Azorlosa JL. The NMDA antagonist MK-801 blocks the extinction of Pavlovian fear conditioning. Behav Neurosci. 1996;110:618–620. doi: 10.1037//0735-7044.110.3.618. [DOI] [PubMed] [Google Scholar]
- Bamji SX, Rico B, Kimes N, Reichardt LF. BDNF mobilizes synaptic vesicles and enhances synapse formation by disrupting cadherin-beta catenin interactions. J Cell Biol. 2006;174:289–299. doi: 10.1083/jcb.200601087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannerman DM, Grubb M, Deacon RM, Yee BK, Feldon J, Rawlins JN. Ventral hippocampal lesions affect anxiety but not spatial learning. Behav Brain Res. 2003;139:197–213. doi: 10.1016/s0166-4328(02)00268-1. [DOI] [PubMed] [Google Scholar]
- Barot SK, Chung A, Kim JJ, Bernstein IL. Functional imaging of stimulus convergence in amygdalar neurons during Pavlovian fear conditioning. PLoS ONE. 2009;4:e6156. doi: 10.1371/journal.pone.0006156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barria A, Derkach V, Soderling T. Identification of the Ca2+/calmodulin dependent protein kinase II regulatory phosphorylation site in the alpha-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate-type glutamate receptor. J Biol Chem. 1997;272:32727–32730. doi: 10.1074/jbc.272.52.32727. [DOI] [PubMed] [Google Scholar]
- Bast T, Zhang WN, Feldon J. The ventral hippocampus and fear conditioning in rats. Different anterograde amnesias of fear after tetrodotoxin inactivation and infusion of the GABA(A) agonist muscimol. Exp Brain Res. 2001;139:39–52. doi: 10.1007/s002210100746. [DOI] [PubMed] [Google Scholar]
- Bauer EP, Schafe GE, LeDoux JE. NMDA receptors and L-type voltage gated calcium channels contribute to long-term potentiation and different components of fear memory formation in the lateral amygdala. J Neurosci. 2002;22:5239–5249. doi: 10.1523/JNEUROSCI.22-12-05239.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer KU, De Koninck P, Leonard AS, Hell JW, Schulman H. Interaction with the NMDA receptor locks CaMKII in an active conformation. Nature. 2001;411:801–805. doi: 10.1038/35081080. [DOI] [PubMed] [Google Scholar]
- Berman DE, Dudai Y. Memory extinction, learning anew, and learning the new: dissociations in the molecular machinery of learning in cortex. Science. 2001;291:2417–2419. doi: 10.1126/science.1058165. [DOI] [PubMed] [Google Scholar]
- Bernard JF, Alden M, Besson JM. The organization of the efferent projections from the pontine parabrachial area to the amygdaloid complex: a Phaseolus vulgaris leucoagglutinin (PHA-L) study in the rat. J Comp Neurol. 1993;329:201–229. doi: 10.1002/cne.903290205. [DOI] [PubMed] [Google Scholar]
- Berretta S, Pantazopoulos H, Caldera M, Pantazopoulos P, Pare D. Infralimbic cortex activation increases c-Fos expression in intercalated neurons of the amygdala. Neuroscience. 2005;132:943–953. doi: 10.1016/j.neuroscience.2005.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevilaqua LR, Bonini JS, Rossato JI, Izquierdo LA, Cammarota M, Izquierdo I. The entorhinal cortex plays a role in extinction. Neurobiol Learn Mem. 2006;85:192–197. doi: 10.1016/j.nlm.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Biedenkapp JC, Rudy JW. Hippocampal and extrahippocampal systems compete for control of contextual fear: role of ventral subiculum and amygdala. Learn Mem. 2009;16:38–45. doi: 10.1101/lm.1099109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissiere S, Humeau Y, Luthi A. Dopamine gates LTP induction in lateral amygdala by suppressing feedforward inhibition. Nat Neurosci. 2003;6:587–592. doi: 10.1038/nn1058. [DOI] [PubMed] [Google Scholar]
- Bissiere S, Zelikowsky M, Ponnusamy R, Jacobs NS, Blair HT, Fanselow MS. Electrical synapses control hippocampal contributions to fear learning and memory. Science. 2011;331:87–91. doi: 10.1126/science.1193785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair HT, Schafe GE, Bauer EP, Rodrigues SM, LeDoux JE. Synaptic plasticity in the lateral amygdala: a cellular hypothesis of fear conditioning. Learn Mem. 2001;8:229–242. doi: 10.1101/lm.30901. [DOI] [PubMed] [Google Scholar]
- Blanhard DC, Blanchard RJ. Innate and conditioned reactions to threat in rats with amygdaloid lesions. J Comp Physiol Psychol. 1972;81:281–290. doi: 10.1037/h0033521. [DOI] [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Bliss TV, Gardner-Medwin AR. Long-lasting potentiation of synaptic transmission in the dentate area of the unanaestetized rabbit following stimulation of the perforant path. J Physiol. 1973;232:357–374. doi: 10.1113/jphysiol.1973.sp010274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss TV, Lomo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol. 1973;232:331–356. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum S, Hebert AE, Dash PK. A role for the prefrontal cortex in recall of recent and remote memories. Neuroreport. 2006;17:341–344. doi: 10.1097/01.wnr.0000201509.53750.bc. [DOI] [PubMed] [Google Scholar]
- Bolles RC. Species-specific defense reactions and avoidance learning. Psychological Review. 1970;77:32–48. [Google Scholar]
- Bordi F, LeDoux J. Sensory tuning beyond the sensory system: an initial analysis of auditory response properties of neurons in the lateral amygdaloid nucleus and overlying areas of the striatum. J Neurosci. 1992;12:2493–2503. doi: 10.1523/JNEUROSCI.12-07-02493.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borowski TB, Kokkinidis L. The effects of cocaine, amphetamine, and the dopamine D1 receptor agonist SKF 38393 on fear extinction as measured with potentiated startle: implications for psychomotor stimulant psychosis. Behav Neurosci. 1998;112:952–965. doi: 10.1037//0735-7044.112.4.952. [DOI] [PubMed] [Google Scholar]
- Bourtchuladze R, Frenguelli B, Blendy J, Cioffi D, Schutz G, Silva AJ. Deficient long-term memory in mice with a targeted mutation of the cAMP responsive element-binding protein. Cell. 1994;79:59–68. doi: 10.1016/0092-8674(94)90400-6. [DOI] [PubMed] [Google Scholar]
- Bouton ME. Context and ambiguity in the extinction of emotional learning: implications for exposure therapy. Behav Res Ther. 1988;26:137–149. doi: 10.1016/0005-7967(88)90113-1. [DOI] [PubMed] [Google Scholar]
- Bouton ME. Context, time and memory retrieval in the interference paradigms of Pavlovian learning. Psychol Bull. 1993;114:80–99. doi: 10.1037/0033-2909.114.1.80. [DOI] [PubMed] [Google Scholar]
- Bouton ME. Context and behavioral processes in extinction. Learn Mem. 2004;11:485–494. doi: 10.1101/lm.78804. [DOI] [PubMed] [Google Scholar]
- Bouton ME, Bolles RC. Contexts, event-memories and extinction. Context and Learning. 1985:133–166. [Google Scholar]
- Bouton ME, Swartzentruber D. Slow reacquisition following extinction: Context, encoding, and retrieval mechanisms. Journal of Experimental Psychology: Animal Behavior Processes. 1989;15:43–53. [Google Scholar]
- Bouton ME, Vurbic D, Woods AM. D-cycloserine facilitates context-specific fear extinction learning. Neurobiol Learn Mem. 2008;90:504–510. doi: 10.1016/j.nlm.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady JV, Schreiner L, Geller I, Kling A. Subcortical mechanisms in emotional behavior; the effect of rhinencephalic injury upon the acquisition and retention of a conditioned avoidance response in cats. J Comp Physiol Psychol. 1954;47:179–186. doi: 10.1037/h0055426. [DOI] [PubMed] [Google Scholar]
- Brambilla R, Gnesutta N, Minichiello L, White G, Roylance AJ, Herron CE, Ramsey M, Wolfer DP, Cestari V, Rossi-Arnaud C, Grant SG, Chapman PF, Lipp HP, Sturani E, Klein R. A role for the Ras signalling pathway in synaptic transmission and long-term memory. Nature. 1997;390:281–286. doi: 10.1038/36849. [DOI] [PubMed] [Google Scholar]
- Bredy TW, Wu H, Crego C, Zellhoefer J, Sun YE, Barad M. Histone modifications around individual BDNF gene promoters in prefrontal cortex are associated with extinction of conditioned fear. Learn Mem. 2007;14:268–276. doi: 10.1101/lm.500907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown S, Schäfer A. An investigation into the function of the occipital and temporal lobes of the monkey’s brain. Philos Trans R Soc London Ser B. 1888;179:303–327. [Google Scholar]
- Brunzell DH, Kim JJ. Fear conditioning to tone, but not to context, is attenuated by lesions of the insular cortex and posterior extension of the intralaminar complex in rats. Behav Neurosci. 2001;115:365–375. [PubMed] [Google Scholar]
- Burgos-Robles A, Vidal-Gonzalez I, Quirk GJ. Sustained conditioned responses in prelimbic prefrontal neurons are correlated with fear expression and extinction failure. J Neurosci. 2009;29:8474–8482. doi: 10.1523/JNEUROSCI.0378-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgos-Robles A, Vidal-Gonzalez I, Santini E, Quirk GJ. Consolidation of fear extinction requires NMDA receptor-dependent bursting in the ventromedial prefrontal cortex. Neuron. 2007;53:871–880. doi: 10.1016/j.neuron.2007.02.021. [DOI] [PubMed] [Google Scholar]
- Busquet P, Hetzenauer A, Sinnegger-Brauns MJ, Striessnig J, Singewald N. Role of L-type Ca2+ channel isoforms in the extinction of conditioned fear. Learn Mem. 2008;15:378–386. doi: 10.1101/lm.886208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cain CK, Blouin AM, Barad M. L-type voltage-gated calcium channels are required for extinction, but not for acquisition or expression, of conditional fear in mice. J Neurosci. 2002;22:9113–9121. doi: 10.1523/JNEUROSCI.22-20-09113.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cain CK, Godsil BP, Jami S, Barad M. The L-type calcium channel blocker nifedipine impairs extinction, but not reduced contingency effects, in mice. Learn Mem. 2005;12:277–284. doi: 10.1101/lm.88805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campeau S, Davis M. Involvement of the central nucleus and basolateral complex of the amygdala in fear conditioning measured with fear potentiated startle in rats trained concurrently with auditory and visual conditioned stimuli. J Neurosci. 1995;15:2301–2311. doi: 10.1523/JNEUROSCI.15-03-02301.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campeau S, Miserendino MJ, Davis M. Intra-amygdala infusion of the N methyl-D-aspartate receptor antagonist AP5 blocks acquisition but not expression of fear-potentiated startle to an auditory conditioned stimulus. Behav Neurosci. 1992;106:569–574. doi: 10.1037//0735-7044.106.3.569. [DOI] [PubMed] [Google Scholar]
- Cannich A, Wotjak CT, Kamprath K, Hermann H, Lutz B, Marsicano G. CB1 cannabinoid receptors modulate kinase and phosphatase activity during extinction of conditioned fear in mice. Learn Mem. 2004;11:625–632. doi: 10.1101/lm.77904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canteras NS, Swanson LW. Projections of the ventral subiculum to the amygdala, septum, and hypothalamus: a PHAL anterograde tract-tracing study in the rat. J Comp Neurol. 1992;324:180–194. doi: 10.1002/cne.903240204. [DOI] [PubMed] [Google Scholar]
- Cenquizca LA, Swanson LW. Spatial organization of direct hippocampal field CA1 axonal projections to the rest of the cerebral cortex. Brain Res Rev. 2007;56:1–26. doi: 10.1016/j.brainresrev.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan WY, Leung HT, Westbrook RF, McNally GP. Effects of recent exposure to a conditioned stimulus on extinction of Pavlovian fear conditioning. Learn Mem. 2010;17:512–521. doi: 10.1101/lm.1912510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CH, Berke JD, Maren S. Single-unit activity in the medial prefrontal cortex during immediate and delayed extinction of fear in rats. PLoS ONE. 2010;5:e11971. doi: 10.1371/journal.pone.0011971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CH, Maren S. Early extinction after fear conditioning yields a context independent and short-term suppression of conditional freezing in rats. Learn Mem. 2009;16:62–68. doi: 10.1101/lm.1085009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CH, Maren S. Medial prefrontal cortex activation facilitates re extinction of fear in rats. Learn Mem. 2011;18:221–225. doi: 10.1101/lm.2070111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhuri A. Neural activity mapping with inducible transcription factors. Neuroreport. 1997;8:iii–vii. [PubMed] [Google Scholar]
- Chhatwal JP, Davis M, Maguschak KA, Ressler KJ. Enhancing cannabinoid neurotransmission augments the extinction of conditioned fear. Neuropsychopharmacology. 2005a;30:516–524. doi: 10.1038/sj.npp.1300655. [DOI] [PubMed] [Google Scholar]
- Chhatwal JP, Gutman AR, Maguschak KA, Bowser ME, Yang Y, Davis M, Ressler KJ. Functional interactions between endocannabinoid and CCK neurotransmitter systems may be critical for extinction learning. Neuropsychopharmacology. 2009;34:509–521. doi: 10.1038/npp.2008.97. [DOI] [PubMed] [Google Scholar]
- Chhatwal JP, Myers KM, Ressler KJ, Davis M. Regulation of gephyrin and GABAA receptor binding within the amygdala after fear acquisition and extinction. J Neurosci. 2005b;25:502–506. doi: 10.1523/JNEUROSCI.3301-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chhatwal JP, Ressler KJ. Modulation of fear and anxiety by the endogenous cannabinoid system. CNS Spectr. 2007;12:211–220. doi: 10.1017/s1092852900020939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chhatwal JP, Stanek-Rattiner L, Davis M, Ressler KJ. Amygdala BDNF signaling is required for consolidation but not encoding of extinction. Nat Neurosci. 2006;9:870–872. doi: 10.1038/nn1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho YH, Friedman E, Silva AJ. Ibotenate lesions of the hippocampus impair spatial learning but not contextual fear conditioning in mice. Behav Brain Res. 1999;98:77–87. doi: 10.1016/s0166-4328(98)00054-0. [DOI] [PubMed] [Google Scholar]
- Ciocchi S, Herry C, Grenier F, Wolff SBE, Letzkus JJ, Vlachos I, Ehrlich I, Sprenfel R, Deisseroth K, Stadler MB, Muller C, Luthi A. Encoding of conditioned fear in central amygdala inhibitory circuits. Nature. 2010;468:277–284. doi: 10.1038/nature09559. [DOI] [PubMed] [Google Scholar]
- Clem RL, Huganir RL. Calcium-permeable AMPA receptor dynamics mediate fear memory erasure. Science. 2010;330:1108–1112. doi: 10.1126/science.1195298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clugnet MC, LeDoux JE. Synaptic plasticity in fear conditioning circuits: induction of LTP in the lateral nucleus of the amygdala by stimulation of the medial geniculate body. J Neurosci. 1990;10:2818–2824. doi: 10.1523/JNEUROSCI.10-08-02818.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clugnet MC, LeDoux JE, Morrison SF. Unit responses evoked in the amygdala and striatum by electrical stimulation of the medial geniculate body. J Neurosci. 1990;10:1055–1061. doi: 10.1523/JNEUROSCI.10-04-01055.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colbran RJ, Brown AM. Calcium/calmodulin-dependent protein kinase II and synaptic plasticity. Curr Opin Neurobiol. 2004;14:318–327. doi: 10.1016/j.conb.2004.05.008. [DOI] [PubMed] [Google Scholar]
- Collingridge GL, Kehl SJ, McLennan H. The antagonism of amino acid induced excitations of rat hippocampal CA1 neurones in vitro. J Physiol. 1983;334:19–31. doi: 10.1113/jphysiol.1983.sp014477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran KA, Desmond TJ, Frey KA, Maren S. Hippocampal inactivation disrupts the acquisition and contextual encoding of fear extinction. J Neurosci. 2005;25:8978–8987. doi: 10.1523/JNEUROSCI.2246-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran KA, Maren S. Hippocampal inactivation disrupts contextual retrieval of fear memory after extinction. J Neurosci. 2001;21:1720–1726. doi: 10.1523/JNEUROSCI.21-05-01720.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran KA, Maren S. Factors regulating the effects of hippocampal inactivation on renewal of conditional fear after extinction. Learn Mem. 2004;11:598–603. doi: 10.1101/lm.78704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran KA, Quirk GJ. Activity in prelimbic cortex is necessary for the expression of learned, but not innate, fears. J Neurosci. 2007;27:840–844. doi: 10.1523/JNEUROSCI.5327-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox J, Westbrook RF. The NMDA receptor antagonist MK-801 blocks acquisition and extinction of conditioned hypoalgesic responses in the rat. Q J Exp Psychol B. 1994;47:187–210. [PubMed] [Google Scholar]
- Cruikshank SJ, Edeline JM, Weinberger NM. Stimulation at a site of auditory-somatosensory convergence in the medial geniculate nucleus is an effective unconditioned stimulus for fear conditioning. Behav Neurosci. 1992;106:471–483. doi: 10.1037//0735-7044.106.3.471. [DOI] [PubMed] [Google Scholar]
- Dalton GL, Wang YT, Floresco SB, Phillips AG. Disruption of AMPA receptor endocytosis impairs the extinction, but not acquisition of learned fear. Neuropsychopharmacology. 2008;33:2416–2426. doi: 10.1038/sj.npp.1301642. [DOI] [PubMed] [Google Scholar]
- Davis M, Rainnie D, Cassell M. Neurotransmission in the rat amygdala related to fear and anxiety. Trends Neurosci. 1994;17:208–214. doi: 10.1016/0166-2236(94)90106-6. [DOI] [PubMed] [Google Scholar]
- Davis M, Whalen PJ. The amygdala: vigilance and emotion. Mol Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- Debiec J, Doyere V, Nader K, Ledoux JE. Directly reactivated, but not indirectly reactivated, memories undergo reconsolidation in the amygdala. Proc Natl Acad Sci U S A. 2006;103:3428–3433. doi: 10.1073/pnas.0507168103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debiec J, LeDoux JE, Nader K. Cellular and systems reconsolidation in the hippocampus. Neuron. 2002;36:527–538. doi: 10.1016/s0896-6273(02)01001-2. [DOI] [PubMed] [Google Scholar]
- Delamater AR. Experimental extinction in Pavlovian conditioning: behavioural and neuroscience perspectives. Q J Exp Psychol B. 2004;57:97–132. doi: 10.1080/02724990344000097. [DOI] [PubMed] [Google Scholar]
- Denniston JC, Miller RR. The role of temporal variables in inhibition produced through extinction. Learn Behav. 2003;31:35–48. doi: 10.3758/bf03195969. [DOI] [PubMed] [Google Scholar]
- Depaulis A, Helfer V, Deransart C, Marescaux C. Anxiogenic-like consequences in animal models of complex partial seizures. Neurosci Biobehav Rev. 1997;21:767–774. doi: 10.1016/s0149-7634(96)00060-7. [DOI] [PubMed] [Google Scholar]
- Derkach V, Barria A, Soderling TR. Ca2+/calmodulin-kinase II enhances channel conductance of alpha-amino-3-hydroxy-5-methyl-4 isoxazolepropionate type glutamate receptors. Proc Natl Acad Sci U S A. 1999;96:3269–3274. doi: 10.1073/pnas.96.6.3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong H, Swanson LW, Chen L, Fanselow MS, Toga AW. Genomic anatomic evidence for distinct functional domains in hippocampal field CA1. Proc Natl Acad Sci. 2009;106:11794–11799. doi: 10.1073/pnas.0812608106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doron NN, Ledoux JE. Organization of projections to the lateral amygdala from auditory and visual areas of the thalamus in the rat. J Comp Neurol. 1999;412:383–409. [PubMed] [Google Scholar]
- Doyere V, Debiec J, Monfils MH, Schafe GE, LeDoux JE. Synapse-specific reconsolidation of distinct fear memories in the lateral amygdala. Nat Neurosci. 2007;10:414–416. doi: 10.1038/nn1871. [DOI] [PubMed] [Google Scholar]
- Duvarci S, Nader K. Characterization of fear memory reconsolidation. J Neurosci. 2004;24:9269–9275. doi: 10.1523/JNEUROSCI.2971-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvarci S, Nader K, LeDoux JE. Activation of extracellular signal-regulated kinase-mitogen-activated protein kinase cascade in the amygdala is required for memory reconsolidation of auditory fear conditioning. Eur J Neurosci. 2005;21:283–289. doi: 10.1111/j.1460-9568.2004.03824.x. [DOI] [PubMed] [Google Scholar]
- Ehrlich I, Humeau Y, Grenier F, Ciocchi S, Herry C, Luthi A. Amygdala inhibitory circuits and the control of fear memory. Neuron. 2009;62:757–771. doi: 10.1016/j.neuron.2009.05.026. [DOI] [PubMed] [Google Scholar]
- El-Ghundi M, O’Dowd BF, George SR. Prolonged fear responses in mice lacking dopamine D1 receptor. Brain Res. 2001;892:86–93. doi: 10.1016/s0006-8993(00)03234-0. [DOI] [PubMed] [Google Scholar]
- English JD, Sweatt JD. Activation of p42 mitogen-activated protein kinase in hippocampal long term potentiation. J Biol Chem. 1996;271:24329–24332. doi: 10.1074/jbc.271.40.24329. [DOI] [PubMed] [Google Scholar]
- English JD, Sweatt JD. A requirement for the mitogen-activated protein kinase cascade in hippocampal long term potentiation. J Biol Chem. 1997;31:19103–19106. doi: 10.1074/jbc.272.31.19103. [DOI] [PubMed] [Google Scholar]
- Esclassan F, Coutureau E, Di Scala G, Marchand AR. Differential contribution of dorsal and ventral hippocampus to trace and delay fear conditioning. Hippocampus. 2009;19:33–44. doi: 10.1002/hipo.20473. [DOI] [PubMed] [Google Scholar]
- Falls WA, Miserendino MJ, Davis M. Extinction of fear-potentiated startle: blockade by infusion of an NMDA antagonist into the amygdala. J Neurosci. 1992;12:854–863. doi: 10.1523/JNEUROSCI.12-03-00854.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow M. Associative vs. topographical accounts of the immediate shock freezing deficit in rats: implications for the response selection rules governing species-specific defensive reactions. Learn Motiv. 1986;17:16–39. [Google Scholar]
- Fanselow M. Factors governing one-trial contextual conditioning. Animal Learn Behav. 1990;18:264–270. [Google Scholar]
- Fanselow MS. From contextual fear to a dynamic view of memory systems. Trends Cogn Sci. 2010;14:7–15. doi: 10.1016/j.tics.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow MS, Dong H. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron. 2010;65:7–19. doi: 10.1016/j.neuron.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow MS, Kim JJ. Acquisition of contextual Pavlovian fear conditioning is blocked by application of an NMDA receptor antagonist D,L-2-amino-5 phosphonovaleric acid to the basolateral amygdala. Behav Neurosci. 1994;108:210–212. doi: 10.1037//0735-7044.108.1.210. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, Poulos AM. The neuroscience of mammalian associative learning. Annu Rev Psychol. 2005;56:207–234. doi: 10.1146/annurev.psych.56.091103.070213. [DOI] [PubMed] [Google Scholar]
- Farinelli M, Deschaux O, Hugues S, Thevenet A, Garcia R. Hippocampal train stimulation modulates recall of fear extinction independently of prefrontal cortex synaptic plasticity and lesions. Learn Mem. 2006;13:329–334. doi: 10.1101/lm.204806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fendt M, Fanselow MS. The neuroanatomical and neurochemical basis of conditioned fear. Neurosci Biobehav Rev. 1999;23:743–760. doi: 10.1016/s0149-7634(99)00016-0. [DOI] [PubMed] [Google Scholar]
- Fernadez Espejo E. Prefrontocortical dopamine loss in rats delays long-term extinction of contextual conditioned fear, and reduces social interaction without affecting short-term social interaction memory. Neuropsychopharmacol. 2003;28:490–498. doi: 10.1038/sj.npp.1300066. [DOI] [PubMed] [Google Scholar]
- Fischer A, Radulovic M, Schrick C, Sananbenesi F, Godovac-Zimmermann J, Radulovic J. Hippocampal Mek/Erk signaling mediates extinction of contextual freezing behavior. Neurobiol Learn Mem. 2007;87:149–158. doi: 10.1016/j.nlm.2006.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonberg E. Control of emotional behavior through the hypothalamus and amygdaloid complex. Ciba Foundation symposium. 1972;8:131–150. doi: 10.1002/9780470719916.ch7. [DOI] [PubMed] [Google Scholar]
- Frankland PW, Bontempi B, Talton LE, Kaczmarek L, Silva AJ. The involvement of the anterior cingulate cortex in remote contextual fear memory. Science. 2004;304:881–883. doi: 10.1126/science.1094804. [DOI] [PubMed] [Google Scholar]
- Frohardt RJ, Guarraci FA, Bouton ME. The effects of neurotoxic hippocampal lesions on two effects of context after fear extinction. Behav Neurosci. 2000;114:227–240. doi: 10.1037//0735-7044.114.2.227. [DOI] [PubMed] [Google Scholar]
- Fukazawa Y, Saitoh Y, Ozawa F, Ohta Y, Mizuno K, Inokuchi K. Hippocampal LTP is accompanied by enhanced F-actin content within the dendritic spine that is essential for late LTP maintenance in vivo. Neuron. 2003;38:447–460. doi: 10.1016/s0896-6273(03)00206-x. [DOI] [PubMed] [Google Scholar]
- Gale GD, Anagnostaras SG, Godsil BP, Mitchell S, Nozawa T, Sage JR, Wiltgen B, Fanselow MS. Role of the basolateral amygdala in the storage of fear memories across the adult lifetime of rats. J Neurosci. 2004;24:3810–3815. doi: 10.1523/JNEUROSCI.4100-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia R, Chang CH, Maren S. Electrolytic lesions of the medial prefrontal cortex do not interfere with long-term memory of extinction of conditioned fear. Learn Mem. 2006;13:14–17. doi: 10.1101/lm.60406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gass P, Wolfer DP, Balschun D, Rudolph D, Frey U, Lipp HP, Schutz G. Deficits in memory tasks of mice with CREB mutations depend on gene dosage. Learn Mem. 1998;5:274–288. [PMC free article] [PubMed] [Google Scholar]
- Giese KP, Fedorov NB, Filipkowski RK, Silva AJ. Autophosphorylation at Thr286 of the alpha calcium-calmodulin kinase II in LTP and learning. Science. 1998;279:870–873. doi: 10.1126/science.279.5352.870. [DOI] [PubMed] [Google Scholar]
- Gisquet-Verrier P, Dutrieux G, Richer P, Doyere V. Effects of lesions to the hippocampus on contextual fear: evidence for a disruption of freezing and avoidance behavior but not context conditioning. Behav Neurosci. 1999;113:507–522. doi: 10.1037//0735-7044.113.3.507. [DOI] [PubMed] [Google Scholar]
- Goddard GV. FUNCTIONS OF THE AMYGDALA. Psychol Bull. 1964;62:89–109. doi: 10.1037/h0044853. [DOI] [PubMed] [Google Scholar]
- Gogolla N, Caroni P, Luthi A, Herry C. Perineuronal nets protect fear memories from erasure. Science. 2009;325:1258–1261. doi: 10.1126/science.1174146. [DOI] [PubMed] [Google Scholar]
- Golstein ML. Effects of hippocampal, amygdala, hypothalamic and parietal lesions on a classically conditioned fear response. Psychol Rep. 1965;16:211–219. doi: 10.2466/pr0.1965.16.1.211. [DOI] [PubMed] [Google Scholar]
- Goosens KA, Holt W, Maren S. A role for amygdaloid PKA and PKC in the acquisition of long-term conditional fear memories in rats. Behav Brain Res. 2000;114:145–152. doi: 10.1016/s0166-4328(00)00224-2. [DOI] [PubMed] [Google Scholar]
- Goosens KA, Maren S. Contextual and auditory fear conditioning are mediated by the lateral, basal, and central amygdaloid nuclei in rats. Learn Mem. 2001;8:148–155. doi: 10.1101/lm.37601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goosens KA, Maren S. Long-term potentiation as a substrate for memory: evidence from studies of amygdaloid plasticity and Pavlovian fear conditioning. Hippocampus. 2002;12:592–599. doi: 10.1002/hipo.10099. [DOI] [PubMed] [Google Scholar]
- Goosens KA, Maren S. Pretraining NMDA receptor blockade in the basolateral complex, but not the central nucleus, of the amygdala prevents savings of conditional fear. Behav Neurosci. 2003;117:738–750. doi: 10.1037/0735-7044.117.4.738. [DOI] [PubMed] [Google Scholar]
- Goosens KA, Maren S. NMDA receptors are essential for the acquisition, but not expression, of conditional fear and associative spike firing in the lateral amygdala. Eur J Neurosci. 2004;20:537–548. doi: 10.1111/j.1460-9568.2004.03513.x. [DOI] [PubMed] [Google Scholar]
- Goshen I, Brodsky M, Prakash R, Wallace J, Gradinaru V, Ramakrishnan C, Deisseroth K. Dynamics of retrieval strategies for remote memories. Cell. 2011;147:678–689. doi: 10.1016/j.cell.2011.09.033. [DOI] [PubMed] [Google Scholar]
- Grace AA, Rosenkranz JA. Regulation of conditioned responses of basolateral amygdala neurons. Physiol Behav. 2002;77:489–493. doi: 10.1016/s0031-9384(02)00909-5. [DOI] [PubMed] [Google Scholar]
- Gunther LM, Denniston JC, Miller RR. Conducting exposure treatment in multiple contexts can prevent relapse. Behav Res Ther. 1998;36:75–91. doi: 10.1016/s0005-7967(97)10019-5. [DOI] [PubMed] [Google Scholar]
- Guzowski JF, Lyford GL, Stevenson GD, Houston FP, McGaugh JL, Worley PF, Barnes CA. Inhibition of activity-dependent arc protein expression in the rat hippocampus impairs the maintenance of long-term potentiation and the consolidation of long-term memory. J Neurosci. 2000;20:3993–4001. doi: 10.1523/JNEUROSCI.20-11-03993.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzowski JF, Timlin JA, Roysam B, McNaughton BL, Worley PF, Barnes CA. Mapping behaviorally relevant neural circuits with immediate-early gene expression. Curr Opin Neurobiol. 2005;15:599–606. doi: 10.1016/j.conb.2005.08.018. [DOI] [PubMed] [Google Scholar]
- Guzowski JF, Worley PF. Cellular compartment analysis of temporal activity by fluorescence in situ hybridization (catFISH) Curr Protoc Neurosci. 2001;Chapter 1(Unit 1 8) doi: 10.1002/0471142301.ns0108s15. [DOI] [PubMed] [Google Scholar]
- Hall J, Thomas KL, Everitt BJ. Cellular imaging of zif268 expression in the hippocampus and amygdala during contextual and cued fear memory retrieval: selective activation of hippocampal CA1 neurons during the recall of contextual memories. J Neurosci. 2001;21:2186–2193. doi: 10.1523/JNEUROSCI.21-06-02186.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han JH, Kushner SA, Yiu AP, Cole CJ, Matynia A, Brown RA, Neve RL, Guzowski JF, Silva AJ, Josselyn SA. Neuronal competition and selection during memory formation. Science. 2007;316:457–460. doi: 10.1126/science.1139438. [DOI] [PubMed] [Google Scholar]
- Harris JA, Westbrook RF. Evidence that GABA transmission mediates context-specific extinction of learned fear. Psychopharm. 1998;140:105–115. doi: 10.1007/s002130050745. [DOI] [PubMed] [Google Scholar]
- Hartley CA, Phelps EA. Changing fear: the neurocircuitry of emotion regulation. Neuropsychopharmacology. 2010;35:136–146. doi: 10.1038/npp.2009.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haubensak W, Kunwar PS, Cai H, Ciocchi S, Wall NR, Ponnusamy R, Biag J, Dong H, Deisseroth K, Callawau EM, Fanselow MS, Luthi A, Anderson DJ. Genetic dissection of an amygdala microcircuit that gates conditioned fear. Nature. 2010;468:270–278. doi: 10.1038/nature09553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi Y, Shi SH, Esteban JA, Piccini A, Poncer JC, Malinow R. Driving AMPA receptors into synapses by LTP and CaMKII: requirement for GluR1 and PDZ domain interaction. Science. 2000;287:2262–2267. doi: 10.1126/science.287.5461.2262. [DOI] [PubMed] [Google Scholar]
- Hefner K, Whittle N, Juhasz J, Norcross M, Karlsson RM, Saksida LM, Bussey TJ, Singewald N, Holmes A. Impaired fear extinction learning and cortico-amygdala circuit abnormalities in a common genetic mouse strain. J Neurosci. 2008;28:8074–8085. doi: 10.1523/JNEUROSCI.4904-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldt SA, Ressler KJ. Training-induced changes in the expression of GABAA-associated genes in the amygdala after the acquisition and extinction of Pavlovian fear. Eur J Neurosci. 2007;26:3631–3644. doi: 10.1111/j.1460-9568.2007.05970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldt SA, Stanek L, Chhatwal JP, Ressler KJ. Hippocampus-specific deletion of BDNF in adult mice impairs spatial memory and extinction of aversive memories. Mol Psychiatry. 2007;12:656–670. doi: 10.1038/sj.mp.4001957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, Little MD, Johnson MR, Melvin LS, de Costa BR, Rice KC. Cannabinoid receptor localization in brain. Proc Natl Acad Sci U S A. 1990;87:1932–1936. doi: 10.1073/pnas.87.5.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez AI, Blace N, Crary JF, Serrano PA, Leitges M, Libien JM, Weinstein G, Tcherapanov A, Sacktor TC. Protein kinase M zeta synthesis from a brain mRNA encoding an independent protein kinase C zeta catalytic domain. Implications for the molecular mechanism of memory. J Biol Chem. 2003;278:40305–40316. doi: 10.1074/jbc.M307065200. [DOI] [PubMed] [Google Scholar]
- Hernandez PJ, Abel T. The role of protein synthesis in memory consolidation: progress amid decades of debate. Neurobiol Learn Mem. 2008;89:293–311. doi: 10.1016/j.nlm.2007.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez PJ, Sadeghian K, Kelley AE. Early consolidation of instrumental learning requires protein synthesis in the nucleus accumbens. Nat Neurosci. 2002;5:1327–1331. doi: 10.1038/nn973. [DOI] [PubMed] [Google Scholar]
- Herry C, Ciocchi S, Senn V, Demmou L, Muller C, Luthi A. Switching on and off fear by distinct neuronal circuits. Nature. 2008;454:600–606. doi: 10.1038/nature07166. [DOI] [PubMed] [Google Scholar]
- Herry C, Ferraguti F, Singewald N, Letzkus JJ, Ehrlich I, Luthi A. Neuronal circuits of fear extinction. Eur J Neurosci. 2010;31:599–612. doi: 10.1111/j.1460-9568.2010.07101.x. [DOI] [PubMed] [Google Scholar]
- Herry C, Garcia R. Prefrontal cortex long-term potentiation, but not longterm depression, is associated with the maintenance of extinction of learned fear in mice. J Neurosci. 2002;22:577–583. doi: 10.1523/JNEUROSCI.22-02-00577.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herry C, Mons N. Resistance to extinction is associated with impaired immediate early gene induction in medial prefrontal cortex and amygdala. Eur J Neurosci. 2004;20:781–790. doi: 10.1111/j.1460-9568.2004.03542.x. [DOI] [PubMed] [Google Scholar]
- Herry C, Trifilieff P, Micheau J, Luthi A, Mons N. Extinction of auditory fear conditioning requires MAPK/ERK activation in the basolateral amygdala. Eur J Neurosci. 2006;24:261–269. doi: 10.1111/j.1460-9568.2006.04893.x. [DOI] [PubMed] [Google Scholar]
- Hikind N, Maroun M. Microinfusion of the D1 antagonist, SCH23390 into the IL but not the BLA impairs consolidation of extinction of auditory fear conditioning. Neurobio Learn Mem. 2008;90:217–222. doi: 10.1016/j.nlm.2008.03.003. [DOI] [PubMed] [Google Scholar]
- Hobin JA, Goosens KA, Maren S. Context-dependent neuronal activity in the lateral amygdala represents fear memories after extinction. J Neurosci. 2003;23:8410–8416. doi: 10.1523/JNEUROSCI.23-23-08410.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobin JA, Ji J, Maren S. Ventral hippocampal muscimol disrupts context specific fear memory retrieval after extinction in rats. Hippocampus. 2006;16:174–182. doi: 10.1002/hipo.20144. [DOI] [PubMed] [Google Scholar]
- Holtzman-Assif O, Laurent V, Westbrook RF. Blockade of dopamine activity in the nucleus accumbens impairs learning extinction of conditioned fear. Learn Mem. 2010;17:71–75. doi: 10.1101/lm.1668310. [DOI] [PubMed] [Google Scholar]
- Hoover WB, Vertes RP. Anatomical analysis of afferent projections to the medial prefrontal cortex in the rat. Brain Struct Funct. 2007;212:149–179. doi: 10.1007/s00429-007-0150-4. [DOI] [PubMed] [Google Scholar]
- Huang F, Chotiner JK, Steward O. Actin polymerization and ERK phosphorylation are required for Arc/Arg3.1 mRNA targeting to activated synaptic sites on dendrites. J Neurosci. 2007;27:9054–9067. doi: 10.1523/JNEUROSCI.2410-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YY, Kandel ER. Postsynaptic induction and PKA-dependent expression of LTP in the lateral amygdala. Neuron. 1998;21:169–178. doi: 10.1016/s0896-6273(00)80524-3. [DOI] [PubMed] [Google Scholar]
- Hugues S, Chessel A, Lena I, Marsault R, Garcia R. Prefrontal infusion of PD098059 immediately after fear extinction training blocks extinction associated prefrontal synaptic plasticity and decreases prefrontal ERK2 phosphorylation. Synapse. 2006;60:280–287. doi: 10.1002/syn.20291. [DOI] [PubMed] [Google Scholar]
- Hugues S, Deschaux O, Garcia R. Postextinction infusion of a mitogen activated protein kinase inhibitor into the medial prefrontal cortex impairs memory of the extinction of conditioned fear. Learn Mem. 2004;11:540–543. doi: 10.1101/lm.77704. [DOI] [PubMed] [Google Scholar]
- Huh KH, Guzman YF, Tronson NC, Guedea AL, Gao C, Radulovic J. Hippocampal Erk mechanisms linking prediction error to fear extinction: roles of shock expectancy and contextual aversive valence. Learn Mem. 2009;16:273–278. doi: 10.1101/lm.1240109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humeau Y, Herry C, Kemp N, Shaban H, Fourcaudot E, Bissiere S, Luthi A. Dendritic spine heterogeneity determines afferent-specific Hebbian plasticity in the amygdala. Neuron. 2005;45:119–131. doi: 10.1016/j.neuron.2004.12.019. [DOI] [PubMed] [Google Scholar]
- Humeau Y, Shaban H, Bissiere S, Luthi A. Presynaptic induction of heterosynaptic associative plasticity in the mammalian brain. Nature. 2003;426:841–845. doi: 10.1038/nature02194. [DOI] [PubMed] [Google Scholar]
- Impey S, Mark M, Villacres EC, Poser S, Chavkin C, Storm DR. Induction of CRE-mediated gene expression by stimuli that generate long-lasting LTP in area CA1 of the hippocampus. Neuron. 1996;16:973–982. doi: 10.1016/s0896-6273(00)80120-8. [DOI] [PubMed] [Google Scholar]
- Impey S, Obrietan K, Storm DR. Making new connections: role of ERK/MAP kinase signaling in neuronal plasticity. Neuron. 1999;23:11–14. doi: 10.1016/s0896-6273(00)80747-3. [DOI] [PubMed] [Google Scholar]
- Impey S, Obrietan K, Wong ST, Poser S, Yano S, Wayman G, Deloulme JC, Chan G, Storm DR. Cross talk between ERK and PKA is required for Ca2+ stimulation of CREB-dependent transcription and ERK nuclear translocation. Neuron. 1998a;21:869–883. doi: 10.1016/s0896-6273(00)80602-9. [DOI] [PubMed] [Google Scholar]
- Impey S, Smith DM, Obrietan K, Donahue R, Wade C, Storm DR. Stimulation of cAMP response element (CRE)-mediated transcription during contextual learning. Nat Neurosci. 1998b;1:595–601. doi: 10.1038/2830. [DOI] [PubMed] [Google Scholar]
- Inda MC, Muravieva EV, Alberini CM. Memory retrieval and the passage of time: from reconsolidation and strengthening to extinction. J Neurosci. 2011;31:1635–1643. doi: 10.1523/JNEUROSCI.4736-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata J, LeDoux JE, Meeley MP, Arneric S, Reis DJ. Intrinsic neurons in the amygdaloid field projected to by the medial geniculate body mediate emotional responses conditioned to acoustic stimuli. Brain Res. 1986;383:195–214. doi: 10.1016/0006-8993(86)90020-x. [DOI] [PubMed] [Google Scholar]
- Jasnow AM, Shi C, Israel JE, Davis M, Huhman KL. Memory of social defeat is facilitated by cAMP response element-binding protein overexpression in the amygdala. Behav Neurosci. 2005;119:1125–1130. doi: 10.1037/0735-7044.119.4.1125. [DOI] [PubMed] [Google Scholar]
- Jay TM, Witter MP. Distribution of hippocampal CA1 and subicular efferents in the prefrontal cortex of the rat studied by means of anterograde transport of Phaseolus vulgaris-leucoagglutinin. J Comp Neurol. 1991;313:574–886. doi: 10.1002/cne.903130404. [DOI] [PubMed] [Google Scholar]
- Josselyn SA. Continuing the search for the engram: examining the mechanism of fear memories. J Psychiatry Neurosci. 2010;35:221–228. doi: 10.1503/jpn.100015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josselyn SA, Kida S, Silva AJ. Inducible repression of CREB function disrupts amygdala-dependent memory. Neurobiol Learn Mem. 2004;82:159–163. doi: 10.1016/j.nlm.2004.05.008. [DOI] [PubMed] [Google Scholar]
- Josselyn SA, Shi C, Carlezon WA, Jr, Neve RL, Nestler EJ, Davis M. Longterm memory is facilitated by cAMP response element-binding protein overexpression in the amygdala. J Neurosci. 2001;21:2404–2412. doi: 10.1523/JNEUROSCI.21-07-02404.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungling K, Seidenbecher T, Sosulina L, Lesting J, Sangha S, Clark SD, Okamura N, Duangdao DM, Xu YL, Reinscheid RK, Pape HC. Neuropeptide S-mediated control of fear expression and extinction: role of intercalated GABAergic neurons in the amygdala. Neuron. 2008;59:298–310. doi: 10.1016/j.neuron.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamprath K, Wotjak CT. Nonassociative learning processes determine expression and extinction of conditioned fear in mice. Learn Mem. 2004;11:770–786. doi: 10.1101/lm.86104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamprath K, Marsicano G, Tang J, Monory K, Bisogno T, Di Marzo V, Lutz B, Wotjak CT. Cannabinoid CB1 receptor mediates fear extinction via habituation-like processes. J Neurosci. 2006;26:6677–6686. doi: 10.1523/JNEUROSCI.0153-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel ER. The molecular biology of memory storage: a dialog between genes and synapses. Biosci Rep. 2001;21:565–611. doi: 10.1023/a:1014775008533. [DOI] [PubMed] [Google Scholar]
- Kang HJ, Schuman EM. Neurotrophin-induced modulation of synaptic transmission in the adult hippocampus. J Physiol Paris. 1995;89:11–22. doi: 10.1016/0928-4257(96)80547-x. [DOI] [PubMed] [Google Scholar]
- Katona I, Rancz EA, Acsady L, Ledent C, Mackie K, Hajos N, Freund TF. Distribution of CB1 cannabinoid receptors in the amygdala and their role in the control of GABAergic transmission. J Neurosci. 2001;21:9506–9518. doi: 10.1523/JNEUROSCI.21-23-09506.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellicutt MH, Schwartzbaum JS. Formation of a conditioned emotional response (CER) folowing lesions of the amygdaloid complex in rats. Psychological Reports. 1963;12:351–358. [Google Scholar]
- Kessels HW, Malinow R. Synaptic AMPA receptor plasticity and behavior. Neuron. 2009;61:340–350. doi: 10.1016/j.neuron.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kida S, Josselyn SA, Pena de Ortiz S, Kogan JH, Chevere I, Masushige S, Silva AJ. CREB required for the stability of new and reactivated fear memories. Nat Neurosci. 2002;5:348–355. doi: 10.1038/nn819. [DOI] [PubMed] [Google Scholar]
- Kim J, Lee S, Park K, Hong I, Song B, Son G, Park H, Kim WR, Park E, Choe HK, Kim H, Lee C, Sun W, Kim K, Shin KS, Choi S. Amygdala depotentiation and fear extinction. Proc Natl Acad Sci U S A. 2007;104:20955–20960. doi: 10.1073/pnas.0710548105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Hamlin AS, Richardson R. Fear extinction across development: the involvement of the medial prefrontal cortex as assessed by temporary inactivation and immunohistochemistry. J Neurosci. 2009;29:10802–10808. doi: 10.1523/JNEUROSCI.0596-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Richardson R. A developmental dissociation in reinstatement of an extinguished fear response in rats. Neurobiol Learn Mem. 2007a;88:48–57. doi: 10.1016/j.nlm.2007.03.004. [DOI] [PubMed] [Google Scholar]
- Kim JH, Richardson R. A developmental dissociation of context and GABA effects on extinguished fear in rats. Behav Neurosci. 2007b;121:131–139. doi: 10.1037/0735-7044.121.1.131. [DOI] [PubMed] [Google Scholar]
- Kim JH, Richardson R. The effect of temporary amygdala inactivation on extinction and reextinction of fear in the developing rat: unlearning as a potential mechanism for extinction early in development. J Neurosci. 2008;28:1282–1290. doi: 10.1523/JNEUROSCI.4736-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Richardson R. New findings on extinction of conditioned fear early in development: theoretical and clinical implications. Biol Psychiatry. 2010;67:297–303. doi: 10.1016/j.biopsych.2009.09.003. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Fanselow MS. Modality-specific retrograde amnesia of fear. Science. 1992;256:675–677. doi: 10.1126/science.1585183. [DOI] [PubMed] [Google Scholar]
- Kluver, Bucy PC. "Psychic blindness" and other symotoms following bilateral temporal lobectomy in rhesus monkeys. American Journal of Physiology. 1937;119:352–353. [Google Scholar]
- Knapska E, Maren S. Reciprocal patterns of c-Fos expression in the medial prefrontal cortex and amygdala after extinction and renewal of conditioned fear. Learn Mem. 2009;16:486–493. doi: 10.1101/lm.1463909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogan JH, Frankland PW, Blendy JA, Coblentz J, Marowitz Z, Schutz G, Silva AJ. Spaced training induces normal long-term memory in CREB mutant mice. Curr Biol. 1997;7:1–11. doi: 10.1016/s0960-9822(06)00022-4. [DOI] [PubMed] [Google Scholar]
- Konorski J. Integrative activity of the brain. Chicago, IL: University of Chicago Press; 1967. [Google Scholar]
- Koo JW, Han JS, Kim JJ. Selective neurotoxic lesions of basolateral and central nuclei of the amygdala produce differential effects on fear conditioning. J Neurosci. 2004;24:7654–7662. doi: 10.1523/JNEUROSCI.1644-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korte M, Carroll P, Wolf E, Brem G, Thoenen H, Bonhoeffer T. Hippocampal long-term potentiation is impaired in mice lacking brain derived neurotrophic factor. Proc Natl Acad Sci U S A. 1995;92:8856–8860. doi: 10.1073/pnas.92.19.8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korte M, Kang H, Bonhoeffer T, Schuman E. A role for BDNF in the late phase of hippocampal long-term potentiation. Neuropharmacology. 1998;37:553–559. doi: 10.1016/s0028-3908(98)00035-5. [DOI] [PubMed] [Google Scholar]
- Krettek JE, Price JL. A description of the amygdaloid complex in the rat and cat with observations on intra-amygdaloid axonal connections. J Comp Neurol. 1978;178:255–280. doi: 10.1002/cne.901780205. [DOI] [PubMed] [Google Scholar]
- Kwapis JL, Jarome TJ, Lonergan ME, Helmstetter FJ. Protein kinase Mzeta maintains fear memory in the amygdala but not in the hippocampus. Behav Neurosci. 2009;123:844–850. doi: 10.1037/a0016343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang EJ, Pare D. Synaptic and synaptically activated intrinsic conductances underlie inhibitory potentials in cat lateral amygdaloid projection neurons in vivo. J Neurophysiol. 1997;77:353–363. doi: 10.1152/jn.1997.77.1.353. [DOI] [PubMed] [Google Scholar]
- Lang EJ, Pare D. Synaptic responsiveness of interneurons of the cat lateral amygdaloid nucleus. Neuroscience. 1998;83:877–889. doi: 10.1016/s0306-4522(97)00420-x. [DOI] [PubMed] [Google Scholar]
- Langton JM, Kim JH, Nicholas J, Richardson R. The effect of the NMDA receptor antagonist MK-801 on the acquisition and extinction of learned fear in the developing rat. Learn Mem. 2007;14:665–668. doi: 10.1101/lm.692407. [DOI] [PubMed] [Google Scholar]
- Langton JM, Richardson R. D-cycloserine facilitates extinction the first time but not the second time: an examination of the role of NMDA across the course of repeated extinction sessions. Neuropsychopharmacology. 2008;33:3096–3102. doi: 10.1038/npp.2008.32. [DOI] [PubMed] [Google Scholar]
- Langton JM, Richardson R. The effect of D-cycloserine on immediate vs. delayed extinction of learned fear. Learn Mem. 2010;17:547–551. doi: 10.1101/lm.1927310. [DOI] [PubMed] [Google Scholar]
- Lanuza E, Moncho-Bogani J, Ledoux JE. Unconditioned stimulus pathways to the amygdala: effects of lesions of the posterior intralaminar thalamus on foot-shock-induced c-Fos expression in the subdivisions of the lateral amygdala. Neuroscience. 2008;155:959–968. doi: 10.1016/j.neuroscience.2008.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanuza E, Nader K, Ledoux JE. Unconditioned stimulus pathways to the amygdala: effects of posterior thalamic and cortical lesions on fear conditioning. Neuroscience. 2004;125:305–315. doi: 10.1016/j.neuroscience.2003.12.034. [DOI] [PubMed] [Google Scholar]
- Laurent V, Marchand AR, Westbrook RF. The basolateral amygdala is necessary for learning but not relearning extinction of context conditioned fear. Learn Mem. 2008;15:304–314. doi: 10.1101/lm.928208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebron K, Milad MR, Quirk GJ. Delayed recall of fear extinction in rats with lesions of ventral medial prefrontal cortex. Learn Mem. 2004;11:544–548. doi: 10.1101/lm.78604. [DOI] [PubMed] [Google Scholar]
- Ledgerwood L, Richardson R, Cranney J. Effects of D-cycloserine on extinction of conditioned freezing. Behav Neurosci. 2003;117:341–349. doi: 10.1037/0735-7044.117.2.341. [DOI] [PubMed] [Google Scholar]
- Ledgerwood L, Richardson R, Cranney J. D-cycloserine and the facilitation of extinction of conditioned fear: consequences for reinstatement. Behav Neurosci. 2004;118:505–513. doi: 10.1037/0735-7044.118.3.505. [DOI] [PubMed] [Google Scholar]
- Ledgerwood L, Richardson R, Cranney J. D-cycloserine facilitates extinction of learned fear: effects on reacquisition and generalized extinction. Biol Psychiatry. 2005;57:841–847. doi: 10.1016/j.biopsych.2005.01.023. [DOI] [PubMed] [Google Scholar]
- LeDoux JE, Cicchetti P, Xagoraris A, Romanski LM. The lateral amygdaloid nucleus: sensory interface of the amygdala in fear conditioning. J Neurosci. 1990;10:1062–1069. doi: 10.1523/JNEUROSCI.10-04-01062.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE, Iwata J, Pearl D, Reis DJ. Disruption of auditory but not visual learning by destruction of intrinsic neurons in the rat medial geniculate body. Brain Res. 1986;371:395–399. doi: 10.1016/0006-8993(86)90383-5. [DOI] [PubMed] [Google Scholar]
- LeDoux JE, Ruggiero DA, Reis DJ. Projections to the subcortical forebrain from anatomically defined regions of the medial geniculate body in the rat. J Comp Neurol. 1985;242:182–213. doi: 10.1002/cne.902420204. [DOI] [PubMed] [Google Scholar]
- LeDoux JE, Sakaguchi A, Reis DJ. Subcortical efferent projections of the medial geniculate nucleus mediate emotional responses conditioned to acoustic stimuli. J Neurosci. 1984;4:683–698. doi: 10.1523/JNEUROSCI.04-03-00683.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JL. Memory reconsolidation mediates the strengthening of memories by additional learning. Nat Neurosci. 2008;11:1264–1266. doi: 10.1038/nn.2205. [DOI] [PubMed] [Google Scholar]
- Lee JL, Everitt BJ, Thomas KL. Independent cellular processes for hippocampal memory consolidation and reconsolidation. Science. 2004;304:839–843. doi: 10.1126/science.1095760. [DOI] [PubMed] [Google Scholar]
- Lee JL, Milton AL, Everitt BJ. Reconsolidation and extinction of conditioned fear: inhibition and potentiation. J Neurosci. 2006;26:10051–10056. doi: 10.1523/JNEUROSCI.2466-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Walker D, Davis M. Lack of a temporal gradient of retrograde amnesia following NMDA-induced lesions of the basolateral amygdala assessed with the fear-potentiated startle paradigm. Behav Neurosci. 1996;110:836–839. doi: 10.1037//0735-7044.110.4.836. [DOI] [PubMed] [Google Scholar]
- Lehmann H, Lacanilao S, Sutherland RJ. Complete or partial hippocampal damage produces equivalent retrograde amnesia for remote contextual fear memories. Eur J Neurosci. 2007;25:1278–1286. doi: 10.1111/j.1460-9568.2007.05374.x. [DOI] [PubMed] [Google Scholar]
- Lessmann V, Gottmann K, Malcangio M. Neurotrophin secretion: current facts and future prospects. Prog Neurobiol. 2003;69:341–374. doi: 10.1016/s0301-0082(03)00019-4. [DOI] [PubMed] [Google Scholar]
- Li XF, Armony JL, LeDoux JE. GABAA and GABAB receptors differentially regulate synaptic transmission in the auditory thalamo-amygdala pathway: an in vivo microiontophoretic study and a model. Synapse. 1996;24:115–124. doi: 10.1002/(SICI)1098-2396(199610)24:2<115::AID-SYN3>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Li Y, Li H, Liu X, Bao G, Tao Y, Wu Z, Xia P, Wu C, Li B, Ma L. Regulation of amygdalar PKA by beta-arrestin-2/phosphodiesterase-4 complex is critical for fear conditioning. Proc Natl Acad Sci U S A. 2009;106:21918–21923. doi: 10.1073/pnas.0906941106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Likhtik E, Pelletier JG, Paz R, Pare D. Prefrontal control of the amygdala. J Neurosci. 2005;25:7429–7437. doi: 10.1523/JNEUROSCI.2314-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Likhtik E, Popa D, Apergis-Schoute J, Fidacaro GA, Pare D. Amygdala intercalated neurons are required for expression of fear extinction. Nature. 2008;454:642–645. doi: 10.1038/nature07167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CH, Lee CC, Gean PW. Involvement of a calcineurin cascade in amygdala depotentiation and quenching of fear memory. Mol Pharmacol. 2003a;63:44–52. doi: 10.1124/mol.63.1.44. [DOI] [PubMed] [Google Scholar]
- Lin CH, Yeh SH, Leu TH, Chang WC, Wang ST, Gean PW. Identification of calcineurin as a key signal in the extinction of fear memory. J Neurosci. 2003b;23:1574–1579. doi: 10.1523/JNEUROSCI.23-05-01574.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CH, Yeh SH, Lu HY, Gean PW. The similarities and diversities of signal pathways leading to consolidation of conditioning and consolidation of extinction of fear memory. J Neurosci. 2003c;23:8310–8317. doi: 10.1523/JNEUROSCI.23-23-08310.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin HC, Mao SC, Chen PS, Gean PW. Chronic cannabinoid administration in vivo compromises extinction of fear memory. Learn Mem. 2008;15:867–884. doi: 10.1101/lm.1081908. [DOI] [PubMed] [Google Scholar]
- Lin HC, Mao SC, Gean PW. Block of gamma-aminobutyric acid-A receptor insertion in the amygdala impairs extinction of conditioned fear. Biol Psychiatry. 2009a;66:665–673. doi: 10.1016/j.biopsych.2009.04.003. [DOI] [PubMed] [Google Scholar]
- Lin HC, Mao SC, Su CL, Gean PW. The role of prefrontal cortex CB1 receptors in the modulation of fear memory. Cereb Cortex. 2009b;19:165–175. doi: 10.1093/cercor/bhn075. [DOI] [PubMed] [Google Scholar]
- Lin HC, Mao SC, Su CL, Gean PW. Alterations of excitatory transmission in the lateral amygdala during expression and extinction of fear memory. Int J Neuropsychopharmacol. 2010;13:335–345. doi: 10.1017/S1461145709990678. [DOI] [PubMed] [Google Scholar]
- Ling DS, Benardo LS, Sacktor TC. Protein kinase Mzeta enhances excitatory synaptic transmission by increasing the number of active postsynaptic AMPA receptors. Hippocampus. 2006;16:443–452. doi: 10.1002/hipo.20171. [DOI] [PubMed] [Google Scholar]
- Ling DS, Benardo LS, Serrano PA, Blace N, Kelly MT, Crary JF, Sacktor TC. Protein kinase Mzeta is necessary and sufficient for LTP maintenance. Nat Neurosci. 2002;5:295–296. doi: 10.1038/nn829. [DOI] [PubMed] [Google Scholar]
- Linke R, Braune G, Schwegler H. Differential projection of the posterior paralaminar thalamic nuclei to the amygdaloid complex in the rat. Exp Brain Res. 2000;134:520–532. doi: 10.1007/s002210000475. [DOI] [PubMed] [Google Scholar]
- Lisman J, Schulman H, Cline H. The molecular basis of CaMKII function in synaptic and behavioural memory. Nat Rev Neurosci. 2002;3:175–190. doi: 10.1038/nrn753. [DOI] [PubMed] [Google Scholar]
- Liu JL, Li M, Dang XR, Wang ZH, Rao ZR, Wu SX, Li YQ, Wang W. A NMDA receptor antagonist, MK-801 impairs consolidating extinction of auditory conditioned fear responses in a Pavlovian model. PLoS ONE. 2009;4:e7548. doi: 10.1371/journal.pone.0007548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu KT, Walker DL, Davis M. Mitogen-activated protein kinase cascade in the basolateral nucleus of amygdala is involved in extinction of fear potentiated startle. J Neurosci. 2001;21:RC162. doi: 10.1523/JNEUROSCI.21-16-j0005.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luthi A, Van der Putten H, Botteri FM, Mansuy IM, Meins M, Frey U, Sansig G, Portet C, Schmutz M, Schroder M, Nitsch C, Laurent JP, Monard D. Endogenous serine protease inhibitor modulates epileptic activity and hippocampal long-term potentiation. J Neurosci. 1997;17:4688–4699. doi: 10.1523/JNEUROSCI.17-12-04688.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean P. Psychosomatic disease and the visceral brain; recent developments bearing on the Papez theory of emotion. Psychosom Med. 1949;11:338–353. doi: 10.1097/00006842-194911000-00003. [DOI] [PubMed] [Google Scholar]
- Mahanty NK, Sah P. Calcium-permeable AMPA receptors mediate long-term potentiation in interneurons in the amygdala. Nature. 1998;394:683–687. doi: 10.1038/29312. [DOI] [PubMed] [Google Scholar]
- Mahanty NK, Sah P. Excitatory synaptic inputs to pyramidal neurons of the lateral amygdala. Eur J Neurosci. 1999;11:1217–1222. doi: 10.1046/j.1460-9568.1999.00528.x. [DOI] [PubMed] [Google Scholar]
- Makino H, Malinow R. AMPA receptor incorporation into synapses during LTP: the role of lateral movement and exocytosis. Neuron. 2009;64:381–390. doi: 10.1016/j.neuron.2009.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malkani S, Wallace KJ, Donley MP, Rosen JB. An egr-1 (zif268) antisense oligodeoxynucleotide infused into the amygdala disrupts fear conditioning. Learn Mem. 2004;11:617–624. doi: 10.1101/lm.73104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamiya N, Fukushima H, Suzuki A, Matsuyama Z, Homma S, Frankland PW, Kida S. Brain region-specific gene expression activation required for reconsolidation and extinction of contextual fear memory. J Neurosci. 2009;29:402–413. doi: 10.1523/JNEUROSCI.4639-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S. Neurotoxic basolateral amygdala lesions impair learning and memory but not the performance of conditional fear in rats. J Neurosci. 1999a;19:8696–8703. doi: 10.1523/JNEUROSCI.19-19-08696.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S. Neurotoxic or electrolytic lesions of the ventral subiculum produce deficits in the acquisition and expression of Pavlovian fear conditioning in rats. Behav Neurosci. 1999b;113:283–290. doi: 10.1037//0735-7044.113.2.283. [DOI] [PubMed] [Google Scholar]
- Maren S. Neurobiology of Pavlovian fear conditioning. Annu Rev Neurosci. 2001;24:897–931. doi: 10.1146/annurev.neuro.24.1.897. [DOI] [PubMed] [Google Scholar]
- Maren S. The amygdala, synaptic plasticity, and fear memory. Ann N Y Acad Sci. 2003;985:106–113. doi: 10.1111/j.1749-6632.2003.tb07075.x. [DOI] [PubMed] [Google Scholar]
- Maren S. Synaptic mechanisms of associative memory in the amygdala. Neuron. 2005;47:783–786. doi: 10.1016/j.neuron.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Maren S. Pavlovian fear conditioning as a behavioral assay for hippocampus and amygdala function: cautions and caveats. Eur J Neurosci. 2008;28:1661–1666. doi: 10.1111/j.1460-9568.2008.06485.x. [DOI] [PubMed] [Google Scholar]
- Maren S. Seeking a spotless mind: extinction, depotentiation, and erasure of fear memory. Neuron. 2011;70:83–845. doi: 10.1016/j.neuron.2011.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, Aharonov G, Fanselow MS. Retrograde abolition of conditional fear after excitotoxic lesions in the basolateral amygdala of rats: absence of a temporal gradient. Behav Neurosci. 1996a;110:718–726. doi: 10.1037//0735-7044.110.4.718. [DOI] [PubMed] [Google Scholar]
- Maren S, Aharonov G, Fanselow MS. Neurotoxic lesions of the dorsal hippocampus and Pavlovian fear conditioning in rats. Behav Brain Res. 1997;88:261–274. doi: 10.1016/s0166-4328(97)00088-0. [DOI] [PubMed] [Google Scholar]
- Maren S, Aharonov G, Stote DL, Fanselow MS. N-methyl-D-aspartate receptors in the basolateral amygdala are required for both acquisition and expression of conditional fear in rats. Behav Neurosci. 1996b;110:1365–1374. doi: 10.1037//0735-7044.110.6.1365. [DOI] [PubMed] [Google Scholar]
- Maren S, Chang CH. Recent fear is resistant to extinction. Proc Natl Acad Sci U S A. 2006;103:18020–18025. doi: 10.1073/pnas.0608398103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, Fanselow MS. Synaptic plasticity in the basolateral amygdala induced by hippocampal formation stimulation in vivo. J Neurosci. 1995;15:7548–7564. doi: 10.1523/JNEUROSCI.15-11-07548.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, Fanselow MS. Electrolytic lesions of the fimbria/fornix, dorsal hippocampus, or entorhinal cortex produce anterograde deficits in contextual fear conditioning in rats. Neurobiol Learn Mem. 1997;67:142–149. doi: 10.1006/nlme.1996.3752. [DOI] [PubMed] [Google Scholar]
- Maren S, Ferrario CR, Corcoran KA, Desmond TJ, Frey KA. Protein synthesis in the amygdala, but not the auditory thalamus, is required for consolidation of Pavlovian fear conditioning in rats. Eur J Neurosci. 2003;18:3080–3088. doi: 10.1111/j.1460-9568.2003.03063.x. [DOI] [PubMed] [Google Scholar]
- Maren S, Hobin JA. Hippocampal regulation of context-dependent neuronal activity in the lateral amygdala. Learn Mem. 2007;14:318–324. doi: 10.1101/lm.477007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, Holt WG. Hippocampus and Pavlovian fear conditioning in rats: muscimol infusions into the ventral, but not dorsal, hippocampus impair the acquisition of conditional freezing to an auditory conditional stimulus. Behav Neurosci. 2004;118:97–110. doi: 10.1037/0735-7044.118.1.97. [DOI] [PubMed] [Google Scholar]
- Maren S, Poremba A, Gabriel M. Basolateral amygdaloid multi-unit neuronal correlates of discriminative avoidance learning in rabbits. Brain Res. 1991;549:311–316. doi: 10.1016/0006-8993(91)90473-9. [DOI] [PubMed] [Google Scholar]
- Maren S, Quick GJ. Neuronal signalling of fear memory. Nat Rev Neurosci. 2004;5:844–852. doi: 10.1038/nrn1535. [DOI] [PubMed] [Google Scholar]
- Maren S, Tocco G, Standley S, Baudry M, Thompson RF. Postsynaptic factors in the expression of long-term potentiation (LTP): increased glutamate receptor binding following LTP induction in vivo. Proc Natl Acad Sci U S A. 1993;90:9654–9658. doi: 10.1073/pnas.90.20.9654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsicano G, Lutz B. Expression of the cannabinoid receptor CB1 in distinct neuronal subpopulations in the adult mouse forebrain. Eur J Neurosci. 1999;11:4213–4225. doi: 10.1046/j.1460-9568.1999.00847.x. [DOI] [PubMed] [Google Scholar]
- Marsicano G, Wotjak CT, Azad SC, Bisogno T, Rammes G, Cascio MG, Hermann H, Tang J, Hofmann C, Zieglgansberger W, Di Marzo V, Lutz B. The endogenous cannabinoid system controls extinction of aversive memories. Nature. 2002;418:530–534. doi: 10.1038/nature00839. [DOI] [PubMed] [Google Scholar]
- Matsuzaki M, Honkura N, Ellis-Davies GC, Kasai H. Structural basis of long-term potentiation in single dendritic spines. Nature. 2004;429:761–766. doi: 10.1038/nature02617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matus-Amat P, Higgins EA, Barrientos RM, Rudy JW. The role of the dorsal hippocampus in the acquisition and retrieval of context memory representations. J Neurosci. 2004;24:2431–2439. doi: 10.1523/JNEUROSCI.1598-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayford M, Bach ME, Huang YY, Wang L, Hawkins RD, Kandel ER. Control of memory formation through regulated expression of a CAMKII transgene. Science. 1996;274:1678–1683. doi: 10.1126/science.274.5293.1678. [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Cytoarchitecture of the central amygdaloid nucleus of the rat. J Comp Neurol. 1982a;208:401–418. doi: 10.1002/cne.902080409. [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Neurons of the lateral and basolateral amygdaloid nuclei: a Golgi study in the rat. J Comp Neurol. 1982b;212:293–312. doi: 10.1002/cne.902120307. [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Immunohistochemical identification of gamma-aminobutyric acid-containing neurons in the rat basolateral amygdala. Neurosci Lett. 1985;53:203–207. doi: 10.1016/0304-3940(85)90186-7. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Mascagni F, Guo L. Projections of the medial and lateral prefrontal cortices to the amygdala: a Phaseolus vulgaris leucoagglutinin study in the rat. Neuroscience. 1996;71:55–75. doi: 10.1016/0306-4522(95)00417-3. [DOI] [PubMed] [Google Scholar]
- McKernan MG, Shinnick-Gallagher P. Fear conditioning induces a lasting potentiation of synaptic currents in vitro. Nature. 1997;390:607–611. doi: 10.1038/37605. [DOI] [PubMed] [Google Scholar]
- McKinney BC, Murphy GG. The L-Type voltage-gated calcium channel Cav1.3 mediates consolidation, but not extinction, of contextually conditioned fear in mice. Learn Mem. 2006;13:584–589. doi: 10.1101/lm.279006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinney BC, Sze W, White JA, Murphy GG. L-type voltage-gated calcium channels in conditioned fear: a genetic and pharmacological analysis. Learn Mem. 2008;15:326–334. doi: 10.1101/lm.893808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally GP. Facilitation of fear extinction by midbrain periaqueductal gray infusions of RB101(S), an inhibor of enkephalin-degrading enzymes. Behav Neurosci. 2005a;119:1672–1677. doi: 10.1037/0735-7044.119.6.1672. [DOI] [PubMed] [Google Scholar]
- McNally GP, Cole S. Opiod receptors in the midbrain periaqueductal gray regulate prediction errors during Pavlovian fear conditioning. Behav Neurosci. 2006;120:313–323. doi: 10.1037/0735-7044.120.2.313. [DOI] [PubMed] [Google Scholar]
- McNally GP, Lee BW, Chiem JY, Choi EA. The midbrain periaqueductal gray and fear extinction: opiod receptor subtype and roles of cyclic, AMP, protein kinase A, and mitogen-activated protein kinase. Behav Neurosci. 2005b;119:1023–1033. doi: 10.1037/0735-7044.119.4.1023. [DOI] [PubMed] [Google Scholar]
- McNally GP, Pigg M, Weidemann G. Opioid receptors in the midbrain periaqueductal gray regulate extinction of pavlovian fear conditioning. J Neurosci. 2004;24:6912–6919. doi: 10.1523/JNEUROSCI.1828-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally GP, Westbrook RF. Opioid receptors regulate the extinction of Pavlovian fear conditioning. Behav Neurosci. 2003;117:1292–1301. doi: 10.1037/0735-7044.117.6.1292. [DOI] [PubMed] [Google Scholar]
- McNaughton BL, Douglas RM, Goddard GV. Synaptic enhancement in fascia dentata: cooperativity among coactive afferents. Brain Res. 1978;157:277–293. doi: 10.1016/0006-8993(78)90030-6. [DOI] [PubMed] [Google Scholar]
- McSweeney FK, Swindell S. Common processes may contribute to extinction and habituation. J Gen Psychol. 2002;129:364–400. doi: 10.1080/00221300209602103. [DOI] [PubMed] [Google Scholar]
- Meins M, Herry C, Muller C, Ciocchi S, Moreno E, Luthi A, Monard D. Impaired fear extinction in mice lacking protease nexin-1. Eur J Neurosci. 2010;31:2033–2042. doi: 10.1111/j.1460-9568.2010.07221.x. [DOI] [PubMed] [Google Scholar]
- Merino SM, Maren S. Hitting Ras where it counts: Ras antagonism in the basolateral amygdala inhibits long-term fear memory. Eur J Neurosci. 2006;23:196–204. doi: 10.1111/j.1460-9568.2005.04546.x. [DOI] [PubMed] [Google Scholar]
- Messaoudi E, Kanhema T, Soule J, Tiron A, Dagyte G, da Silva B, Bramham CR. Sustained Arc/Arg3.1 synthesis controls long-term potentiation consolidation through regulation of local actin polymerization in the dentate gyrus in vivo. J Neurosci. 2007;27:10445–10455. doi: 10.1523/JNEUROSCI.2883-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messaoudi E, Ying SW, Kanhema T, Croll SD, Bramham CR. Brain derived neurotrophic factor triggers transcription-dependent, late phase long-term potentiation in vivo. J Neurosci. 2002;22:7453–7461. doi: 10.1523/JNEUROSCI.22-17-07453.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002;420:70–74. doi: 10.1038/nature01138. [DOI] [PubMed] [Google Scholar]
- Milad MR, Rauch SL, Pitman RK, Quirk GJ. Fear extinction in rats: implications for human brain imaging and anxiety disorders. Biol Psychol. 2006;73:61–71. doi: 10.1016/j.biopsycho.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Milad MR, Vidal-Gonzalez I, Quirk GJ. Electrical stimulation of medial prefrontal cortex reduces conditioned fear in a temporally specific manner. Behav Neurosci. 2004;118:389–394. doi: 10.1037/0735-7044.118.2.389. [DOI] [PubMed] [Google Scholar]
- Miller RR, Barnet RC, Grahame NJ. Assessment of the Rescorla-Wagner model. Psychol Bull. 1995;117:363–386. doi: 10.1037/0033-2909.117.3.363. [DOI] [PubMed] [Google Scholar]
- Millhouse OE. The intercalated cells of the amygdala. J Comp Neurol. 1986;247:246–271. doi: 10.1002/cne.902470209. [DOI] [PubMed] [Google Scholar]
- Mineka S, Ohman A. Phobias and preparedness: the selective, automatic, and encapsulated nature of fear. Biol Psychiatry. 2002;52:927–937. doi: 10.1016/s0006-3223(02)01669-4. [DOI] [PubMed] [Google Scholar]
- Miserendino MJ, Sananes CB, Melia KR, Davis M. Blocking of acquisition but not expression of conditioned fear-potentiated startle by NMDA antagonists in the amygdala. Nature. 1990;345:716–718. doi: 10.1038/345716a0. [DOI] [PubMed] [Google Scholar]
- Miyashita T, Kubik S, Lewandowski G, Guzowski JF. Networks of neurons, networks of genes: an integrated view of memory consolidation. Neurobiol Learn Mem. 2008;89:269–284. doi: 10.1016/j.nlm.2007.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monfils MH, Cowansage KK, Klann E, LeDoux JE. Extinction reconsolidation boundaries: key to persistent attenuation of fear memories. Science. 2009;324:951–955. doi: 10.1126/science.1167975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monfils MH, Cowansage KK, LeDoux JE. Brain-derived neurotrophic factor: linking fear learning to memory formation. Mol Pharmacol. 2007;72:235–237. doi: 10.1124/mol.107.038232. [DOI] [PubMed] [Google Scholar]
- Mueller D, Bravo-Rivera C, Quirk GJ. Infralimbic D2 receptors are necessary for fear extinction and extinction-related tone responses. Biol Psychiatry. 2010;68:1055–1060. doi: 10.1016/j.biopsych.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller D, Porter JT, Quirk GJ. Noradrenergic signaling in infralimbic cortex increases cell excitability and strengthens memory for fear extinction. J Neurosci. 2008;28:369–375. doi: 10.1523/JNEUROSCI.3248-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muigg P, Hetzenauer A, Hauer G, Hauschild M, Gaburro S, Frank E, Landgraf R, Singewald N. Impaired extinction of learned fear in rats selectively bred for high anxiety--evidence of altered neuronal processing in prefrontal amygdala pathways. Eur J Neurosci. 2008;28:2299–2309. doi: 10.1111/j.1460-9568.2008.06511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers KM, Davis M. Mechanisms of fear extinction. Mol Psychiatry. 2007;12:120–150. doi: 10.1038/sj.mp.4001939. [DOI] [PubMed] [Google Scholar]
- Myers KM, Ressler KJ, Davis M. Different mechanisms of fear extinction dependent on length of time since fear acquisition. Learn Mem. 2006;13:216–223. doi: 10.1101/lm.119806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nader K, LeDoux J. The dopaminergic modulation of fear: quinpirole impairs the recall of emotional memories in rats. Behav Neurosci. 1999;113:152–165. doi: 10.1037//0735-7044.113.1.152. [DOI] [PubMed] [Google Scholar]
- Nader K, Schafe GE, Le Doux JE. Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature. 2000;406:722–726. doi: 10.1038/35021052. [DOI] [PubMed] [Google Scholar]
- Nakazawa T, Komai S, Watabe AM, Kiyama Y, Fukaya M, Arima-Yoshida F, Horai R, Sudo K, Ebine K, Delawary M, Goto J, Umemori H, Tezuka T, Iwakura Y, Watanabe M, Yamamoto T, Manabe T. NR2B tyrosine phosphorylation modulates fear learning as well as amygdaloid synaptic plasticity. EMBO J. 2006;25:2867–2877. doi: 10.1038/sj.emboj.7601156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohman A, Mineka S. Fears, phobias, and preparedness: toward an evolved module of fear and fear learning. Psychol Rev. 2001;108:483–522. doi: 10.1037/0033-295x.108.3.483. [DOI] [PubMed] [Google Scholar]
- Ohno M, Frankland PW, Chen AP, Costa RM, Silva AJ. Inducible, pharmacogenetic approaches to the study of learning and memory. Nat Neurosci. 2001;4:1238–1243. doi: 10.1038/nn771. [DOI] [PubMed] [Google Scholar]
- Ohno M, Frankland PW, Silva AJ. A pharmacogenetic inducible approach to the study of NMDA/alphaCaMKII signaling in synaptic plasticity. Curr Biol. 2002;12:654–656. doi: 10.1016/s0960-9822(02)00767-4. [DOI] [PubMed] [Google Scholar]
- Orsini CA, Kim JH, Knapska E, Maren S. Hippocampal and prefrontal projections to the basal amygdala mediate contextual regulation of fear after extinction. J Neurosci. 2011;23:17269–17277. doi: 10.1523/JNEUROSCI.4095-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osten P, Valsamis L, Harris A, Sacktor TC. Protein synthesis-dependent formation of protein kinase Mzeta in long-term potentiation. J Neurosci. 1996;16:2444–2451. doi: 10.1523/JNEUROSCI.16-08-02444.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou LC, Gean PW. Regulation of amygdala-dependent learning by brain derived neurotrophic factor is mediated by extracellular signal-regulated kinase and phosphatidylinositol-3-kinase. Neuropsychopharmacology. 2006;31:287–296. doi: 10.1038/sj.npp.1300830. [DOI] [PubMed] [Google Scholar]
- Ou LC, Gean PW. Transcriptional regulation of brain-derived neurotrophic factor in the amygdala during consolidation of fear memory. Mol Pharmacol. 2007;72:350–358. doi: 10.1124/mol.107.034934. [DOI] [PubMed] [Google Scholar]
- Pamplona FA, Prediger RD, Pandolfo P, Takahashi RN. The cannabinoid receptor agonist WIN 55,212-2 facilitates the extinction of contextual fear memory and spatial memory in rats. Psychopharmacology (Berl) 2006;188:641–649. doi: 10.1007/s00213-006-0514-0. [DOI] [PubMed] [Google Scholar]
- Pape HC, Pare D. Plastic synaptic networks of the amygdala for the acquisition, expression, and extinction of conditioned fear. Physiol Rev. 2010;90:419–463. doi: 10.1152/physrev.00037.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papez JW. A proposed mechanism of emotion. Archives of Neurology and Psychiatry. 1937;38:725–743. [Google Scholar]
- Pare D, Smith Y. The intercalated cell masses project to the central and medial nuclei of the amygdala in cats. Neuroscience. 1993;57:1077–1090. doi: 10.1016/0306-4522(93)90050-p. [DOI] [PubMed] [Google Scholar]
- Pare D, Smith Y. Intrinsic circuitry of the amygdaloid complex: common principles of organization in rats and cats. Trends Neurosci. 1998;21:240–241. doi: 10.1016/s0166-2236(98)01240-5. [DOI] [PubMed] [Google Scholar]
- Parsons RG, Davis M. Temporary disruption of fear-potentiated startle following PKMzeta inhibition in the amygdala. Nat Neurosci. 14:295–296. doi: 10.1038/nn.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons RG, Gafford GM, Helmstetter FJ. Translational control via the mammalian target of rapamycin pathway is critical for the formation and stability of long-term fear memory in amygdala neurons. J Neurosci. 2006;26:12977–12983. doi: 10.1523/JNEUROSCI.4209-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul S, Olausson P, Venkitaramani DV, Ruchkina I, Moran TD, Tronson N, Mills E, Hakim S, Salter MW, Taylor JR, Lombroso PJ. The striatal enriched protein tyrosine phosphatase gates long-term potentiation and fear memory in the lateral amygdala. Biol Psychiatry. 2007;61:1049–1061. doi: 10.1016/j.biopsych.2006.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlov IP. Conditioned Reflexes: An investigation of the Physiological Activity of the Cerebral Cortex. London: Oxford University Press; 1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce JM, Hall G. A model for Pavlovian learning: variations in the effectiveness of conditioned but not of unconditioned stimuli. Psychol Rev. 1980;87:532–552. [PubMed] [Google Scholar]
- Pedreira ME, Maldonado H. Protein synthesis subserves reconsolidation or extinction depending on reminder duration. Neuron. 2003;38:863–869. doi: 10.1016/s0896-6273(03)00352-0. [DOI] [PubMed] [Google Scholar]
- Peters J, Dieppa-Perea LM, Melendez LM, Quirk GJ. Induction of fear extinction with hippocampal-infralimbic BDNF. Science. 2010;328:1288–1290. doi: 10.1126/science.1186909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps EA, LeDoux JE. Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron. 2005;48:175–187. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci. 1992;106:274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- Pitkanen A, Pikkarainen M, Nurminen N, Ylinen A. Reciprocal connections between the amygdala and the hippocampal formation, perirhinal cortex, and postrhinal cortex in rat. A review. Ann N Y Acad Sci. 2000;911:369–391. doi: 10.1111/j.1749-6632.2000.tb06738.x. [DOI] [PubMed] [Google Scholar]
- Pitkanen A, Savander V, LeDoux JE. Organization of intra-amygdaloid circuitries in the rat: an emerging framework for understanding functions of the amygdala. Trends Neurosci. 1997;20:517–523. doi: 10.1016/s0166-2236(97)01125-9. [DOI] [PubMed] [Google Scholar]
- Plath N, Ohana O, Dammermann B, Errington ML, Schmitz D, Gross C, Mao X, Engelsberg A, Mahlke C, Welzl H, Kobalz U, Stawrakakis A, Fernandez E, Waltereit R, Bick-Sander A, Therstappen E, Cooke SF, Blanquet V, Wurst W, Salmen B, Bosl MR, Lipp HP, Grant SG, Bliss TV, Wolfer DP, Kuhl D. Arc/Arg3.1 is essential for the consolidation of synaptic plasticity and memories. Neuron. 2006;52:437–444. doi: 10.1016/j.neuron.2006.08.024. [DOI] [PubMed] [Google Scholar]
- Ploski JE, Park KW, Ping J, Monsey MS, Schafe GE. Identification of plasticity-associated genes regulated by Pavlovian fear conditioning in the lateral amygdala. J Neurochem. 2010;112:636–650. doi: 10.1111/j.1471-4159.2009.06491.x. [DOI] [PubMed] [Google Scholar]
- Ploski JE, Pierre VJ, Smucny J, Park K, Monsey MS, Overeem KA, Schafe GE. The activity-regulated cytoskeletal-associated protein (Arc/Arg3.1) is required for memory consolidation of pavlovian fear conditioning in the lateral amygdala. J Neurosci. 2008;28:12383–12395. doi: 10.1523/JNEUROSCI.1662-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponnusamy R, Nissim HA, Barad M. Systemic blockade of D2-like dopamine receptors facilitates extinction of conditioned fear in mice. Learn Mem. 2005;12:399–406. doi: 10.1101/lm.96605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponnusamy R, Poulos AM, Fanselow MS. Amygdala-dependent and amygdala-independent pathways for contextual fear conditioning. Neuroscience. 2007;147:919–927. doi: 10.1016/j.neuroscience.2007.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulos AM, Li V, Sterlace SS, Tokushige F, Ponnusamy R, Fanselow MS. Persistance of fear memory across time requires the basolateral amygdala complex. Proc Natl Acad Sci USA. 2009;106:11737–11741. doi: 10.1073/pnas.0905257106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulos AM, Ponnusamy R, Dong HW, Fanselow MS. Compensation in the neural circuitry of fear conditioning awakens learning circuits in the bed nuclei of the stria terminalis. Proc Natl Acad Sci USA. 2010;107:14881–14886. doi: 10.1073/pnas.1005754107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ. Memory for extinction of conditioned fear is long-lasting and persists following spontaneous recovery. Learn Mem. 2002;9:402–407. doi: 10.1101/lm.49602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Armony JL, LeDoux JE. Fear conditioning enhances different temporal components of tone-evoked spike trains in auditory cortex and lateral amygdala. Neuron. 1997;19:613–624. doi: 10.1016/s0896-6273(00)80375-x. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Likhtik E, Pelletier JG, Pare D. Stimulation of medial prefrontal cortex decreases the responsiveness of central amygdala output neurons. J Neurosci. 2003;23:8800–8807. doi: 10.1523/JNEUROSCI.23-25-08800.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Mueller D. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology. 2008;33:56–72. doi: 10.1038/sj.npp.1301555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Repa C, LeDoux JE. Fear conditioning enhances short-latency auditory responses of lateral amygdala neurons: parallel recordings in the freely behaving rat. Neuron. 1995;15:1029–1039. doi: 10.1016/0896-6273(95)90092-6. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Russo GK, Barron JL, Lebron K. The role of ventromedial prefrontal cortex in the recovery of extinguished fear. J Neurosci. 2000;20:6225–6231. doi: 10.1523/JNEUROSCI.20-16-06225.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racine RJ, Milgram NW, Hafner S. Long-term potentiation phenomena in the rat limbic forebrain. Brain Res. 1983;260:217–231. doi: 10.1016/0006-8993(83)90676-5. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Farb CR, He Y, Janssen WG, Rodrigues SM, Johnson LR, Hof PR, LeDoux JE, Morrison JH. Distribution of NMDA and AMPA receptor subunits at thalamo-amygdaloid dendritic spines. Brain Res. 2007;1134:87–94. doi: 10.1016/j.brainres.2006.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radulovic J, Kammermeier J, Spiess J. Relationship between fos production and classical fear conditioning: effects of novelty, latent inhibition, and unconditioned stimulus preexposure. J Neurosci. 1998;18:7452–7461. doi: 10.1523/JNEUROSCI.18-18-07452.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radwanska K, Nikolaev E, Knapska E, Kaczmarek L. Differential response of two subdivisions of lateral amygdala to aversive conditioning as revealed by c-Fos and P-ERK mapping. Neuroreport. 2002;13:2241–2246. doi: 10.1097/00001756-200212030-00015. [DOI] [PubMed] [Google Scholar]
- Rasmusson AM, Charney DS. Animal models of relevance to PTSD. Ann N Y Acad Sci. 1997;821:332–351. doi: 10.1111/j.1749-6632.1997.tb48290.x. [DOI] [PubMed] [Google Scholar]
- Rattiner LM, Davis M, French CT, Ressler KJ. Brain-derived neurotrophic factor and tyrosine kinase receptor B involvement in amygdala-dependent fear conditioning. J Neurosci. 2004;24:4796–4806. doi: 10.1523/JNEUROSCI.5654-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raught B, Gingras AC, Sonenberg N. The target of rapamycin (TOR) proteins. Proc Natl Acad Sci U S A. 2001;98:7037–7044. doi: 10.1073/pnas.121145898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repa JC, Muller J, Apergis J, Desrochers TM, Zhou Y, LeDoux JE. Two different lateral amygdala cell populations contribute to the initiation and storage of memory. Nat Neurosci. 2001;4:724–731. doi: 10.1038/89512. [DOI] [PubMed] [Google Scholar]
- Rescorla RA. Pavlovian conditioning. It's not what you think it is. Am Psychol. 1988;43:151–160. doi: 10.1037//0003-066x.43.3.151. [DOI] [PubMed] [Google Scholar]
- Rescorla RA. Spontaneous recovery. Learn Mem. 2004;11:501–509. doi: 10.1101/lm.77504. [DOI] [PubMed] [Google Scholar]
- Rescorla RA, Heth CD. Reinstatement of fear to an extinguished conditioned stimulus. J Exp Psychol Anim Behav Process. 1975;1:88–96. [PubMed] [Google Scholar]
- Rescorla RA, Wagner AR. A theory of Pavlovian conditioning: Variations in the effectiveness of reinforcement and nonreinforcement. In: Black AH, Prokasy WF, editors. Classical Conditioning II, Appleton-Century Crofts. 1972. pp. 64–99. [Google Scholar]
- Ressler KJ, Paschall G, Zhou XL, Davis M. Regulation of synaptic plasticity genes during consolidation of fear conditioning. J Neurosci. 2002;22:7892–7902. doi: 10.1523/JNEUROSCI.22-18-07892.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ressler KJ, Rothbaum BO, Tannenbaum L, Anderson P, Graap K, Zimand E, Hodges L, Davis M. Cognitive enhancers as adjuncts to psychotherapy: use of D-cycloserine in phobic individuals to facilitate extinction of fear. Arch Gen Psychiatry. 2004;61:1136–1144. doi: 10.1001/archpsyc.61.11.1136. [DOI] [PubMed] [Google Scholar]
- Richmond MA, Yee BK, Pouzet B, Veenman L, Rawlins JN, Feldon J, Bannerman DM. Dissociating context and space within the hippocampus: effects of complete, dorsal, and ventral excitotoxic hippocampal lesions on conditioned freezing and spatial learning. Behav Neurosci. 1999;113:1189–1203. doi: 10.1037/0735-7044.113.6.1189. [DOI] [PubMed] [Google Scholar]
- Robinson E. EFFECT OF AMYGDALECTOMY ON FEAR-MOTIVATED BEHAVIOR IN RATS. J Comp Physiol Psychol. 1963;56:814–820. doi: 10.1037/h0045711. [DOI] [PubMed] [Google Scholar]
- Roche M, O'Connor E, Diskin C, Finn DP. The effect of CB(1) receptor antagonism in the right basolateral amygdala on conditioned fear and associated analgesia in rats. Eur J Neurosci. 2007;26:2643–2653. doi: 10.1111/j.1460-9568.2007.05861.x. [DOI] [PubMed] [Google Scholar]
- Rodrigues SM, Farb CR, Bauer EP, LeDoux JE, Schafe GE. Pavlovian fear conditioning regulates Thr286 autophosphorylation of Ca2+/calmodulin dependent protein kinase II at lateral amygdala synapses. J Neurosci. 2004;24:3281–3288. doi: 10.1523/JNEUROSCI.5303-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues SM, Schafe GE, LeDoux JE. Intra-amygdala blockade of the NR2B subunit of the NMDA receptor disrupts the acquisition but not the expression of fear conditioning. J Neurosci. 2001;21:6889–6896. doi: 10.1523/JNEUROSCI.21-17-06889.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez JJ, Davies HA, Silva AT, De Souza IE, Peddie CJ, Colyer FM, Lancashire CL, Fine A, Errington ML, Bliss TV, Stewart MG. Long-term potentiation in the rat dentate gyrus is associated with enhanced Arc/Arg3.1 protein expression in spines, dendrites and glia. Eur J Neurosci. 2005;21:2384–2396. doi: 10.1111/j.1460-9568.2005.04068.x. [DOI] [PubMed] [Google Scholar]
- Rogan MT, Leon KS, Perez DL, Kandel ER. Distinct neural signatures for safety and danger in the amygdala and striatum of the mouse. Neuron. 2005;46:309–320. doi: 10.1016/j.neuron.2005.02.017. [DOI] [PubMed] [Google Scholar]
- Rogan MT, Staubli UV, LeDoux JE. AMPA receptor facilitation accelerates fear learning without altering the level of conditioned fear acquired. J Neurosci. 1997a;17:5928–5935. doi: 10.1523/JNEUROSCI.17-15-05928.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogan MT, Staubli UV, LeDoux JE. Fear conditioning induces associative long-term potentiation in the amygdala. Nature. 1997b;390:604–607. doi: 10.1038/37601. [DOI] [PubMed] [Google Scholar]
- Romanski LM, Clugnet MC, Bordi F, LeDoux JE. Somatosensory and auditory convergence in the lateral nucleus of the amygdala. Behav Neurosci. 1993;107:444–450. doi: 10.1037//0735-7044.107.3.444. [DOI] [PubMed] [Google Scholar]
- Romanski LM, LeDoux JE. Equipotentiality of thalamo-amygdala and thalamo-cortico-amygdala circuits in auditory fear conditioning. J Neurosci. 1992;12:4501–4509. doi: 10.1523/JNEUROSCI.12-11-04501.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosas JM, Bouton ME. Context change and retention interval can have additive, rather than interactive, effects after taste aversion extinction. Psychonomic Bulletin & Review. 1998;5:79–83. [Google Scholar]
- Rosenkranz JA, Grace AA. Dopamine attenuates prefrontal cortical suppression of sensory inputs to the basolateral amygdala of rats. J Neurosci. 2001;21:4090–4103. doi: 10.1523/JNEUROSCI.21-11-04090.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenkranz JA, Moore H, Grace AA. The prefrontal cortex regulates lateral amygdala neuronal plasticity and responses to previously conditioned stimuli. J Neurosci. 2003;23:11054–11064. doi: 10.1523/JNEUROSCI.23-35-11054.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royer S, Martina M, Pare D. An inhibitory interface gates impulse traffic between the input and output stations of the amygdala. J Neurosci. 1999;19:10575–10583. doi: 10.1523/JNEUROSCI.19-23-10575.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royer S, Martina M, Pare D. Bistable behavior of inhibitory neurons controlling impulse traffic through the amygdala: role of a slowly deinactivating K+ current. J Neurosci. 2000;20:9034–9039. doi: 10.1523/JNEUROSCI.20-24-09034.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royer S, Pare D. Bidirectional synaptic plasticity in intercalated amygdala neurons and the extinction of conditioned fear responses. Neuroscience. 2002;115:455–462. doi: 10.1016/s0306-4522(02)00455-4. [DOI] [PubMed] [Google Scholar]
- Rudy JW, Barrientos RM, O’Reilly RC. Hippocampal formation supports conditioning to memory of a context. Behav Neurosci. 2002;116:530–538. doi: 10.1037//0735-7044.116.4.530. [DOI] [PubMed] [Google Scholar]
- Rudy JW, O’Reilly RC. Contextual fear conditioning, conjunctive representations, pattern completion, and the hippocampus. Behav Neurosci. 1999;113:867–880. doi: 10.1037//0735-7044.113.5.867. [DOI] [PubMed] [Google Scholar]
- Rumpel S, LeDoux J, Zador A, Malinow R. Postsynaptic receptor trafficking underlying a form of associative learning. Science. 2005;308:83–88. doi: 10.1126/science.1103944. [DOI] [PubMed] [Google Scholar]
- Sacktor TC, Osten P, Valsamis H, Jiang X, Nail MU, Sublette E. Persistent activation of the zeta isoform of protein kinase C in the maintenance of long-term potentiation. Proc Natl Acad Sci USA. 1993;90:8342–8346. doi: 10.1073/pnas.90.18.8342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacktor TC. PKMzeta, LTP maintenance, and the dynamic molecular biology of memory storage. Prog Brain Res. 2008;169:27–40. doi: 10.1016/S0079-6123(07)00002-7. [DOI] [PubMed] [Google Scholar]
- Sah P, Faber ES, Lopez De Armentia M, Power J. The amygdaloid complex: anatomy and physiology. Physiol Rev. 2003;83:803–834. doi: 10.1152/physrev.00002.2003. [DOI] [PubMed] [Google Scholar]
- Samson RD, Pare D. Activity-dependent synaptic plasticity in the central nucleus of the amygdala. J Neurosci. 2005;25:1847–1855. doi: 10.1523/JNEUROSCI.3713-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santini E, Ge H, Ren K, Pena de Ortiz S, Quirk GJ. Consolidation of fear extinction requires protein synthesis in the medial prefrontal cortex. J Neurosci. 2004;24:5704–5710. doi: 10.1523/JNEUROSCI.0786-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santini E, Muller RU, Quirk GJ. Consolidation of extinction learning involves transfer from NMDA-independent to NMDA-dependent memory. J Neurosci. 2001;21:9009–9017. doi: 10.1523/JNEUROSCI.21-22-09009.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santini E, Quirk GJ, Porter JT. Fear conditioning and extinction differentially modify the intrinsic excitability of infralimbic neurons. J Neurosci. 2008;28:4028–4036. doi: 10.1523/JNEUROSCI.2623-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sara SJ. Strengthening the shaky trace through retrieval. Nat Rev Neurosci. 2000;1:212–213. doi: 10.1038/35044575. [DOI] [PubMed] [Google Scholar]
- Schafe GE, Atkins CM, Swank MW, Bauer EP, Sweatt JD, LeDoux JE. Activation of ERK/MAP kinase in the amygdala is required for memory consolidation of pavlovian fear conditioning. J Neurosci. 2000;20:8177–8187. doi: 10.1523/JNEUROSCI.20-21-08177.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafe GE, Doyere V, LeDoux JE. Tracking the fear engram: the lateral amygdala is an essential locus of fear memory storage. J Neurosci. 2005;25:10010–10014. doi: 10.1523/JNEUROSCI.3307-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafe GE, LeDoux JE. Memory consolidation of auditory pavlovian fear conditioning requires protein synthesis and protein kinase A in the amygdala. J Neurosci. 2000;20:RC96. doi: 10.1523/JNEUROSCI.20-18-j0003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafe GE, Nadel NV, Sullivan GM, Harris A, LeDoux JE. Memory consolidation for contextual and auditory fear conditioning is dependent on protein synthesis, PKA, and MAP kinase. Learn Mem. 1999;6:97–110. [PMC free article] [PubMed] [Google Scholar]
- Schafe GE, Nader K, Blair HT, LeDoux JE. Memory consolidation of Pavlovian fear conditioning: a cellular and molecular perspective. Trends Neurosci. 2001;24:540–546. doi: 10.1016/s0166-2236(00)01969-x. [DOI] [PubMed] [Google Scholar]
- Schafe GE, Swank MW, Rodrigues SM, Debiec J, Doyere V. Phosphorylation of ERK/MAP kinase is required for long-term potentiation in anatomically restricted regions of the lateral amygdala in vivo. Learn Mem. 2008;15:55–62. doi: 10.1101/lm.746808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schinder AF, Poo M. The neurotrophin hypothesis for synaptic plasticity. Trends Neurosci. 2000;23:639–645. doi: 10.1016/s0166-2236(00)01672-6. [DOI] [PubMed] [Google Scholar]
- Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. J Neurol Neurosurg Psychiatry. 1957;20:11–21. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selcher JC, Nekrasova T, Paylor R, Landreth GE, Sweatt JD. Mice lacking the ERK1 isoform of MAP kinase are unimpaired in emotional learning. Learn Mem. 2001;8:11–19. doi: 10.1101/lm.37001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selden NR, Everitt BJ, Jarrard LE, Robbins TW. Complementary roles for the amygdala and hippocampus in aversive conditioning to explicit and contextual cues. Neuroscience. 1991;42:335–350. doi: 10.1016/0306-4522(91)90379-3. [DOI] [PubMed] [Google Scholar]
- Serrano P, Friedman EL, Kenney J, Taubenfeld SM, Zimmerman JM, Hanna J, Alberini C, Kelley AE, Maren S, Rudy JW, Yin JC, Sacktor TC, Fenton AA. PKMzeta maintains spatial, instrumental, and classically conditioned long-term memories. PLoS Biol. 2008;6:2698–2706. doi: 10.1371/journal.pbio.0060318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano P, Yao Y, Sacktor TC. Persistent phosphorylation by protein kinase Mzeta maintains late-phase long-term potentiation. J Neurosci. 2005;25:1979–1984. doi: 10.1523/JNEUROSCI.5132-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi C, Davis M. Pain pathways involved in fear conditioning measured with fear-potentiated startle: lesion studies. J Neurosci. 1999;19:420–430. doi: 10.1523/JNEUROSCI.19-01-00420.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi CJ, Cassell MD. Cascade projections from somatosensory cortex to the rat basolateral amygdala via the parietal insular cortex. J Comp Neurol. 1998;399:469–491. doi: 10.1002/(sici)1096-9861(19981005)399:4<469::aid-cne3>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Sierra-Mercado D, Jr, Corcoran KA, Lebron-Milad K, Quirk GJ. Inactivation of the ventromedial prefrontal cortex reduces expression of conditioned fear and impairs subsequent recall of extinction. Eur J Neurosci. 2006;24:1751–1758. doi: 10.1111/j.1460-9568.2006.05014.x. [DOI] [PubMed] [Google Scholar]
- Sierra-Mercado D, Jr, Padilla-Coreno N, Quirk GJ. Dissociable roles of prelimbic and infralimbic cortices, ventral hippocampus, and basolateral amygdala in the expression and extinction of conditioned fear. Neuropsychopharm. 2010;36:529–538. doi: 10.1038/npp.2010.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sledjeski EM, Speisman B, Dierker LC. Does number of lifetime traumas explain the relationship between PTSD and chronic medical conditions? Answers from the National Comorbidity Survey-Replication (NCS-R) Journal of Behavioral Medicine. 2008;31:341–349. doi: 10.1007/s10865-008-9158-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slipczuk L, Bekinschtein P, Katche C, Cammarota M, Izquierdo I, Medina JH. BDNF activates mTOR to regulate GluR1 expression required for memory formation. PLoS ONE. 2009;4:e6007. doi: 10.1371/journal.pone.0006007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva AJ, Stevens CF, Tonegawa S, Wang Y. Deficient hippocampal long-term potentiation in alpha-calcium-calmodulin kinase II mutant mice. Science. 1992;257:201–206. doi: 10.1126/science.1378648. [DOI] [PubMed] [Google Scholar]
- Soliman F, Glatt CE, Bath KG, Levita L, Jones RM, Pattwell SS, Jing D, Tottenham N, Amso D, Somerville LH, Voss HU, Glover G, Ballon DJ, Liston C, Teslovich T, Van Kempen T, Lee FS, Casey BJ. A genetic variant BDNF polymorphism alters extinction learning in both mouse and human. Science. 2010;327:863–866. doi: 10.1126/science.1181886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotres-Bayon F, Bush DE, LeDoux JE. Acquisition of fear extinction requires activation of NR2B-containing NMDA receptors in the lateral amygdala. Neuropsychopharmacology. 2007;32:1929–1940. doi: 10.1038/sj.npp.1301316. [DOI] [PubMed] [Google Scholar]
- Sotres-Bayon F, Diaz-Mataix L, Bush DE, LeDoux JE. Dissociable roles for the ventromedial prefrontal cortex and amygdala in fear extinction: NR2B contribution. Cereb Cortex. 2009;19:474–482. doi: 10.1093/cercor/bhn099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparks FT, Lehmann H, Sutherland RJ. Between-systems memory interference during retrieval. Eur J Neurosci. 2011;34:780–786. doi: 10.1111/j.1460-9568.2011.07796.x. [DOI] [PubMed] [Google Scholar]
- Stanciu M, Radulovic J, Spiess J. Phosphorylated cAMP response element binding protein in the mouse brain after fear conditioning: relationship to Fos production. Brain Res Mol Brain Res. 2001;94:15–24. doi: 10.1016/s0169-328x(01)00174-7. [DOI] [PubMed] [Google Scholar]
- Stork O, Ji FY, Obata K. Reduction of extracellular GABA in the mouse amygdala during and following confrontation with a conditioned fear stimulus. Neurosci Lett. 2002;327:138–142. doi: 10.1016/s0304-3940(02)00387-7. [DOI] [PubMed] [Google Scholar]
- Storsve AB, McNally GP, Richardson R. US habituation, like CS extinction, produces a decrement in conditioned fear responding that is NMDA dependent and subject to renewal and reinstatement. Neurobiol Learn Mem. 2010;93:463–471. doi: 10.1016/j.nlm.2009.12.011. [DOI] [PubMed] [Google Scholar]
- Sutherland R, Lehmann H. Alternative conceptions of memory consolidation and the role of the hippocampus at the systems level in rodents. Curr Opin Neurobiol. 2011;21:446–451. doi: 10.1016/j.conb.2011.04.007. [DOI] [PubMed] [Google Scholar]
- Sutherland RJ, O’Brien J, Lehmann H. Absence of systems consolidation of fear memories after dorsal, ventral, or complete hippocampal damage. Hippocampus. 2008;18:710–718. doi: 10.1002/hipo.20431. [DOI] [PubMed] [Google Scholar]
- Sutherland RJ, Sparks FT, Lehmann H. Hippocampus and retrograde amnesia in the rat model: a modest proposal for the situation of systems consolidation. Neuropsychologia. 2010;48:2357–2369. doi: 10.1016/j.neuropsychologia.2010.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A, Josselyn SA, Frankland PW, Masushige S, Silva AJ, Kida S. Memory reconsolidation and extinction have distinct temporal and biochemical signatures. J Neurosci. 2004;24:4787–4795. doi: 10.1523/JNEUROSCI.5491-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson LW, Petrovich GD. What is the amygdala? Trends Neurosci. 1998;21:323–331. doi: 10.1016/s0166-2236(98)01265-x. [DOI] [PubMed] [Google Scholar]
- Szapiro G, Vianna MR, McGaugh JL, Medina JH, Izquierdo I. The role of NMDA glutamate receptors, PKA, MAPK, and CAMKII in the hippocampus in extinction of conditioned fear. Hippocampus. 2003;13:53–58. doi: 10.1002/hipo.10043. [DOI] [PubMed] [Google Scholar]
- Szinyei C, Heinbockel T, Montagne J, Pape HC. Putative cortical and thalamic inputs elicit convergent excitation in a population of GABAergic interneurons of the lateral amygdala. J Neurosci. 2000;20:8909–8915. doi: 10.1523/JNEUROSCI.20-23-08909.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szinyei C, Narayanan RT, Pape HC. Plasticity of inhibitory synaptic network interactions in the lateral amygdala upon fear conditioning in mice. Eur J Neurosci. 2007;25:1205–1211. doi: 10.1111/j.1460-9568.2007.05349.x. [DOI] [PubMed] [Google Scholar]
- Szinyei C, Stork O, Pape HC. Contribution of NR2B subunits to synaptic transmission in amygdaloid interneurons. J Neurosci. 2003;23:2549–2556. doi: 10.1523/JNEUROSCI.23-07-02549.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takei N, Inamura N, Kawamura M, Namba H, Hara K, Yonezawa K, Nawa H. Brain-derived neurotrophic factor induces mammalian target of rapamycin-dependent local activation of translation machinery and protein synthesis in neuronal dendrites. J Neurosci. 2004;24:9760–9769. doi: 10.1523/JNEUROSCI.1427-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang SJ, Reis G, Kang H, Gingras AC, Sonenberg N, Schuman EM. A rapamycin-sensitive signaling pathway contributes to long-term synaptic plasticity in the hippocampus. Proc Natl Acad Sci U S A. 2002;99:467–472. doi: 10.1073/pnas.012605299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang YP, Shimizu E, Dube GR, Rampon C, Kerchner GA, Zhuo M, Liu G, Tsien JZ. Genetic enhancement of learning and memory in mice. Nature. 1999;401:63–69. doi: 10.1038/43432. [DOI] [PubMed] [Google Scholar]
- Taubenfeld SM, Milekic MH, Monti B, Alberini CM. The consolidation of new but not reactivated memory requires hippocampal C/EBPbeta. Nat Neurosci. 2001;4:813–818. doi: 10.1038/90520. [DOI] [PubMed] [Google Scholar]
- Thomas GM, Huganir RL. MAPK cascade signalling and synaptic plasticity. Nat Rev Neurosci. 2004;5:173–183. doi: 10.1038/nrn1346. [DOI] [PubMed] [Google Scholar]
- Tocco G, Maren S, Shors TJ, Baudry M, Thompson RF. Long-term potentiation is associated with increased [3H]AMPA binding in rat hippocampus. Brain Res. 1992;573:228–234. doi: 10.1016/0006-8993(92)90767-4. [DOI] [PubMed] [Google Scholar]
- Trivedi MA, Coover GD. Lesions of the ventral hippocampus, but not the dorsal hippocampus, impair conditioned fear expression and inhibitory avoidance on the elevated T-maze. Neurobiol Learn Mem. 2004;81:172–184. doi: 10.1016/j.nlm.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Tronel S, Milekic MH, Alberini CM. Linking new information to a reactivated memory requires consolidation and not reconsolidation mechanisms. PLoS Biol. 2005;3:e293. doi: 10.1371/journal.pbio.0030293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronson NC, Taylor JR. Molecular mechanisms of memory reconsolidation. Nat Rev Neurosci. 2007;8:262–275. doi: 10.1038/nrn2090. [DOI] [PubMed] [Google Scholar]
- Tronson NC, Wiseman SL, Olausson P, Taylor JR. Bidirectional behavioral plasticity of memory reconsolidation depends on amygdalar protein kinase A. Nat Neurosci. 2006;9:167–169. doi: 10.1038/nn1628. [DOI] [PubMed] [Google Scholar]
- Tsvetkov E, Carlezon WA, Benes FM, Kandel ER, Bolshakov VY. Fear conditioning occludes LTP-induced presynaptic enhancement of synaptic transmission in the cortical pathway to the lateral amygdala. Neuron. 2002;34:289–300. doi: 10.1016/s0896-6273(02)00645-1. [DOI] [PubMed] [Google Scholar]
- Turner BH, Herkenham M. Thalamoamygdaloid projections in the rat: a test of the amygdala's role in sensory processing. J Comp Neurol. 1991;313:295–325. doi: 10.1002/cne.903130208. [DOI] [PubMed] [Google Scholar]
- Tye K, Prakash R, Kim SY, Fenno LE, Grosenick L, Zaraki H, Thompson KR, Gradinaru V, Ramakrishnan C, Deisseroth K. Amygdala circuitry mediating reversible and bidirectional control of anxiety. Nature. 2011;17:358–362. doi: 10.1038/nature09820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler WJ, Pozzo-Miller L. Miniature synaptic transmission and BDNF modulate dendritic spine growth and form in rat CA1 neurones. J Physiol. 2003;553:497–509. doi: 10.1113/jphysiol.2003.052639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Eden CG, Uylings HB. Cytoarchitectonic development of the prefrontal cortex in the rat. J Comp Neurol. 1985a;241:253–267. doi: 10.1002/cne.902410302. [DOI] [PubMed] [Google Scholar]
- Van Eden CG, Uylings HB. Postnatal volumetric development of the prefrontal cortex in the rat. J Comp Neurol. 1985b;241:268–274. doi: 10.1002/cne.902410303. [DOI] [PubMed] [Google Scholar]
- Vertes RP. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse. 2004;51:32–58. doi: 10.1002/syn.10279. [DOI] [PubMed] [Google Scholar]
- Vianna MR, Szapiro G, McGaugh JL, Medina JH, Izquierdo I. Retrieval of memory for fear-motivated training initiates extinction requiring protein synthesis in the rat hippocampus. Proc Natl Acad Sci U S A. 2001;98:12251–12254. doi: 10.1073/pnas.211433298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal-Gonzalez I, Vidal-Gonzalez B, Rauch SL, Quirk GJ. Microstimulation reveals opposing influences of prelimbic and infralimbic cortex on the expression of conditioned fear. Learn Mem. 2006;13:728–733. doi: 10.1101/lm.306106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DL, Davis M. The role of amygdala glutamate receptors in fear learning, fear-potentiated startle, and extinction. Pharmacol Biochem Behav. 2002;71:379–392. doi: 10.1016/s0091-3057(01)00698-0. [DOI] [PubMed] [Google Scholar]
- Walker DL, Ressler KJ, Lu KT, Davis M. Facilitation of conditioned fear extinction by systemic administration or intra-amygdala infusions of D cycloserine as assessed with fear-potentiated startle in rats. J Neurosci. 2002;22:2343–2351. doi: 10.1523/JNEUROSCI.22-06-02343.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace TL, Stellitano KE, Neve RL, Duman RS. Effects of cyclic adenosine monophosphate response element binding protein overexpression in the basolateral amygdala on behavioral models of depression and anxiety. Biol Psychiatry. 2004;56:151–160. doi: 10.1016/j.biopsych.2004.04.010. [DOI] [PubMed] [Google Scholar]
- Waltereit R, Mannhardt S, Nescholta S, Maser-Gluth C, Bartsch D. Selective and protracted effect of nifedipine on fear memory extinction correlates with induced stress response. Learn Mem. 2008;15:348–356. doi: 10.1101/lm.808608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Luo W, Reiser G. Trypsin and trypsin-like proteases in the brain: proteolysis and cellular functions. Cell Mol Life Sci. 2008;65:237–252. doi: 10.1007/s00018-007-7288-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeber EJ, Atkins CM, Selcher JC, Varga AW, Mirnikjoo B, Paylor R, Leitges M, Sweatt JD. A role for the beta isoform of protein kinase C in fear conditioning. J Neurosci. 2000;20:5906–5914. doi: 10.1523/JNEUROSCI.20-16-05906.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiskrantz L. Behavioral changes associated with ablation of the amygdaloid complex in monkeys. J Comp Physiol Psychol. 1956;49:381–391. doi: 10.1037/h0088009. [DOI] [PubMed] [Google Scholar]
- Weisskopf MG, Bauer EP, LeDoux JE. L-type voltage-gated calcium channels mediate NMDA-independent associative long-term potentiation at thalamic input synapses to the amygdala. J Neurosci. 1999;19:10512–10519. doi: 10.1523/JNEUROSCI.19-23-10512.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilensky AE, Schafe GE, Kristensen MP, LeDoux JE. Rethinking the fear circuit: the central nucleus of the amygdala is required for the acquisition, consolidation, and expression of Pavlovian fear conditioning. J Neurosci. 2006;26:12387–12396. doi: 10.1523/JNEUROSCI.4316-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JT, Christie MJ, Manzoni O. Cellular and synaptic adaptations mediating opiod dependence. Physiol Rev. 2001;81:299–343. doi: 10.1152/physrev.2001.81.1.299. [DOI] [PubMed] [Google Scholar]
- Willick ML, Kokkinidis L. Cocaine enhances the expression of fear potentiated startle: evaluation of state-dependent extinction and the shock sensitization of acoustic startle. Behav Neurosci. 1995;109:929–938. doi: 10.1037//0735-7044.109.5.929. [DOI] [PubMed] [Google Scholar]
- Wilson A, Brooks DC, Bouton ME. The role of the rat hippocampal system in several effects of context in extinction. Behav Neurosci. 1995;109:828–836. doi: 10.1037//0735-7044.109.5.828. [DOI] [PubMed] [Google Scholar]
- Wilson DA. A comparison of the postnatal development of post-activation potentiation in the neocortex and dentate gyrus of the rat. Brain Res. 1984;318:61–68. doi: 10.1016/0165-3806(84)90063-4. [DOI] [PubMed] [Google Scholar]
- Wilson YM, Murphy M. A discrete population of neurons in the lateral amygdala is specifically activated by contextual fear conditioning. Learn Mem. 2009;16:357–361. doi: 10.1101/lm.1361509. [DOI] [PubMed] [Google Scholar]
- Wiltgen BJ, Sanders MJ, Anagnostaras SG, Sage JR, Fanselow MS. Context fear learning in the absence of the hippocampus. J Neurosci. 2006;17:5484–5491. doi: 10.1523/JNEUROSCI.2685-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiltgen BJ, Godsil BP, Peng Z, June HL, Linn ML, Houser CR, O’Dell TJ, Homanics GF, Fanselow MS. The alpha1 subunit of the GABA(A) receptor modulates fear learning and plasticity in the lateral amygdala. Front Behav Neurosci. 2009;3:1–12. doi: 10.3389/neuro.08.037.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods AM, Bouton ME. D-cycloserine facilitates extinction but does not eliminate renewal of the conditioned emotional response. Behav Neurosci. 2006;120:1159–1162. doi: 10.1037/0735-7044.120.5.1159. [DOI] [PubMed] [Google Scholar]
- Wu GY, Deisseroth K, Tsien RW. Spaced stimuli stabilize MAPK pathway activation and its effects on dendritic morphology. Nat Neurosci. 2001;4:151–158. doi: 10.1038/83976. [DOI] [PubMed] [Google Scholar]
- Xu YL, Reinsched RK, Huitron-Resendiz S, Clark SD, Wang Z, Lin SH, Brucher FA, Zeing J, Ly NK, Henriksen SJ. Neuropeptide S: a neuropeptide promoting arousal and anxiolytic-like effects. Neuron. 2004;43:487–497. doi: 10.1016/j.neuron.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Xu YL, Gall CM, Jackson VR, Civelli O, Reinscheid RK. Distribution of neuropeptide S receptor mRNA and neurochemical characteristics of neuropeptide S-expressing neurons in the rat brain. J Comp Neurol. 2007;500:84–102. doi: 10.1002/cne.21159. [DOI] [PubMed] [Google Scholar]
- Yamada D, Zushida K, Wada K, Sekiguchi M. Pharmacological discrimination of extinction and reconsolidation of contextual fear memory by a potentiator of AMPA receptors. Neuropsychopharmacology. 2009;34:2574–2584. doi: 10.1038/npp.2009.86. [DOI] [PubMed] [Google Scholar]
- Yang YL, Lu KT. Facilitation of conditioned fear extinction by d-cycloserine is mediated by mitogen-activated protein kinase and phosphatidylinositol 3 kinase cascades and requires de novo protein synthesis in basolateral nucleus of amygdala. Neuroscience. 2005;134:247–260. doi: 10.1016/j.neuroscience.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Zelikowsky M, Pham DL, Fanselow MS. Temporal factors control hippocampal contributions to fear renewal after extinction. Hippocampus. 2011 doi: 10.1002/hipo.20954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang WN, Bast T, Feldon J. The ventral hippocampus and fear conditioning in rats: different anterograde amnesias of fear after infusion of N-methyl-D-aspartate or its noncompetitive antagonist MK-801 into the ventral hippocampus. Behav Brain Res. 2001;126:159–174. doi: 10.1016/s0166-4328(01)00256-x. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Won J, Karlsson MG, Zhou M, Rogerson T, Balaji J, Neve R, Poirazi P, Silva AJ. CREB regulates excitability and the allocation of memory to subsets of neurons in the amygdala. Nat Neurosci. 2009;12:1438–1443. doi: 10.1038/nn.2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu JJ, Qin Y, Zhao M, Van Aelst L, Malinow R. Ras and Rap control AMPA receptor trafficking during synaptic plasticity. Cell. 2002;110:443–455. doi: 10.1016/s0092-8674(02)00897-8. [DOI] [PubMed] [Google Scholar]
- Zimmerman JM, Maren S. The central nucleus of the amygdala is essential for acquiring and expressing conditioned fear after overtraining. Learn Mem. 2007;14:634–644. doi: 10.1101/lm.607207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman JM, Maren S. NMDA receptor antagonism in the basolateral but not central amygdala blocks the extinction of Pavlovian fear conditioning in rats. Eur J Neurosci. 2010;31:1664–1670. doi: 10.1111/j.1460-9568.2010.07223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]