Abstract
Previous findings indicate treatment with a selective serotonin reuptake inhibitor (SSRI) facilitates behavioral flexibility when conditions require inhibition of a learned response pattern. The present experiment investigated whether acute treatment with the SSRI, escitalopram, affects behavioral flexibility when conditions require inhibition of a naturally-biased response pattern (elevated conflict test) and/or reversal of a learned response pattern (spatial reversal learning). An additional experiment was carried out to determine whether escitalopram, at doses that affected behavioral flexibility, also reduced anxiety as tested in the elevated plus-maze. In each experiment, Long-Evans rats received an intraperitoneal injection of either saline or escitalopram (0.03, 0.3 or 1.0 mg/kg) 30 minutes prior to behavioral testing. Escitalopram, at all doses tested, enhanced acquisition in the elevated conflict test, but did not affect performance in the elevated plus-maze. Escitalopram (0.3 and 1.0 mg/kg) did not alter acquisition of the spatial discrimination, but facilitated reversal learning. In the elevated conflict and spatial reversal learning test, escitalopram enhanced the ability to maintain the relevant strategy after being initially selected. The present findings suggest that enhancing serotonin transmission with a SSRI facilitates inhibitory processes when conditions require a shift away from either a naturally-biased response pattern or a learned choice pattern.
Keywords: serotonin, reversal learning, escitalopram, rats, anxiety, reward
Introduction
Individuals with various psychiatric and developmental disorders, i.e. major depressive disorder, schizophrenia and autism spectrum disorder, exhibit distinct impairments in generating appropriate strategies and/or inhibiting inappropriate responses that adversely impact daily living (Lopez et al., 2005; Solomon et al., 2009; Withall et al., 2009). These deficits are commonly observed in probabilistic reversal learning tests in which an initial response pattern associated with positive feedback in a highly probable manner, is reversed such that an alternative response becomes associated with positive feedback in a highly probably manner (Dickstein et al., 2009; Leeson et al., 2009; Waltz and Gold, 2007; Withall et al, 2009). Individuals with schizophrenia and autism spectrum disorder also exhibit deficits in which a naturally-biased, prepotent response is context-inappropriate and must be supplanted by an alternative response (Agam et al. 2010; Christ et al., 2007; Harris et al., 2009; Mosconi et al., 2009). For example, these patient populations are impaired on an anti-saccade test in which individuals must inhibit a prepotent bias of generating an eye movement toward a visual target and instead execute a saccade away from the visual target (Munoz and Everling, 2004). Thus, patients with these disorders can experience deficits in withholding both learned and naturally-occurring prepotent responses. Understanding whether similar neural mechanisms underlie the behavioral flexibility required for inhibition of a learned and naturally-occurring prepotent response is critical in the development of effective therapies for treating various clinical disorders.
Investigations into the neural mechanisms underlying cognitive flexibility deficits have focused on neurochemical mechanisms that support inhibitory processes (Eagle and Baunez, 2010). The neurotransmitter serotonin (5-HT) may be critical for inhibitory processes related to flexible responding (Winstanley et al., 2003, 2004). For example, depletion of 5-HT in the marmoset prefrontal cortex with 5,7-DHT produces reversal learning impairments (Clarke et al., 2005; 2007). In humans, depletion of the amino acid precursor of 5-HT, tryptophan selectively impairs probabilistic reversal learning (Finger et al., 2007). Similarly, depletion of 5-HT content in the rat frontal cortex or multiple rat forebrain regions also produces reversal learning deficits (Bari et al., 2010; Lapiz-Bluhm et al., 2009). Conversely, enhancing 5-HT transmission with the selective serotonin reuptake inhibitor (SSRI), citalopram, improves probabilistic reversal learning in rats (Bari et al., 2010). Citalopram facilitates reversal learning by decreasing the probability that a subject switches their choice pattern to the less reinforced choice after receiving no reinforcement on a correct trial (Bari et al., 2010). These findings with SSRI treatment indicate that inhibiting 5-HT reuptake facilitates reversal of a learned prepotent response by modulating negative feedback sensitivity. Taken together, the 5-HT depletion findings and SSRI results suggest that intact 5-HT transmission in the forebrain is critical for reversal of a learned response pattern
SSRI treatment enhancement of probabilistic reversal learning may result from increased 5-HT transmission augmenting inhibitory processes that allows a more rapid shift in response patterns. Alternatively, SSRI treatment may enhance behavioral flexibility, in part, by reducing anxiety. Administration of a SSRI is effective in treating general anxiety disorder (Goodman et al., 2005; Stein et al., 2005) and panic disorder (Stahl et al., 2003). Furthermore, acute escitalopram treatment in rats also reduces anxiety measures in the elevated T-maze test and following electrical stimulation of the dorsolateral periacqueductal gray; a purported model of panic disorder (Hogg et al., 2006; Lim et al., 2010; Pinheiro et al., 2008). Moreover, SSRIs or targeted 5-HT receptor agonists facilitate learning or memory under conditions that have a fear or anxiety component, e.g. fear conditioning or inhibitory avoidance (Burghardt et al., 2004; Hashimoto et al. 2007; Izquierdo et al., 1998; Meneses et al., 2007; Montezinho et al., 2010). The effects of SSRIs on anxiety raise the possibility that enhancing 5-HT transmission with SSRI treatment does not selectively affect behavioral flexibility, but when a response pattern is no longer reinforced this increases anxiety which SSRI treatment alleviates and allows a more rapid switch in response patterns. Because SSRI treatment also affects aversive learning still another possibility is that SSRI treatment may more selectively affect memory consolidation or retrieval processes of a learned strategy that subsequently affects reversal of this acquired strategy. However, because past studies investigating the behavioral effects of SSRIs have usually focused on one aspect of behavior, i.e. learning or anxiety, as opposed to examining the effects of SSRIs on multiple behavioral processes, unknown is whether any of these alternative mechanisms may underlie SSRI effects on behavioral flexibility.
In understanding how SSRI treatment may contribute to behavioral flexibility, another issue is that previous studies have investigated the effects of SSRI treatment on reversal of a learned response pattern, but unknown, is whether SSRI treatment also facilitates inhibition of a more naturally-biased prepotent response. Standard reversal learning paradigms represent tests of inhibiting a learned response pattern that are commonly carried out in an enclosed operant chamber or in mazes in which the arms have side walls. However, tests have not been used to study inhibition of an automatic or naturally-biased, prepotent response pattern. Rodents exhibit a prepotent bias in entering closed arms versus open arms when tested in elevated mazes (Montgomery, 1958; Pellow et al., 1985). These tasks are commonly used as tests to measure anxiety (Graeff et al., 1993; Pellow et al., 1985). Some studies report that citalopram or escitalopram reduce anxiety measures in these tests (Pinheiro et al., 2008; Pollier et al., 2000). Because these tests have both an anxiety and a prepotent response bias component, we wanted to use an elevated maze to determine whether acute escitalopram treatment affects inhibition of a naturally-biased prepotent response to obtain a food reward. Specifically, we used an elevated T-maze in which the open arm was reinforced with a food reward at a high probability, while the closed arm was reinforced at a low probability (Experiment 1). A probabilistic learning procedure was used because, as noted above, probabilistic reversal learning deficits are commonly observed in clinical populations (Dickstein et al., 2009; Leeson et al., 2009; Waltz and Gold, 2007; Withall et al, 2009) and probabilistic reinforcement learning tests may be more ecologically relevant (Bitterman, 1975; Sonsino, 1997; Tsuchida et al., 2010). If SSRI treatment enhances acquisition in the elevated conflict test, this may result from a SSRI principally reducing anxiety. To determine this, Experiment 2 determined the effect of acute escitalopram treatment in the elevated plus-maze. Alternatively, escitalopram may not have a general effect in reducing anxiety, but decreases anxiety when there is a salient motivational condition, i.e. obtaining a food reward. Experiment 3 investigated whether acute escitalopram treatment reduces the latency to obtain a food reward when the only choice is to enter an open arm. To compare the effects of SSRI in inhibition of a naturally-occurring prepotent bias with reversal of a learned prepotent response pattern, Experiment 4 investigated the effect of acute escitalopram treatment on acquisition and reversal learning of a spatial discrimination. Finally, Experiment 5 examined whether post-acquisition escitalopram treatment affected memory consolidation of the originally learned discrimination when tested in reversal learning or in a subsequent retrieval session. Taken together, this set of experiments investigated whether acute SSRI treatment affects behavioral flexibility in naturally-biased and learned prepotent conditions and whether these results are due to effects on anxiety and/or memory processes.
Materials and Methods
Subjects
A total of 161 male Long-Evans rats (Harlan, Indianapolis, IN) served as subjects. Rats weighed between 300–360 g at the time of experiments. A separate cohort of naïve rats were used for each experiment. Rats were individually housed in plastic cages (26.5 cm × 50 cm × 20 cm) in a temperature (22 C) and humidity (30%) controlled environment. Rats were placed on a 12 hour light/dark cycle (lights on at 7:00am). Animals were food restricted to 85–90% of their ad libitum body weight during the course of the experiment but had access to water ad libitum while in their home cage. Animal care was in accordance with the National Institutes for Health’s Guide for the Care and Use of Laboratory Animals and approved by the University of Illinois Institutional Laboratory Animal Care and Use Committee.
Figure 1 diagrams the experimental design for each experiment described below.
Figure 1.
Experimental procedures for each study that illustrate the order in which treatments were administered and behavioral testing occurred. SAL = saline and ESC = escitalopram.
Experiment 1: The effect of escitalopram on prepotent response inhibition in the elevated conflict test
The purpose of Experiment 1 was to determine whether acute escitalopram treatment affected inhibition of naturally-biased prepotent response in the elevated conflict test.
Apparatus
In the training phase, a cross-maze was used in which each maze arm was 55 cm long and 10 cm wide with 15 cm high side walls and a 15 cm high back wall. A food well was located in the distal end of each maze arm 5 cm from then end of each arm. For testing in the elevated conflict test, the cross-maze was modified. One choice arm was changed to an open arm with no side walls or a back wall. The width of this open arm was reduced to 5 cm. This arm was narrower to increase a rat’s prepotent bias to enter the closed arm and avoid the open arm. The walls located on the opposing closed arm were replaced by walls that were each 30 cm high during testing. The other two arms were used as start arms and were identical to those used in training. The maze was placed on a table that was elevated 72 cm above the floor. The open arm extended completely off the table and was supported by a metal stand (3 cm wide, 50 cm long and 71 cm high).
Behavioral Training
Prior to testing, each rat was exposed to the cross-maze and trained to obtain cereal pieces in the food wells. Before training each rat was food restricted as described above. Subsequently, rats were exposed to a cross-maze in which they learned to obtain a half piece of Froot Loop’s cereal (Kellogg, Battle Creek, MI) from each food well. During training, a rat was also picked up after consuming a cereal piece and placed into a different maze arm. This acclimated a rat to being picked up in the maze as occurred in the test phase. After a rat consumed all four cereal pieces from each food well, it was placed in a holding chamber near the maze. The food wells were rebaited and a new trial was started. This phase of training continued until a rat completed a minimum of five trials in 15 min across two consecutive days. Subsequently, a final day of training occurred in which a black plastic block (9 cm wide × 13 cm high × 1 cm thick) was placed at the entrance of one arm giving the maze a T-shape. A rat was placed in the stem arm and allowed to enter either choice arm to obtain a cereal piece. After the initial choice, a rat was placed back in the stem arm. If a rat chose the same arm as the initial choice, it was returned to the stem arm until it chose the other arm. Once a rat had selected both arms it was placed on top of its home cage while the two choice arms were rebaited. The session ended after a rat had completed seven of these trials as in previous experiments (Brown et al., 2010, McCool et al., 2008). Testing occurred in the following session. On average, training required five sessions.
Behavioral Testing
In the elevated conflict test the maze was modified to have an open choice arm and a closed choice arm as described above. In this test, a rat was pseudorandomly started from one of two different start arms so that a start arm was not used for more than two consecutive trials. The two start arms were opposite from each other. The entrance to the arm directly opposite to the start arm was blocked with a black acrylic piece (9 cm wide × 30 cm high × 1 cm thick) to give the maze a T-shape. This allowed entry into either an open or closed arm. The open arm was designated as the correct arm and contained a half piece of cereal on 80% of trials. On the other 20% of trials, the incorrect arm (closed) was baited with a half piece of cereal. The first two trials of the test always contained food reinforcement in the correct arm. The open and closed arms were baited with a cereal reinforcement pseudorandomly such that the open arm was baited a maximum of six consecutive trials. On any individual trial, only one of the choice-arms was baited. A rat was allowed to choose an arm, eat the cereal piece if available, and was promptly placed in a holding chamber near the maze. If a chosen arm was not baited, then a rat was allowed to locomote to the empty food well and was then promptly removed from the maze. Between trials, the maze and black acrylic piece were wiped down with 2% quaternary ammonium chloride solution to minimize the use of olfactory cues. Arms were dried before the following trial. A correct response was recorded if a rat entered the open arm. The inter-trial interval was approximately 15 seconds. This procedure was repeated until a rat chose the open arm on ten consecutive trials. This criterion was selected based upon similar procedures used in previous experiments (Brown et al., 2010, McCool et al., 2008). Testing was completed in a single test session.
Treatment
Rats received an intraperitoneal (i.p.) injection 30 minutes prior to testing in a volume of 1.0 ml/kg. Each rat was randomly assigned to one of the following treatment groups (with the sample size for each group represented in parentheses): 1) Saline (n=8); 2) Escitalopram 0.03 mg/kg (n=6); 3) Escitalopram 0.3 mg/kg (n=10); 4) Escitalopram 1.0 mg/kg (n=6). Escitalopram was mixed in sterile saline. Escitalopram oxalate was obtained from American Customs Chemical Corporation, San Diego, CA, USA.
Analysis of Errors
An analysis of errors was conducted to determine whether escitalopram treatment affected the initial inhibition of a prepotent choice pattern as measured by perseverative errors and/or the maintenance of a new choice after being initially selected as measured by regressive errors (McCool et al., 2008; Ragozzino et al., 2002; 2003). To determine the number of perseverative errors, trials were separated into consecutive blocks of four trials. Perseveration was defined as initially selecting the closed arm in three or four trials in a block. Thus, if a rat chose the closed arm on the majority of trials in a block it was considered to be perseverating on the prepotent choice. Once a rat made two or more choices in the open arm in a block, perseveration was no longer considered to occur. All subsequent entries into the closed arm were defined as regressive errors. Perseveration is considered a measure of the inability to initially inhibit a prepotent choice pattern. Regressive errors determine the ability to maintain a new choice pattern after being initially selected.
As carried out by Bari et al. (2010), an analysis was also performed to determine whether escitalopram altered the sensitivity to reinforcement or no reinforcement on correct trials. A rat’s choices in the test were analyzed based on the outcome (reinforcement or no reinforcement) of each preceding trial and expressed as a ratio. For correct trials, a win-stay ratio was determined by the number of times a rat received a reinforcement in the correct arm and then chose the same correct arm on the subsequent trial, divided by the total number of reinforced trials for the correct trials only. The lose-shift ratio was determined by the number of times a rat changed its choice after not receiving reinforcement in the correct arm on the previous trial, divided by the total number of non-reinforced trials for only correct trials.
Experiment 2: The effect of escitalopram treatment on the elevated plus-maze
The purpose of Experiment 2 was to determine whether acute escitalopram treatment reduced a prepotent bias of avoiding an open arm even when there was no food reward.
Apparatus
The maze consisted of two opposing open (55 cm × 10 cm) arms and two opposing closed arms (55 × 10 × 30 cm) with 15 cm high back walls. The maze was elevated 72 cm above the floor. Open arms were supported by metal stands as in the elevated conflict test. Arms were connected with a (10 × 10 cm) black acrylic square.
Behavioral Testing
The effect of escitalopram treatment on the elevated plus-maze was investigated in Experiment 2. If escitalopram treatment affects acquisition in the elevated conflict test, this may occur by principally modifying anxiety, as opposed to directly affecting an inhibitory response process. To determine this, a separate set of rats received either escitalopram or saline prior to the elevated plus-maze test. No maze training occurred prior to the test. However, all rats were handled and food restricted similar to that in Experiment 1. At task onset, rats were placed in the center square facing an open arm and were allowed to explore the maze for five minutes. The duration spent in open and closed arms, as well as the numbers of entries in open and closed arms were recorded. After each rat was tested, the maze was thoroughly cleaned with a 2% quaternary ammonium chloride solution and allowed to dry for at least 30 minutes.
Treatment
Rats received an i.p. injection of either saline or escitalopram 30 minutes prior to the test session. Each rat was randomly assigned to either the Saline (n = 7) or Escitalopram 1.0 mg/kg (n = 6) group. Escitalopram at 1.0 mg/kg was chosen because this was the highest dose of the SSRI that was effective in the elevated conflict test.
Experiment 3: The effect of escitalopram on the single open arm test
The purpose of Experiment 3 was to determine whether acute escitalopram treatment reduced a prepotent bias in avoiding an open arm when there was a food reward. Thus, this study determined whether escitalopram affected entry into an open arm when adding an appetitive motivational component was introduced.
Apparatus
Rats were trained in the cross maze as described in Experiment 1. For the test phase, the same maze set-up in the elevated conflict test (Experiment 1) was used. The only exception was that a rat could only enter the open arm to receive a food reinforcement. A block was placed at the entrance of the closed arm throughout the test session.
Behavioral Training and Testing
Experiment 2 determined whether escitalopram affected anxiety as tested in the elevated plus-maze. In the elevated plus-maze a rat enters an open arm, but there is no food reward. One possibility is that escitalopram does not have a general effect in reducing anxiety, but reduces anxiety when an explicit reward can be obtained. To determine this, Experiment 3 addressed whether escitalopram altered anxiety by reducing the time to enter an open arm when rats were motivated to obtain a food reward. In this test, rats were trained in a cross maze as described in Experiment 1. As in Experiment 1, two arms, opposite each other, were used as start arms during testing. In each trial, the arm opposite the start arm and the closed arm were blocked. A rat was placed in a start arm and had to navigate to the end of the open arm to receive a half piece of cereal. A rat was pseudorandomly started from one of two different start arms so that any one start arm was not used for more than two consecutive trials. Testing was completed once rats retrieved a cereal reinforcement within 20 s on a single trial for ten consecutive trials. This criterion was used because based in Experiment 1 a rat had an average latency of approximately 20 s when it chose the correct arm in the final ten trials of testing. The test criterion was used to make it comparable to that used in Experiment 1.
Treatment
Rats were randomly assigned to either the saline (n = 6) or escitalopram 1.0 mg/kg (n = 6) group. Treatments were administered i.p. 30 minutes prior to testing. Escitalopram at 1.0 mg/kg was chosen because this was the highest dose of the SSRI that was effective in the elevated conflict test.
Experiment 4: The effect of escitalopram on acquisition and reversal learning of a spatial discrimination
To compare the effects of SSRI in inhibition of a naturally-occurring prepotent bias with reversal of a learned prepotent response pattern, this experiment investigated the effect of acute escitalopram treatment on acquisition and reversal learning of a spatial discrimination.
Apparatus
In the spatial discrimination test, training and testing occurred in a cross-maze identical to the maze used for training in the elevated conflict test (Experiment 1). Therefore, each arm was 55 cm long and 10 cm wide with 15 cm high side walls and a 15 cm high back wall.
Behavioral Training and Testing
In Experiment 4, the effect of escitalopram on acquisition and reversal learning of a spatial discrimination was conducted. Each rat was tested on acquisition and reversal learning of a spatial discrimination over two consecutive days. A similar testing procedure was used as in previous studies (Brown et al., 2010; Ragozzino & Choi, 2004) except that here, a probabilistic learning procedure was used in both test sessions. In the acquisition phase, one choice arm was designated as the correct arm and contained a half piece of cereal on 80% of the trials. On the other 20% of trials, the incorrect arm was baited with a half piece of cereal. The first two trials of the test always contained a reinforcement in the correct arm. Acquisition criterion was achieved when a rat entered the correct arm for ten consecutive trials. Thus, a rat had to learn to always enter the same maze arm based on spatial location for ten consecutive trials.
On the second day of testing (reversal learning), the correct and incorrect arms were reversed from those on acquisition such that a rat was required to enter the arm opposite to that on acquisition. Thus, the new correct arm was reinforced on 80% of the trials and the new incorrect arm was reinforced on 20% of the trials. The first two trials of the test always contained a reinforcement in the correct arm. The criterion for reversal learning was ten consecutive trials for entering the new correct arm.
Treatment
Each rat received an i.p. injection 30 minutes prior to the acquisition phase and reversal learning phase. Rats were randomly assigned to one of the following treatment groups (acquisition treatment- reversal learning treatment): 1) Saline- Saline (n=11); 2) Escitalopram 0.3 mg/kg- Saline (n=11); 3) Escitalopram 1.0 mg/kg- Saline (n=11); 4) Saline- Escitalopram 0.03 mg/kg (n=11); 5) Saline- Escitalopram 0.3 mg/kg (n=11); 6) Saline- Escitalopram 1.0 mg/kg (n=11); 7) Escitalopram 1.0 mg/kg- Escitalopram 1.0 mg/kg (n=7). Group 1 served as the control group. Groups 2–3 determined whether various doses of escitalopram affected acquisition. Groups 4–6 determined whether various doses of escitalopram affected reversal learning. Group 7 determined whether escitalopram at the high dose led to state dependent learning.
Analysis of Errors
Similar to that in the elevated conflict test, an analysis of errors was conducted to determine whether escitalopram treatment affected perseverative and regressive errors in reversal learning. Perseverative and regressive errors were analyzed similar to Experiment 1. In addition, an analysis was performed to determine whether escitalopram altered the sensitivity to reinforcement (win-stay) or no reinforcement (lose-shift) on correct trials. The specific analysis was the same as described for the elevated conflict test (Experiment 1).
Experiment 5A: The effect of post-acquisition escitalopram on spatial reversal learning
In Experiment 4, escitalopram 1.0 mg/kg given prior to acquisition improved spatial reversal learning 24 hours later. To determine whether this dose of escitalopram might possibly decrease memory consolidation and thus enhance reversal learning, the drug was administered post-acquisition. If this is the case, then a post-acquisition injection of escitalopram should enhance spatial reversal learning.
Behavioral Training and Testing
The same apparatus used in Experiment 4 was used in this study. The training and testing procedure was identical to that described above for Experiment 4. However, rats were not injected prior to acquisition, but administered either saline or escitalopram immediately following acquisition. On the second day of testing (reversal learning), rats were injected with saline 30 minutes prior to testing.
Treatment
Rats were assigned to either the Saline - Saline (n=9) or Escitalopram 1.0 mg/kg - Saline (n=10) group.
Experiment 5B: The effect of post-acquisition escitalopram on retrieval of a learned spatial discrimination
Comparable to Experiment 5A, the goal of Experiment 5B was to determine whether a post-acquisition injection of escitalopram decreased memory consolidation. However, in this experiment, rats received retrieval trials in the subsequent test session as opposed to a reversal learning procedure. If escitalopram impairs memory consolidation of a learned spatial discrimination, then rats receiving this treatment should be impaired in retrieving the previously learned discrimination.
Behavioral Training and Testing
The cross-maze used in Experiment 4 was also used in this study The training procedure was identical to that described in Experiment 4. As in Experiment 5A, saline or escitalopram 1.0 mg/kg was administered immediately following acquisition. The following day rats received a retrieval test. Thirty-minutes prior to the retrieval test, each rat received an i.p. injection of saline. In the retrieval session, rats were tested on the same discrimination they had learned previously. Thus, the correct arm remained correct, and again was only reinforced on 80% of the trials, and the incorrect arm was reinforced on 20% of the trials. Retrieval testing was completed when a rat chose the correct arm for 10 consecutive trials.
Treatment
Each rat was assigned to either the Saline - Saline (n=8) or Escitalopram 1.0 mg/kg- Saline (n=6) group.
Statistical Analysis
For the elevated conflict test (Experiment 1) and spatial discrimination test (Experiment 4), a one-way analysis of variance (ANOVA) determined whether there was a significant treatment effect on trials to criterion. In the spatial discrimination test, a separate ANOVA was conducted for the acquisition phase and reversal learning phase. In both the elevated conflict test and spatial reversal learning test, ANOVA tests were conducted to examine differences among the groups on both perseverative and regressive errors. In these same studies, ANOVA tests were conducted to determine a treatment effect on win-stay and lose-shift performance. A significant treatment effect was followed by a Newman-Keuls post-hoc test to determine significant differences between treatment groups. An alpha level of 0.05 was set for significance in all of the statistical analyses.
In the elevated plus-maze experiment, an analysis of the percent open arm duration and percent open arm entries was conducted. Percent open arm duration was determined by dividing the open arm time by the total of the open arm time + closed arm time. The percent open arm entries was determined by dividing the number of open arm entries by the total number of arm entries. Unpaired t-tests determined whether there were significant differences in percent open arm durations or percent open arm entries between the groups. In Experiments 3, 5A and 5B, t-tests were performed to determine differences between the groups on the number of trials to criterion on acquisition, reversal learning and/or retrieval. In addition for Experiment 3, an ANOVA with repeated measures on the latency to complete a trial for the first and last block of 10 trials was carried out. For these analyses an alpha level of 0.05 was set for significance.
Results
Experiment 1: The effect of escitalopram on prepotent response inhibition in the elevated conflict test
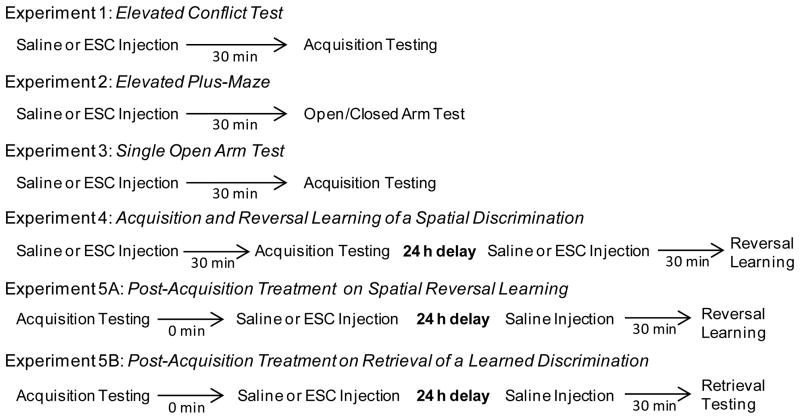
The results in the elevated conflict test are shown in Figure 2. Saline-treated rats required approximately 80 trials to achieve criterion in this task. Escitalopram treatment reduced the trials to criterion in this task ranging from a mean score of 59.6 ± 5.2 SEM to 40.3 ± 1.8 SEM. There was a significant difference in trials to criterion among the groups (F3,29 = 5.85, P < 0.01). Escitalopram at all doses significantly facilitated acquisition compared to that of saline treatment (P’s < 0.05).
Figure 2.
Effect of escitalopram on acquisition in the elevated conflict test. Each rat received an i.p. injection of either saline or escitalopram 30 minutes prior to testing. A) Mean (± SEM) trials to criterion on acquisition. Escitalopram treatment at 0.03, 0.3 and 1.0 mg/kg significantly reduced the number of trials to criterion. * = P < 0.05 vs. SAL. ** = P < 0.01 vs. SAL B) Mean (± SEM) perseverative errors committed during acquisition. Escitalopram treatment did not affect the number of perseverative errors. C) Mean (± SEM) regressive errors committed during acquisition. Escitalopram, 0.03, 0.3 and 1.0 mg/kg significantly reduced regressive errors. * = P < 0.05 vs. saline. ** = P < 0.01 vs. saline. D) Mean (± SEM) percent probabilities of win-stay performance. Escitalopram treatment did not affect win-stay performance. E) Mean (± SEM) percent probabilities of lose-shift performance. Escitalopram treatment did not affect lose-shift performance. SAL = saline and ESC = escitalopram.
In acquiring the elevated conflict test, saline controls perseverated and chose the closed arm an average of nine trials (see Figure 2B). Escitalopram-treated groups tended to perseverate less with scores ranging from a mean of 3.3 ± 1.9 to 6.0 ± 1.4. However, there was no significant difference in perseverative errors among the groups (F3,29= 0.94, P > 0.05). In contrast, there was a significant group effect for regressive errors (F3,29 = 4.91, P < 0.01; see Figure 2C). Rats administered escitalopram at all doses tested committed significantly fewer regressive errors compared to that of saline controls (P’s < 0.05).
An analysis of the win-stay/lose-shift performance indicated that escitalopram treatment did not affect win-stay or lose-shift performance (see Figures 2D and 2E). All groups exhibited an approximately 60% probability of win-stay choices and a range of 40–60% probability for lose-stay choices. There was no significant difference in win-stay probabilities among the groups (F3,29 = 0.80, P > 0.05). In a similar manner, there was not a significant difference among the groups in lose-shift probability (F3,29 = 2.85, P > 0.05).
Experiment 2: The effect of escitalopram treatment on the elevated plus-maze
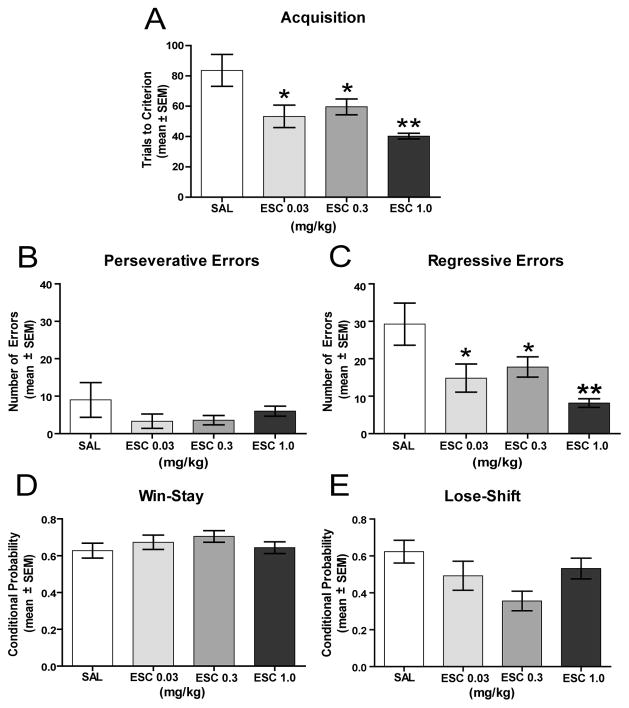
Escitalopram treatment did not affect the time spent in the open arms or the number of open arm entries (see Figures 3A and 3B). The saline group spent a mean of 26.8 ± 6.0% of time in the open arms, while the escitalopram (1.0 mg/kg) group spent 18.3 ± 5.2% of time in the open arms. There was not a significant difference in the percentage of time spent in open arms between the groups (t(12) = 1.07, P > 0.05). For open arm entries, both groups chose an open arm approximately one-third of the time. There was no significant difference in the percent of open arm entries between the groups (t(12) = 0.15, P > 0.05). Thus, escitalopram did not affect either measure in the elevated plus-maze.
Figure 3.
Effect of escitalopram on open arm entries and open arm duration in the elevated plus-maze. Each rat received an i.p. injection of either saline or escitalopram 1.0 mg/kg 30 minutes prior to testing. A) Mean (± SEM) percent open arm entries. Escitalopram treatment did not affect the percentage of open arm entries. B) Mean (± SEM) percent open arm duration. Escitalopram treatment did not affect the percentage of time spent in the open arms. SAL = saline and ESC = escitalopram.
Experiment 3: The effect of escitalopram on the single open arm test
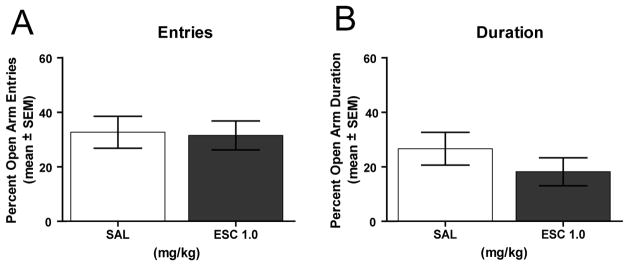
This experiment determined whether escitalopram may reduce anxiety in an elevated maze in order to obtain a reward. If escitalopram reduces anxiety under conditions in which a food reward can be obtained, then escitalopram treatment should enhance acquisition in the single open arm test. The results from the single open arm test are shown in Figure 4. There was not a significant difference in trials to criterion between the saline and escitalopram groups (t(10) = 0.31, P > 0.05). A further analysis on the latency to obtain food from the open arm in first and last blocks of trials revealed there was not a treatment effect (F1,20 = 0.11, P >0.05), but there was a significant block effect (F1,20 = 16.08, P < 0.05) reflecting a decrease in latencies from the first to last block. There was not a significant treatment × block interaction (F1,20 = 0.03, P >0.05). Thus, escitalopram did not alter the latency to retrieve a food reward from the open arm.
Figure 4.
Effect of escitalopram on performance in the single open arm test. Each rat received an i.p. injection of either saline or escitalopram 1.0 mg/kg 30 minutes prior to testing. A) Mean (± SEM) number of trials to reach criterion. Escitalopram treatment did not affect the number of trials to criterion in the single open arm test. B) Mean (± SEM) latency to obtain a food reward in the open in the first and last block of trials. Each block represents 10 trials. Escitalopram treatment did not affect the latency to obtain a food reward. SAL = saline and ESC = escitalopram.
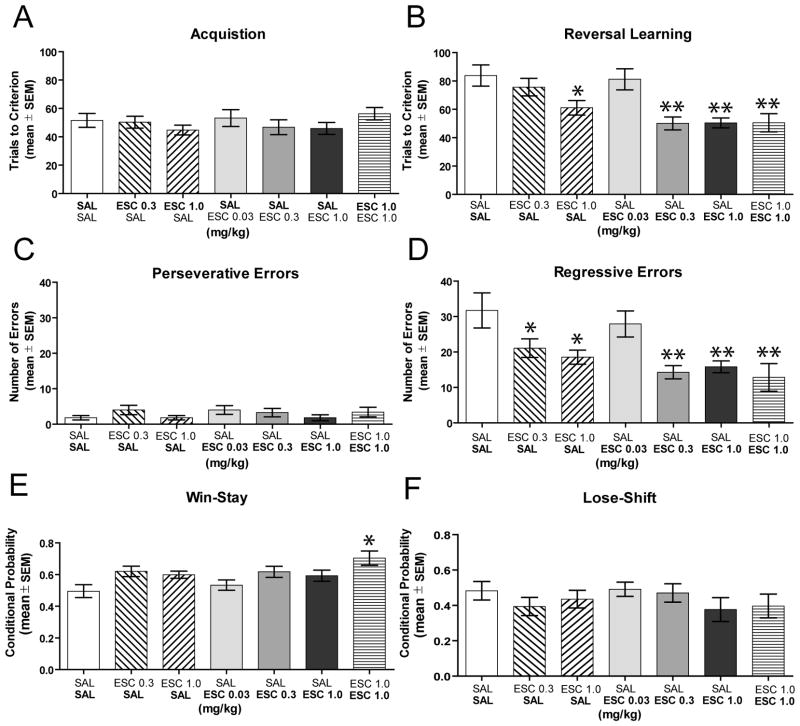
Experiment 4: The effect of escitalopram on acquisition and reversal learning of a spatial discrimination
Acquisition of the spatial discrimination required approximately 50 trials to achieve criterion in both saline-treated and escitalopram-treated rats (see Figure 5A). An ANOVA indicated that there was not a significant treatment effect on spatial acquisition (F6,66 = 0.65, P > 0.05). In contrast to acquisition, the difference in trials to criterion during reversal learning among the groups was significant (F6,66 = 6.20, P < 0.01). As shown in Figure 5B, treatment with escitalopram at 0.3 and 1.0 mg/kg significantly enhanced reversal learning performance compared to that of the saline- saline and saline- escitalopram 0.03 mg/kg groups (P’s < 0.05). The low dose of escitalopram (0.03 mg/kg) administered in the reversal learning session did not affect performance compared to that of the saline- saline and escitalopram 0.3 mg/kg – saline groups (P’s > 0.05). Rats that received escitalopram 1.0 mg/kg on acquisition and saline on reversal learning required significantly fewer trials in reversal learning compared to that of the saline- saline and saline- escitalopram 0.03 mg/kg groups (P’s < 0.05).
Figure 5.
Effect of escitalopram treatment on spatial acquisition and reversal learning. Each rat received an i.p. injection of either saline or escitalopram 30 minutes prior to each test session. The treatments on the x-axis represent the treatment received prior to acquisition (top) and prior to reversal learning (bottom). A) Mean (± SEM) trials to criterion on place acquisition. Escitalopram treatment did not affect acquisition. B) Mean (± SEM) trials to criterion on reversal learning. Escitalopram 1.0 mg/kg – saline, saline – escitalopram 0.3 mg/kg, saline – escitalopram 1.0 mg/kg, escitalopram 1.0 mg/kg – escitalopram 1.0 mg/kg significantly enhanced reversal learning. ** = P < 0.01 vs. SAL - SAL and SAL - ESC 0.03 and ESC 0.3 – SAL. * = P < 0.05 vs. SAL - SAL and SAL - ESC 0.03. C) Mean (± SEM) perseverative errors committed during reversal learning. Escitalopram treatment did not affect the number of perseverative errors. D) Mean (± SEM) regressive errors committed during reversal learning. Escitalopram 0.3 and 1.0 mg/kg administered prior to acquisition, reversal learning, or both significantly reduced regressive errors. * = P < 0.05 vs. SAL - SAL. ** = P < 0.01 vs. SAL - SAL and SAL - ESC 0.03 mg/kg. E) Mean (± SEM) percent probabilities of win-stay performance. The ESC 1.0- ESC 1.0 mg/kg group had enhanced win-stay probabilities. * = P < 0.05 vs. SAL - SAL and SAL - ESC 0.03 mg/kg. F) Mean (± SEM) percent probabilities of lose-shift performance. Escitalopram treatment did not affect lose-shift performance. SAL = saline and ESC = escitalopram.
Escitalopram 1.0 mg/kg treatment on acquisition that subsequently led to improved reversal learning, might reflect state-dependent learning. This is because the SSRI injected in the acquisition phase might lead to learning under the drug state. When this group was administered saline just prior to reversal learning it showed facilitation because it was no longer under the drug state and thus would not be biased in using the originally learned choice pattern. To control for this, another treatment group received escitalopram 1.0 mg/kg prior to the acquisition and reversal learning session. If escitalopram, at this dose, led to state-dependent learning, then this group should be impaired or certainly not exhibit facilitation in the reversal learning phase. To the contrary, this group exhibited a significant reduction in trials to criterion during reversal learning compared to that of the saline- saline, saline- escitalopram 0.03 mg/kg and escitalopram 0.3 mg/kg – saline groups (P’s < 0.05). Furthermore, escitalopram 1.0 mg/kg – escitalopram 1.0 mg/kg treatment was not significantly different in reversal learning performance compared to that of saline- escitalopram 0.3 mg/kg or saline- escitalopram 1.0 mg/kg treatment (P’s > 0.05).
In reversal learning, all groups commonly committed perseverative errors in the first block of trials, but subsequently began to choose the new correct spatial location (see Figure 5C). There was no significant difference in perseverative errors among the groups (F6,66 = 0.46, P > 0.05). There was a significant group effect for the number of regressive errors (F6,66 = 5.10, P < 0.01). As illustrated in Figure 5D, the groups that received escitalopram 0.3 or 1.0 mg/kg in acquisition had significantly fewer regressive errors in reversal learning compared to that of the saline- saline group (P’s < 0.05). Escitalopram at 1.0 mg/kg administered during acquisition and reversal learning, as well as escitalopram administered at 0.3 or 1.0 mg/kg prior to the reversal learning session, also significantly reduced regressive errors compared to that of the saline-escitalopram 0.03 mg/kg and saline- saline groups (P’s < 0.05).
In reversal learning, all groups had a win-stay probability of 50–70% and approximately 50% lose-shift probability (Figures 5E and 5F). There was a significant group effect on win-stay probability scores (F6,66= 3.22, P <0.05). The escitalopram 1.0 mg/kg - escitalopram 1.0 mg/kg group exhibited a significantly greater win-stay choice pattern than that of the saline- saline group and the saline- escitalopram 0.03 mg/kg group (P’s < 0.05). There was no significant difference in lose-shift probabilities among the groups (F6,66 = 0.76, P > 0.05).
Experiment 5A: The effect of post-acquisition escitalopram on spatial reversal learning
Because escitalopram 1.0 mg/kg administered prior to acquisition improved reversal learning performance, subsequent experiments were carried out to determine whether escitalopram treatment on acquisition may facilitate reversal learning by impairing memory consolidation. If escitalopram impairs memory consolidation leading to an enhancement of reversal learning, then escitalopram administered immediately following spatial acquisition should enhance reversal learning performance. The groups that received a post-acquisition injection of saline or escitalopram 1.0 mg/kg exhibited a similar acquisition rate with mean scores of 62.8 ± 11.9 and 53.5 ± 7.6, respectively. The difference in acquisition rates between the groups was not significant (t(17) = 0.67, P > 0.05). A post-acquisition injection of escitalopram 1.0 mg/kg (mean = 69.2 ± 8.0) produced a modest improvement in reversal learning performance compared to that of saline controls (mean = 82.7 ± 7.2), but the difference between the groups was not significant (t(17) = 1.24, P > 0.05).
Experiment 5B: The effect of post-acquisition escitalopram on retrieval of a learned spatial discrimination
If escitalopram impairs memory consolidation, then escitalopram administered immediately following spatial acquisition should impair retention of the learned discrimination. The results from this test indicated that the saline and escitalopram groups required a comparable number of trials to achieve criterion in the acquisition phase (means = 60.3 ± 7.5 and 61.3 ± 4.7, respectively). The difference in trials to criterion between the groups was not significant (t(12) = 0.11, P > 0.05). Post-acquisition injection of escitalopram 1.0 mg/kg or saline led to scores in the retention test 24 hours later that were comparable, means = 24.8 ± 5.1 and 24.0 ± 4.1, respectively. The difference in retention scores between the groups was not significant (t(12) = 0.13, P > 0.05).
Discussion
The present experiments demonstrated that acute treatment with the SSRI, escitalopram, enhances behavioral flexibility when rats must shift away from either a learned prepotent response pattern (spatial reversal learning) or a naturally-occurring prepotent response pattern (elevated conflict test). Past studies have focused on the role of 5-HT in behavioral flexibility employing reversal learning or set-shifting tests. The depletion of brain 5-HT or chronic intermittent stress, which reduces frontal cortex 5-HT release (Lapiz-Bluhm et al., 2009), impairs reversal learning (Bondi et al., 2008; Clarke et al., 2005,2007; Lapiz-Bluhm et al., 2009; Man et al., 2010). Furthermore, SSRI treatment has been reported to enhance reversal learning (Bari et al., 2010) or alleviate a stress-induced set-shifting deficit (Bondi et al., 2008). However, the findings from the elevated conflict test are the first to demonstrate that an SSRI (escitalopram) can also facilitate inhibition of a naturally-occurring response pattern when an alternative choice pattern is optimal. In support of the idea that rats exhibit a natural-bias in preferring the closed arm over the open arm in the elevated conflict test, control rats averaged about 10 trials before first entering the open arm. In contrast, when having a choice between two closed arms as in the spatial discrimination test, rats do not exhibit a similar avoidance for initially entering either arm (data not shown). Furthermore, the trials to criterion in the elevated conflict test was achieved in approximately 80 trials for the control group while acquisition in the spatial discrimination test was achieved in around 50 trials for the control group. Thus, the results suggest that escitalopram enhanced performance in the elevated conflict test by inhibiting a naturally-biased prepotent response pattern.
All the escitalopram doses tested enhanced performance in the elevated conflict test. This includes the lowest dose of 0.03 mg/kg. Past studies suggest that even this low dose of escitalopram can result in plasma concentrations of the drug that correspond with high occupancy of the of the 5-HT transporter (Bundgaard et al., 2006; Sanchez et al., 2003). Moreover, this dose of escitalopram has been shown to increase 5-HT release in the brain (Ceglia et al., 2004). Thus, the present findings in the elevated conflict and spatial reversal learning test suggest that acute SSRI treatment enhances brain 5-HT release to facilitate multiple conditions that require behavioral flexibility.
The elevated conflict test is a task that has a significant “anxiety” component similar to that in the elevated plus-maze. Thus, escitalopram enhancing acquisition in the elevated conflict maze may have resulted from principally reducing anxiety. However, escitalopram at a dose that enhanced performance on the elevated conflict test did not alter performance in the elevated plus-maze. This finding is consistent with other studies showing that citalopram or escitalopram does not affect open arm entries or duration in the elevated plus-maze (Bondi et al., 2008; Sun et al., 2010). Although, acute escitalopram treatment does reduce fear-like responses induced by electrical stimulation of the dorsolateral periaqueductal gray (Lim et al., 2010) and for entering an open arm in the elevated T-maze (Pinheiro et al., 2008). However, the doses used in these studies were higher (2–10 mg/kg) than the highest dose (1 mg/kg) used in the present experiments. Thus, the different findings with escitalopram on measures of anxiety may be explained, at least in part, due to the different drug doses used. Alternatively, escitalopram may reduce anxiety to overcome a naturally-biased prepotent response pattern when there is a significant motivational component, e.g. food reward. Experiment 3 tested for this by determining whether escitalopram affected acquisition in the single open arm test. Escitalopram at 1 mg/kg had no effect on acquiring this test. Taken together, the findings suggest that escitalopram treatment facilitated performance on the elevated conflict test by principally affecting inhibitory processes and not by reducing anxiety.
In the elevated conflict test, escitalopram improved the ability to maintain a response pattern into the open arm after being initially selected. This was observed by a significant reduction in regressive errors. A similar pattern of results was observed in the spatial reversal learning test in which escitalopram facilitated reversal learning by reducing regressive errors. Thus, escitalopram did not affect the initial inhibition of a naturally-biased prepotent response or initial inhibition of a learned prepotent response, but selectively enhanced the ability to reliably execute a new choice pattern after the initial selection. In order to successfully switch choice patterns a subject must initially inhibit the previously correct choice pattern and switch to an alternative choice pattern, but must also maintain that switch by actively inhibiting selection of the previously correct choice pattern. Although escitalopram facilitated the maintenance of the shift in response patterns this did not result from an altered sensitivity to positive reinforcement (win-stay) or negative reinforcement (lose-shift) on correct trials. Previous studies using sustained attention or stop-signal tests have shown that increased brain 5-HT activity reduces impulsive or premature choices (Homberg et al., 2007), while decreasing brain 5-HT release increases impulsive choices (Fletcher et al., 2009; Winstanley et al., 2004). One possible explanation for the present results is that enhancing 5-HT release with escitalopram improved response inhibition of the naturally-biased or learned prepotent response that facilitated the maintenance of a new choice pattern.
Previous work from our laboratory has shown that the dorsomedial striatum and parafascicular thalamic nucleus, two brain regions that are interconnected (Lapper & Bolam, 1992), are important for maintaining a choice pattern when conditions require a change in strategies (Brown et al., 2010; Ragozzino & Choi, 2004). Both the striatum and parafascicular thalamic nucleus receive serotonergic input from the dorsal raphe nucleus (Sim & Joseph, 1992; Vertes, 1991, 2010). One possibility is that escitalopram treatment modulates activity in this circuit to enhance an accurate selection of a response pattern. More specifically, SSRI treatment may facilitate striatal acetylcholine release to enhance behavioral flexibility. Several studies have demonstrated that an increase in acetylcholine release from the dorsomedial striatum is critical for facilitating reversal learning by augmenting the ability to maintain a new choice after being selected (Brown et al., 2010; Palencia & Ragozzino, 2006; Ragozzino & Choi, 2004; Ragozzino et al., 2009). Furthermore, excitatory input from the parafascicular thalamic nucleus is critical for stimulating acetylcholine release in the dorsomedial striatum during reversal learning (Brown et al., 2010). Moreover, past studies have shown that direct brain infusions or systemic injections of a SSRI enhance acetylcholine release from various brain regions (Consolo et al., 1994; Egashira et al., 2006; Yamaguchi et al., 1997). Thus, SSRI treatment may enhance 5-HT release in the parafascicular thalamic nucleus and dorsomedial striatum to increase striatal acetylcholine output facilitating a flexible shift in response patterns.
Escitalopram may alternatively or additionally modify 5-HT release in the orbitofrontal cortex to facilitate the maintenance of a new response pattern after a switch away from a prepotent response pattern. A 5-HT depletion in the orbitofrontal cortex of marmosets impairs reversal learning (Clarke et al., 2007) and drugs that target specific 5-HT receptors infused into the rat orbitofrontal cortex enhance reversal learning (Boulougouris & Robbins, 2010). Orbitofrontal cortex lesions or inactivation often lead to perserverative responding in reversal learning (Chudasama & Robbins, 2003; Kim & Ragozzino, 2005). However, under certain conditions, i.e. increased level of difficulty, the orbitofrontal cortex is important for maintaining a newly selected response pattern during reversal learning (Kim & Ragozzino, 2005). The orbitofrontal cortex projects to the dorsomedial striatum (Berendse et al., 1992) and receives input from intralaminar nuclei that include the parafascicular thalamic nucleus (Berendse & Groenewegen, 1991). This raises the possibility that SSRI treatment increases 5-HT release to modulate a frontal cortex –basal ganglia –thalamic circuit to maintain a shift in a response pattern away from a prepotent choice pattern.
In the spatial discrimination test, escitalopram at the 1.0 mg/kg dose administered prior to acquisition did not affect initial learning, but facilitated reversal learning. Treatment with escitalopram, at similar doses as used in the present experiments, rapidly elevates brain extracellular 5-HT levels which remains elevated for more than two hours (Ceglia et al., 2004; Mork et al., 2003). The spatial acquisition session commonly lasted between 20–40 minutes. Thus, brain 5-HT levels would still be elevated after the completion of acquisition testing, which could have affected the consolidation of the learned discrimination. Specifically, an elevation of 5-HT levels post-acquisition may have impaired memory consolidation that subsequently facilitated reversal learning. However, contrary to the idea that escitalopram at 1.0 mg/kg impaired memory storage, a post-acquisition injection of escitalopram did not affect memory retrieval. Thus, the present findings suggest that escitalopram did not alter memory consolidation to affect reversal learning.
An alternative possibility is that escitalopram leads to state-dependent learning. In this scenario, if a rat initially learned the spatial discrimination under escitalopram and received saline during reversal learning a facilitation should be observed. Conversely, if a rat received escitalopram on acquisition and reversal learning, it should be impaired on reversal learning. Escitalopram at 1.0 mg/kg did not lead to state-dependent learning, because escitalopram administered in the acquisition and reversal learning phase still led to enhanced reversal learning performance. Another possibility is that an acute injection of escitalopram led to plastic changes in the brain that outlasted the time in which the drug was effective, such that beneficial effects of the drug on behavioral flexibility were still observed 24 hours later. However, if escitalopram has a longer lasting effect on behavioral flexibility, the present findings suggest that it is somewhat limited. Escitalopram treatment administered post-acquisition was not as effective in facilitating reversal learning as compared to administration just prior to the reversal learning session.
There is accumulating evidence that abnormalities in the 5-HT transporter and behavioral flexibility impairments are associated with conditions such as obsessive compulsive disorder and autism spectrum disorders (Baumgarten and Grozdanovic, 1998; Doughery et al., 2004; El Mansari and Blier, 2006; Owley et al., 2005; Zitterl et al., 2008; 2009). These among other factors have resulted in individuals with obsessive-compulsive disorder and autism spectrum disorder being treated with SSRIs (Denys et al., 2007; Owley et al., 2010). The present findings suggest that treatment with a SSRI such as escitalopram may be effective in reducing impairments in cognitive flexibility when conditions require inhibition of a learned response pattern, as well as inhibition of a prepotent response pattern. Interestingly, acute administration of the SSRI fluvoxamine significantly decreases repetitive behaviors in autistic individuals (McDougle et al., 1996a). Conversely, short term tryptophan depletion increases repetitive behaviors in autistic adults (McDougle et al., 1996b). These findings suggest that 5-HT transmission influences repetitive behaviors in autism which can be modified by treatments which block reuptake of 5-HT. The present results suggest that SSRI treatment may also be effective in reducing other “insistence on sameness” symptoms in autism spectrum disorder particularly when cognitive demands require inhibition of a learned or an automatic response pattern.
The present experiment only investigated the effects of an acute dose of escitalopram. Future experiments that involve chronic treatment with escitalopram will be important in determining whether repeated treatment with this SSRI is also effective in improving behavioral flexibility. In addition, the effects observed with escitalopram were in normal rats. It is unclear whether SSRI treatment would have similar effects in animal models of psychiatric disorders that exhibit behavioral flexibility deficits as in obsessive-compulsive disorder and autism spectrum disorder. Although, a recent study reported that SSRI treatment reduces social deficits in a mouse model of autism (Chadman, 2011). Furthermore, clinical trials have used atypical antipsychotics, e.g. risperidone or olanzapine, in combination with a SSRI to treat individuals with obsessive-compulsive disorder (Hollander et al., 2003; Koran et al., 2000). These atypical anti-psychotics are known to have 5-HT2 antagonistic properties and have been shown to reduce obsessive thoughts (McDougle et al., 2000). In a recent experiment, we demonstrated that blockade of the 5-HT2A but not 5-HT2C, receptors, improved behavioral flexibility in a strategy switching test (Baker et al., 2011). One possibility is that a combined therapy of a SSRI and 5-HT2A receptor antagonist may be an effective treatment in alleviating cognitive flexibility deficits in conditions such as obsessive compulsive disorder and autism spectrum disorder.
Acknowledgments
This research was supported by NIH grant P50 HD055751 (JAS, MER). We appreciate the assistance of Monika Plonka, Chiali Lee and Punam Rajyaguru with behavioral testing.
Footnotes
Disclosure/Conflict of Interest
John A. Sweeney serves a consultant for Pfizer Pharmaceuticals and has an investigator initiated grant from Janssen Pharmaceuticals. Michael E. Ragozzino has received an investigator initiated grant from Epix Pharmaceuticals
References
- Agam Y, Joseph RM, Barton JJS, Manoach DS. Reduced cognitive control of response inhibition by the anterior cingulate cortex in autism spectrum disorders. Neuroimage. 2010;52:336–347. doi: 10.1016/j.neuroimage.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker PM, Thompson JL, Sweeney JA, Ragozzino ME. Differential effects of 5-HT(2A) and 5-HT(2C) receptor blockade on strategy-switching. Behav Brain Res. 2011;219:123–31. doi: 10.1016/j.bbr.2010.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bari A, Theobald DE, Caprioli D, Mar AC, Aidoo-Micah A, Dalley JW, Robbins TW. Serotonin modulates sensitivity to reward and negative feedback in a probabilistic reversal learning task in rats. Neuropsychopharm. 2010;35:1290–1301. doi: 10.1038/npp.2009.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgarten HG, Grozdanovic Z. Role of serotonin in obsessive-compulsive disorder. Br J Psychiatry. 1998;35:13–20. [PubMed] [Google Scholar]
- Berendse HW, Groenewegen HJ. Restricted cortical termination fields of the midline and intralaminar thalamic nuclei in the rat. Neurosci. 1991;42:73–102. doi: 10.1016/0306-4522(91)90151-d. [DOI] [PubMed] [Google Scholar]
- Berendse HW, Galis-de Graaf Y, Groenewegen HJ. Topographical organization and relationship with ventral striatal compartments of prefrontal corticostriatal projections in the rat. J Comp Neurol. 1992;316:314–347. doi: 10.1002/cne.903160305. [DOI] [PubMed] [Google Scholar]
- Bitterman ME. The comparative analysis of learning. Science. 1975;188:699–709. doi: 10.1126/science.188.4189.699. [DOI] [PubMed] [Google Scholar]
- Bondi CO, Rodriguez G, Gould GG, Frazer A, Morilak DA. Chronic unpredictable stress induces a cognitive deficit and anxiety-like behavior in rats that is prevented by chronic antidepressant drug treatment. Neuropsychopharm. 2008;33:320–331. doi: 10.1038/sj.npp.1301410. [DOI] [PubMed] [Google Scholar]
- Boulougouris V, Robbins TW. Enhancement of spatial reversal learning by 5-HT2C receptor antagonism is neuroanatomically specific. J Neurosci. 2010;30:930–938. doi: 10.1523/JNEUROSCI.4312-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown HD, Baker PM, Ragozzino ME. The parafascicular thalamic nucleus concomitantly influences behavioral flexibility and dorsomedial striatal acetylcholine output in rats. J Neurosci. 2010;30:14390–14398. doi: 10.1523/JNEUROSCI.2167-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bundgaard C, Larsen F, Jørgensen M, Gabrielsson J. Mechanistic model of acute autoinhibitory feedback action after administration of SSRIs in rats: application to escitalopram-induced effects on brain serotonin levels. Eur J Pharm Sci. 2006;29:394–404. doi: 10.1016/j.ejps.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Burghardt NS, Sullivan GM, McEwen BS, Gorman JM, LeDoux JE. The selective serotonin reuptake inhibitor citalopram increases fear after acute treatment but reduces fear with chronic treatment: a comparison with tianeptine. Biol Psychiatry. 2004;55:1171–1178. doi: 10.1016/j.biopsych.2004.02.029. [DOI] [PubMed] [Google Scholar]
- Ceglia I, Acconcia S, Fracasso C, Colovic M, Caccia S, Invernizzi RW. Effects of chronic treatment with escitalopram or citalopram on extracellular 5-HT in the prefrontal cortex of rats: role of 5-HT1A receptors. Br J Psychiatry. 2004;142:469–478. doi: 10.1038/sj.bjp.0705800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadman KK. Fluoxetine but not risperidone increases sociability in the BTBR mouse model of autism. Pharmacol Biochem Behav. 2011;97:586–594. doi: 10.1016/j.pbb.2010.09.012. [DOI] [PubMed] [Google Scholar]
- Christ SE, Holt DD, White DA, Green L. Inhibitory control in children with autism spectrum disorder. J Autism Dev Disord. 2007;37:1155–65. doi: 10.1007/s10803-006-0259-y. [DOI] [PubMed] [Google Scholar]
- Chudasama Y, Robbins TW. Dissociable contributions of the orbitofrontal and infralimbic cortex to pavlovian autoshaping and discrimination reversal learning: further evidence for the functional heterogeneity of the rodent frontal cortex. J Neurosci. 2003;23:8771–8780. doi: 10.1523/JNEUROSCI.23-25-08771.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke HF, Walker SC, Crofts HS, Dalley JW, Robbins TW, Roberts AC. Prefrontal serotonin depletion affects reversal learning but not attentional set shifting. J Neurosci. 2005;25:532–538. doi: 10.1523/JNEUROSCI.3690-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke HF, Walker SC, Dalley JW, Robbins TW, Roberts AC. Cognitive inflexibility after prefrontal serotonin depletion is behaviorally and neurochemically specific. Cereb Cortex. 2007;17:18–27. doi: 10.1093/cercor/bhj120. [DOI] [PubMed] [Google Scholar]
- Consolo S, Bertorelli R, Russi G, Zambelli M, Ladinsky H. Serotonergic facilitation of acetylcholine release in vivo from rat dorsal hippocampus via serotonin 5-HT3 receptors. J Neurochem. 1994;62:2254–2261. doi: 10.1046/j.1471-4159.1994.62062254.x. [DOI] [PubMed] [Google Scholar]
- Denys D, Van Nieuwerburgh F, Deforce D, Westenberg HG. Prediction of response to paroxetine and venlafaxine by serotonin-related genes in obsessive-compulsive disorder in a randomized, double-blind trial. J Clin Psychiatry. 2007;68:747–753. doi: 10.4088/jcp.v68n0512. [DOI] [PubMed] [Google Scholar]
- Dickstein DP, Finger EC, Brotman MA, Rich BA, Pine DS, Blair JR, Leibenluft E. Impaired probabilistic reversal learning in youths with mood and anxiety disorders. Psychol Med. 2009;12:1–12. doi: 10.1017/S0033291709991462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty DD, Rauch SL, Jenike MA. Pharmacotherapy for obsessive-compulsive disorder. J Clin Psychol. 2004;60:1195–1202. doi: 10.1002/jclp.20083. [DOI] [PubMed] [Google Scholar]
- Eagle DM, Baunez C. Is there an inhibitory-response-control system in the rat? Evidence from anatomical and pharmacological studies of behavioral inhibition. Neurosci Biobehav Rev. 2010;34:50–72. doi: 10.1016/j.neubiorev.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egashira N, Matsumoto Y, Mishima K, Iwasaki K, Fujioka M, Matsushita M, Shoyama Y, Nishimura R, Fujiwara M. Low dose citalopram reverses memory impairment and electroconvulsive shock-induced immobilization. Pharmacol Biochem Behav. 2006;83:161–167. doi: 10.1016/j.pbb.2006.01.006. [DOI] [PubMed] [Google Scholar]
- El Mansari M, Blier P. Mechanisms of action of current and potential pharmacotherapies of obsessive-compulsive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:362–373. doi: 10.1016/j.pnpbp.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Finger EC, Marsh AA, Buzas B, Kamel N, Rhodes R, Vythilingham M, Pine DS, Goldman D, Blair JR. The impact of tryptophan depletion and 5-HTTLPR genotype on passive avoidance and response reversal instrumental learning tasks. Neuropsychopharm. 2007;32:206–215. doi: 10.1038/sj.npp.1301182. [DOI] [PubMed] [Google Scholar]
- Fletcher PJ, Chambers JW, Rizos Z, Chintoh AF. Effects of 5-HT depletion in the frontal cortex or nucleus accumbens on response inhibition measured in the 5-choice serial reaction time test and on a DRL schedule. Behav Brain Res. 2009;201:88–98. doi: 10.1016/j.bbr.2009.01.036. [DOI] [PubMed] [Google Scholar]
- Goodman WK, Bose A, Wang Q. Treatment of generalized anxiety disorder with escitalopram: pooled results from double-blind, placebo controlled trials. J Affect Disord. 2005;87:161–167. doi: 10.1016/j.jad.2004.11.011. [DOI] [PubMed] [Google Scholar]
- Graeff FG, Viana MB, Tomaz C. The elevated T maze, a new experimental model of anxiety and memory: Effect of diazepam. Braz J Med Biol Res. 1993;26:67–70. [PubMed] [Google Scholar]
- Harris MS, Reilly JL, Thase ME, Keshavan MS, Sweeney JA. Response suppression deficits in treatment-naïve first-episode patients with schizophrenia, psychotic bipolar disorder and psychotic major depression. Psychiatry Res. 2009;170:150–156. doi: 10.1016/j.psychres.2008.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto K, Fujita Y, Iyo M. Phencyclidine-induced cognitive deficits in mice are improved by subsequent subchronic administration of fluvoxamine: role of sigma-1 receptors. Neuropsychopharmacol. 2007;32:514–521. doi: 10.1038/sj.npp.1301047. [DOI] [PubMed] [Google Scholar]
- Hogg S, Michan L, Jessa M. Prediction of anti-panic properties of escitalopram in the dorsal periaqueductal grey model of panic anxiety. Neuropharm. 2006;51:141–145. doi: 10.1016/j.neuropharm.2006.03.009. [DOI] [PubMed] [Google Scholar]
- Hollander E, Baldini Rossi N, Sood E, Pallanti S. Risperidone augmentation in treatment-resistant obsessive-compulsive disorder: a double-blind, placebo-controlled study. Int J Neuropsychopharm. 2003;6:397–401. doi: 10.1017/S1461145703003730. [DOI] [PubMed] [Google Scholar]
- Homberg JR, Pattij T, Janssen MC, Ronken E, De Boer SF, Schoffelmeer AN, Cuppen E. Serotonin transporter deficiency in rats improves inhibitory control but not behavioural flexibility. Eur J Neurosci. 2007;26:2066–2073. doi: 10.1111/j.1460-9568.2007.05839.x. [DOI] [PubMed] [Google Scholar]
- Izquierdo I, Medina JH, Izquierdo LA, Barros DM, de Souza MM, Mello e Souza T. Short- and long-term memory are differentially regulated by monoaminergic systems in the rat brain. Neurobiol Learn Mem. 1998;69:219–224. doi: 10.1006/nlme.1998.3825. [DOI] [PubMed] [Google Scholar]
- Kim J, Ragozzino ME. The involvement of the orbitofrontal cortex in learning under changing task contingencies. Neurobiol Learn Mem. 2005;83:125–133. doi: 10.1016/j.nlm.2004.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koran LM, Ringold AL, Elliott MA. Olanzapine augmentation for treatment-resistant obsessive-compulsive disorder. J Clin Psychiatry. 2000;61:514–517. doi: 10.4088/jcp.v61n0709. [DOI] [PubMed] [Google Scholar]
- Lapiz-Bluhm MD, Soto-Piña AE, Hensler JG, Morilak DA. Chronic intermittent cold stress and serotonin depletion induce deficits of reversal learning in an attentional set-shifting test in rats. Psychopharm (Berl) 2009;202:329–341. doi: 10.1007/s00213-008-1224-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapper SR, Bolam JP. Input from the frontal cortex and the parafascicular nucleus to cholinergic interneurons in the dorsal striatum of the rat. Neurosci. 1992;51:533–545. doi: 10.1016/0306-4522(92)90293-b. [DOI] [PubMed] [Google Scholar]
- Leeson VC, Robbins TW, Matheson E, Hutton SB, Ron MA, Barnes TR, Joyce EM. Discrimination learning, reversal, and set-shifting in first-episode schizophrenia: stability over six years and specific associations with medication type and disorganization syndrome. Biol Psychiatry. 2009;66:586–593. doi: 10.1016/j.biopsych.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim LW, Blokland A, Tan S, Vlamings R, Sesia T, Aziz-Mohammadi M, Visser-Vandewalle V, Steinbusch HW, Schruers K, Temel Y. Attenuation of fear-like response by escitalopram treatment after electrical stimulation of the midbrain dorsolateral periaqueductal gray. Exp Neurol. 2010;226:293–300. doi: 10.1016/j.expneurol.2010.08.035. [DOI] [PubMed] [Google Scholar]
- Lopez BR, Lincoln AJ, Ozonoff S, Lai Z. Examining the relationship between executive functions and restricted, repetitive symptoms of Autistic Disorder. J Autism Dev Disord. 2005;35:445–460. doi: 10.1007/s10803-005-5035-x. [DOI] [PubMed] [Google Scholar]
- Man MS, Dalley JW, Roberts AC. Opposing effects of 5,7-DHT infusions into the orbitofrontal cortex and amygdala on flexible responding. Cereb Cortex. 2010;20:1668–1675. doi: 10.1093/cercor/bhp236. [DOI] [PubMed] [Google Scholar]
- McCool MF, Patel S, Talati R, Ragozzino ME. Differential involvement of M1-type and M4-type muscarinic cholinergic receptors in the dorsomedial striatum in task switching. Neurobiol Learn Mem. 2008;89:114–124. doi: 10.1016/j.nlm.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougle CJ, Naylor ST, Cohen DJ, Aghajanian GK, Heninger GR, Price LH. Effects of tryptophan depletion in drug-free adults with autistic disorder. Arch Gen Psychiatry. 1996a;53:993–1000. doi: 10.1001/archpsyc.1996.01830110029004. [DOI] [PubMed] [Google Scholar]
- McDougle CJ, Naylor ST, Cohen DJ, Volkmar FR, Heninger GR, Price LH. A double-blind, placebo-controlled study of fluvoxamine in adults with autistic disorder. Arch Gen Psychiatry. 1996b;53:1001–1008. doi: 10.1001/archpsyc.1996.01830110037005. [DOI] [PubMed] [Google Scholar]
- McDougle CJ, Epperson CN, Pelton GH, Wasylink S, Price LH. A double-blind, placebo-controlled study of risperidone addition in serotonin reuptake inhibitor-refractory obsessive-compulsive disorder. Arch Gen Psychiatry. 2000;57:794–801. doi: 10.1001/archpsyc.57.8.794. [DOI] [PubMed] [Google Scholar]
- Meneses A. A pharmacological analysis of an associative learning task: 5-HT(1) to 5-HT(7) receptor subtypes function on a pavlovian/instrumental autoshaped memory. Learn Mem. 2007;10:363–72. doi: 10.1101/lm.60503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery KM. The relation between fear induced by novel stimulation and exploratory behavior. J Comp Physiol Psychol. 1958;48:254–260. doi: 10.1037/h0043788. [DOI] [PubMed] [Google Scholar]
- Montezinho LP, Miller S, Plath N, Jensen NH, Karlsson JJ, Witten L, Mørk A. The effects of acute treatment with escitalopram on the different stages of contextual fear conditioning are reversed by atomoxetine. Psychopharm. 2010;212:131–143. doi: 10.1007/s00213-010-1917-5. [DOI] [PubMed] [Google Scholar]
- Mørk A, Kreilgaard M, Sánchez C. The R-enantiomer of citalopram counteracts escitalopram-induced increase in extracellular 5-HT in the frontal cortex of freely moving rats. Neuropharm. 2003;45:167–173. doi: 10.1016/s0028-3908(03)00138-2. [DOI] [PubMed] [Google Scholar]
- Mosconi MW, Kay M, D’Cruz AM, Seidenfeld A, Guter S, Stanford LD, Sweeney JA. Impaired inhibitory control is associated with higher-order repetitive behaviors in autism spectrum disorders. Psychol Med. 2009;39:1559–1566. doi: 10.1017/S0033291708004984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz DP, Everling S. Look away: the anti-saccade task and the voluntary control of eye movement. Nat Rev Neurosci. 2004;5:218–228. doi: 10.1038/nrn1345. [DOI] [PubMed] [Google Scholar]
- Owley T, Walton L, Salt J, Guter SJ, Jr, Winnega M, Leventhal BL, Cook EH., Jr An open-label trial of escitalopram in pervasive developmental disorders. J Am Acad Child Adolesc Psychiatry. 2005;44:343–348. doi: 10.1097/01.chi.0000153229.80215.a0. [DOI] [PubMed] [Google Scholar]
- Owley T, Brune CW, Salt J, Walton L, Guter S, Ayuyao N, Gibbons RD, Leventhal BL, Cook EH. A pharmacogenetic study of escitalopram in autism spectrum disorders. Autism Res. 2010;3:1–7. doi: 10.1002/aur.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palencia CA, Ragozzino ME. The effect of N-methyl-D-aspartate receptor blockade on acetylcholine efflux in the dorsomedial striatum during response reversal learning. Neurosci. 2006;143:671–678. doi: 10.1016/j.neuroscience.2006.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellow S, Chopin P, File SE, Briley M. Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods. 1985;14:149–67. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- Pinheiro SN, Del-Ben CM, Zangrossi H, Jr, Graeff FG. Anxiolytic and panicolytic effects of escitalopram in the elevated T-maze. J Psychopharmacol. 2008;22:132–137. doi: 10.1177/0269881107079866. [DOI] [PubMed] [Google Scholar]
- Pollier F, Sarre S, Aguerre S, Ebinger G, Mormède P, Michotte Y, Chaouloff F. Serotonin reuptake inhibition by citalopram in rat strains differing for their emotionality. Neuropsychopharm. 2000;22:64–76. doi: 10.1016/S0893-133X(99)00092-5. [DOI] [PubMed] [Google Scholar]
- Ragozzino ME, Ragozzino KE, Mizumori SJ, Kesner RP. Role of the dorsomedial striatum in behavioral flexibility for response and visual cue discrimination learning. Behav Neurosci. 2002;116:105–115. doi: 10.1037//0735-7044.116.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragozzino ME, Kim J, Hassert D, Minniti N, Kiang C. The contribution of the rat prelimbic-infralimbic areas to different forms of task switching. Behav Neurosci. 2003;117:1054–1065. doi: 10.1037/0735-7044.117.5.1054. [DOI] [PubMed] [Google Scholar]
- Ragozzino ME, Choi D. Dynamic changes in acetylcholine output in the medial striatum during place reversal learning. Learn Mem. 2004;11:70–77. doi: 10.1101/lm.65404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragozzino ME, Mohler EG, Prior M, Palencia CA, Rozman S. Acetylcholine activity in selective striatal regions supports behavioral flexibility. Neurobiol Learn Mem. 2009;91:13–22. doi: 10.1016/j.nlm.2008.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez C, Bergqvist PB, Brennum LT, Gupta S, Hogg S, Larsen A, Wiborg O. Escitalopram, the S-(+)-enantiomer of citalopram, is a selective sterotonin reuptake inhibitor with potent effects in animal models predictive of antidepressant and anxiolytic activities. Psychopharm (Berl) 2003;167:353–362. doi: 10.1007/s00213-002-1364-z. [DOI] [PubMed] [Google Scholar]
- Sim LJ, Joseph SA. Serotonin and substance P afferents to parafascicular and central medial nuclei. Peptides. 1992;13:171–6. doi: 10.1016/0196-9781(92)90159-z. [DOI] [PubMed] [Google Scholar]
- Solomon M, Ozonoff SJ, Ursu S, Ravizza S, Cummings N, Ly S, Carter CS. The neural substrates of cognitive control deficits in autism spectrum disorders. Neuropsychologia. 2009;47:2515–2526. doi: 10.1016/j.neuropsychologia.2009.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonsino D. Learning to learn, pattern recognition, and Nash equilibrium. Games & Econ Behav. 1997;18:286–331. [Google Scholar]
- Stahl SM, Gergel I, Li D. Escitalopram in the treatment of panic disorder: a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2003;64:1322–1327. doi: 10.4088/jcp.v64n1107. [DOI] [PubMed] [Google Scholar]
- Stein DJ, Andersen HF, Goodman WK. Escitalopram for the treatment of GAD: efficacy across different subgroups and outcomes. Ann Clin Psychiatry. 2005;17:71–75. doi: 10.1080/10401230590932335. [DOI] [PubMed] [Google Scholar]
- Sun T, He W, Hu G, Li M. Anxiolytic-like property of risperidone and olanzapine as examined in multiple measures of fear in rats. Pharmacol Biochem Behav. 2010;95:298–307. doi: 10.1016/j.pbb.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchida A, Doll BB, Fellows LK. Beyond reversal: a critical role for human orbitofrontal cortex in flexible learning from probabilistic feedback. J Neurosci. 2010;30:16868–75. doi: 10.1523/JNEUROSCI.1958-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertes RP. A PHA-L analysis of ascending projections of the dorsal raphe nucleus in the rat. J Comp Neurol. 1991;313:643–668. doi: 10.1002/cne.903130409. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Linley SB, Hoover WB. Pattern of distribution of serotonergic fibers to the thalamus of the rat. Brain Struct Funct. 2010;215:1–28. doi: 10.1007/s00429-010-0249-x. [DOI] [PubMed] [Google Scholar]
- Waltz JA, Gold JM. Probabilistic reversal learning impairments in schizophrenia: further evidence of orbitofrontal dysfunction. Schizophr Res. 2007;93:296–303. doi: 10.1016/j.schres.2007.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Withall A, Harris LM, Cumming SR. The relationship between cognitive function and clinical and functional outcomes in major depressive disorder. Psychol Med. 2009;39:393–402. doi: 10.1017/S0033291708003620. [DOI] [PubMed] [Google Scholar]
- Winstanley CA, Chudasama Y, Dalley JW, Theobald DE, Glennon JC, Robbins TW. Intra-prefrontal 8-OH-DPAT and M100907 improve visuospatial attention and decrease impulsivity on the five-choice serial reaction time task in rats. Psychopharm (Berl) 2003;167:304–314. doi: 10.1007/s00213-003-1398-x. [DOI] [PubMed] [Google Scholar]
- Winstanley CA, Theobald DE, Dalley JW, Glennon JC, Robbins TW. 5-HT2A and 5-HT2C receptor antagonists have opposing effects on a measure of impulsivity: interactions with global 5-HT depletion. Psychopharm (Berl) 2004;176:376–385. doi: 10.1007/s00213-004-1884-9. [DOI] [PubMed] [Google Scholar]
- Yamaguchi T, Suzuki M, Yamamoto M. Facilitation of acetylcholine release in rat frontal cortex by indeloxazine hydrochloride: involvement of endogenous serotonin and 5-HT4 receptors. Naunyn-Schmiedeberg’s Arch Pharmacol. 1997;356:712–ss720. doi: 10.1007/pl00005110. [DOI] [PubMed] [Google Scholar]
- Zitterl W, Aigner M, Stompe T, Zitterl-Eglseer K, Gutierrez-Lobos K, Wenzel T, Zettinig G, Hornik K, Pirker W, Thau K. Changes in thalamus-hypothalamus serotonin transporter availability during clomipramine administration in patients with obsessive-compulsive disorder. Neuropsychopharm. 2008;33:3126–3134. doi: 10.1038/npp.2008.35. [DOI] [PubMed] [Google Scholar]
- Zitterl W, Stompe T, Aigner M, Zitterl-Eglseer K, Ritter K, Zettinig G, Hornik K, Asenbaum S, Pirker W, Thau K. Diencephalic serotonin transporter availability predicts both transporter occupancy and treatment response to sertraline in obsessive-compulsive checkers. Biol Psychiatry. 2009;66:1115–1122. doi: 10.1016/j.biopsych.2009.07.009. [DOI] [PubMed] [Google Scholar]