Abstract
Psychosocial dysfunction in older children and adolescents is common and may lead to non-adherence to HIV treatments. Poor adherence leads to HIV treatment failure and the development of resistant virus. In resource-limited settings where treatment options are typically limited to only one or two available lines of therapy, identification of individuals at highest risk of failure before failure occurs is of critical importance. Rapid screening tools for psychosocial dysfunction may allow for identification of those children and adolescents who are most likely to benefit from limited psychosocial support services targeted at preventing HIV treatment failure. The Pediatric Symptom Checklist (PSC) is used in high resource settings for rapid identification of at-risk youth. In 692 HIV-infected treated children (ages of 8-<17 years) in Botswana, having a high score on the PSC was associated with having virologic failure (OR 1.7, 95% CI 1.1–2.6). The PSC may be a useful screening tool in pediatric HIV.
Keywords: HIV, adolescents, treatment failure, psychosocial dysfunction, Africa
Introduction
More than two million children worldwide are infected with HIV, approximately 90% of whom live in sub-Saharan Africa (UNAIDS, 2009; UNICEF, 2010). Among children who have aged into adolescence with HIV in sub-Saharan Africa, psychosocial problems and poor drug adherence are cited as the greatest challenges to their continued success (Ferrand R, 2010). When compared to adults and younger children, HIV-infected adolescents demonstrate the highest rates of poor medication adherence and treatment failure (Appleby et al., 2005; Crockett, Weinman, Hankins, & Marteau, 2009; DeLaMora, Aledort, & Stavola, 2006; Khan et al., 2009; Ledlie, 2001; Mellins, Brackis-Cott, Dolezal, & Abrams, 2004; Murphy et al., 2005; Murphy et al.; Murphy et al., 2003; Nachega et al., 2009; Reisner et al., 2009; Vijayan, Benin, Wagner, Romano, & Andiman, 2009). Higher rates of HIV treatment non-adherence in youth are often related to psychosocial problems (Bauman, Silver, Draimin, & Hudis, 2007; Cluver, Gardner, & Operario, 2007; DeLaMora et al., 2006). As older children and adolescents gain autonomy over their medication-taking, their psychosocial problems may play an increasing role in influencing treatment outcomes (Ledlie, 2001; Mellins et al., 2004). If so, by measuring psychosocial dysfunction in these youth we might identify those at highest risk of treatment failure. This is of particular importance in busy clinics in resource-limited settings where limited psychosocial support resources need to target patients at highest risk (Prince M et al., 2007).
We therefore sought to determine whether a simple screening test for psychosocial problems holds promise for identifying older children and adolescents at greatest risk of HIV treatment failure in busy clinics in resource-limited settings in Botswana treated according to national guidelines (Botswana National HIV AIDS Treatment Guidelines, 2008) including non-nucleoside reverse transcriptase inhibitor-based regimens as first line and boosted protease inhibitor based regimens as second line if first line fails. Located in Southern Africa, Botswana has approximately 1.7 million people with a large burden of HIV (Stover, Fidzani, Molomo, Moeti, & Musuka, 2008). Antiretroviral therapy has resulted in HIV-infected infants surviving through adolescence, even in resource-limited settings (Bolton-Moore et al., 2007; Kline et al., 2004). UNAIDS estimates that Botswana has ~16,000 HIV infected children aged 0–14 years (UNAIDS, 2011).
Given limited resources to support HIV-infected adolescents, in whom adherence is the greatest challenge, we need to identify those at greatest risk of treatment failure to intervene before HIV resistance emerges and they clinically deteriorate. Rapid screening tests can be used to identify populations at greatest risk of problems related to mental health and social difficulties and have been used extensively in the U.S. (Eisert, Sturner, & Mabe, 1991; Sturner, 1991). The Pediatric Symptom Checklist (PSC) was developed in the U.S. to allow for rapid identification of individuals between the ages of 6 and 16 years who would benefit most from detailed evaluation and treatment of emotional and behavioral problems (Jellinek & Murphy, 1988; Jellinek, Murphy, & Burns, 1986; Jellinek et al., 1988). The PSC is a 35 item parent-report tool with symptom ratings of never, sometimes or often present (scored 0, 1, or 2) and graded by simple addition of items. It takes less than 10 minutes to complete and can be administered without assistance to parents and caregivers with elementary-level education. A briefer, 17-item version is also available (Borowsky, Mozayeny, & Ireland, 2003; Duke, Ireland, & Borowsky, 2005; Gall, Pagano, Desmond, Perrin, & Murphy, 2000; Gardner, Lucas, Kolko, & Campo, 2007).
The PSC has been used in many different cultural contexts and has been translated into other languages including Japanese, Spanish, German and Dutch (Ishizaki, 2000; Jutte, Burgos, Mendoza, Ford, & Huffman, 2003; Reijneveld, Vogels, Hoekstra, & Crone, 2006; Thun-Hohenstein & Herzog, 2008). Translated versions have been tested in the appropriate ethnic groups with different score cut-offs proving appropriate in different settings. For the Japanese version, the optimal cut-off score is 17 (Ishizaki, 2000). For the Dutch version, a cut-off of 25 is recommended (Reijneveld et al., 2006). Part of our study included determining the most appropriate cut-off score for the Setswana PSC (Lowenthal et al., 2011). We now report on the association between high scores on the PSC and virologic outcomes among HIV-infected older children and adolescents in Botswana.
Methods
We culturally-adapted a Setswana translation of the PSC utilizing a team of 8 bilingual (Setswana-English) professionals. We then piloted the tool in a population similar to the study population and confirmed the tool’s internal consistency and factor structure in Batswana children and adolescents. We determined the optimal score cutoff for the Setswana PSC using receiver operator characteristic (ROC) analyses (Hanley & McNeil, 1982). ROC curves were generated by determining the sensitivity and specificity of each potential PSC score cut-off with respect to dichotomized parent reports of concern about the child. Binomial exact 95% confidence intervals were generated for sensitivity and specificity at each cut point and areas under the ROC curves were calculated.
We then sought to determine whether having a high PSC score was associated with virologic treatment failure among HIV-infected children and adolescents in this African setting. We targeted HIV-infected children on combination antiretroviral therapy (cART) for ≥6 months since those who would achieve complete viral suppression should do so by this time. Virologic failure was defined as a confirmed HIV-1 viral load >400 copies/ml after 6 months on first-line treatment. We assessed whether children with virologic failure had higher PSC scores using Wilcoxon rank sum test in unadjusted analyses and using linear regression to adjust for potential confounders, including demographic and clinic characteristics. We assessed the association between having a PSC score above the cut-off value and having virologic failure using a chi-squared test in unadjusted analyses and using logistic regression to adjust for potential confounders. We also assessed each individual PSC question for a trend of higher likelihood of failure with increasing score using a non-parametric test of trend.
Results
We enrolled 692 participants all of whom initiated cART with non-nucleoside reverse transcriptase-based therapy at least 6 months prior to enrollment (median age=11.9 years (IQR 10.2–13.6, range 8–16.9); median treatment duration=56 months (IQR 37–72, range 6–134); 50.3% female) from Francistown and Maun, Botswana. More than 90% of subjects were perinatally HIV-infected with 12 (1.7%) reportedly infected via breastfeeding and 1 (0.1%) infected via rape. Orphan status was common (52.6%) with 102 (28%) being double-orphans, 261 (72%) being single-orphans, but only 18 (5%) residing in an orphanage. Most children (98.7%) had been enrolled in school with 96% being within 2 years of the age-appropriate grade level. The median age at initiation of cART was 7.4 (IQR 5.4–9.3) years. At baseline 48% were CDC immune category 3 and 6.8% were CDC immune category 3 at the time of enrollment in our study. Virologic failure had occurred in 161 (23.3%) patients at some time in their treatment history.
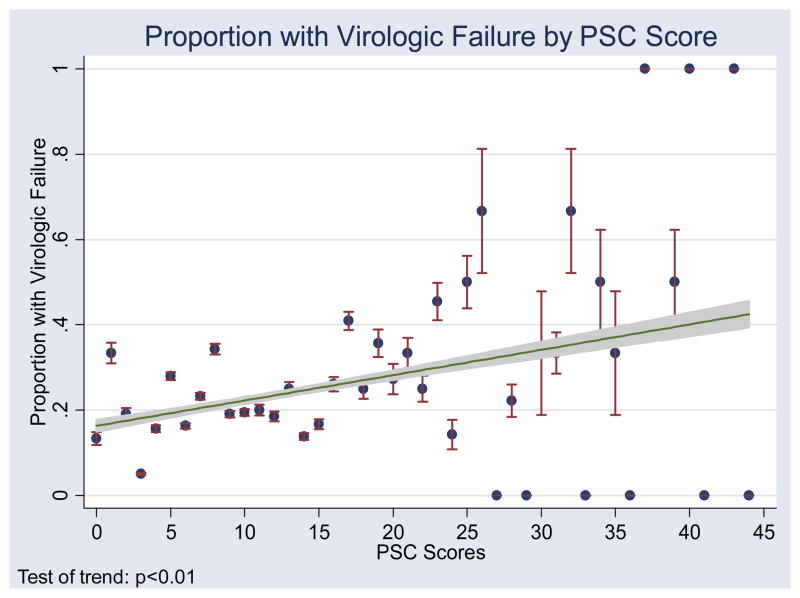
A score cut-off of 20 on the PSC was chosen based on the ROC curve analysis. The chosen cut-off gave us an accuracy of 92% for correctly classifying subjects according to our standard measure. Using the cut-off score of 20, 120 (17.3%) patients had high scores. The median PSC score for those with virologic failure was 12 (IQR 7–19) and the median for those without virologic failure was 10 (IQR 5–16, p<0.01). A scatter plot with a fitted line showing the proportion of subjects with each PSC score who had virologic failure demonstrates increasing proportions with virologic failure among those with higher PSC scores (Figure 1). Virologic failure was more common among those with a PSC score ≥20 (31.7%) than among those with a PSC score of <20 (21.5%, unadjusted OR 1.7, 95% CI 1.1–2.6; sensitivity 23%, specificity 85%, PPV 32%, NPV 78%). The results were not significantly altered by controlling for demographic or clinical factors, including age, age at initiation of cART, sex, nadir CD4, orphan status, primary caregiver, and HIV disclosure status.
Figure 1.
Proportion of subjects with virologic failure by PSC score
From the full-length (35 question) PSC, 9 questions had a statistically significant test of trend for a higher likelihood of failure in those with higher scores at the 0.05 level and 6 questions had a statistically significant test of trend at the 0.01 level. Table 1 shows the distribution of test scores and results from the test of trend for the 17 questions that make up the brief-report version of the PSC. Questions which tended to be scored higher among patients with virologic failure were mainly related to attention/executive dysfunction, and depressive symptoms.
Table 1.
Distribution of PSC brief report item scores
| PSC Question | Number (percent) without (N=530) and with (N=162) virologic failure who answered never (0), sometimes (1), or often (2) for each PSC question | Test of Trend p-value | ||
|---|---|---|---|---|
| No Virologic Failure | Virologic Failure | |||
| Worries a lot | (0) | 424 (80) | 109 (67) | 0.008 |
| (1) | 64 (12) | 37 (23) | ||
| (2) | 42 (8) | 16 (10) | ||
| Seems to be having less fun | (0) | 446 (84) | 122 (75) | 0.005 |
| (1) | 64 (13) | 29 (18) | ||
| (2) | 16 (3) | 11 (7) | ||
| Feels sad, unhappy | (0) | 389 (73) | 102 (63) | 0.003 |
| (1) | 120 (23) | 46 (28) | ||
| (2) | 21 (4) | 14 (9) | ||
| Feels bad about him or her self | (0) | 470 (89) | 109 (67) | 0.002 |
| (1) | 41 (8) | 37 (23) | ||
| (2) | 19 (4) | 16 (10) | ||
| Does not listen to rules | (0) | 465 (88) | 127 (78) | 0.002 |
| (1) | 50 (9) | 24 (15) | ||
| (2) | 15 (3) | 11 (7) | ||
| Distracted easily | (0) | 348 (66) | 83 (51) | 0.012 |
| (1) | 118 (22) | 57 (35) | ||
| (2) | 64 (12) | 22 (14) | ||
| Daydreams too much | (0) | 354 (67) | 92 (56) | 0.013 |
| (1) | 130 (25) | 48 (30) | ||
| (2) | 46 (8) | 22 (14) | ||
| Does not understand other people’s feelings | (0) | 391 (74) | 105 (65) | NS |
| (1) | 59 (11) | 28 (17) | ||
| (2) | 80 (15) | 29 (18) | ||
| Teases others | (0) | 458 (86) | 140 (86) | NS |
| (1) | 54 (10) | 17 (11) | ||
| (2) | 18 (4) | 5 (3) | ||
| Blames others for his or her troubles | (0) | 471 (89) | 140 (86) | NS |
| (1) | 47 (9) | 18 (11) | ||
| (2) | 12 (2) | 4 (3) | ||
| Takes things that do not belong to him or her | (0) | 471 (89) | 140 (86) | NS |
| (1) | 43 (8) | 19 (12) | ||
| (2) | 16 (3) | 3 (2) | ||
| Refuses to share | (0) | 395 (74) | 118 (73) | NS |
| (1) | 46 (9) | 20 (12) | ||
| (2) | 89 (17) | 24 (15) | ||
| Fidgety, unable to sit still | (0) | 417 (79) | 131 (81) | NS |
| (1) | 87 (16) | 22 (14) | ||
| (2) | 26 (5) | 9 (5) | ||
| Acts as if driven by motor | (0) | 451 (85) | 134 (83) | NS |
| (1) | 54 (10) | 21 (13) | ||
| (2) | 25 (5) | 7 (4) | ||
| Feels hopeless | (0) | 464 (88) | 137 (85) | NS |
| (1) | 34 (6) | 17 (10) | ||
| (2) | 32 (6) | 8 (5) | ||
| Has trouble concentrating | (0) | 353 (67) | 95 (59) | NS |
| (1) | 110 (21) | 39 (24) | ||
| (2) | 67 (13) | 28 (17) | ||
| Fights with other children | (0) | 467 (88) | 147 (91) | NS |
| (1) | 45 (9) | 10 (6) | ||
| (2) | 18 (3) | 5 (3) | ||
Discussion
Psychosocial dysfunction as measured by the PSC is related to treatment failure among HIV-infected youth in Botswana. Because of our study’s cross-sectional design, we cannot comment on causality. Psychosocial dysfunction may have played a role in causing treatment failure, may be the result of treatment failure, or both. If psychosocial dysfunction is found in longitudinal studies to precede treatment failure, then by screening for psychosocial dysfunction, those children and adolescents at highest risk of virologic failure in resource-limited settings might be identified. Because the sensitivity and specificity were insufficiently high, the PSC score cannot be used to either rule out or rule in the diagnosis of virologic failure, and is not a substitute for virologic monitoring. However, if found in longitudinal studies to be a predictor of virologic failure, the PSC could easily be utilized in resource-limited settings to allow for prioritization of scarce psychosocial support resources to target children and adolescents at the highest risk of treatment failure.
Further studies need to be done to assess whether high scores on the PSC can predict poor adherence and treatment failure before poor outcomes are recognizable. If proven to be a useful predictor of poor outcomes, translation and cultural-adaptation of the test for use in other linguistic and cultural groups would be recommended. Another major limitation of our study is that other factors contributing to failure were not investigated. The “low risk group” still had a >20% virologic failure rate, suggesting that further studies to address the factors contributing to poor outcomes among older children and adolescents in resource-limited settings are urgently needed (Ferrand R, 2010).
Acknowledgments
The authors would like to thank the clinic staff, patients and families who helped to make this study possible. This research was supported by a grant for the Penn Center for AIDS Research (CFAR), an NIH-funded program (P30 AI 045008).
References
- Appleby PR, Marks G, Ayala A, Miller LC, Murphy S, Mansergh G. Consideration of future consequences and unprotected anal intercourse among men who have sex with men. J Homosex. 2005;50(1):119–133. doi: 10.1300/J082v50n01_06. [DOI] [PubMed] [Google Scholar]
- Bauman LJ, Silver EJ, Draimin BH, Hudis J. Children of mothers with HIV/AIDS: unmet needs for mental health services. Pediatrics. 2007;120(5):e1141–1147. doi: 10.1542/peds.2005-2680. [DOI] [PubMed] [Google Scholar]
- Bolton-Moore C, Mubiana-Mbewe M, Cantrell RA, Chintu N, Stringer EM, Chi BH, et al. Clinical outcomes and CD4 cell response in children receiving antiretroviral therapy at primary health care facilities in Zambia. JAMA. 2007;298(16):1888–1899. doi: 10.1001/jama.298.16.1888. [DOI] [PubMed] [Google Scholar]
- Borowsky IW, Mozayeny S, Ireland M. Brief psychosocial screening at health supervision and acute care visits. Pediatrics. 2003;112(1 Pt 1):129–133. doi: 10.1542/peds.112.1.129. [DOI] [PubMed] [Google Scholar]
- Botswana National HIV AIDS Treatment Guidelines. 2008 Retrieved October 4, 2011, from http://www.moh.gov.bw/templates/moh/File/BOTSWANA%20HIVAIDS%20TREATMENT%20%20GUIDELINES%20%28November%201%202008%29.pdf.
- Cluver L, Gardner F, Operario D. Psychological distress amongst AIDS-orphaned children in urban South Africa. J Child Psychol Psychiatry. 2007;48(8):755–763. doi: 10.1111/j.1469-7610.2007.01757.x. [DOI] [PubMed] [Google Scholar]
- Crockett RA, Weinman J, Hankins M, Marteau T. Time orientation and health-related behaviour: measurement in general population samples. Psychol Health. 2009;24(3):333–350. doi: 10.1080/08870440701813030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLaMora P, Aledort N, Stavola J. Caring for adolescents with HIV. Curr HIV/AIDS Rep. 2006;3(2):74–78. doi: 10.1007/s11904-006-0021-2. [DOI] [PubMed] [Google Scholar]
- Duke N, Ireland M, Borowsky IW. Identifying psychosocial problems among youth: factors associated with youth agreement on a positive parent-completed PSC-17. Child Care Health Dev. 2005;31(5):563–573. doi: 10.1111/j.1365-2214.2005.00551.x. [DOI] [PubMed] [Google Scholar]
- Eisert DC, Sturner RA, Mabe PA. Questionnaires in behavioral pediatrics: guidelines for selection and use. J Dev Behav Pediatr. 1991;12(1):42–50. [PubMed] [Google Scholar]
- Ferrand RLS, Whande B, Munaiwa L, Langhaug L, Cowan F, Mugurungi O, Gibb D, Munyati S, Williams BG, Corbett EL. Survey of children accessing HIV services in a high prevalence setting: time for adolescents to count? Bull World Health Organization. 2010;88(6):428–434. doi: 10.2471/BLT.09.066126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gall G, Pagano ME, Desmond MS, Perrin JM, Murphy JM. Utility of psychosocial screening at a school-based health center. J Sch Health. 2000;70(7):292–298. doi: 10.1111/j.1746-1561.2000.tb07254.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner W, Lucas A, Kolko DJ, Campo JV. Comparison of the PSC-17 and alternative mental health screens in an at-risk primary care sample. J Am Acad Child Adolesc Psychiatry. 2007;46(5):611–618. doi: 10.1097/chi.0b013e318032384b. [DOI] [PubMed] [Google Scholar]
- Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143(1):29–36. doi: 10.1148/radiology.143.1.7063747. [DOI] [PubMed] [Google Scholar]
- Ishizaki Y, Fukai Y, Kobayashi Y, Ozawa K. Validation and cutoff score of the Japanese version of the Pediatric Symptom Checklist: Screening of school-aged children with psychosocial and psychosomatic disorders (in Japanese) The Journal of the Japan Pediatric Society. 2000;104:831–840. [Google Scholar]
- Jellinek MS, Murphy JM. Screening for psychosocial disorders in pediatric practice. Am J Dis Child. 1988;142(11):1153–1157. doi: 10.1001/archpedi.1988.02150110031013. [DOI] [PubMed] [Google Scholar]
- Jellinek MS, Murphy JM, Burns BJ. Brief psychosocial screening in outpatient pediatric practice. J Pediatr. 1986;109(2):371–378. doi: 10.1016/s0022-3476(86)80408-5. [DOI] [PubMed] [Google Scholar]
- Jellinek MS, Murphy JM, Robinson J, Feins A, Lamb S, Fenton T. Pediatric Symptom Checklist: screening school-age children for psychosocial dysfunction. J Pediatr. 1988;112(2):201–209. doi: 10.1016/s0022-3476(88)80056-8. [DOI] [PubMed] [Google Scholar]
- Jutte DP, Burgos A, Mendoza F, Ford CB, Huffman LC. Use of the Pediatric Symptom Checklist in a low-income, Mexican American population. Arch Pediatr Adolesc Med. 2003;157(12):1169–1176. doi: 10.1001/archpedi.157.12.1169. [DOI] [PubMed] [Google Scholar]
- Khan M, Song X, Williams K, Bright K, Sill A, Rakhmanina N. Evaluating adherence to medication in children and adolescents with HIV. Arch Dis Child. 2009;94(12):970–973. doi: 10.1136/adc.2008.156232. [DOI] [PubMed] [Google Scholar]
- Kline MW, Matusa RF, Copaciu L, Calles NR, Kline NE, Schwarzwald HL. Comprehensive pediatric human immunodeficiency virus care and treatment in Constanta, Romania: implementation of a program of highly active antiretroviral therapy in a resource-poor setting. Pediatr Infect Dis J. 2004;23(8):695–700. doi: 10.1097/01.inf.0000135454.46188.83. [DOI] [PubMed] [Google Scholar]
- Ledlie SW. The psychosocial issues of children with perinatally acquired HIV disease becoming adolescents: a growing challenge for providers. AIDS Patient Care STDS. 2001;15(5):231–236. doi: 10.1089/10872910152050748. [DOI] [PubMed] [Google Scholar]
- Lowenthal E, Lawler K, Harari N, Moamogwe L, Masunge J, Masedi M, et al. Validation of the Pediatric Symptom Checklist in HIV-infected Batswana. Journal of Child and Adolescent Mental Health. 2011;23(1):17–28. doi: 10.2989/17280583.2011.594245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellins CA, Brackis-Cott E, Dolezal C, Abrams EJ. The role of psychosocial and family factors in adherence to antiretroviral treatment in human immunodeficiency virus-infected children. Pediatr Infect Dis J. 2004;23(11):1035–1041. doi: 10.1097/01.inf.0000143646.15240.ac. [DOI] [PubMed] [Google Scholar]
- Murphy DA, Belzer M, Durako SJ, Sarr M, Wilson CM, Muenz LR. Longitudinal antiretroviral adherence among adolescents infected with human immunodeficiency virus. Arch Pediatr Adolesc Med. 2005;159(8):764–770. doi: 10.1001/archpedi.159.8.764. [DOI] [PubMed] [Google Scholar]
- Murphy DA, Lam P, Naar-King S, Robert Harris D, Parsons JT, Muenz LR. Health literacy and antiretroviral adherence among HIV-infected adolescents. Patient Educ Couns. 79(1):25–29. doi: 10.1016/j.pec.2009.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy DA, Sarr M, Durako SJ, Moscicki AB, Wilson CM, Muenz LR. Barriers to HAART adherence among human immunodeficiency virus-infected adolescents. Arch Pediatr Adolesc Med. 2003;157(3):249–255. doi: 10.1001/archpedi.157.3.249. [DOI] [PubMed] [Google Scholar]
- Nachega JB, Hislop M, Nguyen H, Dowdy DW, Chaisson RE, Regensberg L, et al. Antiretroviral therapy adherence, virologic and immunologic outcomes in adolescents compared with adults in southern Africa. J Acquir Immune Defic Syndr. 2009;51(1):65–71. doi: 10.1097/QAI.0b013e318199072e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince M, Patel V, Saxena S, Maj M, Maselko J, MR P, et al. No health without mental health. Lancet. 2007;370(9590):859–877. doi: 10.1016/S0140-6736(07)61238-0. [DOI] [PubMed] [Google Scholar]
- Reijneveld SA, Vogels AG, Hoekstra F, Crone MR. Use of the Pediatric Symptom Checklist for the detection of psychosocial problems in preventive child healthcare. BMC Public Health. 2006;6:197. doi: 10.1186/1471-2458-6-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisner SL, Mimiaga MJ, Skeer M, Perkovich B, Johnson CV, Safren SA. A review of HIV antiretroviral adherence and intervention studies among HIV-infected youth. Top HIV Med. 2009;17(1):14–25. [PMC free article] [PubMed] [Google Scholar]
- Stover J, Fidzani B, Molomo BC, Moeti T, Musuka G. Estimated HIV trends and program effects in Botswana. PLoS One. 2008;3(11):e3729. doi: 10.1371/journal.pone.0003729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturner RA. Parent questionnaires: basic office equipment? J Dev Behav Pediatr. 1991;12(1):51–54. [PubMed] [Google Scholar]
- Thun-Hohenstein L, Herzog S. The predictive value of the pediatric symptom checklist in 5-year-old Austrian children. Eur J Pediatr. 2008;167(3):323–329. doi: 10.1007/s00431-007-0494-z. [DOI] [PubMed] [Google Scholar]
- UNAIDS. AIDS Epidemic Update 2009. Journal. 2009 Retrieved from http://data.unaids.org/pub/Report/2009/JC1700_Epi_Update_2009_en.pdf.
- UNAIDS. Country Spotlight Botswana. 2011 Retrieved January 23, 2011, from http://www.unaids.org/en/regionscountries/countries/botswana/
- UNICEF. Children and AIDS: 5th Stocktaking Report. Geneva: 2010. [Google Scholar]
- Vijayan T, Benin AL, Wagner K, Romano S, Andiman WA. We never thought this would happen: transitioning care of adolescents with perinatally acquired HIV infection from pediatrics to internal medicine. AIDS Care. 2009;21(10):1222–1229. doi: 10.1080/09540120902730054. [DOI] [PMC free article] [PubMed] [Google Scholar]