Abstract
Mothers in numerous species exhibit heightened aggression in defense of their young. This shift typically coincides with the duration of lactation in nonhuman mammals, which suggests that human mothers may display similarly accentuated aggressiveness while breast feeding. Here we report the first behavioral evidence for heightened aggression in lactating humans. Breast-feeding mothers inflicted louder and longer punitive sound bursts on unduly aggressive confederates than did formula-feeding mothers or women who had never been pregnant. Maternal aggression in other mammals is thought to be facilitated by the buffering effect of lactation on stress responses. Consistent with the animal literature, our results showed that while lactating women were aggressing, they exhibited lower systolic blood pressure than did formula-feeding or never-pregnant women while they were aggressing. Mediation analyses indicated that reduced arousal during lactation may disinhibit female aggression. Together, our results highlight the contributions of breast feeding to both protecting infants and buffering maternal stress.
Keywords: aggressive behavior, fear, evolutionary psychology, stress reactions
As the adage prescribes, one should “never come between a mother bear and her cubs.” The ferocity with which mothers—including human mothers—are prone to defend their offspring is widely appreciated, although the underlying psychological mechanisms that enable human maternal aggression have never been specified. In the research reported here, we investigated the vital role of breast feeding in mediating maternal aggression postpartum.
Lactating macaques display more aggression than females at any other reproductive stage (Maestripieri, 1994; Schino, D’Amato, & Troisi, 2004; Troisi, D’Amato, Carnera, & Trinca, 1988). Similar upsurges of aggression during the course of lactation have been observed in rats and mice (Lonstein & Gammie, 2002), prairie voles (Ylänen & Horne, 2002), hamsters (Giordano, Siegel, & Rosenblatt, 1984), lions (Grinnell & McComb, 1996), deer (Smith, 1987), domestic cats (Schneirla, Rosenblatt, & Tobach, 1963), rabbits (Ross, Sawin, Zarrow, & Denenberg, 1963), squirrels (J. C. Taylor, 1966), and domestic sheep (Hersher, Richmond, & Moore, 1963). Several studies of rodents have shown maternal defense to be integral to the survival of young (Heise & Lippke, 1997; Ylänen & Horne, 2002). In animal species, mothers’ aggressive behaviors typically are manifest when agents deemed threatening approach the nesting site or behave in potentially dangerous ways toward the mother or infant.
Although research has demonstrated the prevalence and adaptive significance of heightened aggression during lactation in other mammals, the topic remains virtually unexplored in humans. Two studies, however, have found heightened self-reported hostility among mothers 5 days after parturition (Ledesma, de Luis, Montejo, Llorca, & Perez-Urdaniz, 1988; Mastrogiacomo et al., 1983), suggesting that maternal defense may indeed extend to humans. In the behavioral study reported here, we investigated whether there is an increase in aggression in mothers postpartum and, if so, whether this increased aggression is linked to breast feeding.
Previous research in rodents indicates that lactation enables heightened defensive aggression by dampening fear. Researchers have suggested that lactation is accompanied by a down-regulation of the stress response because aggressive tendencies normally curtailed by fear are disinhibited (Gammie, D’Anne, Lee, & Stevenson, 2008; Hansen & Ferreira, 1986; Hansen, Ferreira, & Selart, 1985). Numerous studies of both lactating rodents and lactating human mothers have found that they exhibit lower physiological arousal, via greater parasympathetic control, in response to a variety of stressors (for a review, see Mezzacappa, 2004). Fear constrains aggression, thereby typically inducing flight or freezing behaviors instead of fighting behaviors (Boccia & Pedersen, 2001; Erskine, Barfield, & Goldman, 1978; Maestripieri & D’Amato, 1991). Extrapolating from the animal literature, we predicted that lactating women would exhibit lower levels of arousal during an aggressive encounter than would nonlactating women, and that arousal and aggression would be inversely correlated.
Method
We recruited 20 exclusively breast-feeding and 20 formula-feeding mothers (10 nonlactating and 10 mixed-feeding mothers) with infants between 3 and 6 months old. Mothers were recruited via telephone from the Utah County birth records. Mothers in the breast-feeding group had exclusively breast-fed since giving birth. The formula-feeding group included both entirely nonlactating mothers and those who fed with both formula and breast milk (mixed feeding). The mixed-feeding mothers were instructed to abstain from breast feeding for at least 12 hr prior to their experimental session. Mothers received $20 in compensation for participation in this study. In addition, 20 undergraduate women who had never given birth (nulliparous women) were recruited in exchange for course credit. After withdrawals resulting from infant distress (n = 3) and women’s suspicion during the aggression paradigm (n = 3), the final sample consisted of 18 exclusively breast-feeding mothers, 17 formula-feeding mothers (10 nonlactating and 7 mixed-feeding mothers), and 19 nulliparous women. The women were predominantly from middle-class backgrounds (90% Caucasian, 3% Asian, 4% American Indian, and 3% Latina/Hispanic; see Table 1 for means and standard deviations of demographic characteristics). Two participants declined the blood pressure (BP) measure, and 1 participant’s data were lost because of an equipment malfunction; complete BP samples were available for 17 exclusively breast-feeding mothers, 16 formula-feeding mothers (10 nonlactating and 6 mixed-feeding mothers), and 18 nulliparous women.
Table 1.
Characteristics of the Subjects in the Study
| Group | n | Age (mean in years) |
Income bracket (mean) |
Married (%) | Employed (%) | Number of children (mean) |
Age of infant (mean in months) |
|---|---|---|---|---|---|---|---|
| Exclusively breast-feeding mothers | 18 | 26.8a (4.04) | 3.59a (2.43) | 100 | 6a | 2.52 (1.12) | 3.93 (0.58) |
| Formula-feeding mothers | 17 | 26.1a (5.07) | 3.28a (1.67) | 100 | 50b | 2.52 (1.12) | 4.30 (1.06) |
| Nulliparous women | 19 | 21.1b (1.78) | 1.84b (1.92) | 26 | 84b | — | — |
Note: Standard deviations are shown in parentheses. Each subject’s household income was categorized into one of eight brackets (1 = under $10,000; 2 = $10,000–$24,999; 3 = $25,000–$39,999; 4 = $40,000–$54,999; 5 = $55,000–$69,999; 6 = $70,000–$84,999; 7 = $85,000–$100,000; 8 = over $100,000). Employment is reported as the percentage of people in each group who were employed outside the home at the time of the experiment. Within each column, values with different subscripts are significantly different (p < .05).
Before the testing session, participants were sent an online survey asking about their infants’ feeding habits (e.g., percentage of the diet consisting of breast milk), as well as the participants’ personality, relationships, and household income. In the testing session, we measured aggression and BP. To determine whether the time elapsed since breast feeding influences aggression, we asked each participant to complete the aggression paradigm (see details in the next section) both before and after a feeding period; this procedure allowed us to make within-subjects comparisons. During the feeding period, exclusively breast-feeding mothers breast-fed their infants, nonlactating and mixed-feeding mothers fed their infants formula, and nulliparous women were given a 10-min break.
Behavioral measure of aggression
To obtain a behavioral measure of aggression, we had participants follow a paradigm that has been validated as a measure of physical aggression (Bushman, 2002). First, each participant met a female confederate who posed as a fellow research participant and was an ostensible opponent in a competitive reaction time task. The rules of the contest and reaction time task were explained to both the participant and the confederate during a training period. Then the subject and the confederate were taken into separate rooms, where they would supposedly compete via linked computer terminals. The ersatz competition consisted of a dot-probe task (Pourtois, Grandjean, Sander, & Vuilleumier, 2004). Participants were told that the winner of each round (i.e., the competitor who responded more quickly) would choose the volume and duration of an aversive sound burst administered to the loser. In actuality, participants interacted with a standardized computer program that presented the same pattern of wins, losses, and sound bursts to each participant.
Aggression was operationalized as the average volume and duration of sound bursts chosen by participants during the confrontation. The prefeeding and postfeeding confrontations were each composed of eight rounds. Participants chose the sound-burst volume before each round began and controlled the duration of sound after rounds they “won” by depressing a red button for a maximum of 5 s (i.e., the sound was supposedly administered for as long as they held down the button within the 5-s window). Duration scores were then converted from milliseconds into continuous scores ranging from 0 to 10. Volume levels ranged from a minimum of 60 dB (Level 1) to a maximum of 105 dB (Level 10). A silent setting (Level 0) was also available. Volume and duration scores were highly correlated with each other (r = .89, p < .001) and were therefore averaged to create a more reliable aggression measure (see Bushman, 2002). Regardless of who won a given round, participants were shown the volume their competitor had ostensibly chosen. The volume and duration patterns presented were equivalently aggressive during the prefeeding and post-feeding confrontations.
Three female research assistants acted as confederates. Pilot testing revealed that the unduly aggressive sound bursts were not believable unless the confederate appeared rude during the initial meeting with a participant. Therefore, confederates were trained to ignore participants, chew gum, and check their cell phones for 20 s while the experimenter spoke during the training period. The program Inquisit (Version 3.0.1.0; Millisecond Software Co., Seattle, WA) was used to present all stimuli.
Measures of autonomic reactivity
Measures of BP were taken three times during a 10-min baseline period before the confrontation (at 5 min, 8 min 30 s, and 10 min) and three times during both the prefeeding and the postfeeding confrontations (at 30 s, 3 min, and 6 min). The three readings for each epoch were then averaged to increase reliability (Kamarck, Debsk, & Manuck, 1992). A Dinamap Model Pro 100 monitor (Critikon Corp., Tampa, FL) was used to measure BP.
Self-reported trait aggression
An aggression questionnaire (Buss & Perry, 1992) was included in the initial online surveys so that we could rule out the possibility that self-selecting factors somehow lead women with more aggressive dispositions to breast-feed and less aggressive women to feed their infants formula. This 29-item scale assesses the cognitive, affective, and behavioral aspects of trait aggression and includes subscale measures of Anger, Hostility, Physical Aggression, and Verbal Aggression. Confirmatory factor analyses have provided support for the dimensional structure of this scale (Buss & Perry, 1992). Further, the retest reliability for this questionnaire over 9 weeks is satisfactory (correlations range from .72 for Anger to .80 for Physical Aggression and for the total score; Buss & Perry, 1992), which means that this scale taps a relatively stable trait. The Cronbach’s alpha for the scale as a whole was .88. The Cronbach’s alphas for the subscales were as follows—Anger: α = .76; Hostility; α = .77; Physical Aggression: α =.68; and Verbal Aggression: α =.77.
Results
Preliminary analyses
Analyses of variance revealed that the three groups differed in age, income, employment status, and romantic-relationship status (single/not dating/no boyfriend, dating/boyfriend, cohabitating with partner, engaged, or married; Table 1). Therefore, we conducted a linear regression to identify potential predictors of aggression. The model indicated that having a more committed romantic partner (β = −0.39, p < .025) or having a higher income (β = −0.35, p < .05) diminished aggression; these variables were therefore entered as covariates into the subsequent aggression analyses. The three groups did not differ on self-reported trait aggression or on any of the aggression subscales, so these scores were not included as covariates in the analysis predicting aggressive behavior. Further, the breast-feeding and formula-feeding mothers did not differ in the ages of their infants or the number of children they had (see Table 1).
Preliminary linear regression analyses identified only romantic-relationship status as an important contributor to both systolic BP (SBP; β = 0.37, p < .05) and diastolic BP (DBP; β = 0.46, p < .05). Therefore, relationship status was entered as a covariate in all BP analyses.
All statistical tests reported are two-tailed. Means and standard deviations reported are adjusted for covariates unless otherwise noted.
Primary analyses
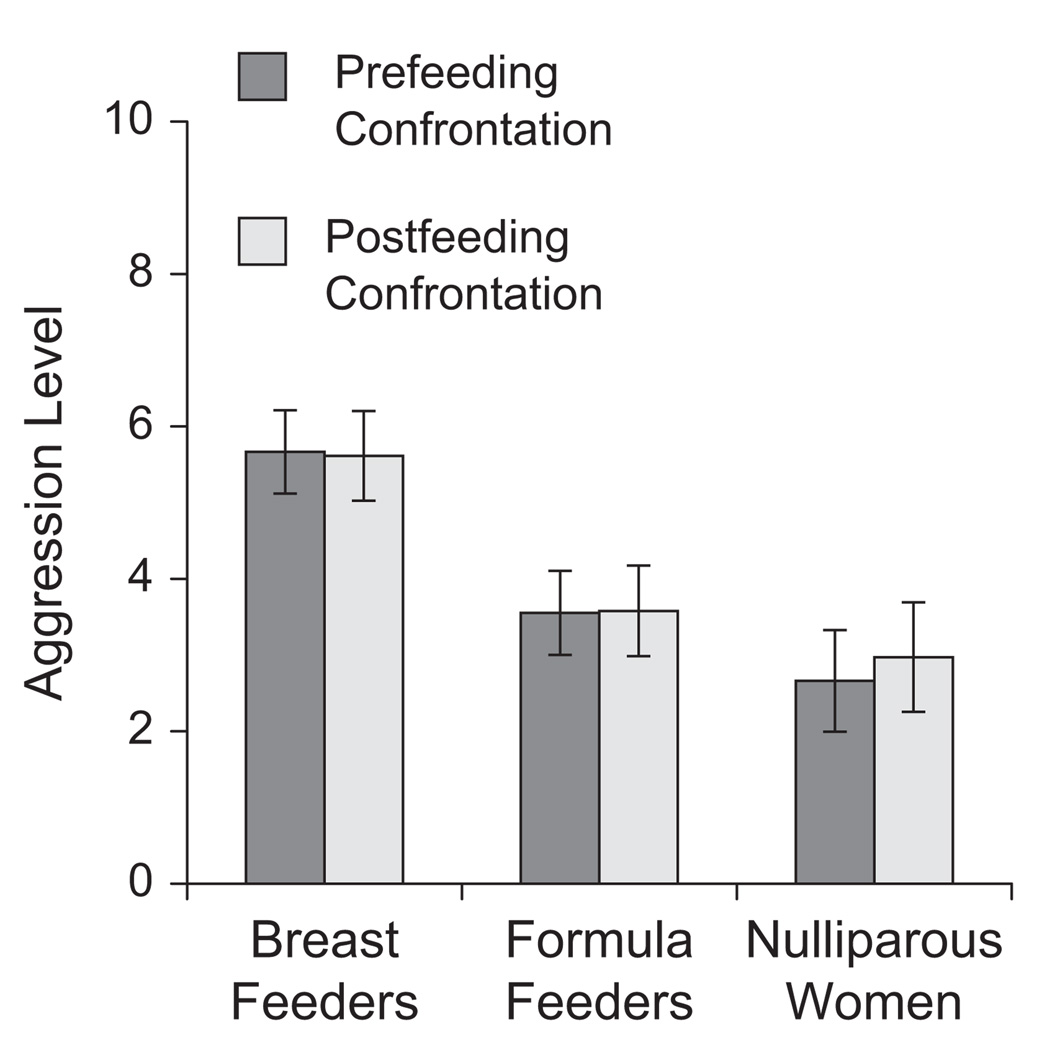
To determine whether exclusively breast-feeding mothers were more aggressive than formula-feeding mothers or nulliparous women, and to identify what role, if any, time elapsed since feeding had on aggression, we performed a repeated measures analysis of covariance (ANCOVA). This test revealed a significant difference in aggression levels across the three groups of women, F = (2, 51) = 6.72, p < .005, η2 = .17 (Fig. 1). As predicted, planned contrasts revealed that exclusively breast-feeding mothers (M = 5.6, SD = 2.3) were more aggressive than either formula-feeding mothers (M = 3.6, SD = 2.3, p < .01) or nulliparous women (M = 2.8, SD = 2.9, p < .01). Nulliparous women and formula-feeding mothers did not differ in aggression levels (p = .46). These results suggest that it is lactation, and not motherhood or other changes related to pregnancy, that promotes heightened postpartum aggression in humans. Across groups, there was no difference between aggression levels before versus after the feeding period (a rest period for nonmothers), F(1, 52) = 0.44, p = 0.70, η2 < .001. Similarly, the interaction between the time of aggression measurement (prefeeding vs. postfeeding) and group was not significant, F(4, 49) = 0.53, p = .72, η2 = .021; aggression levels neither went up nor went down directly after breast feeding or formula feeding. Consequently, prefeeding and postfeeding aggression scores were collapsed into an average aggression score for each participant in all subsequent aggression analyses.
Fig. 1.
Mean aggression before and after the feeding period in the three groups of women (exclusively breast feeding, formula feeding, and nulliparous; the latter group took a break during the feeding period). Error bars represent standard errors of the mean.
Follow-up analyses were conducted to test whether the two subtypes of formula-feeding mothers (nonlactating, n = 10, and mixed feeding, n = 7) both differed from exclusively breast-feeding mothers (n = 18) in level of aggression. If lactation influences aggression in humans, then exclusively breast-feeding mothers should be more aggressive than nonlactating mothers, and mixed-feeding mothers’ scores should fall between the scores of the two other groups. As predicted, the mean aggression level of the exclusively breast-feeding women was the highest (M = 4.9, SD = 2.0), followed by the level of the mixed-feeding women (M = 3.0, SD = 2.1), and then the level of the nonlactating mothers (M = 2.6, SD = 2.0).1 An ANCOVA with planned contrasts revealed a significant difference across groups, F(2, 32) = 4.75, p < .02, η2 = .21. Exclusively breast-feeding mothers were significantly more aggressive than both mixed-feeding (p < .05) and nonlactating (p < .01) mothers. There was not a significant difference between mixed-feeding and nonlactating mothers’ aggression (p = .75). Further analyses revealed a significant correlation between the percentage of the infant’s diet that was made up by breast milk among lactating women and how much aggression they displayed during the experiment (r = .42, p < .05).
Another aim of this study was to test whether lactation would dampen autonomic arousal during an aggressive encounter and whether low arousal would correlate with increased aggression. To assess whether lactation dampened stress responsiveness to the aggressive confrontation, we performed separate repeated measures ANCOVAs on SBP and DBP. Across groups, there was a significant increase in BP from baseline to the confrontations—SBP: F(1, 49) = 89.07, p < .001, η2 = .58; DBP: F(1, 49) = 48.30, p < .001, η2 = .45. Thus, the aggressive encounters were somewhat stressful, as we intended.
At baseline, exclusively breast-feeding mothers had lower SBP (M = 97.6 mmHg, SD = 6.6) than formula-feeding mothers (M = 102.4 mmHg, SD = 7.1), F(2, 47) = −4.8, p < .05. There were no other significant differences in BP at baseline. Exclusively breast-feeding women’s change in BP from baseline to the confrontations (SBP: M = 53.8 mmHg, SD = 8.3; DBP: M = 32.7 mmHg, SD = 6.4) tended to be smaller than the BP change of nulliparous women (SBP: M = 63.2 mmHg, SD = 10.2, p < .05; DBP: M = 39.5 mmHg, SD = 8.1, p < .05) and formula-feeding women (SBP: M = 58.1 mmHg, SD = 8.4, p < .10; DBP: M = 34.6 mmHg, SD = 6.4, p = .35).
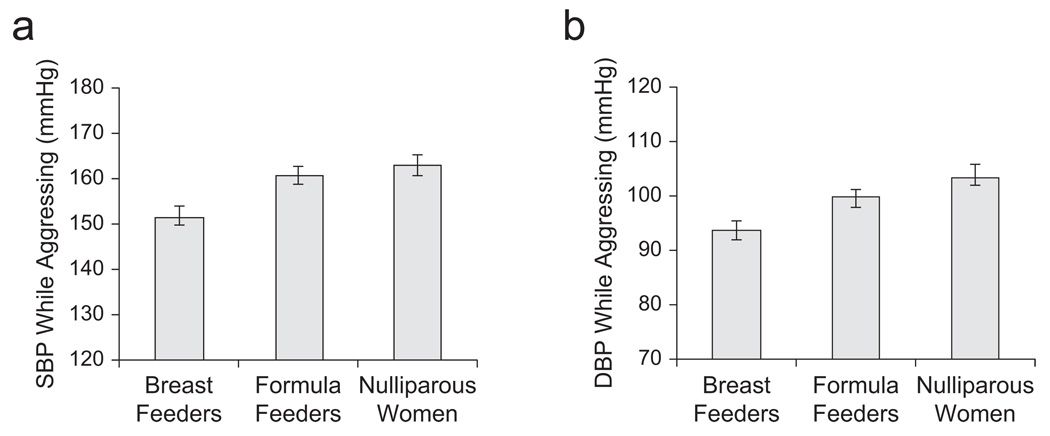
There was a significant difference in BP during the confrontations as a function of group—SBP: F(2, 47) = 3.7, p < .05, η2 = .13; DBP: F(2, 47) = 3.46, p < .05, η2 = .11. As predicted, exclusively breast-feeding mothers had lower SBP during the confrontations (M = 151.6 mmHg, SD = 12.2) than did both formula-feeding women (M = 160.6 mmHg, SD = 12.1, p < .05) and nulliparous women (M = 162.8 mmHg, SD = 15.0, p < .05; Fig. 2a). Exclusively breast-feeding mothers also had lower DBP (M = 94.0 mmHg, SD = 9.4) than did nulliparous women (M = 104.1 mmHg, SD = 11.7, p < .05) and formula-feeding women (M = 100.49 mmHg, SD = 16.8, p < .05) during the aggressive encounters (see Fig. 2b). Further, there was a significant inverse correlation between the percentage of the infant’s diet that was made up by breast milk among lactating women and their SBP during the confrontations (r = −.39, p < .025). Together, these findings corroborate the role of lactation in decreasing autonomic arousal during aggressive confrontations.
Fig. 2.
Mean (a) systolic blood pressure (SBP) and (b) diastolic blood pressure (DBP) during the two aggressive encounters in the three groups of women (exclusively breast feeding, formula feeding, and nulliparous). Error bars represent standard errors of the mean.
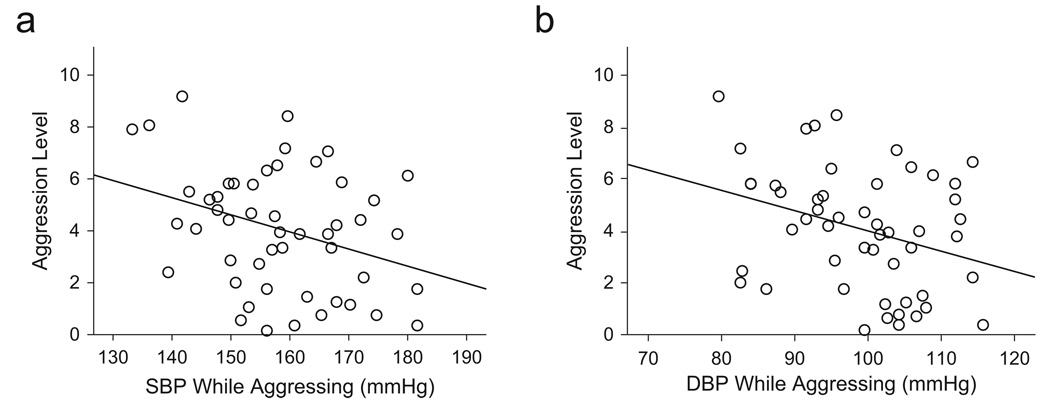
To examine whether lowered autonomic arousal promoted heightened aggression, we calculated Pearson’s correlations (two-tailed) between BP and aggression scores during the confrontations. Aggression was inversely correlated with SBP across groups, r(51) = −.351, p < .02), and there was a similar trend for DBP, r(51) = −.27, p < .06); women who had lower BP during the confrontations tended to be more aggressive Fig. 3). When this finding associating lower BP with higher aggression is coupled with our finding that exclusively breast-feeding women had lower SBP than formula-feeding (mixed-feeding and nonlactating) and nulliparous women, it appears plausible that decreased autonomic arousal (which is consistent with diminished stress) disinhibited aggressive responses in exclusively breast-feeding mothers.
Fig. 3.
Scatter plots (with best-fitting regression lines) showing the association between aggression and mean (a) systolic blood pressure (SBP) and (b) diastolic blood pressure (DBP) during the aggression paradigm.
Mediation analyses
We tested whether SBP during the aggressive encounters mediated the relationship between breast feeding and aggression (controlling for income and relationship status) using Preacher and Hayes’s (2008) bootstrapping procedure recommended for small sample sizes. Regressions revealed that more breast feeding (0 = no breast feeding, which included nulliparous women and nonlactating mothers; 1 = mixed feeding; 2 = exclusive breast feeding) predicted both more aggression (β = 0.31, p < .05) and lower SBP (β = −0.33, p < .05). Simultaneous regression of aggression on breast-feeding status and SBP suggested that physiological arousal mediated the effect of breast feeding on aggression. Specifically, the effect of breast-feeding status was not significant when SBP was included in the model (β = 0.21, p = .17), whereas SBP remained a significant predictor (β = −0.31, p < .05), F(4, 46) = 3.17, p < .05, R2 = .22. A bootstrap test with 5,000 replications indicated a significant indirect effect of breast feeding on aggression via SBP (95% confidence interval = [0.02, 0.75]). These findings are consistent with the hypothesis that breast feeding increases aggressive behavior by attenuating physiological arousal.
Discussion
This study provides the first behavioral evidence of heightened aggression in breast-feeding women. Mothers who exclusively breast-fed their infants were almost twice as aggressive as formula-feeding mothers and nulliparous women. However, this study did not show mothers as a group to be more aggressive than nonmothers—formula-feeding mothers did not demonstrate more aggressive behavior than nonmothers. In addition, lactation-related decreases in autonomic stress response mediated the effect of breast feeding on aggression. Exclusively breast-feeding mothers had lower BP during the aggressive encounters relative to the other groups, and BP correlated inversely with aggressive behavior. Together, these findings suggest that in humans, as in many other mammalian species, lactating mothers are more likely to aggress against hostile conspecifics than are nonlactating mothers or nulliparous women, at least in part because they experience dampened arousal in response to stressful aggressive encounters.
In a sense, humans are born “prematurely” relative to their mammalian counterparts: Humans’ large and complex brains take many years to mature, and during this time, humans face an extended period of vulnerability to hazards (Johnson, 2005). Parental protection of children is therefore a fundamental selection pressure for humans that is likely to have promoted the evolution of mechanisms to facilitate defensive responses (Hahn-Holbrook, Holbrook, & Haselton, 2011). The reduction in fear of aggressing that is attendant to lactation appears to be one such time-matched proximate mechanism, bolstering maternal protection at the point in the human life span when offspring are most vulnerable. We are not proposing, however, that lactation-induced aggression engenders hostile behavior indiscriminately or promotes offensive aggression directed at goals such as access to mates or social dominance. From an adaptive-functional perspective, one would not expect mothers to have evolved mechanisms that lead them to initiate potentially dangerous aggressive encounters beyond the realm of defense during the point at which their young are dependent on their mother’s milk for survival (Campbell, 1999; S. E. Taylor et al., 2000). Rather, as research from nonhuman mammals documents, lactation is likely to boost aggression primarily in contexts in which either the mother or her offspring are in jeopardy (Archer, 1988).
Breast-feeding mothers may respond to hostile provocations with heightened aggression even when infants are not immediately present. The breast-feeding mothers in our study, for example, were more aggressive than non-breast-feeding mothers and nonmothers in the absence of a direct threat to their infants, who were in an adjacent room. This finding parallels numerous findings that rodent mothers aggress when a hostile conspecific is introduced, even when the pups are removed before the encounter (Lonstein & Gammie, 2002). By responding more aggressively to perceived threats, whether directed specifically at infants or at themselves, lactating mothers in the ancestral past may have deterred predators and hostile conspecifics. Further research is needed to assess the impact of the proximity of infants to ostensibly hostile persons on human maternal defense. In addition, contextual factors such as the sex or relative formidability of the hostile persons, or the quality of mother-infant attachment, may influence maternal defense (Hahn-Holbrook et al., 2011).
It remains to be seen whether the links among lactation, stress reactivity, and aggression translate into substantive differences in the well-being of children or mothers in modern industrialized societies. Conceivably, breast feeding may help mothers muster assertiveness in hostile social exchanges (e.g., with romantic partners, at work, or during recreation), some of which may directly pertain to infant welfare. Crucially, however, we are not suggesting that formula-feeding mothers or mothers with older children do not also confront hazards to defend their children, or that lactation constitutes the sole pathway through which maternal defense manifests itself. We propose only that lactation is an important pathway for promoting aggression toward hostile interlopers, and that it has this effect by reducing otherwise prohibitive levels of maternal fear.
One limitation of the current study, and any study of lactation in humans, is that we could not experimentally manipulate conception or the choice to breast-feed. Therefore, we cannot claim that lactation is the causal factor facilitating the observed increase in aggressive behavior in our sample. We were, however, able to rule out a number of alternative explanations. For instance, lactation remained an important predictor of aggression after we controlled for variables that may potentially differ as a function of infant feeding method (e.g., romantic-relationship status, age of mother, income, and work status). Nevertheless, variables such as maternal attachment could covary with both willingness to aggress and the decision to breast-feed, and must therefore be examined in further research. Future studies might also explore whether lactation-linked hormones, such as oxytocin, are responsible for the heightened aggression observed in breast-feeding women, given that oxytocin buffers BP reactivity to stress (Light et al., 2000) and has been implicated in heightened defensive aggression (see De Dreu et al., 2010).
In conclusion, this research provides the first behavioral evidence linking lactation with heightened postpartum aggression. Moreover, our results correlating reduced autonomic reactivity with greater aggression may provide insight into female forms of aggression more generally. Further research on the mediators of heightened aggression during the lactation period may carry implications both for preventing aggression in problematic contexts and for fostering defensive aggressive behaviors that are socially desirable.
Acknowledgments
We thank Joel Mort and Jesse Bering for facilitating funding for this research. We thank Rachel Olson, Courtney Lisonbee, Paige Pickard, Ben Reece, and Victor Solis for research assistance. We also thank Chris Porter, Ross Flom, and Blake Jones for sharing laboratory space for this project.
Funding
Financial support from the Institute of Cognition and Culture at Queen’s University Belfast, the Women’s Research Institute at Brigham Young University, and the U.S. Air Force, Office of Scientific Research (Grant FA8655-09-1-3065) is gratefully acknowledged. The first author was supported by the MH15750 training fellowship in Biobehavioral Issues in Mental and Physical Health at the University of California, Los Angeles, during work on this project.
Footnotes
Declaration of Conflicting Interests
The authors declared that they had no conflicts of interest with respect to their authorship or the publication of this article.
The adjusted marginal means differ from those presented in the previous analysis because they were adjusted for income but not relationship status because all the mothers in our sample were married, and because nulliparous women were not included in this second analysis. The raw means of aggression for all groups were as follows: breast-feeding mothers (M = 4.8, SD = 1.6), formula-feeding group as a whole (M = 2.8, SD = 2.4), mixed-feeding subset of formula-feeding mothers (M = 3.3, SD = 2.8), exclusively formula-feeding subset of formula-feeding mothers (M = 2.5, SD = 2.2), and nulliparous women (M = 4.0, SD = 2.3).
References
- Archer J. The behavioral biology of aggression. Cambridge, England: Cambridge University Press; 1988. [Google Scholar]
- Boccia ML, Pedersen CA. Brief vs. long maternal separations in infancy: Contrasting relationships with adult maternal behavior and lactation levels of aggression and anxiety. Psychoneuroendocrinology. 2001;26:657–672. doi: 10.1016/s0306-4530(01)00019-1. [DOI] [PubMed] [Google Scholar]
- Bushman BJ. Does venting anger feed or extinguish the flame? Catharsis, rumination, distraction, anger, and aggressive responding. Personality and Social Psychology Bulletin. 2002;24:724–731. [Google Scholar]
- Buss AH, Perry M. The aggression questionnaire. Journal of Personality and Social Psychology. 1992;63:452–459. doi: 10.1037//0022-3514.63.3.452. [DOI] [PubMed] [Google Scholar]
- Campbell A. Staying alive: Evolution, culture, and women’s intrasexual aggression. Behavioral & Brain Sciences. 1999;22:203–252. doi: 10.1017/s0140525x99001818. [DOI] [PubMed] [Google Scholar]
- De Dreu CKW, Greer LL, Handgraaf MJJ, Shalvi S, Van Kleef GA, Baas M, Feith SWW. The neuropeptide oxytocin regulates parochial altruism in intergroup conflict among humans. Science. 2010;328:1408–1411. doi: 10.1126/science.1189047. [DOI] [PubMed] [Google Scholar]
- Erskine MS, Barfield RJ, Goldman BD. Intraspecific fighting during late pregnancy and lactation in rats and effects of litter removal. Behavioral Biology. 1978;23:206–218. doi: 10.1016/s0091-6773(78)91814-x. [DOI] [PubMed] [Google Scholar]
- Gammie SC, D’Anne KL, Lee G, Stevenson SA. Role of corticotropin releasing factor-related peptides in the neural regulation of maternal defense. In: Bridges JS, editor. The neurobiology of the parental brain. Oxford, England: Academic Press; 2008. pp. 103–114. [Google Scholar]
- Giordano AL, Siegel HI, Rosenblatt JS. Effects of mother-litter separation and reunion on maternal aggression and pup mortality in lactating hamsters. Physiology and Behavior. 1984;33:903–906. doi: 10.1016/0031-9384(84)90226-9. [DOI] [PubMed] [Google Scholar]
- Grinnell J, McComb K. Maternal grouping as a defense against infanticide by males: Evidence from field playback experiments on African lions. Behavioral Ecology. 1996;7:55–59. [Google Scholar]
- Hahn-Holbrook JA, Holbrook C, Haselton M. Parental precaution: Neurobiological means and adaptive ends. Neuroscience and Biobehavioral Reviews. 2011;35:1052–1066. doi: 10.1016/j.neubiorev.2010.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen S, Ferreira A. Food intake, aggression, and fear behavior in the mother rat: Control by neural systems concerned with milk ejection and maternal behavior. Behavioral Neuroscience. 1986;100:64–70. doi: 10.1037//0735-7044.100.1.64. [DOI] [PubMed] [Google Scholar]
- Hansen S, Ferreira A, Selart ME. Behavioral similarities between mother rats and benzodiazepine-treated non-maternal animals. Psychopharmacology. 1985;86:344–347. doi: 10.1007/BF00432226. [DOI] [PubMed] [Google Scholar]
- Heise S, Lippke J. Role of female aggression in prevention of infanticidal behavior in male common voles, Microtus arvalis. Aggressive Behavior. 1997;23:293–298. [Google Scholar]
- Hersher L, Richmond JB, Moore AU. Maternal behavior in sheep and goats. In: Rheingold HL, editor. Maternal behavior in mammals. New York, NY: Wiley; 1963. pp. 203–232. [Google Scholar]
- Johnson MH. Developmental cognitive neuroscience. 2nd ed. Malden, MA: Blackwell; 2005. [Google Scholar]
- Kamarck TW, Debsk TT, Manuck SB. Enhancing the laboratory-to-life generalizability of cardiovascular reactivity using multiple occasions of measurement. Psychophysiology. 1992;37:533–542. [PubMed] [Google Scholar]
- Ledesma J, de Luis JM, Montejo AL, Llorca G, Perez-Urdaniz A. Maternal aggression in human beings. New Trends in Experimental Clinical Psychiatry. 1988;4:223–228. [Google Scholar]
- Light KC, Smith TE, Johns JM, Brownley KA, Hofheimer JA, Amico JA. Oxytocin responsivity in mothers of infants: A preliminary study of relationships with blood pressure during laboratory stress and normal ambulatory activity. Health Psychology. 2000;19:560–567. doi: 10.1037//0278-6133.19.6.560. [DOI] [PubMed] [Google Scholar]
- Lonstein JS, Gammie SC. Sensory, hormonal, and neural control of maternal aggression in laboratory rodents. Neuroscience and Biobehavioral Reviews. 2002;26:869–888. doi: 10.1016/s0149-7634(02)00087-8. [DOI] [PubMed] [Google Scholar]
- Maestripieri D. Costs and benefits of maternal aggression in lactating female rhesus macaques. Primates. 1994;35:443–453. [Google Scholar]
- Maestripieri D, D’Amato FR. Anxiety and maternal aggression in house mice (Mus musculus): A look at interindividual variability. Journal of Comparative Psychology. 1991;105:295–301. doi: 10.1037/0735-7036.105.3.295. [DOI] [PubMed] [Google Scholar]
- Mastrogiacomo I, Fava M, Fava GA, Kellner R, Cetera C, Grismondi G. Postpartum hostility and prolactin. International Journal of Psychiatry in Medicine. 1983;12:289–294. doi: 10.2190/6k03-e32r-nja4-9c3f. [DOI] [PubMed] [Google Scholar]
- Mezzacappa ES. Breastfeeding and maternal stress response and health. Nutrition Reviews. 2004;67:261–268. doi: 10.1111/j.1753-4887.2004.tb00050.x. [DOI] [PubMed] [Google Scholar]
- Pourtois G, Grandjean D, Sander D, Vuilleumier P. Electrophysiological correlates of rapid spatial orienting toward fearful faces. Cerebral Cortex. 2004;14:619–633. doi: 10.1093/cercor/bhh023. [DOI] [PubMed] [Google Scholar]
- Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behavior Research Methods. 2008;40:879–891. doi: 10.3758/brm.40.3.879. [DOI] [PubMed] [Google Scholar]
- Ross S, Sawin PB, Zarrow MX, Denenberg VH. Maternal behavior in the rabbit. In: Rheingold HL, editor. Maternal behavior in mammals. New York, NY: Wiley; 1963. pp. 94–121. [Google Scholar]
- Schino G, D’Amato FR, Troisi A. Maternal aggression in lactating female Japanese macaques: Time course and interindividual variation. Canadian Journal of Zoology. 2004;82:1975–1979. [Google Scholar]
- Schneirla TC, Rosenblatt JS, Tobach E. Maternal behavior in the cat. In: Rheingold HL, editor. Maternal behavior in mammals. New York, NY: Wiley; 1963. pp. 122–168. [Google Scholar]
- Smith WP. Maternal defense in Columbian white-tailed deer: When is it worth it? The American Naturalist. 1987;130:310–316. [Google Scholar]
- Taylor JC. Home range and agonistic behavior in the grey squirrel. Symposia of the Zoological Society London. 1966;18:229–234. [Google Scholar]
- Taylor SE, Klein LC, Lewis BP, Gruenewald TL, Gurung RAR, Updegraff JA. Biobehavioral responses to stress in females: Tend-and-befriend, not fight-or-flight. Psychological Review. 2000;107:411–429. doi: 10.1037/0033-295x.107.3.411. [DOI] [PubMed] [Google Scholar]
- Troisi A, D’Amato FR, Carnera A, Trinca L. Maternal aggression by lactating group-living Japanese macaque females. Hormones and Behavior. 1988;22:444–452. doi: 10.1016/0018-506x(88)90049-9. [DOI] [PubMed] [Google Scholar]
- Ylönen H, Horne T. Infanticide and effectiveness of pup protection in bank voles: Does the mother recognize a killer? Acta Ethologica. 2002;4:97–101. [Google Scholar]