Abstract
BACKGROUND
Our previous study showed that prostate cancer cells overexpress and secrete secretory phospholipases A2 group IIa (sPLA2-IIa) and plasma sPLA2-IIa was elevated in prostate cancer patients. The current study further explored the underlying mechanism of sPLA2-IIa overexpression and the potential role of sPLA2-IIa as a prostate cancer biomarker.
METHODS
Plasma and tissue specimens from prostate cancer patients were analyzed for sPLA2-IIa levels. Regulation of sPLA2-IIa expression by Heregulin-α was determined by western blot and reporter assay.
RESULTS
We found that Heregulin-α enhanced expression of the sPLA2-IIa gene via the HER2/HER3-elicited pathway. The EGFR/HER2 dual inhibitor Lapatinib and the NF-kB inhibitor Bortezomib inhibited sPLA2-IIa expression induced by Heregulin-α. Heregulin-α upregulated expression of the sPLA2-IIa gene at the transcriptional level. We further confirmed that plasma sPLA2-IIa secreted by mouse bearing human prostate cancer xenografts reached detectable plasma concentrations. A Receiver Operating Characteristic (ROC) analysis of patient plasma specimens revealed that high levels of plasma sPLA2-IIa, with the optimum cutoff value of 2.0 ng/ml, were significantly associated with high Gleason score (8~10) relative to intermediate Gleason score (6~7) prostate cancers and advanced relative to indolent cancers. The area under the ROC curve (AUC) was 0.73 and 0.74, respectively.
CONCLUSION
We found that Heregulin-α, in addition to EGF, contributes to sPLA2-IIa overexpression in prostate cancer cells. Our findings support the notion that high levels of plasma sPLA2-IIa may serve as a poor prognostic biomarker capable of distinguishing aggressive from indolent prostate cancers, which may improve decision making and optimize patient management.
Keywords: plasma cancer biomarker, indolent and aggressive prostate cancer, poor prognosis, HER/HER2-elicited pathway, Heregulin-α, HER3
INTRODUCTION
Prostate cancer is the second leading cause of cancer death among men in the United States (1). Standard diagnostic tests for prostate cancer include PSA, biopsy, Gleason score, and magnetic resonance imaging (MRI). All of these tests, however, have their limitations. PSA test lacks both sensitivity and specificity for prostate cancer and has not been validated in the setting of prostate cancer surveillance. Prostate biopsies are prone to sampling errors and repeated multiple core biopsies trigger inflammation. Imaging often misses small tumors. To date, the biopsy-dependent Gleason score remains the sole diagnostic modality with confirmed prognostic power, with high scores correlating with negative outcomes. Overcoming these limitations can be a challenge for Urologists and Medical Oncologists. Regrettably, there are no validated plasma biomarkers with diagnostic and prognostic power, which underscores the urgent need for new additional biomarkers for prostate cancer.
Urologic guidelines have established a PSA value of 4.0 ng/ml as the upper limit of normal and at this level, a prostate biopsy is mandatory (2). However, due to the suboptimal sensitivity of the PSA test, more than a half a million men each year will be subjected to unnecessary biopsies (3,4). PSA is tissue-specific but not cancer-specific and has only 21 % sensitivity for predicting prostate cancer (5). Plasma PSA levels are elevated in benign diseases, the most common being BPH. This group of patients constitutes 30~50% of men with elevated PSA tests (6). On the other hand, given the heterogeneous nature of cancer, many aggressive prostate cancers with high Gleason scores do not demonstrate high PSA values and fail to be detected in PSA screening, which underscores the low sensitivity of the test (6–10). Although the PSA test is widely used for active surveillance of indolent (insignificant or favorable) prostate cancer, its significance remains to be validated by clinical trials. Given the heterogeneous nature of prostate cancers, there is a clear unmet clinical need to identify novel plasma biomarkers to improve management of these tumors.
Numerous studies have revealed that elevated HER/HER2-PI3K-Akt-NF-kB signaling and inflammation participate in the development and progression of many cancers, including prostate cancer (11–13). NF-kB is a key link between inflammation and tumorigenesis (14,15). The secretory phospholipases A2 group IIa (sPLA2-IIa) is a phospholipid hydrolase enzyme mediating the release of arachidonic acid (AA) and lysophosphatidylcholine, the precursors of eicosanoids and platelet-activating factor, respectively (16,17). Eicosanoids exert control over many physiologic and pathologic processes, such as inflammation, immunity, and tumorigenesis. Multiple key genes in the eicosanoid biosynthetic pathway, e.g. NF-kB (13), Cox-2 (18), and sPLA2-IIa (19–21), are overexpressed in prostate cancer and are associated with cancer progression. Our group and others found that sPLA2-IIa is a NF-kB target gene (22,23). Several early studies have demonstrated that sPLA2-IIa is overexpressed in almost all human prostate cancer specimens and further elevated levels of sPLA2-IIa are associated with tumor grade, while sPLA2-IIa is undetectable in normal prostate tissue (19–21). sPLA2-IIa remains elevated in androgen-independent prostate cancers failing hormonal therapy (24). Preclinical studies have revealed that high levels of sPLA2-IIa expression are associated with a more aggressive tumor phenotype in the spontaneous TRAMP prostate cancer model (25). sPLA2-IIa stimulates prostate tumor growth (24,26), while inhibition of eicosanoid signaling leads to tumor regression in a mouse xenograft prostate cancer (27).
We previously reported that sPLA2-IIa was overexpressed in androgen-independent prostate cancer LNCaP-AI cells relative to their androgen-dependent LNCaP cell counterparts and was involved in androgen-independent cell growth (23). We were the first to demonstrate that prostate cancer cells secreted sPLA2-IIa and EGF stimulated sPLA2-IIa expression and secretion via EGFR/HER2-PI3K-Akt-NF-kB signaling (23,28). In the current study, we further explored the underlying mechanism of sPLA2-IIa overexpression and the potential role of sPLA2-IIa in prostate cancer. We found that Heregulin-α also stimulates sPLA2-IIa expression via the HER2/HER3-elicited pathway. More importantly, using a mouse prostate cancer xenograft model, we confirmed that prostate tumor secreted detectable levels of sPLA2-IIa into the circulation. To validate plasma sPLA2-IIa as a potential biomarker for prostate cancer, a larger sample size analyzed by ROC analysis revealed that high levels of plasma sPLA2-IIa were associated with high Gleason scores and advanced cancer stage. This finding implicates plasma sPSLA2-IIa as a potential poor prognostic biomarker in prostatic neoplastic disease.
MATERIALS AND METHODS
Plasma samples
134 prostate cancer patients from the University Hospital, Cincinnati, Ohio, were consented, and subsequently plasma and tissue specimens and patient’s medical information were obtained from UC cancer center tissue bank.
Reagents
RPMI 1640 medium was purchased from Invitrogen (Gaithersburg, MD). Fetal bovine serum (FBS) and charcoal/dextran-treated FBS were purchased from HyClone Laboratories (Logan, UT). sPLA2-IIa antibody and sPLA2-IIa ELISA kit were obtained from Cayman Chemical (Ann Arbor, MI). sPLA2-IIa antibody for IHC was from LifeSpan BioSciences (Seattle, WA). P-HER2, P-HER3 antibodies were from Cell Signaling Technology (Danvers, MA). HER2 and HER3 antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Lapatinib and Bortezomib were purchased from Selleck Chemicals LLC. Heregulin-α was purchased from Thermo Fisher Scientific Inc. (Fremont, CA). Prostate disease spectrum tissue array was from US Biomax (Rockville, MD).
Cell culture
The human prostate adenocarcinoma cell line LNCaP was obtained from ATCC (Rockville, MD) and maintained in RPMI-1640 medium supplemented with 10% FBS (complete medium) at 37°C in 5% CO2. LNCaP-AI cells were maintained in RPMI-1640 medium supplemented with 10% charcoal/dextran-treated FBS (stripped medium). LAPC-4 cells, which express wild type AR, were maintained in Iscove’s modified Dulbecco’s medium supplemented with 10% FBS and 10 nmol/L DHT. Transient transfection experiments were performed in stripped medium.
Plasmid
800 bp of sPLA2-IIa promoter fragment was amplified by PCR using LNCaP cell genomic DNA as template. Plasmid sPLA2-IIa(−800)-Luc was generated by insertion of the PCR fragment into pGL5-Luc vector as described previously (23).
Western blot analysis
Western blot analysis was performed as previously described (23). Briefly, aliquots of samples with the same amount of protein, determined using the Bradford assay (BioRad, Hercules, CA), were mixed with loading buffer (final concentrations of 62.5 mM Tris-HCl, pH 6.8, 2.3% SDS, 100 mM dithiothreitol, and 0.005% bromophenol blue), boiled, fractionated in a SDS-PAGE, and transferred onto a 0.45-um nitrocellulose membrane (BioRad). The filters were blocked with 2% fat-free milk in PBS, and probed with primary antibody in PBS containing 0.1% Tween 20 (PBST) and 1% fat-free milk. The membranes were then washed four times in PBST and incubated with horseradish peroxidase-conjugated secondary antibody (BioRad) in PBST containing 1% fat-free milk. After washing four times in PBST, the membranes were visualized using the ECL Western blotting detection system (Amersham Co., Arlington Height, IL).
Reporter assay
Cells (105/well) were seeded in 12 well tissue culture plates. Next day, Optifect-mediated transfection was used for the transient transfection assay according to the protocol provided by Invitrogen/Life Technologies, Inc. The cells were then treated with hormone or drugs in stripped medium for 24 hours. Subsequently, the cell extracts were prepared and luciferase activity was assessed in a Berthold Detection System (Pforzheim, Germany) using a kit (Promega, Madison, WI) following the manufacture’s instruction. For each assay, cell extract (20 ul) was used and the reaction was started by injection of 50 ul of luciferase substrate. Each reaction was measured for 10 seconds in the Luminometer. Luciferase activity was defined as light units/mg protein.
Enzyme-linked immunosorbent assay (ELISA)
sPLA2-IIa level in plasma was determined by ELISA kit (Catalog No. 585000, Cayman Chemical Company). The ELISA was performed according to the protocol provided by Company. All human plasma samples were diluted for ten times for ELISA. The concentration of sPLA2-IIa plasma was tested in duplicate, indicated as pg/ml, and determined against standard curve of each ELISA assay.
Immunohistochemical (IHC) staining
IHC staining was performed as detailed in our previous studies (29). Briefly, paraffin-embedded tissue sections were deparaffinized in xylene, rehydrated in graded alcohol, and transferred to PBS. The slides were treated with a citric acid-based antigen-retrieval buffer (DAKO Co., Carpinteria, CA), followed by 3% H2O2 in methanol, incubated in blocking buffer (5% BSA and 5% horse serum in PBS) and then in the blocking buffer containing first antibody. After washing, the slides were incubated with a biotinylated secondary antibody (BioGenex Laboratories, San Ramon, CA), followed by washing and incubation with the streptavidin-conjugated peroxidase (BioGenex). A positive reaction was visualized by incubating the slides with stable diaminobenzidine and counterstaining with Gill’s hematoxylin (BioGenex) and mounted with Universal Mount mounting medium (Fisher Scientific, Pittsburgh, PA).
Subcutaneous inoculation
LNCaP cells (1X106 cells in 100 uL HBSS) were mixed with 100 ul Matrigel and injected subcutaneously on the left lateral chest wall close to the axilla of male nude mice. Three mice were inoculated with tumor cells, while the mice without tumor cell inoculation were used as control. Tumor growth was monitored twice a week using calipers. By the eighth week of cancer cell inoculation, the tail vein blood samples were collected and plasma samples were prepared and subjected to ELISA assay to determine the human sPLA2-IIa. The concentration of sPLA2-IIa was tested in duplicate and determined against standard curve of the ELISA assay.
Statistical Analysis
Unpaired t-test was performed to evaluate the difference of the mean levels of plasma sPLA2-IIa between stage T1 prostate cancer (n=18) and the stage T2~T4 prostate cancer (n=101) as well as between Gleason score 6~7 (n=85) and Gleason scores 8~10 (n=41) prostate cancer.
A parametric Receiver Operating Characteristic (ROC) analysis was performed to associate a high level of plasma sPLA2-IIa with advanced stage T2~T4 prostate cancer (n=101) relative to early stage T1 cancer (n=18) and with high Gleason scores 8~10 (n=41) prostate cancer relative to intermediate Gleason score 6~7 (n=85) cancer. The optimum cutoff value of plasma sPLA2-IIa was determined, which separated the combined set of sPLA2-IIa values into two groups, such that the number of correctly classified specimens was maximized, and the associated sensitivity, specificity, and AUC were determined.
RESULTS
Prostate tumor secreted sPLA2-IIa into the circulation to a detectable level
We previously demonstrated elevated plasma sPLA2-IIa levels in prostate cancer patients. Given that macrophages also secrete sPLA2-IIa during inflammatory reactions, we determined whether tumors secret sPLA2-IIa into the circulation in a mouse prostate cancer model. One million LNCaP cells were inoculated subcutaneously into the nude mice. The LNCaP tumors reached approximately 2.9 cm3 by the eighth week after LNCaP cell inoculation. The blood samples from the tumor-bearing mice or control non-tumor-bearing mice were collected. We found that plasma human sPLA2-IIa reached approximately 9.73 ± 5.8 ng/ml in the tumor-bearing mice, while it was undetectable in the control mice. This data confirmed that plasma sPLA2-IIa is secreted from cancer cells to reach a detectable level and can potentially serve as a biomarker for prostate cancer.
High levels of plasma sPLA2-IIa were associated with high Gleason score and advanced cancer stage
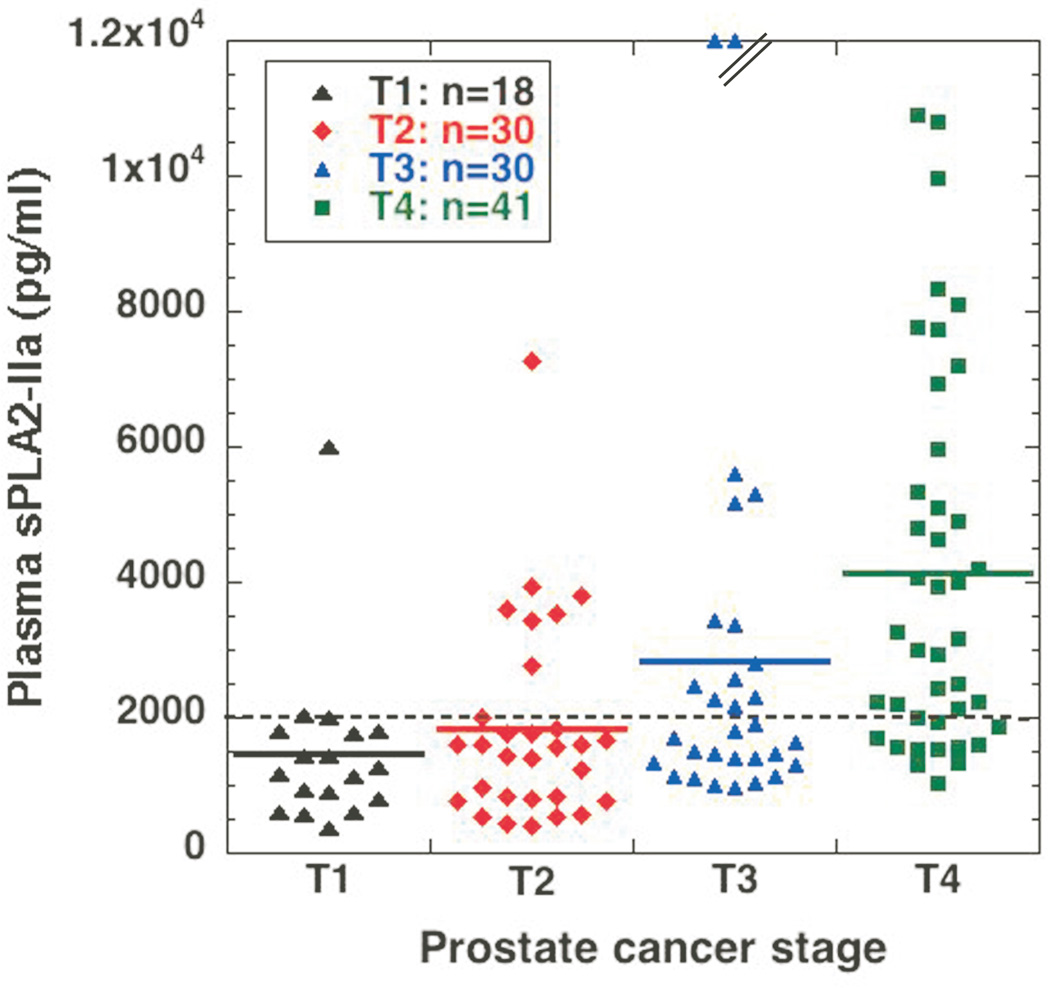
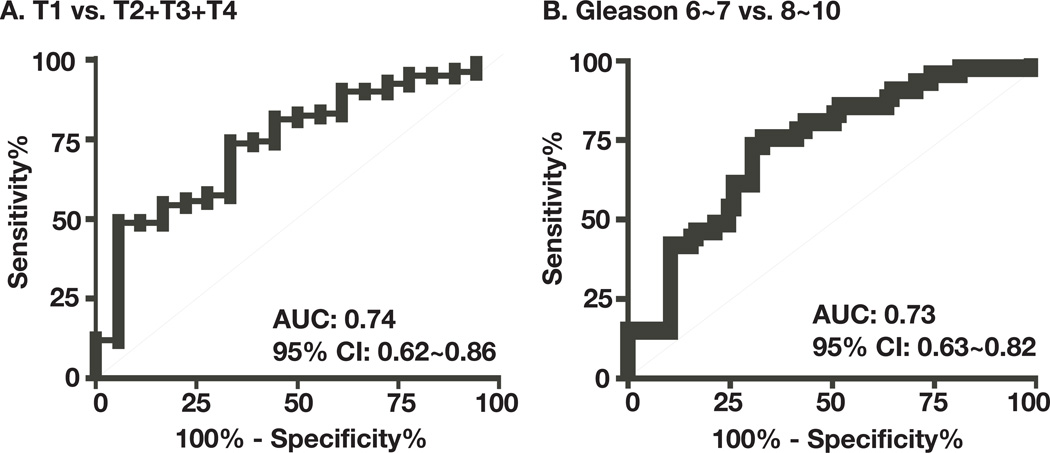
To perform a detailed analysis and potentially correlate high levels of plasma sPLA2-IIa with advanced prostate cancer and high Gleason score using a large statistical significant sample size, we collected an additional 91 plasma samples from prostate cancer patients regardless of treatment. A total 134 samples, including 43 prostate cancer samples published previously (23), were analyzed. We found that the mean levels of plasma sPLA2-IIa were 1479 ± 1246, 1843 ± 1481, 3130 ± 3892, and 4141 ± 2790 pg/ml for stage T1 (n=18), T2 (n=30), T3 (n=30), and T4 (n=41) prostate cancers, respectively, which were increased with prostate cancer progression (Fig. 1 and supplementary table 1). We determined whether high levels of plasma sPLA2-IIa were associated with advanced cancer relative to indolent cancer by the Receiver Operating Characteristic (ROC) analysis. The indolent prostate cancer was defined as stage T1 and Gleason score 6 in this prostate cancer cohort (30–32). The mean value of plasma sPLA2-IIa values from the indolent stage T1 prostate cancer (n=18) and the advanced stage T2~T4 prostate cancer (n=101) were 1480 ± 293 (n=18) and 3159 ± 299 (n=101) pg/ml, respectively. The levels of plasma sPLA2-IIa from patients with advanced prostate cancer were significantly higher than those with indolent cancer (P<0.0001, Unpaired t-test). ROC analysis revealed that an optimum cutoff value for plasma sPLA2-IIa of 2.0 ng/ml predicted an advanced prostate cancer with 50% sensitivity and 83% specificity (Fig. 2A). Area Under Curve (AUC) was 0.74 (95% confidence interval: 0.6204 to 0.8559). The mean ages for stage T1, T2, T3, and T4 prostate cancers were 69.28 ± 9.59, 63.23 ± 6.27, 62.67 ± 8.94, and 65.56 ± 11.22, respectively. Therefore, the age of the patients was not significantly associated with the advanced stage of prostate cancer (P>0.1) and did not contribute to elevated plasma sPLA2-IIa.
Fig. 1. Determination of plasma sPLA2-IIa in prostate cancer patients.
Plasma samples from prostate cancer patients were diluted ten times and then subjected to ELISA analysis in duplicate for each sample. The average was calculated as pg/ml based on the standard curve of each experiment. Two samples from stage T3 prostate cancer are above the scale. The dotted line indicates 2.0 ng/ml cutoff value.
Fig. 2. Association analysis of high levels of plasma sPLA2-IIa with high Gleason score and advanced cancer stage.
18 specimens of stage T1 prostate cancer versus 101 specimens of advanced stage T2~T4 prostate cancer (A) and 85 specimens of Gleason score 6~7 prostate cancer versus 41 specimens of Gleason scores 8~10 prostate cancer (B), were subjected to ROC analysis. Area under the ROC curve (AUC) and 95% confidence interval (95% CI) were determined.
A further analysis demonstrated that the mean plasma sPLA2-IIa values from the intermediate Gleason score (6~7; n=85) and high Gleason scores (8~10; n=41) prostate cancers were 2098 ± 196 and 4063 ± 595 pg/ml, respectively. The levels of plasma sPLA2-IIa were significantly higher in prostate cancers with Gleason scores 8~10 than those with Gleason score 6~7 regardless of treatment (P < 0.0001, Unpaired t-test). ROC analysis revealed that an optimum cutoff value for plasma sPLA2-IIa of 2.0 ng/ml predicted prostate cancer of high Gleason score with 61% sensitivity and 73% specificity (Fig. 2B). The AUC was 0.73 (95% confidence interval: 0.63 to 0.82). The mean ages for prostate cancer patients with intermediate Gleason score and high Gleason score were 66.98 ± 10.18 and 63.41 ± 9.43, respectively. Therefore, the age of the patients was not significantly associated with high Gleason score (P>0.1) and did not contribute to an elevated plasma sPLA2-IIa.
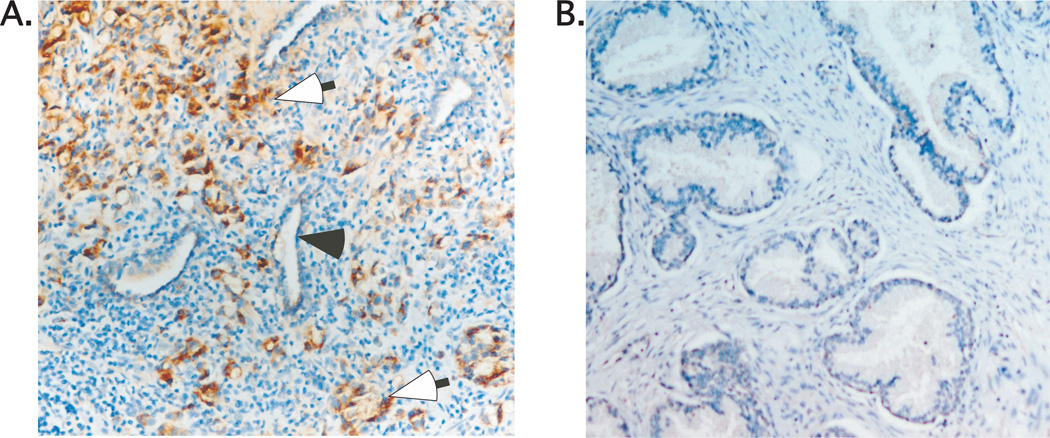
IHC analysis showed an elevated expression of sPLA2-IIa in tumor specimens (23,28) (Fig. 3A), confirming the findings of others (19–21). Further, in a comprehensive analysis of 50 biopsies from both commercial prostate disease tissue arrays and our cancer center tissue bank, we found no significant positive staining for sPLA2-IIa in non-malignant prostate tissue specimens (Fig. 3B).
Fig. 3. Expression analysis of sPLA2-IIa in prostate cancer (A) and BPH (B) specimens.
Open arrow (brown staining) indicates positivity for sPLA2-IIa in prostate cancer cells. Close arrow indicates normal epithelial cells. sPLA2-IIa is undetectable in the epithelial cells of normal acini and BPH.
It was noteworthy that there was no significant correlation between high PSA levels and Gleason scores or cancer stage, since there was a wide range of PSA levels from 0.1 to 3,300 ng/ml among 134 samples. Almost all 134 prostate cancer patients have been treated with a variety of modalities including hormone ablation therapy, radiotherapy, and prostatectomy. It should be recognized that these treatments significantly alter patient's plasma PSA levels. Nevertheless, our data strongly suggest that high levels of plasma sPLA2-IIa are associated with advanced cancer stage and high Gleason score and as such, represent a robust prognostic biomarker for the identification of poor prognosis prostate cancer.
Heregulin-α stimulates sPLA2-IIa expression
To further elucidate the impact of HER/HER2-PI3K-Akt-NF-kB signaling on sPLA2-IIa expression in prostate cancer cells, we determined whether Heregulin-α a ligand predominantly for HER3, regulates expression of the sPLA2-IIa gene in prostate cancer cells (23). LNCaP, LNCaP-AI, and LAPC4 cells were treated with Heregulin-α for 24 hours and the resultant cell extracts were subjected to western blot analysis. Significant phosphorylation of HER3 at the Tyr 1289 site was observed upon Heregulin-α treatment, while its basal phosphorylation level was very low in untreated LNCaP, LNCaP-AI, and LAPC4 cells (Fig. 4). Heregulin-α phosphorylates and activates HER3 and stimulates sPLA2-IIa expression in a dose-dependent manner (Fig. 4A). The basal phosphorylation level of HER2 at Tyr 1248 site was high, while Heregulin-α treatment did not significantly increase HER2 phosphorylation, indicating that HER2 was constitutively active in these cells (Fig. 4). Heregulin-α significantly stimulated sPLA2-IIa expression in LNCaP cells and moderately in LNCaP-AI cells, given that basal expression of sPLA2-IIa was increased in LNCaP-AI cells (23). Furthermore, the EGFR/HER2 dual inhibitor Lapatinib and NF-kB inhibitor Bortezomib blocked Heregulin-α–induced HER3 and HER2 phosphorylation, and consistently downregulated sPLA2-IIa expression at both basal level and in the setting of Heregulin-α-induced expression. These data showed that HER3 is activated upon Heregulin-α treatment and HER2/HER3 activation induced by Heregulin-α stimulation results in enhanced sPLA2-IIa expression. Bortezomib treatment abolished or compromised phosphorylation of HER2 and HER3, but the molecular mechanism remains to be clarified.
Fig. 4. Heregulin-α activates HER3 and stimulates sPLA2-IIa expression in prostate cancer cells.
LNCaP, LNCaP-AI, and LAPC4 cells were starved in 1% stripped medium for 24 hours. The cells were then treated with various concentrations of Heregulin-α for 24 hours (A). The cells were also treated with Lapatinib (10 uM) and Bortezomib (5 uM) without or with Heregulin-α (50 ng/ml) for 24 hours (B-D). The cell extracts were prepared and subjected to western blot analysis for sPLA2-IIa, HER2, P-HER2, HER3, and P-HER3.
This study showed that HER2 is constitutively active in prostate cancer cells, contributing to elevated signaling of the HER/HER2-elicited pathway. Both EGF and Heregulin-α stimulate sPLA2-IIa expression via the HER/HER2-PI3K-Akt-NF-kB pathway in prostate cancer cells.
Heregulin-α stimulates expression of the sPLA2-IIa gene at the transcriptional level
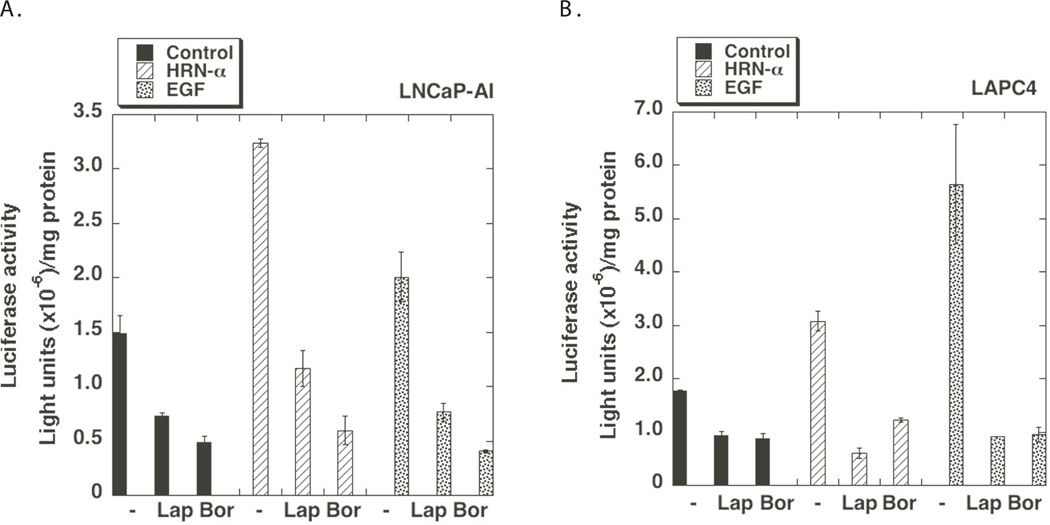
To determine whether Heregulin-α regulates sPLA2-IIa gene expression, we performed reporter assays using sPLA2-IIa(-800)-Luc, which is a luciferase reporter driven by 800 bp promoter of the sPLA2-IIa gene including two NF-kB response elements as reported by us and others previously (22,23). sPLA2-IIa(−800)-Luc reporter was transiently transfected into LNCaP-AI and LAPC4 cells. Subsequently, the cells were treated with EGF or Heregulin-α without or with Lapatinib and Bortezomib for 24 hours. Consistent with sPLA2-IIa protein expression data (Fig. 4), EGF and Heregulin-α significantly stimulated the promoter activity of sPLA2-IIa gene in LNCaP-AI and LAPC4 cells (Fig. 5). Lapatinib and Bortezomib downregulated the promoter activity of the sPLA2-IIa gene both at the basal level and in response to EGF or Heregulin-α stimulation. This data supports the notion that the elevated signaling of the HER/HER2-PI3K-Akt-NF-kB pathway upregulates expression of the sPLA2-IIa gene at the transcriptional level.
Fig. 5. Heregulin-α regulates sPLA2-IIa promoter activity.
LNCaP-AI (A) and LAPC4 (B) cells (105 cells/well) were seeded in a 12 well plate. Next day, the cells were transiently transfected with sPLA2-IIa(−800)-Luc (0.25 ug/well) overnight. The cells were then treated with Lapatinib (10 uM) and Bortezomib (5 uM) without or with EGF (100 ng/ml) or Heregulin-α (50 ng/ml) for 24 hours. Luciferase assay was performed according to a standard protocol with Renilla luciferase as an internal control. Data are presented as the mean (±SD) of duplicate values of a representative experiment that was independently repeated for five times.
DISCUSSION
Previously, we reported that prostate cancer cells secreted sPLA2-IIa and elevated plasma levels of sPLA2-IIa were found in prostate cancer patients. The current study demonstrated that prostate tumors, but not macrophages, secreted detectable levels of sPLA2-IIa. Additional plasma samples were collected and ROC analysis demonstrated that high levels of plasma sPLA2-IIa were associated with high Gleason scores and advanced cancer stage. Furthermore, we found that Heregulin-α, in addition to EGF, stimulated sPLA2-IIa expression via the HER2/HER3-elicited pathway. Elevated HER/HER2-PI3K-Akt-NF-kB signaling contributed to overexpression of sPLA2-IIa. Given that HER/HER2-PI3K-Akt-NF-kB signaling is involved in the development and progression of prostate cancer, our data suggest that plasma sPLA2-IIa may serve as a prognostic biomarker. As such, use of sPLA2-IIA in the clinical setting should significantly assist clinicians with their decision algorithm, enabling them to improve their management of prostate cancer.
Published epidemiologic data indicate that the death rate from prostate cancer is approximately 10~15%, implicating that most prostate cancers are indolent (insignificant or favorable) (30–32). It is also reported that prostate cancer is over-diagnosed by approximately 20% to 66% in the US and in Europe, especially with the use of the PSA test (33,34). The low annual mortality rate from prostate cancer after 15 years from diagnosis does not support aggressive treatment for indolent prostate cancers (30–32), while significant or unfavorable prostate cancers should be approached with aggressive treatments (33,35). Furthermore, although most prostate cancers diagnosed at an early stage are indolent, tumor progression and metastasis may eventually occur (35). Biopsy-dependent Gleason scores remain the sole diagnostic modality with confirmed prognostic power. Although the PSA test is widely used for active surveillance of indolent (insignificant or favorable) prostate cancer, its clinical utility has not been validated in active surveillance trials. In the absence of any alternative, clinicians rely on it as a screening biomarker and a measure of tumor burden in patients undergoing anti-tumor treatment. Given the heterogeneous nature of prostate cancers, identification of a novel plasma biomarker addresses a significant and immediate unmet clinical need. Given that high levels of plasma sPLA2-IIa were associated with high Gleason scores and advanced cancer stage, plasma sPLA2-IIa may potentially be used for the determination of poor prognostic tumors as well as in active surveillance of indolent prostate cancer.
The expression of the sPLA2-IIa gene is not tissue-specific (23). Increased levels of plasma sPLA2-IIa were reported in the setting of bacterial and viral infections, IL-2 infusion (36,37), and in coronary artery disease (38). However, a recent clinical study including more than 500 patients showed that there was no significant alterations in plasma levels of sPLA2-IIa protein among patients with coronary artery disease relative to healthy controls (38). Although macrophages may secrete sPLA2-IIa during an inflammatory response, our xenograft LNCaP tumor experiments demonstrated that tumor cells secrete detectable levels of sPLA2-IIa, confirming that elevated plasma sPLA2-IIa in patients with prostate cancers is the result of their prostate cancer. Furthermore, we found that age does not contribute to an elevated plasma sPLA2-IIa. To support this, we also found elevated plasma levels of sPLA2-IIa in lung cancer patients (unpublished data). An increased basal level of plasma sPLA2-IIa as a result of chronic lung inflammation or benign lung tumors was observed as compared to specimens from healthy controls. Nevertheless, high levels of plasma sPLA2-IIa are associated with advanced lung cancers and a decreased overall lung cancer survival. Validation of plasma sPLA2-IIa as prostate cancer biomarker in several prostate cancer cohorts is an ongoing project.
The EGF family of ligands, including EGF and Heregulins, stimulate the formation of HER (ErbB) receptor homodimers and heterodimers and receptor tyrosine kinase activity. EGF preferentially binds to EGFR and induces EGFR homodimers or EGFR/HER2 heterodimers. Binding to HER3 by Heregulin-α induces the formation of HER2/HER3 heterodimers (39). HER2 does not bind to any ligand with high affinity, but preferentially forms heterodimers with other HER family members for activation. In addition to EGFR and HER2, it was recently reported that HER3 and Heregulin-α were overexpressed in prostate cancer and predicted a poor prognosis (40). Furthermore, androgen receptor (AR) transactivation and cell proliferation induced by Heregulin-α were more potently inhibited by the EGFR/HER2 dual tyrosine kinase inhibitor Lapatinib than the EGFR-specific inhibitor Gefitinib, suggesting that autocrine-activated HER2/HER3 contributes to the proliferation signal (41,42). We found that Heregulin-α enhanced sPLA2-IIa expression in prostate cancer cells, which was blocked by the EGFR/HER2 dual inhibitor Lapatinib and the NF-kB inhibitor Bortezomib. Our findings suggest that an enhanced HER/HER2-PI3K-Akt-NF-kB signaling of the HER network contributes to sPLA2-IIa overexpression and secretion in prostate cancer cells.
We identified the underlying molecular mechanisms of sPLA2-IIa overexpression by the HER/HER2-elicited pathways in prostate cancer. Significant overexpression of sPLA2-IIa was found in prostate cancer specimens, but not in prostate tissues from benign prostatic diseases. Furthermore, high levels of plasma sPLA2-IIa were associated with high Gleason scores and advanced disease stage. Our data strongly suggest that plasma sPLA2-IIa can serve as a poor prognostic biomarker for prostate cancer and is able to distinguish indolent from aggressive prostate tumors. Plasma sPLA2-IIa may also be used in the setting of active surveillance for indolent prostate cancer or for monitoring tumor burden during treatment. Ultimately, the approach described herein could assist clinicians to better manage patients with a spectrum of prostatic malignancies.
Supplementary Material
Table 1.
Characteristics of the patients
| Characteristics | Age | Number of patients |
|---|---|---|
| Prostate cancer | 134 | |
| Mean age year (range) | 65.7 (45~93) | |
| Stage | ||
| I | 18 | |
| II | 30 | |
| III | 30 | |
| IV | 41 | |
| Gleason score 6~7 | 85 | |
| Gleason score 8~10 | 41 | |
| Pretreatment samples | 6 | |
| Treatment samples | 128 |
Acknowledgments
Funding: This work is partially supported by Cancer Center Foundation, University of Cincinnati College of Medicine, NIH grants R01 CA119935 and CA131137.
Footnotes
Disclosure Statement: The authors have no relationships to disclose.
REFERENCES
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics: 2009. CA: a cancer journal for clinicians. 2009;59(4):225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.USNews. Prostate cancer tests. 2010 http://healthusnewscom/usnews/health/cancer/prostate/pros_test_printhtm.
- 3.De Angelis G, Rittenhouse HG, Mikolajczyk SD, Blair Shamel L, Semjonow A. Twenty Years of PSA: From Prostate Antigen to Tumor Marker. Reviews in urology. 2007;9(3):113–123. [PMC free article] [PubMed] [Google Scholar]
- 4.Wolf AM, Wender RC, Etzioni RB, Thompson IM, D'Amico AV, Volk RJ, Brooks DD, Dash C, Guessous I, Andrews K, DeSantis C, Smith RA. American Cancer Society guideline for the early detection of prostate cancer: update 2010. CA: a cancer journal for clinicians. 2010;60(2):70–98. doi: 10.3322/caac.20066. [DOI] [PubMed] [Google Scholar]
- 5.Serdar MA, Oguz O, Olgun A, Seckin B, Ilgan S, Hasimi A, Salih M, Peker F, Kutluay T. Diagnostic approach to prostate cancer using total prostate specific antigen-based parameters together. Ann Clin Lab Sci. 2002;32(1):22–30. [PubMed] [Google Scholar]
- 6.Catalona WJ, Smith DS, Ratliff TL, Dodds KM, Coplen DE, Yuan JJ, Petros JA, Andriole GL. Measurement of prostate-specific antigen in serum as a screening test for prostate cancer. The New England journal of medicine. 1991;324(17):1156–1161. doi: 10.1056/NEJM199104253241702. [DOI] [PubMed] [Google Scholar]
- 7.Roddam AW, Duffy MJ, Hamdy FC, Ward AM, Patnick J, Price CP, Rimmer J, Sturgeon C, White P, Allen NE. Use of prostate-specific antigen (PSA) isoforms for the detection of prostate cancer in men with a PSA level of 2–10 ng/ml: systematic review and meta-analysis. European urology. 2005;48(3):386–399. doi: 10.1016/j.eururo.2005.04.015. discussion 398-389. [DOI] [PubMed] [Google Scholar]
- 8.Catalona WJ, Ramos CG, Carvalhal GF, Yan Y. Lowering PSA cutoffs to enhance detection of curable prostate cancer. Urology. 2000;55(6):791–795. doi: 10.1016/s0090-4295(99)00602-0. [DOI] [PubMed] [Google Scholar]
- 9.Thompson IM, Pauler DK, Goodman PJ, Tangen CM, Lucia MS, Parnes HL, Minasian LM, Ford LG, Lippman SM, Crawford ED, Crowley JJ, Coltman CA., Jr Prevalence of prostate cancer among men with a prostate-specific antigen level< or =4.0 ng per milliliter. The New England journal of medicine. 2004;350(22):2239–2246. doi: 10.1056/NEJMoa031918. [DOI] [PubMed] [Google Scholar]
- 10.Gilbert SM, Cavallo CB, Kahane H, Lowe FC. Evidence suggesting PSA cutpoint of 2.5 ng/mL for prompting prostate biopsy: review of 36,316 biopsies. Urology. 2005;65(3):549–553. doi: 10.1016/j.urology.2004.10.064. [DOI] [PubMed] [Google Scholar]
- 11.Signoretti S, Montironi R, Manola J, Altimari A, Tam C, Bubley G, Balk S, Thomas G, Kaplan I, Hlatky L, Hahnfeldt P, Kantoff P, Loda M. Her-2-neu expression and progression toward androgen independence in human prostate cancer. Journal of the National Cancer Institute. 2000;92(23):1918–1925. doi: 10.1093/jnci/92.23.1918. [DOI] [PubMed] [Google Scholar]
- 12.Craft N, Shostak Y, Carey M, Sawyers CL. A mechanism for hormone-independent prostate cancer through modulation of androgen receptor signaling by the HER-2/neu tyrosine kinase. Nat Med. 1999;5:280–285. doi: 10.1038/6495. [DOI] [PubMed] [Google Scholar]
- 13.Shukla S, MacLennan GT, Fu P, Patel J, Marengo SR, Resnick MI, Gupta S. Nuclear factor-kappaB/p65 (Rel A) is constitutively activated in human prostate adenocarcinoma and correlates with disease progression. Neoplasia (New York, NY) 2004;6(4):390–400. doi: 10.1593/neo.04112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vandermoere F, El Yazidi-Belkoura I, Adriaenssens E, Lemoine J, Hondermarck H. The antiapoptotic effect of fibroblast growth factor-2 is mediated through nuclear factor-kappaB activation induced via interaction between Akt and IkappaB kinase-beta in breast cancer cells. Oncogene. 2005;24(35):5482–5491. doi: 10.1038/sj.onc.1208713. [DOI] [PubMed] [Google Scholar]
- 15.Paradon M, Salvat C, Fan Q, Bereziat G, Olivier JL. An SP1-like 5'-GACCACGCC-3' sequence is critical for activity of the inflammatory phospholipase A2 promoter and binds several non-zinc finger proteins. European journal of biochemistry / FEBS. 1998;258(1):113–122. doi: 10.1046/j.1432-1327.1998.2580113.x. [DOI] [PubMed] [Google Scholar]
- 16.Cummings BS. Phospholipase A2 as targets for anti-cancer drugs. Biochemical pharmacology. 2007;74(7):949–959. doi: 10.1016/j.bcp.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 17.Triggiani M, Granata F, Giannattasio G, Marone G. Secretory phospholipases A2 in inflammatory and allergic diseases: not just enzymes. The Journal of allergy and clinical immunology. 2005;116(5):1000–1006. doi: 10.1016/j.jaci.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 18.Wang W, Bergh A, Damber JE. Cyclooxygenase-2 expression correlates with local chronic inflammation and tumor neovascularization in human prostate cancer. Clin Cancer Res. 2005;11(9):3250–3256. doi: 10.1158/1078-0432.CCR-04-2405. [DOI] [PubMed] [Google Scholar]
- 19.Kallajoki M, Alanen KA, Nevalainen M, Nevalainen TJ. Group II phospholipase A2 in human male reproductive organs and genital tumors. The Prostate. 1998;35(4):263–272. doi: 10.1002/(sici)1097-0045(19980601)35:4<263::aid-pros5>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 20.Jiang J, Neubauer BL, Graff JR, Chedid M, Thomas JE, Roehm NW, Zhang S, Eckert GJ, Koch MO, Eble JN, Cheng L. Expression of group IIA secretory phospholipase A2 is elevated in prostatic intraepithelial neoplasia and adenocarcinoma. The American journal of pathology. 2002;160(2):667–671. doi: 10.1016/S0002-9440(10)64886-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Graff JR, Konicek BW, Deddens JA, Chedid M, Hurst BM, Colligan B, Neubauer BL, Carter HW, Carter JH. Expression of group IIa secretory phospholipase A2 increases with prostate tumor grade. Clin Cancer Res. 2001;7(12):3857–3861. [PubMed] [Google Scholar]
- 22.Antonio V, Brouillet A, Janvier B, Monne C, Bereziat G, Andreani M, Raymondjean M. Transcriptional regulation of the rat type IIA phospholipase A2 gene by cAMP and interleukin -1beta in vascular smooth muscle cells: interplay of the CCAAT/enhancer binding protein (C/EBP), nuclear factor-kappaB and Ets transcription factors. The Biochemical journal. 2002;368(Pt 2):415–424. doi: 10.1042/BJ20020658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dong Z, Liu Y, Scott KF, Levin L, Gaitonde K, Bracken RB, Burk B, Zhai Q, Wang J, Oleksowicz L, Lu S. Secretory phospholipase A2-IIa is involved in prostate cancer progression and may potentially serve as a biomarker for Prostate Cancer. Carcinogenesis. 2010;31:1948–1955. doi: 10.1093/carcin/bgq188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sved P, Scott KF, McLeod D, King NJ, Singh J, Tsatralis T, Nikolov B, Boulas J, Nallan L, Gelb MH, Sajinovic M, Graham GG, Russell PJ, Dong Q. Oncogenic action of secreted phospholipase A2 in prostate cancer. Cancer research. 2004;64(19):6934–6940. doi: 10.1158/0008-5472.CAN-03-3018. [DOI] [PubMed] [Google Scholar]
- 25.Morgenbesser SD, McLaren RP, Richards B, Zhang M, Akmaev VR, Winter SF, Mineva ND, Kaplan-Lefko PJ, Foster BA, Cook BP, Dufault MR, Cao X, Wang CJ, Teicher BA, Klinger KW, Greenberg NM, Madden SL. Identification of genes potentially involved in the acquisition of androgen-independent and metastatic tumor growth in an autochthonous genetically engineered mouse prostate cancer model. The Prostate. 2007;67(1):83–106. doi: 10.1002/pros.20505. [DOI] [PubMed] [Google Scholar]
- 26.Patel MI, Singh J, Niknami M, Kurek C, Yao M, Lu S, Maclean F, King NJ, Gelb MH, Scott KF, Russell PJ, Boulas J, Dong Q. Cytosolic phospholipase A2-alpha: a potential therapeutic target for prostate cancer. Clin Cancer Res. 2008;14(24):8070–8079. doi: 10.1158/1078-0432.CCR-08-0566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Narayanan BA, Narayanan NK, Pittman B, Reddy BS. Regression of mouse prostatic intraepithelial neoplasia by nonsteroidal anti-inflammatory drugs in the transgenic adenocarcinoma mouse prostate model. Clin Cancer Res. 2004;10(22):7727–7737. doi: 10.1158/1078-0432.CCR-04-0732. [DOI] [PubMed] [Google Scholar]
- 28.Dong Z, Liu Y, Levin L, Oleksowicz L, Wang J, Lu S. Vav3 oncogene is involved in regulation of secretory phospholipase A2-IIa expression in prostate cancer. Oncology Reports. 2011;25(6):1511–1516. doi: 10.3892/or.2011.1237. [DOI] [PubMed] [Google Scholar]
- 29.Dong ZY, Liu Y, Lu S, Wang A, Lee K, Wang LH, Revelo M, Lu S. Vav3 oncogene is overexpressed and regulates cell growth and androgen receptor activity in human prostate cancer. Mol Endo. 2006;20:2315–2325. doi: 10.1210/me.2006-0048. [DOI] [PubMed] [Google Scholar]
- 30.Bastian PJ, Carter BH, Bjartell A, Seitz M, Stanislaus P, Montorsi F, Stief CG, Schroder F. Insignificant prostate cancer and active surveillance: from definition to clinical implications. European urology. 2009;55(6):1321–1330. doi: 10.1016/j.eururo.2009.02.028. [DOI] [PubMed] [Google Scholar]
- 31.Dall'Era MA, Cooperberg MR, Chan JM, Davies BJ, Albertsen PC, Klotz LH, Warlick CA, Holmberg L, Bailey DE, Jr, Wallace ME, Kantoff PW, Carroll PR. Active surveillance for early-stage prostate cancer: review of the current literature. Cancer. 2008;112(8):1650–1659. doi: 10.1002/cncr.23373. [DOI] [PubMed] [Google Scholar]
- 32.Klotz L. Active surveillance for favorable risk prostate cancer: rationale, risks, and results. Urol Oncol. 2007;25(6):505–509. doi: 10.1016/j.urolonc.2007.05.021. [DOI] [PubMed] [Google Scholar]
- 33.Albertsen PC, Hanley JA, Fine J. 20-year outcomes following conservative management of clinically localized prostate cancer. Jama. 2005;293(17):2095–2101. doi: 10.1001/jama.293.17.2095. [DOI] [PubMed] [Google Scholar]
- 34.Draisma G, Etzioni R, Tsodikov A, Mariotto A, Wever E, Gulati R, Feuer E, de Koning H. Lead time and overdiagnosis in prostate-specific antigen screening: importance of methods and context. Journal of the National Cancer Institute. 2009;101(6):374–383. doi: 10.1093/jnci/djp001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Johansson JE, Andren O, Andersson SO, Dickman PW, Holmberg L, Magnuson A, Adami HO. Natural history of early, localized prostate cancer. Jama. 2004;291(22):2713–2719. doi: 10.1001/jama.291.22.2713. [DOI] [PubMed] [Google Scholar]
- 36.Wolbink GJ, Schalkwijk C, Baars JW, Wagstaff J, van den Bosch H, Hack CE. Therapy with interleukin-2 induces the systemic release of phospholipase-A2. Cancer Immunol Immunother. 1995;41(5):287–292. doi: 10.1007/BF01517216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Juffrie M, Meer GM, Hack CE, Haasnoot K, Sutaryo, Veerman AJ, Thijs LG. Inflammatory mediators in dengue virus infection in children: interleukin-6 and its relation to C-reactive protein and secretory phospholipase A2. The American journal of tropical medicine and hygiene. 2001;65(1):70–75. doi: 10.4269/ajtmh.2001.65.70. [DOI] [PubMed] [Google Scholar]
- 38.Wootton PT, Drenos F, Cooper JA, Thompson SR, Stephens JW, Hurt-Camejo E, Wiklund O, Humphries SE, Talmud PJ. Tagging-SNP haplotype analysis of the secretory PLA2IIa gene PLA2G2A shows strong association with serum levels of sPLA2IIa: results from the UDACS study. Human molecular genetics. 2006;15(2):355–361. doi: 10.1093/hmg/ddi453. [DOI] [PubMed] [Google Scholar]
- 39.Carpenter G. The EGF Receptor Family: Biologic Mechanisms and Role in Cancer. Elsevier Academic Press; 2004. [Google Scholar]
- 40.Leung HY, Weston J, Gullick WJ, Williams G. A potential autocrine loop between heregulin-alpha and erbB-3 receptor in human prostatic adenocarcinoma. Br J Urol. 1997;79(2):212–216. doi: 10.1046/j.1464-410x.1997.30412.x. [DOI] [PubMed] [Google Scholar]
- 41.Chakrabarty A, Rexer BN, Wang SE, Cook RS, Engelman JA, Arteaga CL. H1047R phosphatidylinositol 3-kinase mutant enhances HER2-mediated transformation by heregulin production and activation of HER3. Oncogene. 2010;29(37):5193–5203. doi: 10.1038/onc.2010.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gregory CW, Whang YE, McCall W, Fei X, Liu Y, Ponguta LA, French FS, Wilson EM, Earp HS., 3rd Heregulin-induced activation of HER2 and HER3 increases androgen receptor transactivation and CWR-R1 human recurrent prostate cancer cell growth. Clin Cancer Res. 2005;11(5):1704–1712. doi: 10.1158/1078-0432.CCR-04-1158. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.