Summary
The advent of intracranial stents has widened the indications for endovascular treatment of broad-necked and fusiform aneurysms. Leo stent is a self-expandable, nitinol, braided stent dedicated to intracranial vessels. The aim of this study is to present our experience in endovascular treatment of broad-necked and fusiform intracranial aneurysms using self-expanding, nitinol Leo stents.
Between February 2004 and November 2006, 25 broad-necked and three fusiform aneurysms in 28 patients were treated using Leo stents in our centre. There were 18 patients who experienced acute subarachnoid haemorrhage due to aneurysm rupture, two patients who experienced SAH at least 12 months ago and in eight patients aneurysms were found incidentally. Aneurysms were located as follows: internal carotid artery15, basilar artery5, basilar tip3, posterior inferior cerebral artery2, M1/M2 segment1, A2 segment1 and vertebral artery1.
There were no difficulties with stent deployment and delivery. All patients after acute SAH (n=18) underwent stent implantation and coil embolization in one procedure. The remaining patients underwent coil embolization in a staged procedure. Immediate aneurysm occlusion of more than 95% was achieved in all patients who underwent stent placement and coil embolization in one procedure. There were three thromboembolic complications encountered in patients in an acute setting of SAH, preloaded only on acetylsalicylic acid. Use of abciximab led to patency within the stent and parent vessel. However, one of these patients presented rebleeding from the aneurysm during administration of abciximab and died.
Application of Leo stents in cases of broadnecked and fusiform intracranial aneurysms is safe and effective with a low complication rate.
Key words: endovascular, intracranial aneurysm, Leo, stent, fusiform
Introduction
The aim of the successful treatment of intracranial aneurysms is to occlude them from the cerebral circulation to prevent their rupture and in cases of already ruptured aneurysms, to prevent their rebleeding giving the possibility for more intensive and safer treatment of symptomatic vasospasm. Prior to the advent of Guglielmi detachable coils (GDC) and their introduction into clinical use in 1990, patients with intracranial aneurysms were offered the only treatment option - surgical clip application1. Endovascular treatment with coils enabled the occlusion of unruptured and ruptured intracranial aneurysms without the need for craniotomy.
The International Subarachnoid Aneurysmal Trial (ISAT) revealed that endovascular coiling is associated with lower morbidity and mortality rates in selected cases compared with neurosurgical clipping2. However, endosaccular packing with coils is limited by the morphological conditions of an aneurysm3 and hence has limitations in the treatment of broad-necked aneurysms due to possible coil protrusion into the parent vessel as well as possible long-term angiographic recurrences4. The advent of new endovascular techniques such as: three-dimensional coils, balloon-assisted remodeling and microstents has overcome these restrictions. Application of the above techniques simplifies effective coil packing via the stent mesh into the aneurysmal sack and hence is an encouraging alternative for the treatment of wide-necked, fusiform or dissecting aneurysms located in the cervical and proximal intracranial arteries5,6,7. The first stent dedicated to intracranial vessels was the self-expanding, nitinol Neuroform device (Boston Scientific/Target, Natick, MA) and its upgraded version Neuroform 2 Treo8. The Leo stent (Balt, Montmorency, France) has several advantages over the Neuroform stents. The major advantages are an innovative distal hook that enables resheathing and repositioning of the stent when necessary, high radial force and ease of delivery8.
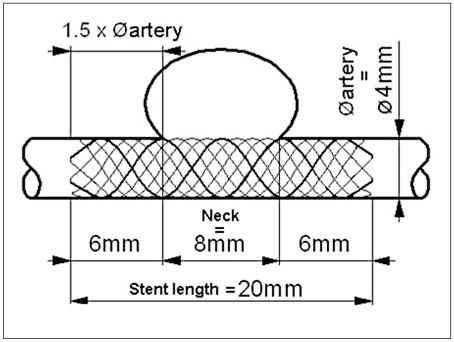
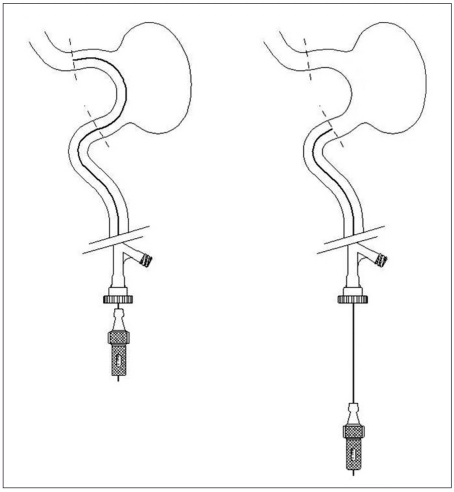
Figure 1.
Scheme of Leo stent.
Figure 2.
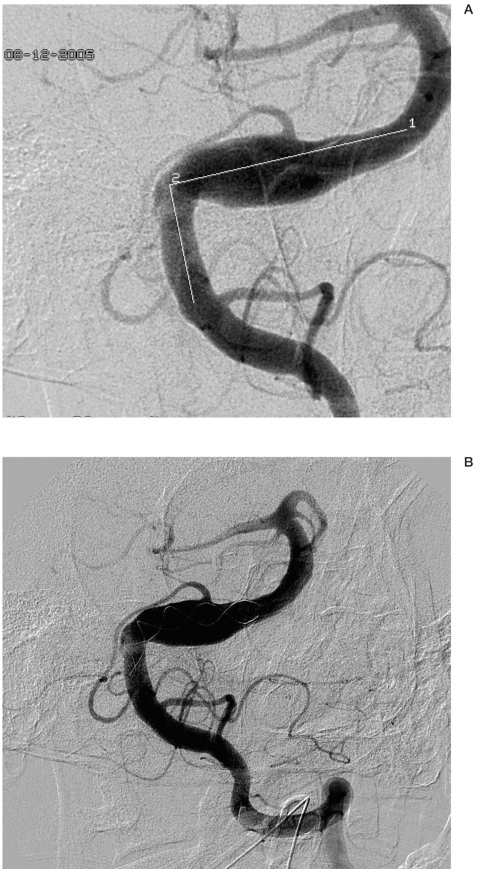
Method of determining the length of the needed stent with the aid of guidewire.
The purpose of this study is to present our single-center early experience with endovascular treatment of wide-necked and fusiform, ruptured and unruptured intracranial aneurysms using the nitinol, self-expandable Leo stent.
Material and Methods
Study population
Between February 2004 and November 2006, there were 319 endovascular treatments of intracranial aneurysms in 293 patients performed at The Department of Neurosurgery and Neurotraumatology, Poznan University of Medical Sciences, Poland.
In 28 of them, a self-expandable Leo stent (Balt, Montmorency, France) was implanted. The present study group consisted of 28 patients with 28 broad-necked or fusiform aneurysms. There were 17 females and 11 males, mean aged 47.5 years (range 18-62). The indications for stent implantation were as follows: broad-neck in 24 cases, fusiform aneurysm in three cases and coil protrusion during the procedure in one case. The locations of the aneurysms were as follows: internal carotid artery 15, basilar artery5, basilar tip3, posterior inferior cerebral artery 2, M1/M2 segment of the middle cerebral artery1, A2 segment of the anterior cerebral artery1 and vertebral artery1 (Table 1). There were 20 patients who experienced subarachnoid haemorrhage (SAH) due to aneurysm rupture (19 broad-necked aneurysms and one fusiform). Of those patients, 18 were treated in the context of acute SAH and two were treated due to recurrence of aneurysm after primary coiling 12 months ago.
Table 1.
Summary of patients data.
| Pa- tient no. |
Age | Sex | Indication | Location | Stent diameter and length |
Morphology | Immediate aneurysms obliteration |
Complications | Pharmacolo- gical treatment |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 54 | F | SAH | ICA | 3.5 x 12 | BN | CO | ASA | |
| 2 | 28 | M | SAH | PICA | 2.5 x 12 | BN | CO | ASA | |
| 3 | 53 | F | SAH | ICA | 3.5 x 18 | BN | CO | ASA | |
| 4 | 56 | M | SAH | BA | 2.5 x 18 | BN | CO | ASA | |
| 5 | 43 | F | SAH | BA tip | 2.5 x 18 | BN | CO | TB | ASA |
| 6 | 62 | F | SAH | ICA | 3.5 x 18 | BN | CO | ASA | |
| 7 | 18 | F | SAH/coils protrusion |
A2 | 2.5 x 18 | BN | CO | TB | - |
| 8 | 53 | F | SAH | BA | 2.5 x 12 | BN | CO | ASA | |
| 9 | 54 | F | SAH | ICA | 4.5 x 25 | BN | INC | ASA | |
| 10 | 50 | M | SAH | M1/M2 | 2.5 x 12 | BN | CO | ASA | |
| 11 | 44 | M | SAH | BA tip | 2.5 x 12 | BN | TB/ death |
ASA | |
| 12 | 25 | F | SAH | ICA | 3.5 x 25 | BN | INC | ASA | |
| 13 | 47 | M | SAH | BA tip | 2.5 x 18 | BN | CO | ASA | |
| 14 | 55 | F | SAH | ICA | 3.5 x 18 | BN | CO | death | ASA |
| 15 | 54 | F | SAH | ICA | 4.5 x 25 | BN | CO | ASA | |
| 16 | 54 | M | SAH | PICA | 2.5 x 12 | BN | CO | ASA | |
| 17 | 51 | M | SAH | VA | 3.5 x 18 | F | NO | ASA | |
| 18 | 45 | M | SAH | BA | 2.5 x 12 | BN | NO | - | |
| 19 | 61 | F | pSAH | ICA | 4.5 x 20 | BN | CO | ASA, ticlo | |
| 20 | 49 | F | pSAH | ICA | 3.5 x 25 | BN | CO | ASA, ticlo | |
| 21 | 44 | M | Inc | BA | 4.5 x 50 | F | NO | ASA, ticlo | |
| 22 | 47 | M | Headache | BA | 4.5 x 50 | F | NO | ASA, ticlo | |
| 23 | 43 | M | Inc | ICA | 4.5 x 20 | BN | CO | ASA, ticlo | |
| 24 | 44 | F | Inc | ICA | 3.5 x 18 | BN | CO | ASA, ticlo | |
| 25 | 54 | F | CNP | ICA | 4.5 x 20 | BN | x | ASA, ticlo | |
| 26 | 57 | F | Inc | ICA | 3.5 x 18 | BN | x | ASA, ticlo | |
| 27 | 47 | F | Inc | ICA | 4.5 x 40 | BN | x | ASA, ticlo | |
| 28 | 49 | F | Headache | ICA | 3.5 x 25 | BN | x | ASA, ticlo | |
|
SAH, Subarachnoid haemorrhage; ICA, internal carotid artery; BN, broad-necked; BA tip, tip of basilar artery; TB, thrombosis ASA, acetylsalicylic acid; PICA, posterior inferior cerebellar artery; BA, basilar artery; INC, incomplete; M1/M2, M1/M2 segment of middle cerebral artery; A2, A2 segment of anterior cerebral artery; VA, vertebral artery; F, fusiform; pSAH, pimary SAH, at least 12 month ago, Inc, incidental; CNP cranial nerve palsy; ticlo, ticlopidine; CNP, cranial nerves palsy; x - coils embolization planned in the future. | |||||||||
There were eight patients with unruptured aneurysms.
Figure 3.
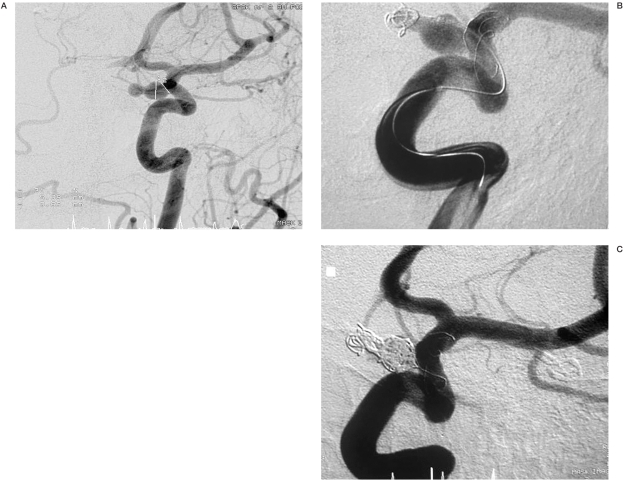
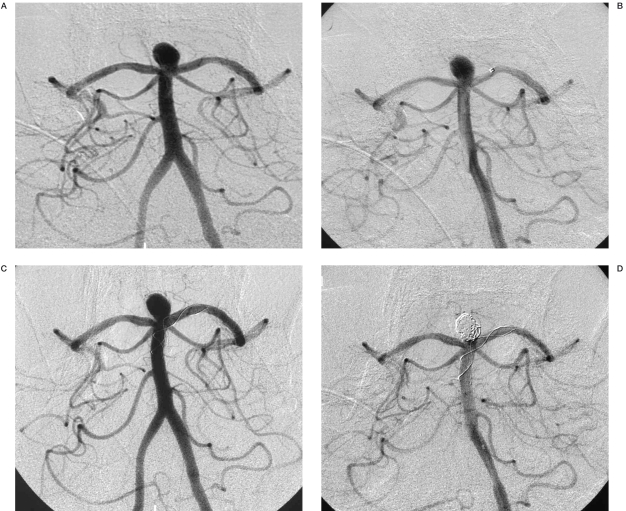
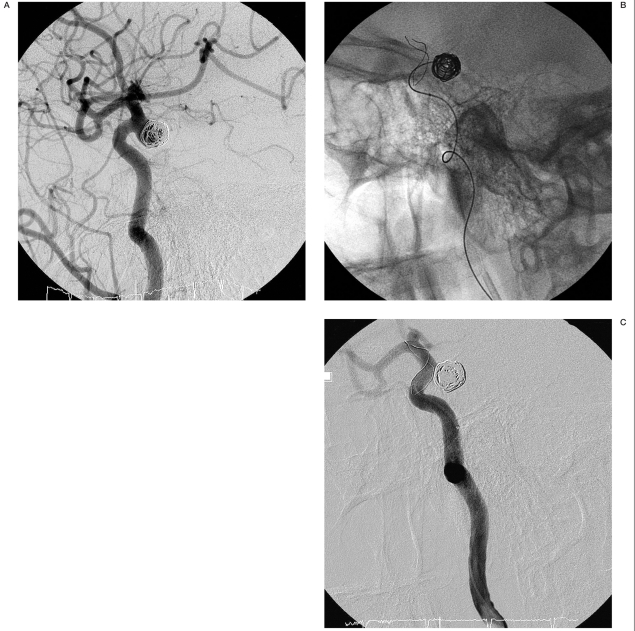
Angiograms of patient no. 1. A) Aneurysm located at the internal carotid artery. B) Partially coiled aneurysm with stent implanted. C) Total occlusion of the aneurysm with coils and stent, immediately after the procedure.
Antiplatelet therapy
In all patients with unruptured aneurysms, medical premedication was started three days prior to the procedure with 2 x 250 mg ticlopidine and 150 mg acetylsalicylic acid (ASA) daily. Patients who experienced SAH at least 12 months ago were treated as patients with unruptured aneurysms. Sixteen patients in acute setting of SAH were given 150 mg ASA orally or in cases of unconscious patients via nasogastric tube directly before the procedure. Two patients (nos. 7 and 18) received no antiplatelet therapy after SAH because stent placement in these patients was not previously planned. All patients were given 5000 units of heparin intravenously during the procedure to maintain activated partial thromboplastin time two to three times the basal value after the femoral sheath was introduced. All patients were continued on fraxiparine 0.3-0.6 ml every 12 hours for the following three days.
Procedural details
All patients underwent cerebral angiography prior to procedures. All procedures were performed under general anaesthesia and via femoral access.
A 6F guiding catheter Casasco (Balt, Montmorency, France) was introduced into the internal carotid artery or vertebral artery. Through the guiding catheter, a 0.21-0.28 Vasco microcatheter (Balt, Montmorency, France) was navigated on a 0.14 guidewire (Balt, Montmorency, France) to the parent vessel distally to the aneurysmal neck. The diameter of the Vasco catheter depended on the stent's diameter. The delivery system was then introduced inside the Vasco microcatheter. The stent is resheathable when up to 90% is deployed. The final detachment was obtained by resheathing the pusher inside the catheter by advancing the catheter.
The stent diameter was determined by selective angiographic examination with measurement of the vessel diameter on the basis of calibration of the guide catheter.
In cases of broad-necked aneurysms, the stent length was determined as follows: 2 x (1.5 x artery diameter) + neck length. In cases of fusiform aneurysms, the stent length was determined as 30% longer than the aneurysm length. When measurements were complicated due to tortuousity of vessels, the length of the needed stent was established with the aid of a guidewire (figure 2).
Figure 4.
Angiograms of patient no. 2. A) Broad-necked aneurysm at the posterior inferior cerebellar artery. B) After stent implantation. C) Total occlusion of the aneurysm with coils and stent.
After stent placement, coil embolization was performed using hydrolytically or mechanically detachable platinum coils (Balt, Montmorency, France). A Vasco +10 microcatheter was navigated through the artery lumen and then through the stent mesh into the aneurysmal sack. A microcatheter was introduced on a SOR 0.09 compatible guidewire (Balt, Montmorency, France).
Sixteen out of 17 patients after acute SAH with broad-necked aneurysms underwent stent implantation and coil embolization in one procedure. Stent placement in Patient no. 7 was performed after coil packing due to coil protrusion into the parent vessel. The case of patient no. 18 was previously described. Patient no. 17 with a fusiform aneurysm after acute SAH was treated with stent alone.
Patients who sustained SAH earlier than 12 months ago (n=2) were treated as patients who did not have SAH i.e. they underwent a staged procedure. Both patients had undergone primary coil embolization at least 12 months previously. In these patients, control angiographic scans revealed recanalization of previously embolized aneurysms and the decision of additional coiling with stent placement was made.
Among eight unruptured aneurysms, two fusiform aneurysms were treated with stent insertion alone. The remaining six broad-necked aneurysms were treated in a staged procedure: first, stent implantation and second, coil packing after approximately three to six months.
Two of these patients had already undergone a staged procedure. Four of them have not undergone coil embolization so far and are scheduled for a further procedure.
Results
Immediate aneurysm occlusion, stent deployment, coiling
A total of 28 stents were successfully deployed in 28 patients. There were no complications noted in relation to stent placement (i.e. stent migration, torsion or fracture). In all cases, there were no difficulties with stent detachment from the delivery system. All stents were deployed successfully at the targeted landing zone. In cases of broad-necked aneurysms, there was no stent shrinkage in size beyond the limits outlined by the manufacturer. In cases of three fusiform aneurysms, stent shrinkage exceeded limits outlined by the manufacturer by 11-14%.
Figure 5.
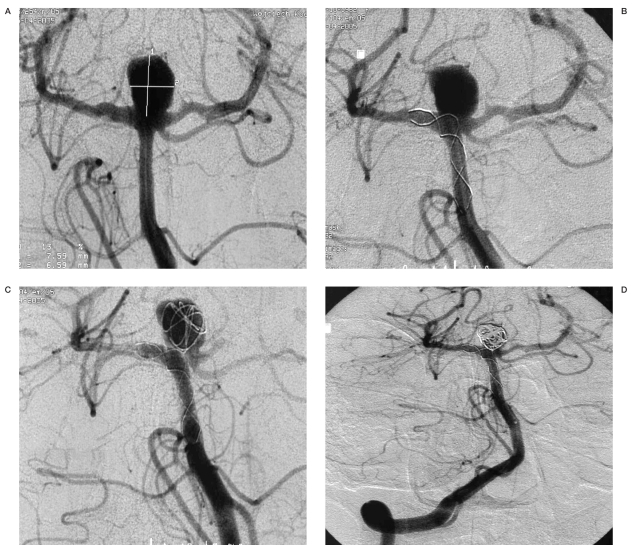
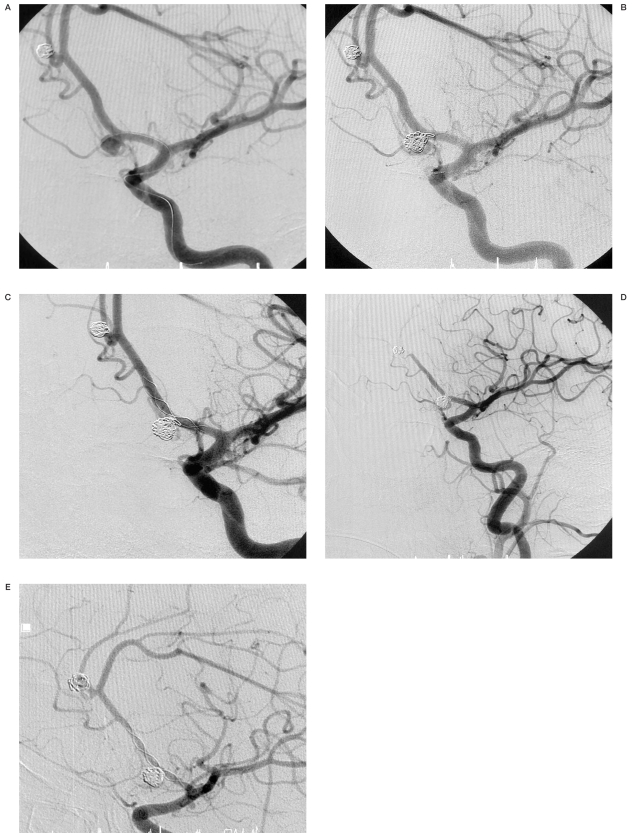
Angiograms of patient no. 13. A) Broad-necked aneurysm at the apex of the basilar artery. B) Radiopaque marker of microcatheter Vasco +21 placed in the posterior cerebral artery and the basilar artery. C) After stent implantation. Stent placed in the left posterior cerebral artery and the basilar artery. D) Total occlusion of the aneurysm with coils and stent.
Only one difficulty with coil embolization after stent placement was observed (patient no. 18). It was impossible to advance the Vasco +10 microcatheter (Balt, Montmorency, France) through the stent mesh. In the end, only the stent was placed and the aneurysm is planned to be packed with coils. After the procedure, angiography revealed decreased intraaneurysmal flow.
Immediate aneurysm occlusion of more than 95% was achieved in all patients who underwent stent placement and coil embolization in one procedure and in two patients who underwent coil packing in a staged procedure.
Stents were implanted alone in three patients with fusiform aneurysms. In all these patients, there was no aneurysm exclusion observed immediately after the procedure.
Coil embolization has not yet been performed in four cases of broad-necked, unruptured aneurysms. The case of patient no. 18 was described above.
Complications/thrombosis and death
There were three thromboembolic complications encountered (patients 5,7,11). All these patients were in an acute setting of SAH and two of them were preloaded only on ASA (nos. 5,11) and one without any antiplatelet pretreatment (no. 7). Patients nos. 5 and 11 with BA tip aneurysms had stents implanted through the bifurcation. In both patients, thrombosis appeared in the opposite posterior cerebral artery supplied through the stent meshes. In these three cases, the smallest stent diameters (2.5 mm) were used.
Figure 6.
Angiograms of patient no. 5. A) Broad-necked aneurysm at the apex of the basilar artery. B) After stent implantation. Stent placed in the right posterior cerebral artery and the basilar artery. C) Partial stent-supported coil embolization of the aneurysm. D) Complete occlusion of the aneurysm with coils and stent.
In these patients, abciximab (ReoPro, Eli Lilly) were used in doses obtained from cardiology practice. In all cases patency within the stent and parent vessel was achieved. However, one patient (no. 11) with an aneurysm on the basilar apex presented rebleeding from the aneurysm during administration of abciximab and died.
The patient underwent the endovascular procedure two days after SAH and was V grade Hunt-Hess scale.
There were two deaths in the present study. The previously described patient (no. 11) died due to rebleeding. The second patient (no. 14) died as a result of her primary SAH complications four days after successful endovascular treatment of the aneurysm. The patient was treated endovascularly 11 days after SAH and was in Hunt and Hess Grade V.
Follow-up
Follow-up angiographic data after six to 12 months were available in 16 patients out of the surviving 26. There was no stent displacement, fracture or torsion observed during this period. In addition, there were no in-stent stenosis and thrombosis demonstrated proximally and distally to the stent. There were no events of haemorrhages. In all cases, follow-up angiography revealed free flow within the stent and parent vessel.
Control angiograms obtained in 13 patients with broad-necked aneurysms, demonstrated more than 95% occlusion in 12 patients and "dog ear" filling in one patient.
Follow-up examination performed in three patients with fusiform aneurysms, after stenting alone, revealed no thrombosis within aneurysms and free flow in the parent vessels in two patients. In the remaining one patient with vertebral artery aneurysm, control angiographic scan performed 12 months after the procedure showed complete remodeling of the parent vessel.
Four patients with unruptured broad-necked aneurysms, who had already had stents implanted, have not yet undergone coil embolization. There were no delayed thromboembolic events or rebleeding noted during the follow-up period.
Discussion
Broad-necked and fusiform aneurysms are a major challenge for interventional radiologists and neurosurgeons. The endovascular treatment of fusiform aneurysms or aneurysms with broad-neck carries a risk of coil protrusion into the parent vessel, incomplete occlusion and long-term angiographic recurrences 4,9. According to Brillstra et Al, the rate of aneurysm recanalization after occlusion with GDC coils is 17% in narrow-neck and 42% in broad-neck aneurysms 10. The application of a stent into the parent vessel has three main advantages. First, it enables dense aneurysm packing with coils without compromising the vessel lumen3,11. Second, it promotes intra-aneurysmal flow stagnation which facilitates thrombosis and reduction of coil compaction in the region of inflow zone and prevents subsequent growth of the aneurysm 3,11,12. Third, the stent mesh may provide a framework for endothelial growth, enabling the remodeling of the aneurysm neck and causing its permanent separation from the parent vessel lumen 3,11.
Figure 7.
Angiograms of patient no. 11. A) Broad-necked aneurysm at the apex of the basilar artery. B) After stent placement into the left posterior cerebral artery and the basilar artery. Complete thrombosis of the basilar artery. C) After intraarterial administration of abciximab and coil embolization. Severe hemorrhage from the aneurysmal sack.
Figure 8.
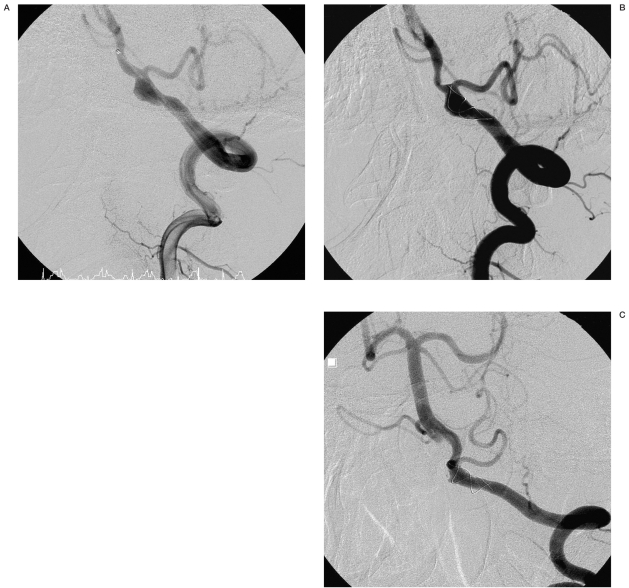
Angiograms of patient no. 8. A) Broad-necked aneurysm of the basilar artery. B) After stent placement. C) Complete occlusion of the aneurysm with coils and stent.
The first stent devices were balloon-expandable coronary stents. Their primary disadvantages were increased rigidity 3 and potential risk of damaging a dysplastic segment of the artery with possible vessel rupture 13,14. The first self-expandable stents dedicated to endovascular surgery of ICA were Neuroform and their upgraded versions Neuroform 2 and Neuroform 2 Treo (Boston Scientific/Target, Natick, MA). The latter have allowed better parent vessel scaffold to avert coil protrusion and their greater flexibility compared with balloon-expandable stents has enabled endoluminal access to distal and tortuous vessels 4. The Leo stent is a self expandable, braided nitinol stent that combines high radial force and simple delivery8. Its visualization (both the length and diameter) is enabled by two platinum threads running throughout the stent. The main advantage of this stent is the possibility of reloading it into a delivering device and replacing when less than 90% of its length is deployed owing to an innovative distal hook 8. The high radial force decreases the potential of stent displacement after deployment 8.
Because metal surfaces of the stent favour thromobogenesis until endothelialization of the stent occurs, the antiplatelet regimen is a prerequisite for stent implantation to prevent thrombus formation and occlusion of the parent vessel13,15. Lopes et Al described cadaveric histological evaluation of carotid artery aneurysm treated with the Neuroform stent four months after stenting. It showed complete endothelialization of the stent and filling of the aneurysmal sack with fibrocellular tissue. Nonetheless, fibrocellular tissue was lacking in the central part of the neck with material resembling thrombus 16. Consequently, antiaggregant therapy should be started prior to the procedure and continued for at least three months thereafter 14. This protocol is obtained from cardiology practice. In the present study, 500 mg ticlopidine and 150 mg ASA per day were administered in all patients with unruptured aneurysms three days before the procedure. The patients who had sustained SAH earlier than 12 months previously, primarily treated with coiling, and requiring an additional embolization due to aneurysm recanalization, were pretreated with ASA and ticlopidine as patients who did not experience SAH. None of these patients experienced thromboembolic complications during or after the procedure. This finding is similar the study of Lubicz et Al who also reported no procedure-related complications in patients with unruptured aneurysms17. Benitez et Al described three thromboembolic complications related to stent (Neuroform) insertion in a study population consisting of 48 patients 18.
Figure 9.
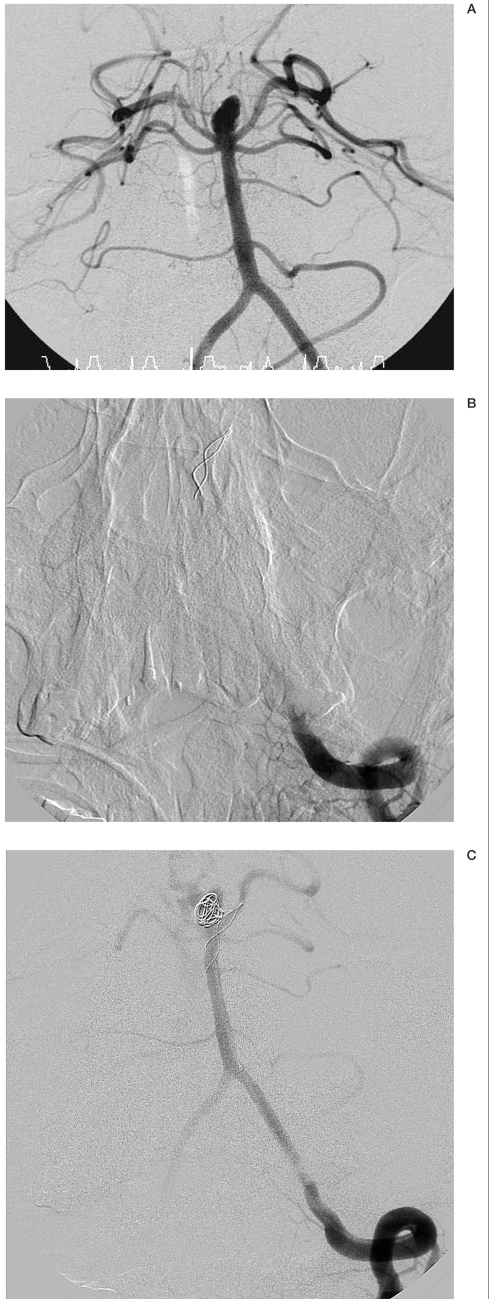
Angiograms of patient no. 19. A) 12 months after primary coils embolization. Recanalization of the aneurysm at the internal carotid artery. B) After stent placement. During coils packing. C) Complete occlusion of the aneurysm with coils and stent.
The application of antithrombotic medications in patients after acute SAH remains a matter of debate. No definite strategy for treatment of these challenging patients has yet been established. In the present study, 15 patients after acute SAH were treated with 150 mg acetylsalicylic acid directly prior the procedure and were given 5000 units of unfractioned heparin intraprocedurally and fraxiparine postprocedurally for three days. Two patients7,18 after SAH were not given any antithrombotic agents before the procedure, because the decision for stent implantation was made during the procedure due to coil protrusion.
Figure 10.
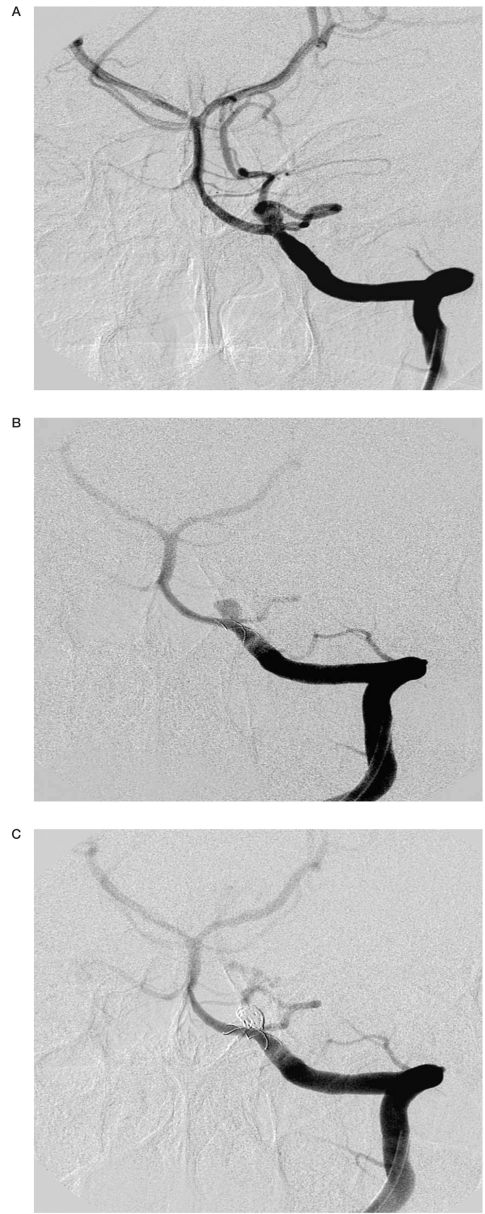
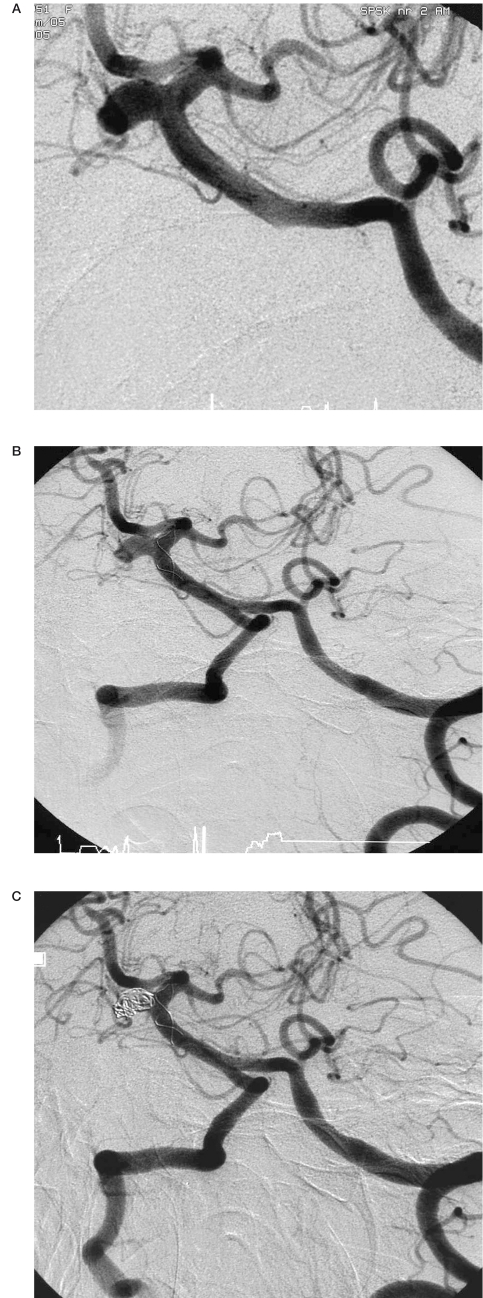
Angiograms of patient no. 7. A) After coil embolization of the aneurysm of the pericallosal artery. Microcatheter Vasco inside the aneurysm of the A2 segment of the anterior cerebral artery. B) Coils protrusion into the parent vessel. C) Complete occlusion of the aneurysm with stent and coils. D) Thrombosis in the distal segment of the anterior cerebral artery. Thrombus formation within the stent at the level of aneurysmal neck. E) After intraarterial administration of abciximab. Complete recanalization of the anterior cerebral artery.
We noted three cases of thrombosis within the stent and the parent artery in patients with ruptured aneurysm within 15-20 minutes after stent deployment (including the above mentioned patient without antiplatelet pretreatment). In all these patients, intra-arterial use of abciximab (ReoPro, Eli Lilly) led to patency within the stent and the parent artery. However, one patient with the aneurysm on the basilar apex presented rebleeding from the aneurysm and died. The remaining 13 patients did not experience either thromboembolic events or rebleeding. This may suggest that the pharmacological therapy protocol we use may be safe and efficient in the treatment of broadnecked aneurysms in patients in the acute setting of SAH.
Sani et Al recommend a staged approach: first, partial coil embolization or surgical clipping, then stent and coil placement after antithrombotic therapy can be initiated13. Fiorella et Al concluded that the staged approach is the safest and most effective strategy15. When a staged approach is not feasible, they advise administration of 650 mg ASA directly after coil placement and initiation of dual antiplatelet therapy the next day11. Benitez et Al highlighted the role of aggressive treatment of hydrocephalus prior to the procedure. They emphasised performing ventriculostomy early rather than reversing anticoagulation 18. The antiaggregative therapy with ticlopidine was continued for six months and ASA for life after the procedure both in patients treated with previously unruptured and ruptured aneurysms.
We did not observe problems with choosing an appropriate stent diameter or length. However, in cases of fusiform aneurysms, the stent length had to be 11-14% larger than the determined measurements. Stent shrinkage exceeded the limits outlined by the manufacturer.
In the present study, all stents were successfully deployed. There was no stent migration, torsion or fracture. Some studies have reported difficulties with deployment of Neuroform stent devices 15,18. Fiorella et Al described problems during an attempt to deploy the stent (Neuroform) in six cases: bending of the microwire, stabilizer and stent delivery catheter.Two cases of stent displacement after deployment were reported in their study11.
Figure 11.
Angiograms of patient no. 21. A) Fusiform aneurysm of the basilar artery. B) Directly after stent placement. No aneurysm occlusion. Free flow within the aneurysm and stent.
We encountered one problem with coil embolization after stenting. The obstacle lay in the difficulty to manipulating the microcatheter into the aneurysmal sack through the stent meshes. Finally, we aborted further attempts to catheterize the aneurysm, and coil embolization in this patient is planned in a delayed manner. However, angiography scan demonstrated significant flow reduction. This prevents possible aneurysm rerupture and further complications.
Immediate angiographic results obtained in patients with ruptured aneurysms, in whom the coil packing was performed in the same procedure, were satisfactory: in all patients >95% occlusion of the aneurysmal sack was achieved. However, in the case of one patient (patient no. 16) with a saccular aneurysm on the basilar artery, coil embolization could not be performed due to difficulties with manipulating the microcatheter into the aneurysm. Benitez et Al achieved 99 to 100% occlusion in 35 cases in a study population consisting of 48 patients18. Fiorella et Al reported 46% completed or nearly completed occlusion rate 15. They explained the difference among these studies with the different anatomical group of aneurysms. He also states that in patients in an acute setting of SAH, there was no emphasis on complete aneurysm occlusion at the initial stage. An aggressive approach to therapy can lead to complications such as stent displacement, due to the lack of radial force of the just deployed Neuroform stent, or the increased severity of possible perforation due to an intensive antithrombotic therapy15. Kis et Al also suggested that dense coil packing is not obligatory because the main goal in patients after acute SAH is to prevent rebleeding by stent deployment and partial coil packing of the aneurysmal sack4.
In our study, despite the fact that we tried to perform and did achieve complete occlusion at one procedure, there were no such complications noted. In the two patients with unruptured aneurysms, in whom a staged procedure was performed, complete immediate postembolization aneurysm occlusion was achieved in both cases.
Figure 12.
Angiograms of patient no. 17. A) Fusiform aneurysm of the vertebral artery prior to the procedure. B) Directly after stent placement. C) Follow-up scan 12 months after the procedure. Complete remodeling of the vertebral artery.
In cases of ruptured aneurysms, the lengths of the stents were kept as short as possible. In our opinion, a shorter length of stent carries a lower risk of in-stent thrombosis: this is extremely important in patients pretreated only with ASA. In such cases, the stent length has to be determined accurately to provide proper stent choice. Stent deployment has to be performed extremely precisely as the stent is only slightly longer than the aneurysmal neck and the risk of inappropriate placement is high. In our study, three out of 16 patients in an acute setting of SAH, treated with stent implantation, presented thrombus formation within the stent immediately after stent insertion. It must be emphasized that thrombosis did not occur in the shortest stents. Our experience with ruptured, broad-neck aneurysms suggests that this strategy may be effective. This relationship was not confirmed in patients with unruptured aneurysms, who were given dual antiplatelet therapy. Nevertheless, Szikora et Al attributed thrombosis to stent misalignment and not to the general characteristic of the device 3.
In 1997, Higashida et Al reported the first case of successful combined endovascular stent placement and coil embolization of a fusiform aneurysm involving the distal vertebral artery and proximal third of the basilar artery19. So far, only small sample size studies have been available regarding the treatment of fusiform aneurysms.
There were three cases of fusiform aneurysms in our study, which were treated with stent insertion alone. In two cases, follow-up angiography revealed that stent implantation did not cause expected aneurysm occlusion. However, aneurysms remained clinically and angiographically stable. The patients were given 500 mg ticlopidine and 150 mg ASA in the time between the procedure and follow-up examination. All these patients are scheduled to undergo a second procedure with another stent implantation - "double stenting" to increase the mesh density and hence further flow reduction.
However, the potential of flow redirection caused by the stent may have reduced the risk of aneurysm enlargement and rupture. Fiorella et Al. achieved favorable results in treating fusiform and dissecting aneurysms with stent implantation alone. They observed progressive remodeling in all five cases in which the Neuroform stents had been deployed 15. In the present study, in one case of fusiform aneurysm, complete (resolution of the aneurysm) remodeling was observed 12 months after the procedure. Lubicz et Al used a different technique in the treatment of fusiform aneurysm. They placed a balloon microcatheter and inflated it within the stent while delivering coils to prevent their prolapse into the vessel lumen17. In our opinion, balloon application is not necessary because the stent acts as a sufficient scaffolding for coil placement. We did not encounter a complication of coil protrusion through the stent meshes so far in cases of broad-necked saccular aneurysms.
Although the sample size is small and further investigations are needed, our study suggests that the application of stents in the treatment of fusiform aneurysms may be advantageous.
Treatment of basilar tip aneurysms is the most challenging. The difficulties result from the fact that despite the small dome to neck ratio, the posterior cerebral arteries and sometimes superior cerebellar arteries may originate directly from the aneurysm base 20. Therefore, the endovascular reconstruction of basilar artery apex aneurysm requires special strategies. Several cases have been reported of this type of aneurysm being treated with the use of Y-configured dual stent-assisted technique20,21. The technique consists of deploying two stents in the basilar and bilateral posterior cerebral arteries in a Y-configuration before coil placement in the aneurysm sack. Thorell et Al reported six cases of successful deployment of both stents (in study population consisting of seven patients with unruptured aneurysms) 20. However, two patients experienced thrombosis. Our approach to these demanding aneurysms differs. We implant only one stent into the basilar and one of the posterior cerebral arteries. There were three cases of basilar tip aneurysms in our study, all aneurysms were ruptured. In two of these cases, thrombosis occurred after stent deployment. Intra-arterial administration of abciximab resulted in complete clot thrombolysis. However, one patient died due to rebleeding from the aneurysm as already described.
Although immediate results of endovascular occlusion of intracranial aneurysms are satisfactory, the long-term efficacy has not yet been established. In our study, follow-up results were available in 16 patients after six to 12 months. There was no stent displacement, fracture or torsion observed during this period. Among 13 broad-necked aneurysms treated both with stent and coils, ten of which were ruptured, complete obliteration at follow-up was observed in 12 and "dog ear" filling in one. Kis et Al encountered recurrences or intended incomplete occlusion on follow-up in four out of 21 aneurysms treated with coils and Leo stent 4. To determine the long-term durability of endovascular treatment of cerebral aneurysms, longer series with larger study populations are required.
Conclusions
The use of self-expandable stents dedicated to intracranial vessels has expanded the indications for endovascular treatment of broadnecked and fusiform aneurysms, which were previously not amenable to this type of treatment. The method of stent-supported coil embolization is safe and effective. However, in patients in acute setting of SAH without complete antiplatelet pretreatment, the possibility of thrombosis must be taken into consideration. Although short-term results are encouraging, long-term follow-up data are required to establish the long-term durability of endovascular treatment of cerebral aneurysms.
References
- 1.Guglielmi G, Vifluela F, et al. Electrothrombosis of saccular aneurysms via endovascular approach. Part 2: Preliminary clinical experience. J Neurosurg. 1991;75:8–14. doi: 10.3171/jns.1991.75.1.0008. [DOI] [PubMed] [Google Scholar]
- 2.Molyneux A, Kerr R, et al. International Subarachnoid Aneurysm Trial (ISAT) Collaborative Group. International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomized trial. Lancet. 2002;360:1267–1274. doi: 10.1016/s0140-6736(02)11314-6. [DOI] [PubMed] [Google Scholar]
- 3.Szikora I, Berentei Z, et al. Endovascular treatment of intracranial aneurysms with parent vessel reconstruction using balloon and self expandable stents. Acta Neurochir (Wien) 2006;148:711–723. doi: 10.1007/s00701-006-0785-6. [DOI] [PubMed] [Google Scholar]
- 4.Kis B, Weber W, et al. Elective treatment of saccular and broad-necked intracranial aneurysms using a closed-cell nitinol stent (Leo) Neurosurgery. 2006;58:443–450. doi: 10.1227/01.NEU.0000197103.10364.0C. [DOI] [PubMed] [Google Scholar]
- 5.Higashida RT, Smith W, et al. Intravascular stent and endovascular coil placement for a ruptured fusiform aneurysm of the basilar artery. Case report and review of the literature. J Neurosurg. 1997;87:944–949. doi: 10.3171/jns.1997.87.6.0944. [DOI] [PubMed] [Google Scholar]
- 6.Lanzino G, Wakhloo AK, et al. Efficacy and current limitations of intravascular stents for intracranial internal carotid, vertebral, and basilar artery aneurysms. J Neurosurg. 1999;91:538–546. doi: 10.3171/jns.1999.91.4.0538. [DOI] [PubMed] [Google Scholar]
- 7.Phatouros CC, Sasaki TY, et al. Stent-supported coil embolization: the treatment of fusiform and wide-neck aneurysms and pseudoaneurysms. Neurosurgery. 2000;47:107–113. doi: 10.1097/00006123-200007000-00023. [DOI] [PubMed] [Google Scholar]
- 8.Pumar JM, Blanco M, et al. Preliminary experience with Leo self-expanding stent for the treatment of intracranial aneurysms. Am J Neuroradiol. 2005;26:2573–2577. [PMC free article] [PubMed] [Google Scholar]
- 9.Byrne JV, Adams CBT, et al. Endosaccular treatment of inoperable intracranial aneurysms with platinum coils. Br J Neurosurg. 1995;9:585–592. doi: 10.1080/02688699550040864. [DOI] [PubMed] [Google Scholar]
- 10.Brillstra EH, Rinkel GJ, et al. Treatment of intracranial aneurysms by embolization with coils: A systematic review. Stroke. 1999;30:470–476. doi: 10.1161/01.str.30.2.470. [DOI] [PubMed] [Google Scholar]
- 11.Fiorella D, Albuquerque F, et al. Preliminary experience using the Neuroform Stent for treatment of cerebral aneurysms. Neurosurgery. 2004;54:6–16. doi: 10.1227/01.neu.0000097194.35781.ea. [DOI] [PubMed] [Google Scholar]
- 12.Lieber BB, Gounis MJ. The physics of endoluminal stenting in the treatment of cerebrovascular aneurysms. Neurol Res. 2002;24(Sup 1):S33–42. doi: 10.1179/016164102101200014. [DOI] [PubMed] [Google Scholar]
- 13.Sani S, Jobe KW, Lopes DK. Treatment of wide-necked cerebral aneurysms with the Neuroform2 Treo stent. A prospective 6-month study. Neurosurg Focus. 2005;18:E4. doi: 10.3171/foc.2005.18.2.5. [DOI] [PubMed] [Google Scholar]
- 14.Leon MB, Baim DS, et al. A clinical trial comparing three antithrombotic-drug regimens after coronaryartery stenting. N Engl J Med. 1998;339:1665–1671. doi: 10.1056/NEJM199812033392303. [DOI] [PubMed] [Google Scholar]
- 15.Fiorella D, Albuquerque FC, et al. Usefulness of the Neuroform stent for the treatment of cerebral aneurysms: results at initial (3-6-mo) follow-up. Neurosurgery. 2005;56:1191–1201. doi: 10.1227/01.neu.0000159645.86823.af. [DOI] [PubMed] [Google Scholar]
- 16.Lopes D, Sani S. Histological postmortem study of an internal carotid artery aneurysm treated with the Neuroform stent. Neurosurgery. 2005;56:E416. doi: 10.1227/01.neu.0000147977.07736.66. [DOI] [PubMed] [Google Scholar]
- 17.Lubicz B, Leclerc X, et al. Retractable self-expandable stent for endovascular treatment of wide-necked intracranial aneurysms: preliminary experience. Neurosurgery. 2006;58:451–457. doi: 10.1227/01.NEU.0000200346.39119.3D. [DOI] [PubMed] [Google Scholar]
- 18.Benitez RP, Silva MT, et al. Endovascular occlusion of wide-necked aneurysms with a new intracranial microstent (Neuroform) and detachable coils. Neurosurgery. 2004;54:1359–1367. doi: 10.1227/01.neu.0000124484.87635.cd. [DOI] [PubMed] [Google Scholar]
- 19.Higashida RT, Smith W, et al. Intravascular stent and endovascular coil placement for a ruptured fusiform aneurysm of the basilar artery. Case report and review of the literature. J Neurosurg. 1997;87:944–999. doi: 10.3171/jns.1997.87.6.0944. [DOI] [PubMed] [Google Scholar]
- 20.Thorell WE, Chow MM, et al. Y-configured dual intracranial stent-assisted coil embolization for the treatment of wide-necked basilar tip aneurysms. Neurosurgery. 2005;56:1035–1040. [PubMed] [Google Scholar]
- 21.Perez-Arjona E, Fessler RD. Basilar artery to bilateral posterior cerebral artery ‘Y stenting’ for endovascular reconstruction of wide-necked basilar apex aneurysms: report of three cases. Neurol Res. 2004;26:276–281. doi: 10.1179/016164104225013969. [DOI] [PubMed] [Google Scholar]