Abstract
Drosophila adults, when placed into a novel open-field arena, initially exhibit an elevated level of activity followed by a reduced stable level of spontaneous activity and spend a majority of time near the arena edge, executing motions along the walls. In order to determine the environmental features that are responsible for the initial high activity and wall-following behavior exhibited during exploration, we examined wild-type and visually impaired mutants in arenas with different vertical surfaces. These experiments support the conclusion that the wall-following behavior of Drosophila is best characterized by a preference for the arena boundary, and not thigmotaxis or centrophobicity. In circular arenas, Drosophila mostly move in trajectories with low turn angles. Since the boundary preference could derive from highly linear trajectories, we further developed a simulation program to model the effects of turn angle on the boundary preference. In an hourglass-shaped arena with convex-angled walls that forced a straight versus wall-following choice, the simulation with constrained turn angles predicted general movement across a central gap, whereas Drosophila tend to follow the wall. Hence, low turn angled movement does not drive the boundary preference. Lastly, visually impaired Drosophila demonstrate a defect in attenuation of the elevated initial activity. Interestingly, the visually impaired w1118 activity decay defect can be rescued by increasing the contrast of the arena's edge, suggesting that the activity decay relies on visual detection of the boundary. The arena boundary is, therefore, a primary object of exploration for Drosophila.
Keywords: Anxiety, centrophobicity, Drosophila, exploratory activity, thigmotaxis
Introduction
Exploratory behaviors are the acts and postures that allow an animal to gather information about a novel environment (Crusio and Van Abeelen 1986). These behaviors have been further classified into diversive and specific exploration dependent on the actuating agent (Berlyne 1966). While diversive exploration is driven by a desire to be stimulated and relieve boredom, specific exploration is induced by novelty and may be driven by anxiety-like responses. Specific exploration was initially called the investigatory reflex by Pavlov when he found that dogs would stop from active behaviors to attend novel stimuli (Pavlov and Anrep 1927). Even though exploratory behaviors are a complex and dynamic response to the novel stimuli, these behaviors are likely to include regular features that depend on properties of the environment. Identifying these physical variables and understanding how they influence exploratory behavior can give significant insights into the mechanisms involved in behavioral responses to external stimuli.
Drosophila melanogaster respond to a novel open-field arena with a high level of initial activity, followed by decay to lower levels of spontaneous activity (Connolly 1967; Meehan and Wilson 1987). In Drosophila, the elevated initial activity has been proposed to represent specific exploration (Liu et al. 2007). Initial activity scales linearly with the circumference of the circular arena, is independent of handling prior to placement within the arena, and is genetically separable; mutations in the kurtz arrestin result specifically in lower levels of initial activity (Liu et al. 2007). Lastly, visually impaired flies are significantly impaired in the attenuation of initial activity, suggesting that visual information is required for the rapid decay from elevated initial activity to spontaneous activity within the novel open-field arena (Liu et al. 2007).
Drosophila species also display strong wall-following behavior in open-field arenas; which has been alternatively interpreted as thigmotaxis (the attraction to the touch of the arena wall) and centrophobicity (center avoidance due to fear) (Gotz and Biesinger 1985; Besson and Martin 2005; Valente et al. 2007). Strong wall-following behavior may be a complex interaction that includes both thigmotactic and centrophobic responses, and in many species may represent a search for safety (Treit and Fundytus 1988; Choleris et al. 2001). In rodents, the avoidance of the central zone depends on external factors such as vision and level of illumination as well as thigmotactic vibrissae stimulation (Morato and Castrechini 1989; Cardenas et al. 2001). Similarly, wall-following behavior in Periplaneta americana relies on both thigmotactic stimulation of the antenna and visual guidance (Creed and Miller 1990). The presence of coupled thigmotactic and visual components has also been proposed for Drosophila open-field behavior (Besson and Martin 2005; Liu et al. 2007).
To determine the environmental features that elicit exploratory and wall-following behaviors, we examined wild type and visually impaired mutants in arenas with different environments. Herein, we show that Drosophila actively explore the arena boundary over other internal environments. Wild-type Drosophila also display a significant preference for darkened corners. The boundary exploration overrides the preference for darkened corners. We propose this preference for darkened corners represents shelter seeking.
Materials and Methods
Fly stocks and husbandry
All stocks were raised and maintained on standard yeast-cornmeal agar food at room temperature. Flies that were used in behavioral assays were two- to five-day-old males raised on standard food at 25°C, 60% humidity, with 12 h of light/day. The norpA7 mutants were obtained from the Bloomington Stock Center.
Behavior assays
The base and walls of all the open-field arenas were made from clear polycarbonate. The ceiling of the arena was made from the lid of a 15-cm polystyrene petri plate (Fisher Scientific, Pittsburgh, PA). A 2-mm hole was drilled in the top of the arena, near the side to allow for the aspiration of a fly into the arena. Since the top of the arena was larger than the bottom, the hole could be shifted out of the active arena area after the fly was added. The flies were typically aspirated into the arena ∼2–3 cm from the boundary, with the starting positions rotated between the four quadrant positions of the arena. The arenas were illuminated by two 23 W compact fluorescent flood lights (R40, 1200 lumens, 5100 K), located 1.15 m above the arena. Arenas were set up in a laboratory that was maintained between 22°C and 24°C. The movement of the fly within the arena was tracked with Ethovision XT v5.0 (Noldus Information Technology, Leesburg, VA). The recording rate of the tracker was set to 30 frames per second. All the arenas were 0.7 cm in height.
Statistical analysis
The collected data were analyzed with Ethovision XT v5.0 (Noldus Information Technology). Before beginning the experiments, it was determined that Canton-S had no significant preferences for individual arena quadrants. To eliminate any biased results due to the starting position of the fly, the starting locations of the fly were equally distributed across different zones used in the analysis. The measured variables included total path length, distance from center, the percentage of time spent in different zones defined by the investigator using the tracking software. In calculating the percentage of time spent in different zones, all flies, regardless of their speed, were included. Each measure was determined for each successive 1-min time bin. The automated video trackers were able to follow the flies for a minimum of 98% of the time. The analyzed data were imported into StatView v5.0.1 (SAS Institute, Cary, NC) or MATLAB (The MathWorks, Inc., Natick, MA) for statistical analysis. In all our statistical analysis, the threshold for P-value was 0.05. In the hourglass-shaped arena, trajectories that passed the horizontal midpoint of the central chasm were counted horizontal transitions (HTs). These trajectories typically result in movement between the chambers. Those trajectories that crossed vertical midpoint in the gap of the 2-cm central chasm were taken as vertical transitions (VTs). A diagonal movement though the chasm was record as both an HT and a VT. The VT index was computed as (number of VT−number of HT)/(total number of transitions).
Turning angle calculation
The Ethovision Tracking system (Noldus Information Technology) records XY position of the fly at 30 frames per second. To calculate turning angles of flies for different sampling rates, we use MATLAB to reconstruct the trajectory of flies at different sampling rates. Three consecutive positions were used to calculate a turn angle using a simple law of cosines rule.
Simulating movement in an open-field arena
The Flymatron simulation software was written in Visual Basic and allows the modeling of the effect of turn angles on the spatial orientation of the fly in arenas of any shape. Flymatron can load any type of arena and outputs the spatial positions of the fly for each iteration. An undirected network of nodes of a fixed size determined by user input (rows and columns) or the by the size of an arena image is first generated. In this network, there are no diagonal links between nodes. The user can alter the size and shape of the arena by making pixels below a fixed luminosity as wall nodes. The user can also input a set of different parameters that control the turn angle and movement distance of the fly. The two main parameters, field of vision and sight distance, limit the amount of turn angle and distance the fly can move in one iteration. Once the grid is created and the fly's starting position and direction of motion are generated randomly, a set of candidate target points is determined based on the input parameters. These candidate target nodes are then examined in the context of the network (environment) to exclude those that are not appropriate, such as if the target node is a wall, is unreachable (e.g., behind a wall), or is outside the network. If there are no candidate target nodes remaining, then the fly executes a random turn until there is a set of available candidate target points. On the availability of candidate target points, the fly resumes its movement as defined by the initial input parameters. The new position of the fly is randomly chosen from the set of the available points. These steps of selecting potential target nodes and choosing the single target node are repeated for a certain number of iteration which is user-defined. For our experiments, 20,000 iterations were run for each simulation.
Results
Corner preferences
Wild-type Canton-S flies will linger in the corners of square arenas (Liu et al. 2007). It is possible that the corners represent increased thigmotactic surfaces that could drive the preference. We examined whether the corner preference would be increased by smaller angles using three parallelogram arenas (Fig. 1). The smaller angled corners in these arenas bring the vertical surfaces closer, increasing their thigmotactic potential. The first arena had a 7.2 cm square base with four 90° corners. The base of the second arena had a 7.2-cm rhomboid base with alternate corners of 60° and 120°. The last parallelogram arena had a base with 7.2-cm sides and alternate corners of 30° and 150°. The time spent in a 1-cm2 area located at equal and opposite corners was determined for each arena. In the square arena, wild-type Canton-S spent roughly 25% of the time in each pair of opposite 90° corners with no significant differences between opposite corner pairs (Fig. 1; t = 0.116, P-value = 0.909, df = 23). Wild-type Canton-S spent significantly more time in the acute 60° corners than the obtuse 120° corners (Fig 1; t = 2.265, P-value = 0.011, df = 23). Lastly, although Canton-S spent more time in the 30° corner than in the 150° corner, the difference was not significant (Fig. 1; t = 1.014, P-value = 0.316, df = 23). The time spent in corners was approximately the same for each of the three parallelogram arenas (∼50%). The obtuse 120° and 150° corners retain an attractive quality for Drosophila since the flies spend considerable time within the proximity of these corners. The absence of a preference for 30° versus 150° corners is not consistent with smaller angles presenting a stronger thigmotactic attraction.
Figure 1.
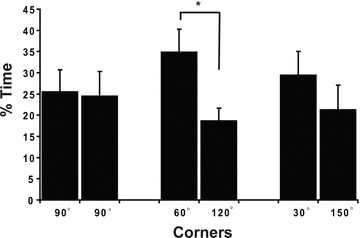
Parallelogram-shaped arenas. Preference for corners is increased by smaller angles at the corners. There are no significant differences between the mean percentage of time spent in 1-cm2 area located at opposite corners with equal angle of 90° (Paired t -test: t = 0.116, P = 0.909) or between the 30° and 150° opposite corners (t = 1.014, P = 0.316). However, the flies spent significantly more time within the acute 60° corners than the 120° corners (t = 2.65, P = 0.011). For each arena n = 24.
We next examined the antecedent for corner preference by placing four 90° corners, formed by two perpendicular intersecting walls extending 3 cm from the center point, in the center of the arena (Fig. 2A). If the corners are strongly preferred thigmotactic surfaces, the flies would leave the boundary and spend more time within the center of the arena. Although the internal corners significantly increased the amount of time in proximity to the center (t = –5.909, P-value < 0.0001, df = 31), the percentage of time spent (∼6%) was far below that of external corners (∼50%; Fig. 2B), suggesting the presumptive preference for the internal corners is less than the preference for the concave arena boundary. In these experiments, we also examined the preference for different combinations of darkened walls. For all the different combinations, the internal corners significantly increased the amount of time in proximity to the center (dark edge and dark corner: t = –3.03, P-value = 0.014, df = 31; dark edge and clear internal corner: t = –4.239, P-value = 0.0003, df = 31; clear edge and dark internal corner: t = –17.587, P-value < 0.0001, df = 31). In the first three conditions, the total time in the arena did not significantly affect the percentage of time spent in proximity to the internal corners (clear edge and clear corner: F9, 620 = 0.736, P-value = 0.676; both edge and corner dark: F9, 620 = 0.442, P-value = 0.912; dark edge and clear corner: F9, 620 = 0.111, P-value = 0.999). However, when the boundary wall is clear and the internal walls are opaque, the flies spend increasingly more time in close proximity to the internal corners as the exploratory activity phase is attenuated (Fig. 2C; F9, 620 = 2.380, P-value = 0.012). Hence, exploration supersedes the strong preference for the darkened internal corner. Drosophila also strongly prefer the arena boundary to the clear internal corners.
Figure 2.
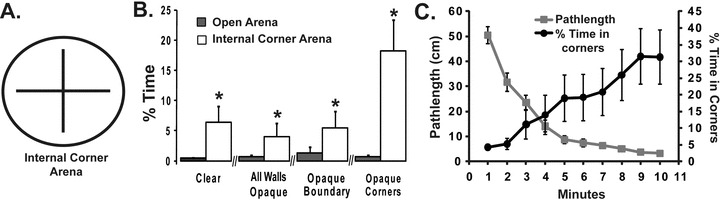
A time-dependent preference for opaque internal corners. (A). An arena was constructed with two intersecting walls that generated four internal corners. (B). The mean time spent in the 4-cm2 sector in the center of the arena was determined with four combinations of opaque internal and external vertical surfaces. In each case, the flies spend more time in the center zone in the arena with internal corners than the control open arena of the same size. When the outer wall was clear and the internal walls were opaque, the flies spent even more time in the center. (C). Only in this last experiment with the opaque internal corners, there was a significant interaction between mean percentage of time spent in the center and time in the arena (F9620 = 2.380, P = 0.012). This time dependence leads to an inverse relationship between amount of specific exploration and percentage of time spent in the corner. n = 32 for each arena.
The basis for the Drosophila corner preference was examined further using a circular arena with a radius of 4.2 cm and a 2.56 cm2 recessed alcove (Fig. 3A). This alcove provided the fly an area further distanced from the arena center, as well as two external 90° corners as additional thigmotactic substrates. This alcove accounts for ∼11.5% of the arena perimeter. If the flies responded neutrally to the cove compared to the rest of the boundary, they would be present within this area approximately 6.9 sec/min. Since there appeared to be a significant effect of wall opacity in driving the fly's behavior in the previous experiment (Fig. 2), we examined the alcove arena with four sequential experiments, altering the vertical surface that was opaque (Fig. 3). Even when the circular edge of the alcove arena is clear, the flies demonstrate a significant preference for the alcove; an even stronger preference for the alcove is seen when the alcove walls are opaque and the circular edge is clear (Fig. 3B). When the circular edge of the arena was darkened, wild-type flies demonstrated little preference for the alcove and the external corners contained therein (Fig. 3B). Similar to the results with the darkened internal corners, there was a significant interaction between time in the arena and the preference for the darkened alcove (Fig. 3B; F9, 1240 = 7.122, P-value < 0.0001). This alcove preference increases as specific exploration of the novel arena decreases. In all four experiments with this cove arena, the flies spend significantly more time at the arena's boundary than in the central zone (data not shown). Since the alcove preference is not expressed during the first minute within the arena, while the flies are still expressing significant wall-following behavior, and the alcove represents the furthest distance from the center, centrophobicity does not account for the dominant wall-following behavior. We also failed to find a difference between the time attending a 1.5 cm black wall arc and an identically sized area at the opposite end of an 8.4 cm circular arena (t = –1.55, P-value = 0.13, df = 31) suggesting that neither the black wall nor the contrast of a black-clear border was preferentially attended.
Figure 3.
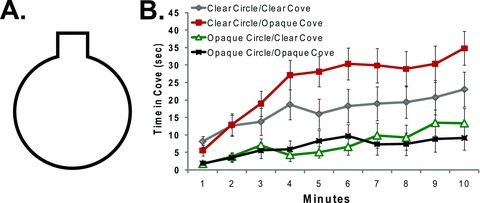
A time-dependent preference for a recessed alcove with opaque walls. (A). Diagram of the arena used in this experiment. Four different arena permutations were tested in which the walls of the arena (circular part) and the alcove were either clear or opaque. (B). The mean time spent in the alcove per minute is shown for each of the four arena permutations. No significant differences were found for the two arenas with opaque circles. Flies spent significantly more time in the alcove in the experiment with the clear circle and dark alcove than in the other three arenas (Bonferroni–Dunn; P < 0.001 for all comparisons). There was also a significant effect of time. n = 32 for each arena. Cove = 11.5% of perimeter, cove neutrality = 6.9 sec/min.
Preference for the arena boundary
In most open-field arenas, the boundary is both the furthest extent of the explorable territory and the only available vertical surface; either of these two features could be responsible for attracting the flies. In the internal corner arena, the flies did attend the internal surfaces, but to a significantly lesser degree than the curved boundary, leaving open the possibility that curved surfaces are generally preferred to straight walls. To address this concern, we have examined the behavior of wild-type Drosophila in arenas having equally spaced internal concentric circular walls (Fig. 4A). The walls in this arena subdivide the space into four concentric zones with different areas. The inner zones also offer walls of greater curvatures, and more proximate thigmotaxis. In this concentric circle arena, with either clear or opaque walls, the flies displayed a significant preference for the outermost zone (Fig. 4B) compared to the expected value based on neutral space (clear walls: χ2 = 91.95, P-value < 0.0001, df = 3; opaque walls: χ2 = 17.2, P-value = 0.0006, df = 3). The neutral expectation is derived from the percent area of each zone (i.e., zone 1 accounts for 45.1% of the total arena area, resulting in an expected percentage of time in zone of 270.6 sec). When the walls were opaque, the flies did spend significantly less time in the outermost zone compared to the transparent walls (zone 1; P-value < 0.01), but still more than expected based on neutral space (χ2 = 17.2, P-value = 0.0006, df = 3). Therefore, the preference was for the arena boundary, and not simply vertical surfaces.
Figure 4.
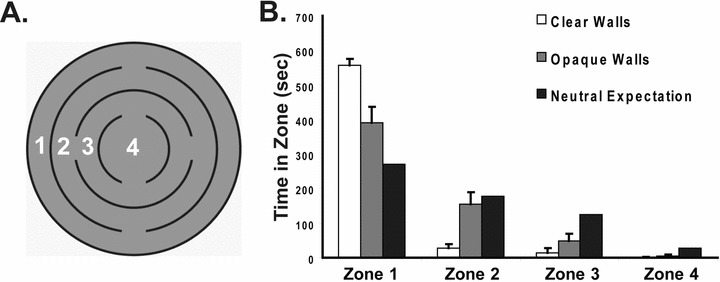
Arena boundary and not vertical surfaces are preferred. (A). An arena was constructed with internal concentric walls. For analysis, the arena was subdivided into four zones. (B). The behavior of flies was examined in the concentric circle arena having either transparent or opaque walls. When the walls were clear, wild-type Canton-S spent 92.7% of the time in the outermost zone. This is significantly more time than when the walls were opaque (65.2%, P <0.0001). The neutral expectation is derived from the percent area of each zone. n = 32 for each experiment.
To test if the potential tiny gaps at the meeting of the ceiling and wall in previous arenas were responsible for boundary preference in previous arenas, a doughnut ceiling arena (Figure not shown) was used. A ceiling with a 2.5-cm diameter hole at the center was firmly affixed to the walls of the arena. A loose lid like the previous circular arena was laid on the top of the doughnut arena to create potential gaps at the edge of central zone. Even in the arena with a doughnut ceiling, flies spent the most amount of time near the edge (Fig. 6B; edge zone: 84.89 ±3.78%, middle zone: 13.98 ± 3.73%, central zone: 0.96 ± 0.26%). A robust boundary preference was retained even in the doughnut ceiling arena.
Figure 6.
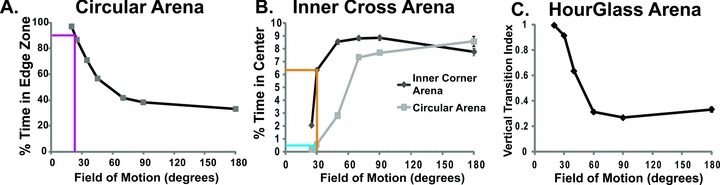
Modeling the effect of turn angle on a fly's position within the arenas. Each data point is the average of 20 simulations ± SEM. (A). Within a circular arena, there is a strong effect of limiting the fly's field of motion (FoM; equals twice the turn angle) on the percentage of time spent in the edge zone (outer one-third) of the arena. A simulated fly that can only move 10° either right or left is largely stuck in the edge zone; while a fly capable of 180° turns spend one-third of its time in each of the three concentric zones. The magenta lines indicate the percentage of time Canton-S spent experimentally in the edge zone (Fig 2; 89.9%), which corresponds to ∼24° FoM. (B). The percentage of time in a centrally located 2-cm2 zone was determined for both the inner cross-arena and a control open-field arena. The time Canton-S spent in this zone experimentally (Fig 2B) is indicated in yellow for the inner cross-arena and cyan for the control open field. Both of these measures correspond to ∼30°FoM. (C). In an hourglass-shaped arena, the numbers of vertical transitions (VTs) and horizontal transitions (HTs) across a central chasm were determined. A VT index is defined as (number of vertical transitions– number of horizontal transitions)/total number of transitions.
Drosophila display low turn angle trajectories
One potential explanation for a robust boundary preference is that the flies have a strong bias for moving in relatively straight lines, resulting in a centrifugal dispersal within the arena (Creed and Miller 1990). Although flies can rotate to make sharp turns of 100° or more in the arena, such instances may be very rare, especially while the fly is in motion (Strauss and Heisenberg 1990). Innate propensities for straight trajectories may represent a specific strategy to escape from distant threats, or may have evolved as a general response to slow and distant predators (Furuichi 2002; Eilam 2005). A physical inability or an innately low propensity to turn while walking would result in the animal being largely limited to the arena's edge. To examine this possibility, we first measured the turning behavior of Canton-S flies within the arena. In central and edge zone, we examined the distribution and median of absolute turn angles of wild-type flies in an 8.4-cm diameter circular arena at 10 different sampling intervals (Supporting information). Different sampling intervals were considered as large sampling intervals can miss significant turning behaviors in the trajectory while small sampling intervals can capture a “wobble” like characteristic caused by changes in the tracking centroid of the fly without significant changes in the orientation during movement.
Both in the edge and central zones, the median turn angle increased as the sampling interval increased (Fig. 5B). In the edge zone, the angle at which the turn angle distribution peaked increased from 3.6° to 12.6° (Supporting information and Fig. 5A) as the sampling interval increased from 0.1 to 1 sec. This indicates that different sampling intervals can give rise to different estimates of turn angles. However, for all of the 10 sampling intervals considered, the peaks of the distributions occur at small turn angles (maximum of 12.6°), which shows that flies prefer to execute small turn angles both in the edge and central zone. Irrespective of the sampling interval, the distribution of turn angle and the median turn angle in the edge zone and central zone were significantly different (Supporting information). This indicates that flies displayed different turn angle behavior in edge and central zone. The dissimilarity is most likely because the movement along the edge is shaped by the curvature of the circular edge. To examine this possibility, the turn angles were calculated for all the move lengths (ranging from 1 to 3 cm) of the fly in the edge zone. The computed turn angles were compared against the corresponding expected turn angles along the curvature of the arena. There was no significant difference between the observed and expected turn angles in the edge zone, which strongly suggests that wall-following behavior affects turning behavior (Supporting information).
Figure 5.
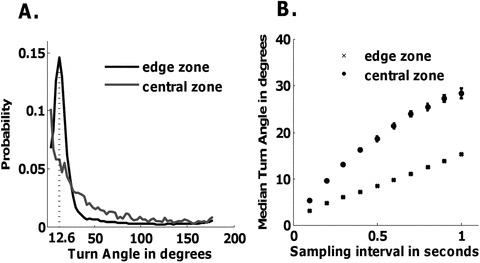
Drosophila display few large-angled turns in circular open-field arenas. Turn angle was estimated in two separate zones within the arena. The central zone is the inner one-third portion of the arena and the edge zone is the outer one-third of the arena. (A). The distribution of the turn angle (histogram bin size of 3.6° was used) is shown with a 1-sec sampling interval. The most frequent turn angle for the edge and central zones are 12.6° and 3.6°, respectively. The turn angle distributions within the two zones are significantly different (χ2 = 43,412, P < 0.0001). (B). The median turn angle increases when sampling interval increases. For each sampling interval, the median turn angles between both zones were significantly different (sampling interval = 1 sec, t = 283.43, P < 0.0001). n = 173 Canton-S males.
The propensity to walk in relatively straight lines may either cause the edge preference or develop as a result of this preference. To determine if the measured propensity for low turn angles is sufficient to account for the observed wall-following behavior, we have used Flymatron to systematically test the effect of field of motion (FoM) on the spatial orientation behavior of simulated flies (Fig. 6). The simulation was run for each arena with 20 pseudo-randomly chosen starting positions by altering the maximum FoM, an FoM of 30° allowed turning angle of 15° to the right and 15° to the left of the fly's direction of movement, and choosing step size randomly as zero to five nodes. In these simulations, we recorded the node visits and movement history within specific areas that matched our previous experimental measurements (Fig. 2). Canton-S will spend ∼90–95% of the time in the outer one-third of an 8.4-cm arena (Liu et al. 2007); this edge preference corresponded to a 24° FoM or 12° turn angle (Fig. 8A), approximately the same value for the peak turn angle of Canton-S within edge zone (Fig. 5A).
Figure 8.
Drosophila visually attend the arena's edge during exploration. Wild-type Canton-S, w1118, and norpA7 were examined in circular arenas that had either a clear or opaque boundary. The activity of the normally sighted Canton-S and the blind norpA7 did not significantly change in the two different arenas. However, the visually impaired w1118 flies demonstrated distinct differences (Bonferroni–Dunn; P < 0.0001). The increased contrast obtained with the darkened arena wall rescued the w1118 activity decay phenotype. n = 24 for each genotype/condition.
The movement of flies was also simulated in the open-field arena with internal corners, while varying the FoM (Fig. 6B). Canton-S will spend ∼6% of the time in the central 2-cm2 zone of the internal corner arena, and 1% of the time in the comparable open-field arena (Fig. 2B). Both of these values were both closely matched by a maximum 30° FoM (15° turn angle) in the Flymatron simulator (Figs. 2B, 6B). The results from the simulated movement in both an open-field arena and in the internal cross-arenas were consistent with a strong effect of constrained turn angle on wall-following behavior of Drosophila.
In order to further examine this hypothesis, we simulated the movement of a fly with different FoM constraints in an hourglass-shaped arena (Creed and Miller 1990). The probative value of this arena comes from a gap that forces a choice between walking straight (vertical crossing) and following the wall (horizontal crossing; Fig. 7A). The minimum VT index was obtained with fields of motion of 90°, and even at 180° the simulations produced significantly greater VTs than HTs (Fig. 6C). If a restricted FoM of 25–30° is responsible for driving the edge preference of Drosophila in open-field arenas, then we predict that in an hourglass arena, Canton-S will display a VT index close to 0.9.
Figure 7.
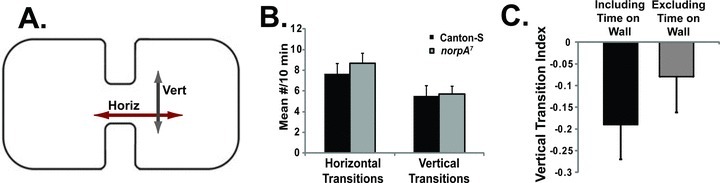
Flies display wall-following behavior in an hourglass-shaped arena. (A). The hourglass arena. This arena is 10 cm long × 5 cm wide, and 0.7 cm in height. A fly walking in this arena may make an HT by following the wall from one chamber into the next, or it may make a VT by crossing the 2 cm central chasm. (B). There were no significant differences between Canton-S and the blind norpA7 in either the number of vertical (F1,57 = 0.280, P = 0.599) or horizontal (F1,57 = 0.0003, P = 0.98) transitions at the chasm. n = 32 for each genotype. The VT indexes are negative for both genotypes. (C). Wall-following behavior does not require walking on the walls in the hourglass arena. Canton-S males were examined for vertical and HTs. The position of the fly, either walking on the wall or walking adjacent to the wall, was recorded for each transition. The VT index was separately determined for all transitions or with the wall-walking transitions excluded. n = 64.
We examined this prediction using both normally sighted Canton-S and blind norpA7 flies in a comparable hourglass arena (Fig. 7A). Both of these genotypes displayed more HTs than VTs (F1,53 = 0.064, P-value = 0.80) suggesting a moderately greater wall-following effect than low turn angle effect in both blind and sighted genotypes (Fig. 7B). The negative VT indexes for Canton-S (–0.195 ± 0.079) and norpA7 (–0.199 ± 0.070) are inconsistent with the simulation results using constrained turn angles (Fig. 6C). There were no significant differences between Canton-S and the blind norpA7 in either the number of vertical (Fig. 7B; F1,57 = 0.280, P-value = 0.599) or horizontal (Fig. 7B; F1,57 = 0.0003, P-value = 0.98) transitions, indicating that the visual detection of gap distance was not a primary factor for choice of direction. These results argue that a simple physical constraint on turning cannot solely explain the wall-following behavior of Drosophila in the hourglass arena. In this analysis, the HTs occurred with the fly walking on the wall, ceiling, or floor of the arena. However, walking alongside the walls and walking on the walls are not equivalent and are expected to produce different trajectories. Therefore, the VT index was computed for normally sighted Canton-S by excluding cases when the fly was walking on the wall (vertical surface; Fig. 7C). The transition index in this case was –0.079 ± 0.08, which was significantly different from simulations (Fig. 6C,7C; t = 0.751, P-value = 0.0011, df = 62). Hence, our conclusion that a simple physical constraint on turning cannot solely explain the wall-following behavior of Drosophila in the hourglass arena still holds even after excluding cases of flies walking on walls.
Visual exploration of the arena boundary
We previously hypothesized that the reduced activity decay in visually impaired flies occurs because they are less able to abrogate the novelty of the arena (Liu et al. 2007). Many insects including Drosophila use visual guidance to direct search patterns (reviewed in Bell 1990; Gotz 1994). Since Drosophila spend most of their time at the arena edge, it is possible that the edge represents a primary object of exploration. The w1118 mutant flies are not blind—they are positively phototactic, but have poor visual acuity due to the absence of pigments in the cells that surround the photoreceptor neurons (Hengstenberg and Gotz 1967). In the w1118 flies, the photoreceptors are activated by tangential light, and as a consequence these flies have very poor visual contrast and cannot perform certain optimotor tasks (Kalmus 1948). Conversely, the norpA7 mutant flies are defective in phospholipase Cβ, fail to form a receptor potential, and are completely blind (Harris and Stark 1977). We examined Canton-S, w1118, and norpA7 flies in arenas with either a clear outer wall or with the outer wall made opaque (Fig. 8). Darkening the arena's edge did not alter the time-dependent activity pattern of either wild-type (F1, 478 = 0.051, P-value = 0.903) or the completely blind norpA7 flies (F1, 478 = 1.364, P-value = 0.244). The increased contrast of the arena boundary did however rescue the activity decay phenotype of the poorly sighted w1118 flies (Fig. 8; w1118 in clear and opaque walls: F1, 518 = 75.341, P-value < 0.0001). This response to a change in the visual representation of the boundary strongly suggests a role for vision in the attenuation of initial activity. Hence, we propose that the decay from the high levels of initial activity to the lower levels of spontaneous activity is a result of the visual exploration of the arena boundary.
Discussion
Drosophila melanogaster explore novel arenas employing a strong wall-following behavior (Gotz and Biesinger 1985; Gotz 1994; Besson and Martin 2005). We demonstrate using various arena environments that the wall-following behavior is actually a strong preference specifically for the arena's boundary and not vertical surfaces in general, and is largely independent of thigmotaxis or centrophobism. The trivial explanation of constrained turning and centrifugal movement is also incapable of accounting for the boundary preference. The arena boundary is however a primary object of exploration, and vision is required to abrogate the novelty presented by the boundary. The expressed boundary preference may be the result of an active search for escape routes. Interestingly, in our new darkened internal corner and darkened cove paradigms, there was a distinct time-dependent preference for the opaque corners located within the arenas. This preference appeared following the attenuation of active exploration, and may represent shelter-seeking behavior.
Not thigmotaxis or centrophobicity
Drosophila's significant boundary preferences, and the absence of preferences for internal walls (straight or curved) in the concentric circle arena and in the internal corner arena, and for 30° corners versus 150° corners, are inconsistent with thigmotaxis as a force for the wall-following behavior (Besson and Martin 2005; Liu et al. 2007; Valente et al. 2007). Additionally, Drosophila do not have extended antennae or vibrissae that maintain contact with the wall during movement. However, Drosophila will walk on the vertical arena boundaries in addition to the floor and ceiling of the arena.
Centrophobicity was previously questioned as a driving force for wall-following behavior since blind flies, incapable of seeing the arena center, also significantly prefer edge zones over central locations (Besson and Martin 2005; Liu et al. 2007). The behavior of flies in the parallelogram arenas and the alcove arena is also inconsistent with a strong centrophobic drive in the strict sense of this term. Wild-type flies demonstrate equal preference for 30° corners and 150° corners, even though the former is much further from the center and more confined space than the latter. Additionally, the flies did not significantly prefer the alcove, the farthest point from the center, during the initial exploration phase in the alcove arena. The strong alcove preference emerged after the specific exploration phase. During exploration of the arena containing an alcove, the flies still display strong wall-following behavior, indicating wall-following and centrophobicity are separable.
Shelter-seeking behavior
There was considerable preference for opaque internal corners over clear walls and for the dark alcove over clear circular boundaries. The absence of preference for a darkened wall section lacking a corner and the waning preferences for clear corners indicate that the predilection is for an emergent quality of the orthogonal darkened walls. Rats avoid bright light in an open-field arena and the plus maze, presumably because bright light increases the chances of being spotted by predators (Ennaceur et al. 2006). We suggest the most parsimonious explanation is that these darkened corners represent shelter. However, this preference for dark corners was evident only when the specific exploration of the boundary waned. In rodents, anxiety induced by novelty is suggested as one of the main driving component of exploratory behavior (Simon et al. 1994; Treit and Fundytus 1988). The need to abrogate novelty with specific exploration can supersede other needs such as hunger, thirst, or even predator avoidance (Hinde 1954; Chance and Mead 1955; Zimbardo and Montgomery 1957). The delayed expression of shelter-seeking behavior in Drosophila indicates that the shelter provided by the darkened corners does not satisfy the need to explore.
Low turn angles are not responsible for arena edge preference
Creed and Miller differentiated between active wall-following behavior, a positive drive toward the wall, and passive wall-following behavior resulting from dominant movement patterns independent of motivation (Creed and Miller 1990). We were able to demonstrate using the Flymatron simulation program that a limitation in large turn angles matches experimental data in both circular and internal corner arenas but not in an hourglass-shaped arena. Unlike in the hourglass arena, in circular arenas there is no requirement for flies to make large-angled turns to follow the wall because the arena walls are concave. In circular arenas, the effect of the curved walls on the turn angle is clearly evident in the shift of the peak of the turn angle from 0° to 12° in the turn angle distribution in the boundary zone. Hence, small turn angle movement is not driving the wall-following behavior rather it is wall-following behavior that shapes the turn angles made by flies.
Exploration of boundary
Our data strongly suggest that the boundary of a circular arena is a primary object of exploration, as demonstrated by the ability of high-contrast walls to rescue the w1118 attenuation of exploration deficit. It remains possible however that the w1118 initial activity attenuation phenotype is not primarily due to poor visual acuity. Mutations in white are pleiotropic, resulting in defects in vision and also reduced levels of dopamine, serotonin, and histamine found with the Drosophila head (Borycz et al. 2008; Sitaraman et al. 2008). These biogenic amine reductions, in theory, may cause hyperactivity or learning deficits independent of visual exploration that could contribute to the w1118 activity attenuation phenotype (Sitaraman et al. 2008). The lower levels of dopamine found in the heads of the w1118 mutants is an unlikely source for the activity attenuation phenotype since reducing dopamine leads to lower levels of spontaneous activity (Liu et al. 2007; Riemensperger et al. 2011). Nevertheless, we believe that the most straightforward explanation for these data is that similar to blind norpA7, glass2, and the white-eyed brown1, scarlet1 double mutant (Liu et al. 2007), the activity attenuation defect in w1118 is due to the poor visual acuity associated with this mutation. Although this is likely due to the absence of screening pigments in the eyes of the w1118 mutants, the visual defect may also result from the reduced histamine found within this genotype since this neurotransmitter is used by photoreceptor neurons (Hardie 1987). In either or both cases, the opaque boundary likely rescues this activity attenuation phenotype due to the increased contrast it provides, allowing the w1118 mutants to detect the boundary and abrogate the novelty.
In the concentric inner circle and the internal corner arenas, the flies were preferentially attending to the arena boundary and not just vertical walls. This suggests that there is a specific feature of the boundary that the flies attend. When the flies are actively exploring the arena boundary, they bypass shelter, suggesting this is not a primary goal for the exploration. Moreover, our turn angle calculations and hourglass experiments indicate that wall-following behavior shapes turn angles in the boundary zone and not vice versa. Our observations strongly suggest other potential reasons for boundary preference. The propensity for straight trajectories in the central zone may be an important clue to identifying these features.
In the central zone, the turn angle distribution peaks at zero degree showing straight trajectories. Mathematical models of predator avoidance indicate that straight trajectories have greatest success against distant and slow-moving predators, while rapid, more convoluted paths have greatest fitness against a close or fast predator (Furuichi 2002). In an open-field arena, the nimble spiny mice will display winding trajectories, while the pedestrian Günther's Voles travel in more straight trajectories and spend less time in the central zones of the arena (Eilam 2003, 2004). Interestingly, these two species display combinations of fleeing and freezing when they respond to barn owl's (Tyto alba) attacks (Edut and Eilam 2004). By analogy, it is possible that relatively low turn angle movement of Drosophila in open-field arenas represents an avoidance/escape behavior. Straight trajectories cause the flies to spend less time in the center by decreasing the amount of time taken to reach the boundary. Experiments with Brachyrhaphis episcopi, the tropical poeciliid fish, indicate that those from high-predation environments have shorter latencies to reach the arena boundary and explore novel areas more than those from low-predation environments (Archard and Braithwaite 2011). Likewise in Drosophila, the arena boundary provides a better source for escape routes compared to internal corners and vertical surfaces present inside the arena. A wall-following behavior interrupted by a few visits to the center of the arena in straight trajectories will result in more time along the walls and less time in the center, which in turn can optimize the chance of finding escape routes along the boundary. This adaptive behavior may significantly enhance fitness through increased dispersal and predatory avoidance.
Acknowledgments
We are grateful to C. Manson-Bishop and R. Goldfeder for technical assistance and helpful discussions. The work was funded by the MH091304 award from the National Institute for Mental Health to GR.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Section 1. Significance between Turn Angel Distributions between Central and Edge zones.
Figure S1. Turning angle distributions in the central and edge zone for two sampling intervals.
Figure S2. Turn angles in the edge zone were driven by the curvature of the circular arena.
Figure S3. Trajectory of a fly in a circular arena.
Please note: Wiley-Blackwell is not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Archard GA, Braithwaite VA. Increased exposure to predators increases both exploration and activity level in Brachyrhaphis episcopi. J. Fish Biol. 2011;78:593–601. doi: 10.1111/j.1095-8649.2010.02880.x. [DOI] [PubMed] [Google Scholar]
- Bell W. Searching behaviour: the behavioural ecology of finding resources. New York: Chapman and Hall Ltd; 1990. [Google Scholar]
- Berlyne DE. Curiosity and exploration. Science. 1966;153:25–33. doi: 10.1126/science.153.3731.25. [DOI] [PubMed] [Google Scholar]
- Besson M, Martin JR. Centrophobism/thigmotaxis, a new role for the mushroom bodies in Drosophila. J. Neurobiol. 2005;62:386–396. doi: 10.1002/neu.20111. [DOI] [PubMed] [Google Scholar]
- Borycz J, Borycz JA, Kubow A, Lloyd V, Meinertzhagen IA. Drosophila ABC transporter mutants white, brown and scarlet have altered contents and distribution of biogenic amines in the brain. J. Exp. Biol. 2008;211:3454–3466. doi: 10.1242/jeb.021162. [DOI] [PubMed] [Google Scholar]
- Cardenas F, Lamprea MR, Morato S. Vibrissal sense is not the main sensory modality in rat exploratory behavior in the elevated plus-maze. Behav. Brain. Res. 2001;122:169–174. doi: 10.1016/s0166-4328(01)00180-2. [DOI] [PubMed] [Google Scholar]
- Chance MRA, Mead AP. Competition between feeding and investigation in the rat. Behaviour. 1955;8:174–181. [Google Scholar]
- Choleris E, Thomas AW, Kavaliers M, Prato FS. A detailed ethological analysis of the mouse open field test: effects of diazepam, chlordiazepoxide and an extremely low frequency pulsed magnetic field. Neurosci. Biobehav. Rev. 2001;25:235–260. doi: 10.1016/s0149-7634(01)00011-2. [DOI] [PubMed] [Google Scholar]
- Connolly K. Locomotor activity in Drosophila. 3. A distinction between activity and reactivity. Anim. Behav. 1967;15:149–152. doi: 10.1016/s0003-3472(67)80026-5. [DOI] [PubMed] [Google Scholar]
- Creed RP, Miller JR. Interpreting animal wall-following behavior. Cel. Mol. Life Sci. 1990;46:758–761. [Google Scholar]
- Crusio WE, Van Abeelen JH. The genetic architecture of behavioural responses to novelty in mice. Heredity. 1986;56:55–63. doi: 10.1038/hdy.1986.8. [DOI] [PubMed] [Google Scholar]
- Edut S, Eilam D. Protean behavior under barn-owl attack: voles alternate between freezing and fleeing and spiny mice flee in alternating patterns. Behav. Brain Res. 2004;155:207–216. doi: 10.1016/j.bbr.2004.04.018. [DOI] [PubMed] [Google Scholar]
- Eilam D. Open-field behavior withstands drastic changes in arena size. Behav. Brain Res. 2003;142:53–62. doi: 10.1016/s0166-4328(02)00382-0. [DOI] [PubMed] [Google Scholar]
- Eilam D. Locomotor activity in common spiny mice (Acomys cahirinuse): the effect of light and environmental complexity. BMC Ecol. 2004;4:16–25. doi: 10.1186/1472-6785-4-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eilam D. Die hard: a blend of freezing and fleeing as a dynamic defense—implications for the control of defensive behavior. Neurosci. Biobehav. Rev. 2005;29:1181–1191. doi: 10.1016/j.neubiorev.2005.03.027. [DOI] [PubMed] [Google Scholar]
- Ennaceur A, Michalikova S, Chazot PL. Models of anxiety: responses of rats to novelty in an open space and an enclosed space. Behav. Brain Res. 2006;171:26–49. doi: 10.1016/j.bbr.2006.03.016. [DOI] [PubMed] [Google Scholar]
- Furuichi N. Dynamics between a predator and a prey switching two kinds of escape motions. J. Theor. Biol. 2002;217:159–166. doi: 10.1006/jtbi.2002.3027. [DOI] [PubMed] [Google Scholar]
- Gotz KG, Biesinger R. Centrophobism in Drosophila melanogaster.2. Physiological approach to search and search control. J. Comp. Physiol. A Sensory Neural Behav. Physiol. 1985;156:329–337. [Google Scholar]
- Gotz KG. Exploratory strategies in Drosophila. Stuttgart: Fischer; 1994. [Google Scholar]
- Hardie RC. Is histamine a neurotransmitter in insect photoreceptors? J. Comp. Physiol. A. 1987;161:201–213. doi: 10.1007/BF00615241. [DOI] [PubMed] [Google Scholar]
- Harris WA, Stark WS. Hereditary retinal degeneration in Drosophila melanogaster. A mutant defect associated with the phototransduction process. J. Gen. Physiol. 1977;69:261–291. doi: 10.1085/jgp.69.3.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hengstenberg R, Gotz KG. Effect of facet-separating pigments on the perception of light and contrast in eye mutants of Drosophila. Kybernetik. 1967;3:276–285. doi: 10.1007/BF00271510. [DOI] [PubMed] [Google Scholar]
- Hinde RA. Factors governing the changes in strength of a partially inborn response, as shown by the mobbing behaviour of the chaffinch (Fringilla coelebs). I. The nature of the response, and an examination of its course. Proc. R Soc. Lond. B Biol. Sci. 1954;142:306–331. doi: 10.1098/rspb.1954.0028. [DOI] [PubMed] [Google Scholar]
- Kalmus H. The optimotor responses of some eye mutants of Drosophila. J. Genet. 1948;45:206–213. [Google Scholar]
- Liu L, Davis RL, Roman G. Exploratory activity in Drosophila requires the kurtz nonvisual arrestin. Genetics. 2007;175:1197–1212. doi: 10.1534/genetics.106.068411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meehan MJ, Wilson R. Locomotor activity in the Tyr-1 mutant of Drosophila melanogaster. Behav. Genet. 1987;17:503–512. doi: 10.1007/BF01073117. [DOI] [PubMed] [Google Scholar]
- Morato S, Castrechini P. Effects of floor surface and environmental illumination on exploratory activity in the elevated plus-maze. Braz. J. Med. Biol. Res. 1989;22:707–710. [PubMed] [Google Scholar]
- Pavlov IP, Anrep GV. Conditioned reflexes; an investigation of the physiological activity of the cerebral cortex. Humphrey Milford, [London]: Oxford Univ. Press; 1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riemensperger T, Isabel G, Coulom H, Neuser K, Seugnet L, Kume K, Iche-Torres M, Cassar M, Strauss R, Preat T, et al. Behavioral consequences of dopamine deficiency in the Drosophila central nervous system. Proc. Natl. Acad. Sci. USA. 2011;108:834–839. doi: 10.1073/pnas.1010930108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon P, Dupuis R, Costentin J. Thigmotaxis as an index of anxiety in mice. Influence of dopaminergic transmissions. Behav Brain Res. 1994;61:59–64. doi: 10.1016/0166-4328(94)90008-6. [DOI] [PubMed] [Google Scholar]
- Sitaraman D, Zars M, Laferriere H, Chen YC, Sable-Smith A, Kitamoto T, Rottinghaus GE, Zars T. Serotonin is necessary for place memory in Drosophila. Proc. Natl. Acad. Sci. USA. 2008;105:5579–5584. doi: 10.1073/pnas.0710168105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss R, Heisenberg M. Coordination of legs during straight walking and turning in Drosophila melanogaster. J. Comp. Physiol. A. 1990;167:403–412. doi: 10.1007/BF00192575. [DOI] [PubMed] [Google Scholar]
- Treit D, Fundytus M. Thigmotaxis as a test for anxiolytic activity in rats. Pharmacol. Biochem. Behav. 1988;31:959–962. doi: 10.1016/0091-3057(88)90413-3. [DOI] [PubMed] [Google Scholar]
- Valente D, Golani I, Mitra PP. Analysis of the trajectory of Drosophila melanogaster in a circular open field arena. PLoS ONE. 2007;2:e1083. doi: 10.1371/journal.pone.0001083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimbardo PG, Montgomery KC. Effects of free-environment rearing upon exploratory-behavior. Psychol. Rep. 1957;3:589–594. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


