Abstract
Previous picture-word interference (PWI) fMRI-paradigms revealed ambiguous mechanisms underlying facilitation and inhibition in healthy subjects. Lexical distractors revealed increased (enhancement) or decreased (suppression) activation in language and monitoring/control areas. Performing a secondary examination and data analysis, we aimed to illuminate the relation between behavioral and neural interference effects comparing target-related distractors (REL) with unrelated distractors (UNREL). We hypothesized that interference involves both (A) suppression due to priming and (B) enhancement due to simultaneous distractor and target processing. Comparisons to UNREL should remain distractor unspecific even at a low threshold. (C) Distractor types with common characteristics should reveal overlapping brain areas. In a 3T MRI scanner, participants were asked to name pictures while auditory words were presented (stimulus onset asynchrony [SOA] = –200 msec). Associatively and phonologically related distractors speeded responses (facilitation), while categorically related distractors slowed them down (inhibition) compared to UNREL. As a result, (A) reduced brain activations indeed resembled previously reported patterns of neural priming. Each target-related distractor yielded suppressions at least in areas associated with vision and conflict/competition monitoring (anterior cingulate cortex [ACC]), revealing least priming for inhibitors. (B) Enhancements concerned language-related but distractor-unspecific regions. (C) Some wider brain regions were commonly suppressed for combinations of distractor types. Overlapping areas associated with conceptual priming were found for facilitatory distractors (inferior frontal gyri), and areas related to phonetic/articulatory processing (precentral gyri and left parietal operculum/insula) for distractors sharing feature overlap. Each distractor with semantic relatedness revealed nonoverlapping suppressions in lexical-phonological areas (superior temporal regions). To conclude, interference combines suppression of areas well known from neural priming and enhancement of language-related areas caused by dual activation from target and distractor. Differences between interference and priming need to be taken into account. The present interference paradigm has the potential to reveal the functioning of word-processing stages, cognitive control, and responsiveness to priming at the same time.
Keywords: Facilitation, fMRI, inhibition, naming, picture-word interference task, semantic priming, visual object priming, word processing
Introduction
In picture-word interference (PWI) paradigms, pictures of simple objects are presented along with lexical distractors, and the participants are instructed to name the pictures. Dependent on their linguistic relation to the picture, distractors may speed up (facilitation) or slow down (inhibition) naming responses (see Fig. 1). Response times (RTs) in PWI paradigms have shown to be speeded up by associatively related and phonologically related distractor words (e.g., target picture dog, distractor bone and fog, respectively) when compared to unrelated words (e.g., roof), and they have been reported to be slowed down by categorically related words (e.g., cat) (e.g., Glaser and Düngelhoff 1984; Schriefers et al. 1990; Damian and Martin 1999; Alario et al. 2000; Starreveld 2000; Jescheniak and Schriefers 2001; Abdel Rahman and Melinger 2007; Mahon et al. 2007). In the few previous functional magnetic resonance imaging (fMRI) studies on PWI, hemodynamic changes corresponding to the behavioral interference effects involved brain regions related to language processing as well as conflict processing comprising conflict/competition monitoring and cognitive control. However, the brain mechanisms underlying facilitation and inhibition in interference paradigms remain equivocal (see Fig. 1). The specific impact of facilitatory distractors on language-related brain activations was either a signal increase (Mechelli et al. 2007; Abel et al. 2009a) or a signal decrease (De Zubicaray et al. 2002; De Zubicaray and McMahon 2009). Likewise, the inhibitory distractors induced either increased language-related brain activations (Abel et al. 2009a) or decreased ones (De Zubicaray and McMahon 2009). Furthermore, increased activation in brain regions related to monitoring/control processes has been reported for facilitation (De Zubicaray et al. 2002) and inhibition (De Zubicaray et al. 2001; Abel et al. 2009a; but cf. De Zubicaray and McMahon 2009). An increase or decrease of activation was primarily determined relative to distractors without a relation to the target picture, other target-related distractors, or a lower control condition.
Figure 1.

Clarification of terms used in the present lexical interference study. The relation between behavioral interference effects, neural interference effects, and underlying cognitive mechanisms is unresolved, as indicated by question marks.
Thus, behavioral facilitation and inhibition effects may lead to the same kind of brain responses, signal increase and decrease. It remains unclear whether all distractor types are associated with suppression as well as enhancement, whether suppressed/enhanced activation patterns are characteristic for each distractor type (i.e., distractor specific), and which underlying mechanisms are responsible for the effects. Further insights into the relation between behavioral interference effects given in a certain distractor type, the neural interference effects, and the underlying cognitive mechanisms are crucial for a reasonable interpretation of respective brain imaging results (see question marks in Fig. 1).
Our previous interference fMRI experiment with auditory distractors (Abel et al. 2009a) revealed that linguistic-processing stages could be segregated by comparing increased activations of target-related distractors, while hemodynamic responses in comparison to unrelated distractors remained distractor unspecific and were therefore rather neglected (see Table 1 and Fig. S1 for previous findings). “Distractor unspecific” refers to the finding that activated areas were not restricted to one distractor type only. At the same time, activations did not overlap for all distractor types either. In the present contribution, we reconsider the contrast of related versus unrelated distractors. Thereby, we reexamine the suppression results of Abel et al. (2009a) in detail (UNREL > REL) and additionally perform secondary data analyses (REL > UNREL, conjunction analyses), in order to test hypotheses on the mechanisms underlying interference effects (see new predictions in Table 1).
Table 1.
Cognitive and neural characteristics of the four distractor conditions: recent findings and new predictions
| Segregation of word-processing stages (results of Abel et al. 2009a) | Predicted for present secondary analysis | |||
|---|---|---|---|---|
| Distractor type | Cognitive mechanism | Neural mechanism in language areas | Function of activated brain region1 | Neural mechanism related to priming |
| Phonological | Facilitation | Dual activation (enhanced, P > other distractor type) | Phonological/phonetic | Priming, incl. conflict processes (suppressed, U > P) |
| Associative | Facilitation | Dual activation (enhanced, A > related distractor type) | Vision/semantics | Priming, incl. conflict processes (suppressed, U > A) |
| Categorical | Inhibition | Dual activation (enhanced, C > related distractor type2) | Vision/lexical semantics | Priming, low for conflict processes (suppressed, U > C) 2 |
| Unrelated | Basis of comparison | Unspecific due to missing distractor/target overlap (U > related distractor) | High demands on the whole naming process, which scarcely outperforms dual activations | Basis of comparison |
Functions of the distractor-specific brain regions (see also Fig. S1) have shown to comply with assumptions about the intersecting cognitive stages.
A brain region related to conflict processes (monitoring in left anterior cingulate cortex) has already shown to be enhanced for categorical compared to phonological distractors (C > P); suppression of brain areas related to priming including conflict processing is nevertheless probable for all distractor types, even though high effort in principle may be reflected by enhancement in comparison to unrelated distractors.
Behavioral interference effects have shown to be a good means of investigating psycholinguistic stages. While the facilitatory effects have been attributed to the beneficial activation of neighboring words, the inhibitory effects have been explained by the effortful need to resolve the extra activation of competing neighbors. In the swinging lexical network model of Abdel Rahman and Melinger (2009), semantic distractors influence conceptual processing due to priming and lexical processing due to competition between lexical entries. We conclude that word priming and monitoring/control are decisive cognitive mechanisms underlying behavioral interference effects. Notably, associative facilitators may turn into inhibitors dependent on the context (Abdel Rahman and Melinger 2007; Sass et al. 2010). Contrary, categorical distractors may turn into facilitators when presented early (stimulus onset asynchrony [SOA] = –400 msec; Glaser and Düngelhoff 1984) or when subliminally processed (masked priming; Finkbeiner and Caramazza 2006). Thus, categorical distractors contain a facilitatory potential. Apart from especially strong demands on monitoring and control processes, they rely on word priming just as facilitatory distractors do (see also Abdel Rahman and Melinger 2009).
The term “priming” has been defined as an “improvement or change in the identification, production or classification of a stimulus as a result of a prior encounter with the same or a related stimulus” (Schacter et al. 2007). A priming effect usually has been associated with reduced brain responses for the primed compared to unprimed stimuli, even though priming-related response increases also have been reported (Henson 2003; for the language domain, e.g., Heim et al. 2009; Koester and Schiller 2011). The literature on neural correlates of priming effects apply the term “response enhancement” to increased and “response suppression” to reduced hemodynamic responses (e.g., Henson 2003; Vuilleumier et al. 2005; Raposo et al. 2006; Kuperberg et al. 2008; Sass et al. 2009; Sachs et al. 2011). Generally speaking, suppression is attributed to the faster or more efficient processing of primed stimuli (see Grill–Spector et al. 2006, for neural models of suppression). On the contrary, any effortful and attention-related processing as well as the forward spread of activation itself have been related to enhancement (Henson 2003; Marinkovic et al. 2003; Abel et al. 2009a). Since the behavioral interference effects have been linked to priming, we adopt the notions of enhanced/suppressed brain responses. However, it is an unresolved question whether the neural patterns of picture naming with interference match those of neural priming in the visual/linguistic domain.
The locus of priming effects in the brain has been shown to depend on the stimuli used and the tasks performed on these stimuli. In the following, we focus on suppression effects of priming studies that are associated with more effective processing. If the task performed on prime and target requires semantic processing (conceptual priming), suppression is usually found in left inferior frontal gyrus (IFG) associated with semantic memory retrieval (Kotz et al. 2002; Matsumoto et al. 2005; Raposo et al. 2006; Wible et al. 2006; Meister et al. 2007). In a transcranial magnetic stimulation (TMS) study, the left IFG has even shown to be the basis of the conceptual priming effect (Wig et al. 2005). Moreover, if the target is preceded by a semantically related stimulus (semantic priming), suppression has been reported to involve middle and/or superior temporal gyrus (STG) attributed to lexical access (Rissman et al. 2003; Giesbrecht et al. 2004; Matsumoto et al. 2005; Wible et al. 2006). Activation in medial temporal cortex also has been shown to be reduced (Rossell et al. 2003; Raposo et al. 2006). If visual objects are repeatedly presented (perceptual priming), repetition suppression is regularly observed in occipitotemporal brain regions linked to visual and conceptual processing (Simons et al. 2003; Wig et al. 2005; Horner and Henson 2008). Moreover, regions related to conflict/competition monitoring (anterior cingulate cortex [ACC]) and/or controlled processing (supplementary-motor area, SMA) were demonstrated to be involved in priming (Simons et al. 2003; Matsumoto et al. 2005; Wible et al. 2006). Activity reductions in priming paradigms were claimed to spare motor areas (Maccotta and Buckner 2004). However, premotor areas have shown to be reduced for semantic priming (e.g., Rissman et al. 2003). Thus, for priming in the visual/linguistic domain, brain areas related to language and conflict processing were found—just as would be expected for lexical interference, and here especially for facilitatory distractors. Our hypothesis A therefore states that reduced brain activations of our lexical interference fMRI-paradigm resemble previously reported patterns of neural priming. Figure 2 gives an overview of the assumptions on lexical interference, including hypothesis A.
Figure 2.

Overview of assumptions on lexical interference in our fMRI-paradigm. The figure depicts the hypotheses A–C and adds previous findings from Abel et al. (2009a) as indicated by asterisks (see also Tab. 1). Priming may occur for both facilitatory (fast naming response) and inhibitory (slow response) distractor types. Especially in brain areas related to conflict processing, enhancement may occur due to more effortful processing (instead of dual activation). REL1 > REL2, more activation for a related distractor type 1 (e.g., associative distractors) to another related distractor type 2 (e.g., phonological distractors).
However, the mechanisms underlying interference appear to be even more complex. Our lexical interference fMRI-paradigm (Abel et al. 2009a) was created to differentiate the brain regions associated with word-processing stages in the Levelt model (Indefrey and Levelt 2004). For the first time, it combined all four above-mentioned lexical distractor types. Each distractor was presented 200 msec before picture onset (SOA = –200 msec). The resulting naming RTs for each distractor type complied with previous reports, revealing specific language-related brain areas only when enhancements comparing target-related distractors were regarded. The standard procedure to investigate the facilitating and inhibiting effects of distractors, that is, the comparison of target-related distractor types (REL) to the unrelated distractor (UNREL), did not reveal brain responses specific to a distractor type. Instead, there was wide but distractor-unspecific repetition suppression (REL < UNREL). Therefore, neural priming effects expected in hypothesis A should be observable for each related condition. Moreover, given our previous conservative threshold (uncorrected voxel P = 0.001 and cluster P = 0.05, or voxel level Z > 4.65) only the phonological condition revealed repetition enhancement (REL > UNREL), namely in supramarginal gyrus (Abel et al. 2009a). We concluded that the unrelated condition places high demands on the whole naming process because there is no overlap between distractor and target that might assist the naming process (Table 1). As a consequence, a comparison to unrelated distractors could not offer a comprehensible and unambiguous localization of networks specific to word-processing stages.
Our hypothesis B therefore claims that distractor-unspecific enhancements could be found for all related distractors (REL > UNREL), when the statistical threshold was less conservative (Fig. 2). In order to comprehend the occurrence of enhancements, the peculiarities of interference need to be considered and its dissimilarities to priming highlighted. In his review on neuroimaging studies of priming, Henson (2003) concluded that enhancement occurs in regions engaged in an additional process for primed compared to unprimed stimuli, and suppression occurs in regions occupied in processes for both primed and unprimed stimuli. In interference paradigms, the pairs of distractor (prime) and target picture are compared between conditions, and therefore all conditions should require the same language processes. Nevertheless, facilitatory interference does not generally lead to suppressed language-related brain activations, just as inhibitory interference does not generally cause increased activations for monitoring/cognitive control.
Thus, there appear to be profound differences between interference (defined as an overlap in processing of prime and target) and priming (defined as beneficial preactivation of the target). In priming paradigms, the interval between prime and target usually varies from seconds to months (Tulving and Schacter 1990). However, if the prime is presented shortly before the target (like in masked priming paradigms, e.g., Rossell et al. 2003), the “event-related hemodynamic response is still an aggregate response to both the prime and target” (Henson 2003). In other words, there is repetition enhancement because the activation of the prime is added to the one of the target (Schnyer et al. 2002). In interference paradigms, the time interval (SOA) between distractor and target is per definition relatively short, which has several important consequences. First, hemodynamic responses can be specifically enhanced for linguistic stages due to the intersection of distractor and word-processing stages as mentioned above (Abel et al. 2009a). The increase of activation due to parallel processing of distractor and target was termed “dual activation” in Abel et al. (2009a). A boost of activation occurs directly at overlapping word-processing stages and indirectly at neighboring stages due to forward spreading of activation. Second, profound and potentially long-term neural changes as mechanism underlying response alterations can be presumed for priming (Henson 2003), but this explanation is implausible for interference. As shown for repeated picture naming, the strengthening of links between pictorial and lexical representation takes time to establish (at least 30s; van Turennout et al. 2000). Third, short SOAs (<250 msec) have been presumed to evoke automatic activation spreading to related representations, while greater SOAs are open to strategies (cf. Neely 1991). To sum, it remains unclear to which extent neural correlates of interference resemble neural priming effects and mirror dual activation, given the short SOAs for the former. In the present manuscript, we presume that both enhancement and suppression are intertwined in a lexical interference task.
Even though comparisons to the unrelated distractor should yield distractor-unspecific brain responses (hypotheses A and B), enhanced/suppressed brain regions may overlap for distractor types that share common characteristics-–constituting our hypothesis C (see Fig. 2). This is much more probable for suppression than for enhancement, because brain activations for related distractors barely exceeded the one for the effortful unrelated distractors, and the related distractors were highly specific (see Abel et al. 2009a). Three combinations of distractor types can be considered:
Both phonologically and associatively related distractors speed picture naming responses; thus, overlapping brain regions especially sensitive to facilitation may be observable when combining both distractor types.
Both phonologically and categorically related distractors entail features of the target picture, either parts of its sounds/phonemes or of its semantic attributes; there may be overlapping brain regions related to lexical features.
And both associatively and categorically related distractors contain semantic relationships to the target, either regarding conceptual-semantic associations or lexical-semantic neighborhoods; there may be overlapping brain areas for semantics in general.
To resume, our previous paper (Abel et al. 2009a) focused on the enhancements given in the comparisons between target-related distractors in order to separate language-processing stages. In contrast, the present work aims at a better understanding what enhanced and suppressed brain responses—featured by comparisons to unrelated distractors—represent, especially if these enhancements/suppressions are distractor unspecific and if suppression mirrors the results previously found in priming (instead of revealing deactivated language areas specific for a certain distractor type). This required reexaminations as well as secondary data analyses on the comparison of target-related distractor types to unrelated distractors in our lexical interference fMRI-paradigm. We presume (1) to find suppression at least in some brain areas predescribed for neural priming including conflict processing. This should occur for facilitatory interference, and to a lower extent also for inhibitory interference of categorical distractors due to their potential role as a prime. (2) Enhanced brain activations found at a less conservative threshold (uncorrected for multiple comparisons, P < 0.001) in language-related areas should be distractor unspecific, and (3) enhanced/suppressed brain regions (uncorrected) may overlap for linguistic distractor types (i.e., for distractors with (i) facilitatory effects (phonologically and associatively related), (ii) feature overlap (phonologically and categorically related), or (iii) semantic relationships (associatively and categorically related)).
Materials and Methods
Participants
Nineteen healthy, right-handed native German speakers with a mean age of 26 years (range 19–36) participated in the experiment. Handedness was determined according to the Edinburgh Inventory (Oldfield 1971). The four female subjects were controlled for their hormonal status. Participants provided their informed consent in accordance with procedures approved by the Freiburg University Ethics Committee.
Materials
For the picture names of 140 concrete black-and-white drawings (Snodgrass and Vanderwart 1980), 140 digitally recorded auditory distractors with speech durations between 400 and 800 msec (mean 600 msec) were created. For each of the four conditions, 35 combinations of a picture and its distractor word were constructed. Picture names and distractors were simplex German words, and each of them occurred only once to avoid repetition effects. There was no difference between pools regarding the following linguistic parameters (one-way analysis of variance [ANOVA], all Fs < 1.0, P > 0.4): Speech duration of distractors, visual complexity and familiarity of pictures (Genzel et al. 1995), as well as spoken lemma frequency (CELEX German database [On–line] 2001) and word length measured by number of phonemes and syllables for distractors and pictures. Pictures were chosen from a diversity of semantic categories and balanced as far as possible (for more details on methods, see Abel et al. 2009a).
The linguistic similarity between distractor word and target picture was varied in four experimental conditions. The distractor had a word form relation (i.e., sharing at least two onset phonemes, the syllable number, and the stress pattern) in the phonological condition (P; distractor Karte/card, target Katze/cat), an associative-semantic relation in the associative condition (A; distractor monkey, target banana), belonged to the same semantic category in the categorical condition (C; distractor lamp, target candle), or had no relation in the unrelated condition (U; distractor kiwi, target bed).
Apparatus
Auditory and visual stimuli were delivered by Presentation 10.0 (http://nbs.neurobs.com). Presentation of auditory distractors and recordings of naming responses were performed via MR-compatible sets of micro- and headphones. The headphones featured efficient gradient noise suppression (MR confon, Magdeburg, Germany; http://www.mr-confon.de). A dual-channel, noise canceling fiber optical microphone system in combination with OptiMRI noise reduction software (Optoacoustics Ltd., Or-Yehuda, Israel; http://www.optoacoustics.com) yielded digital audio files with high signal-to-noise ratio and high speech quality.
Procedure
After a 5-min training session with practice items to get used to the task, two consecutive fMRI sessions of 70 trials (300 image volumes = 11 min) were performed. Each trial started with an auditory distractor that lasted for about 600 msec (mean, range 400–800 msec). Two hundred milliseconds after distractor onset (SOA = –200 msec), a picture was presented for 6 sec, and finally a fixation cross appeared for a jittered duration (mean 3 sec, range 2–4 sec), resulting in an interstimulus interval of approximately 9.2 sec. Subjects were instructed to name each picture as fast and accurately as possible and to attend to the distractor word as it may but need not assist word finding.
RT analysis and interrater reliability
After fMRI sessions, responses were consulted for scoring of each participant's correctness of naming responses and for the analysis of RTs including visual inspection of the waveform (see Rastle and Davis 2002). Contrary to automated analyses, the manual extraction of RTs from the sound files with high signal-to-noise ratio does not depend on such variables as initial phoneme, individual participant characteristics, or breathing into the microphone (see also Discussion section). Initial onsets were adequately balanced across our conditions. In order to control for subjective variability of manual RT extraction, we examined the interrater reliability for four randomly selected subjects assessed by two speech pathologists. Interrater reliability over all conditions was high (r = 0.997, P < 0.001) with a mean difference of 11.8 msec (SE = 1.1 msec).
Image acquisition, processing, and analysis
Anatomical (MPRAGE: data matrix, 256 × 256; TR, 2.2 sec; TE, 2.6 msec; pixel size, 1 mm3) and functional images (EPI sequence: data matrix, 64 × 64; FOV, 19.2 cm; TE, 30 msec; TR, 2.19 sec) were recorded on a 3T Siemens TIM-Trio with an 8-element head coil in a circularly polarized mode. Using continuous acquisition, functional data were acquired from 36 interleaved slices with 3 mm thickness. Images were analyzed with SPM 5 (http://www.fil.ion.ucl.ac.uk/spm). Preprocessing included slice timing, coregistration and segmentation of the anatomical image, normalization using the parameters estimated during segmentation, and smoothing with a 12-mm full-width half-maximum (FWHM). Realignment parameters were only estimated because motion and distortion correction had been performed beforehand by a scanner software (see Zaitsev et al. 2004).
Trials that elicited acceptable naming responses (e.g., the distractor/picture pair Kugel/bowl and Kuchen/cake) were reclassified accordingly (e.g., naming response Torte/tart, reclassified from phonological to unrelated condition; 0.9% of all trials). A total of 4.4% of all trials were discarded because of naming errors. Picture onsets were modeled as the critical event using the canonical hemodynamic response function (HRF), and estimated realignment parameters were applied as multiple regressors in SPM 5. Statistical analyses comprised a calculation of main effects on the first and standard repeated measures ANOVAs on the second level (subtraction and conjunction analyses [conjunction null]). We intended to compare the unrelated distractor condition (UNREL) to the related linguistic distractor conditions (REL). We performed whole-brain analyses (instead of regions of interest analyses) because we wanted to examine the complete patterns of brain activations. We aimed to find (1) suppressed brain responses in the subtraction analysis UNREL > REL, (2) enhanced brain responses in the subtraction analysis of REL > UNREL, and (3) communalities between related distractors in comparison to the unrelated distractor in conjunction analyses. In order to eliminate deactivations of the subtrahend becoming significant because of the subtraction, contrasts were inclusively masked by the minuend with P = 0.05 uncorrected (e.g., Vohn et al. 2007). Activation maxima reaching an α-threshold of 0.05 corrected for multiple comparisons with the false discovery rate (FDR) method (Genovese et al. 2002) and at least 30 contiguous voxels were rendered onto the lateral and/or medial surface of a standard brain and presented in a table. An α-threshold of 0.001 (uncorrected for multiple comparisons) was considered for the subtraction analyses of related > unrelated distractors (for figure and table, ≥5 voxels) and the complex conjunction analyses (for the table, ≥5 voxels). An appropriate identification of resulting brain structures was ascertained by using WFU PickAtlas (http://www.rad.wfubmc.edu/fmri) and Talairach daemon client (http://www.talairach.org), complemented by information about the extent of activation clusters gained from MNISpace (http://www.ihb.spb.ru/~pet_lab/MSU/MSUMain.html).
Results
A full consideration of the behavioral data and the neural responses for comparisons of related distractors can be found in Abel et al. (2009a) (see also Figs. S1, 2; Table 1). Figure 3 presents repetition suppressions as given in the comparison of the unrelated distractor condition to related conditions (see also Table 2). We report peaks and extension of activations. Signal decreases for the phonological distractor condition (Fig. 3A) comprised a large cluster with peaks in left lingual gyrus (LG) (Brodmann area [BA] 18), right middle occipital gyrus, right subgyral frontal area (extending to medial frontal gyrus), as well as left SMA/ACC (BA 32). SMA activation mainly involved pre-SMA, but also extended to SMA-proper. Moreover, a peak in left parahippocampal gyrus (BA 20) was observed. All these areas were deactivated bilaterally and extended to bilateral fusiform gyrus (FG), inferior occipital gyrus, cuneus and precuneus, pre- and postcentral gyrus, thalamus, anterior insula, cerebellum, and brainstem.
Figure 3.
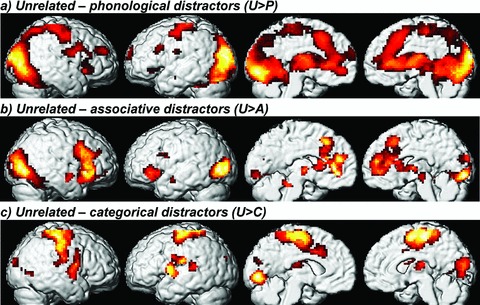
Repetition suppression: areas of significant brain activation (contrasts thresholded at false discovery rate [FDR]P < 0.05 [at least 30 voxels] and masked by the minuend at P < 0.05 uncorrected) when subtracting a related distractor condition from the unrelated distractor condition, rendered onto the lateral and medial surface of a standard brain (see also Table 2).
Table 2.
Response suppressions: decreases in brain activity for the related distractor condition compared to the unrelated condition
| Co-ordinates of maximum | |||||||
|---|---|---|---|---|---|---|---|
| Extent1 | Z-score | Cluster P (unc) | Voxel P (FDR-corrected) | x | y | z | Structure (Brodmann area) |
| Unrelated > phonological distractors (U > P, equivalent to P < U) | |||||||
| 15452 | 6.82 | <0.001 | <0.001 | –12 | –85 | –1 | Left lingual gyrus (18) |
| 6.39 | <0.001 | 36 | –78 | 12 | Right middle occipital gyrus | ||
| 4.69 | <0.001 | 24 | 29 | 1 | Right frontal (sub-gyral) | ||
| 4.60 | <0.001 | –9 | 20 | 43 | Left pre-SMA/ACC (32) | ||
| 39 | 3.49 | 0.510 | 0.003 | –39 | –15 | –17 | Left medial temporal/parahippocampal gyrus (20) |
| Unrelated > associative distractors (U > A, equivalent to A < U) | |||||||
| 1660 | 5.37 | <0.001 | <0.001 | 36 | –70 | 1 | Right inf. occipital gyrus |
| 3070 | 5.27 | <0.001 | <0.001 | 33 | 23 | –4 | Right inf. frontal gyrus (47)/insula |
| 4.36 | 0.002 | –30 | 29 | –1 | Left inf. frontal gyrus (47)/insula | ||
| 3.78 | 0.006 | 6 | 22 | 43 | Right pre-SMA/ACC (32) | ||
| 102 | 4.39 | 0.160 | 0.001 | –39 | –13 | –17 | Left medial temporal/parahippocampal gyrus (20) |
| 629 | 4.35 | 0.002 | 0.002 | –39 | –76 | 4 | Left middle occipital gyrus (19) |
| 95 | 3.72 | 0.174 | 0.006 | –36 | 1 | 22 | Left precentral gyrus |
| 95 | 3.09 | 0.174 | 0.020 | 50 | –6 | –10 | Right superior temporal gyrus |
| 37 | 3.07 | 0.393 | 0.020 | 9 | –24 | –4 | Right brainstem, midbrain |
| 40 | 3.03 | 0.374 | 0.022 | –12 | –39 | –24 | Left cerebellum (culmen) |
| Unrelated > categorical distractors (U > C, equivalent to C<U) | |||||||
| 357 | 5.30 | 0.014 | <0.001 | –9 | –82 | –1 | Left lingual gyrus (18) |
| 3916 | 4.47 | <0.001 | 0.005 | –30 | –17 | 59 | Left precentral gyrus (6) |
| 4.42 | 0.006 | 30 | –18 | 53 | Right precentral gyrus (6) | ||
| 3.45 | 0.013 | –12 | 19 | 32 | Left ACC (32) | ||
| 759 | 4.00 | 0.001 | 0.009 | –27 | –19 | 20 | Left parietal operculum/insula |
| 338 | 3.91 | 0.016 | 0.009 | 15 | –84 | 15 | Right cuneus (18) |
| 88 | 3.61 | 0.184 | 0.013 | –48 | –34 | 13 | Left post. superior temporal gyrus |
| 42 | 3.28 | 0.356 | 0.019 | –21 | –78 | 23 | Left cuneus (18) |
Areas of significant brain activations when subtracting the phonological, associative, or categorical distractor condition from the unrelated distractor condition. Contrasts were inclusively masked by the minuend with P < 0.05 uncorrected and FDR-corrected (P < 0.05, at least 30 voxels) (see also Fig. 3). Coordinates refer to the Talairach space (Talairach and Tournoux, 1998). The present table is partly similar to Table 5 of Abel et al. (2009a); it gives more activation peaks within a cluster to be able to interpret the results in more detail.
For huge clusters (>3000 voxels), maximal three of the highest peaks within an extent are shown on subsequent lines (without information about extent and cluster P) if they are more than 50 mm apart from the maximum.
ACC, anterior cingulate cortex; SMA, supplementary-motor area.
For the associative distractor condition (Fig. 3B), peaks of signal decreases were found in right-hemisphere inferior occipital gyrus, pre-SMA/ACC (BA 32), STG, and brainstem, as well as in left-hemisphere parahippocampal (BA 20) and middle occipital gyrus (BA 19), precentral gyrus, and cerebellum. Just STG was recruited unilaterally, all the other brain regions bilaterally. Activations extended to right FG and to bilateral LG, cuneus, thalamus, and medial frontal gyrus. Furthermore, there were peaks for bilateral IFG (BA 47) in transition to insulae.
Repetition suppression for the categorical distractor condition (Fig. 3C) was found in left LG (BA 18), ACC (BA 32), posterior section of STG, and parietal operculum/insula. Only the latter region was bilaterally suppressed. Moreover, activation decrease was found in precentral gyrus (BA 6) and cuneus (BA 18) bilaterally. Activations also involved bilateral middle occipital gyrus, thalamus, the middle section of STG, postcentral gyrus, and SMA (largely restricted to SMA-proper).
Figure 4 illustrates repetition enhancements realized by subtracting the unrelated condition from each distractor condition at an uncorrected threshold (see also Table 3). In the following, we only report the peaks of activation. As a result, for the phonological distractors signal increases were observed in left inferior parietal lobule (BA 40), middle frontal gyrus (BA 11), and precuneus (BA 7). Moreover, the middle temporal gyrus (MTG) (BA 21) was involved bilaterally. Increased activations for the associative condition were again found in left MTG (BA 21), as well as in inferior (BA 40) and superior (BA 7) parietal lobule. For the categorical condition, an increase of activation was found in left inferior/middle frontal gyrus (BA 11/47).
Figure 4.

Repetition enhancement: areas of significant brain activation (contrasts thresholded at uncorrected P < 0.001 [≥5 voxels] and masked by the minuend at P < 0.05 uncorrected) when subtracting the unrelated distractor condition from the phonological (A, B), associative (C), or categorical (D) distractor condition, rendered onto the lateral surface of a standard brain (see also Table 3).
Table 3.
Response enhancements: increases in brain activity for the related distractor conditions compared to the unrelated distractor condition
| Co-ordinates of maximum | ||||||
|---|---|---|---|---|---|---|
| Extent | Z-score | Voxel P FDR-corrected | x | y | z | Structure (Brodmann area) |
| Phonological > unrelated distractors (P > U) | ||||||
| 180 | 4.98 | 0.002 | –56 | –42 | 41 | Left inferior parietal lobule (40) |
| 31 | 3.68 | 0.026 | 65 | –24 | –11 | Right middle temporal gyrus (21) |
| 6 | 3.60 | 0.031 | –36 | 46 | –15 | Left middle frontal gyrus (11) |
| 26 | 3.42 | 0.050 | –56 | –26 | –6 | Left middle temporal gyrus (21) |
| 5 | 3.32 | 0.064 | –3 | –65 | 36 | Left precuneus (7) |
| Associative > unrelated distractors (A > U) | ||||||
| 29 | 3.94 | 0.489 | –62 | –41 | –5 | Left middle temporal gyrus (21) |
| 8 | 3.50 | 0.489 | –39 | –64 | 50 | Left superior parietal lobule (7) |
| 6 | 3.45 | 0.489 | –53 | –47 | 49 | Left inferior parietal lobule (40) |
| Categorical > unrelated distractors (C > U) | ||||||
| 15 | 3.74 | 1.000 | –45 | 40 | –15 | Left inferior/middle frontal gyrus (11/47) |
Areas of significant brain activation (contrasts thresholded at uncorrected P < 0.001 and masked by the minuend with P < 0.05 uncorrected, ≥5 voxels) when subtracting the unrelated distractor condition from the phonological, associative, or categorical distractor condition (see also Fig. 4). Coordinates refer to the Talairach space (Talairach and Tournoux 1998).
In order to reveal the communalities between related distractors in comparison to the unrelated distractor, we present results of the conjunction analyses in Figure 5 (Table 4). We present the peaks of activation. There was joint enhancement (14 voxels only) for both facilitatory conditions (P > U + A > U) in left inferior parietal lobule (BA 40). However, there was no common enhancement for the two conditions sharing feature overlap (P > U + C > U) or semantic relationships (A > U + C > U). Regarding communalities in repetition suppression, combining the two conditions featuring facilitation revealed a signal decrease in right inferior occipital gyrus (BA 19) and pre-SMA/ACC (BA 32). In the left hemisphere, activation in middle occipital gyrus, more anterior ACC (BA 32), and to a minor extent in parahippocampal gyrus (BA 20) were reduced. Moreover, bilateral IFG/insula were involved. For the two conditions sharing feature overlap, there was a joint decrease of activation in left LG (BA 18), parietal operculum/insula, and to a minor extent ACC (BA 32). Moreover, there were right hemisphere suppressions in cuneus (BA 18), precentral gyrus (BA 4), medial temporal/middle occipital gyrus, and to a minor extent in right thalamus and left precentral gyrus (BA 4). Finally, a minor signal decrease for the two conditions featuring semantic relationships was found in right medial temporal/middle occipital gyrus. The same small cluster was commonly suppressed for all distractor types, while there was no jointly enhanced brain region for them.
Figure 5.

Areas of significant brain activation (conjunction null, threshold at uncorrected P < 0.001, masked with first term at uncorrected P < 0.05) representing the processing of (a) facilitative distractors and (b) distractors with feature overlap, rendered onto the lateral and medial surface of a standard brain (see also Table 4).
Table 4.
Communalities between related distractors: changes in brain activity derived from conjunction analyses involving the unrelated distractor condition
| Co-ordinates of maximum | ||||||
|---|---|---|---|---|---|---|
| Extent | Z-score | Voxel P FDR-cor | x | y | z | Structure (Brodmann area) |
| Enhancement for facilitation: conjunction P > U and A > U | ||||||
| 14 | 3.45 | 0.786 | –53 | –47 | 49 | Left inferior parietal lobule (40) |
| Enhancement for feature overlap: conjunction P > U and C > U | ||||||
| No activation reaching threshold | ||||||
| Enhancement for semantic relationship: conjunction A > U and C > U | ||||||
| No activation reaching threshold | ||||||
| Suppression for facilitation: conjunction U > P and U > A | ||||||
| 894 | 5.37 | <0.001 | 36 | –70 | 1 | Right inf. occipital gyrus (19) |
| 84 | 4.37 | 0.002 | 27 | 23 | –1 | Right inf. frontal gyrus (47)/insula |
| 291 | 4.35 | 0.002 | –39 | –76 | 4 | Left middle occipital gyrus |
| 32 | 3.80 | 0.009 | –15 | 38 | 12 | Left ACC (32) |
| 42 | 3.78 | 0.009 | 6 | 22 | 43 | Right pre-SMA/ACC (32) |
| 6 | 3.49 | 0.016 | –39 | –15 | –17 | Left medial temporal/parahippocampal gyrus (20) |
| 18 | 3.36 | 0.022 | –24 | 29 | –1 | Left inf. frontal gyrus/insula |
| Suppression for feature overlap: conjunction U > P and U > C | ||||||
| 196 | 5.30 | 0.001 | –9 | –82 | –1 | Left lingual gyrus (18) |
| 54 | 3.97 | 0.026 | –27 | –19 | 20 | Left parietal operculum/insula |
| 61 | 3.91 | 0.031 | 15 | –84 | 15 | Right cuneus (18) |
| 22 | 3.66 | 0.053 | 36 | –61 | 3 | Right medial temporal/middle occip. gyrus1 |
| 110 | 3.55 | 0.062 | 39 | –20 | 56 | Right precentral gyrus (4) |
| 11 | 3.45 | 0.070 | –12 | 19 | 32 | Left ACC (32) |
| 9 | 3.42 | 0.075 | 21 | –19 | 18 | Right thalamus |
| 5 | 3.26 | 0.086 | –33 | –20 | 59 | Left precentral gyrus (4) |
| Suppression for semantic relationship: conjunction U > A and U > C | ||||||
| 11 | 3.66 | 0.425 | 36 | –61 | 3 | Right medial temporal/middle occip. gyrus1 |
Areas of significant brain activation (contrasts thresholded at uncorrected P < 0.001 and masked by the minuend with P < 0.05 uncorrected, ≥5 voxels) when performing the conjunction analyses. Coordinates refer to the Talairach space (Talairach and Tournoux 1998).
At this low threshold, the conjunction of U > P + U > C + U > A yields an activation cluster exactly at this coordinate (11 voxels, Z = 3.66, voxel P, FDR-corrected = 0.510) (cf. Abel et al. 2009a). There is no activation at this threshold for P > U + C > U + A > U.
ACC, anterior cingulate cortex; SMA, supplementary-motor area.
Figure 6 presents parameter estimates, that is, the levels of activation, for each condition in selected regions found in the conjunction and subtraction analyses. The relevant areas are left caudal ACC (x, y, z: –12, 19, 32), left rostral ACC (–15, 38, 12), left IFG (–30, 29, –1), and right IFG (33, 23, –4).
Figure 6.

Contrast estimates for selected brain regions
Discussion
We examined the mechanisms of enhancement and suppression in a lexical interference fMRI-paradigm previously used to differentiate cognitive stages of word processing in the brain (Abel et al. 2009a). We contrasted neural activations of target-related distractor types, which comprised a phonological, associative, or categorical relation to the target name, with an unrelated distractor condition. To shortly sum up findings, our prediction that neural correlates of interference resemble neural priming effects was correct (hypothesis A) (for overview, see Table 5). Each related distractor type revealed reduced brain activations (suppression) at least in areas related to vision (occipitotemporal regions) and conflict/competition monitoring (ACC), both of which have previously been shown to be implicated in neural priming. At the same time, increased activations (enhancement) of areas related to language processing were evident for each distractor type (hypothesis B). However, these enhancements were distractor unspecific at our uncorrected threshold. Finally, we found jointly suppressed and—to a lower degree—enhanced brain areas for distractor types (hypothesis C): Regarding suppression, there were communalities for (1) facilitatory distractors in areas related to vision and conflict processing (ACC/pre-SMA), complemented by areas linked to primed semantic memory retrieval (IFG) and memory processes (parahippocampal gyrus). For (2) distractors with feature overlap, areas associated with vision, monitoring (ACC), and phonetic/articulatory processing (precentral gyrus and left parietal operculum/insula) were suppressed. For (3) each distractor with semantic relatedness, nonoverlapping right or bilateral STG were suppressed. The latter may be attributed to automatic, effortless, and efficient spreading of activation to the phonological lexicon. Likewise, automatic spreading of activation to phonetic/articulatory processing may have caused the prominent suppression of bilateral sensory-motor regions for categorical distractors, which at the same time placed strong demands on semantic memory retrieval and cognitive control to inhibit the distractor. This finding offers a neural explanation for a previous cognitive account of the facilitatory potential in categorical distractors (Finkbeiner and Caramazza 2006). All of these neural components have been predescribed to be sensitive to conceptual/semantic priming. Below, we present a detailed discussion of our findings.
Table 5.
Overview of brain areas suppressed for each distractor type organized according to their presumed functions
| Perceptual/visual object priming | Conceptual/semantic priming | Conflict processing | Memory | Word production | |||
|---|---|---|---|---|---|---|---|
| Distractor | Visual (recognition, mental imagery) | Conceptual | Retrieval/encoding | Phonetic/articulatory | Errors/effort | ||
| Phonological | LG (B), IOG and MOG (B), Cuneus (B), Precuneus (B) | FG (B) | - | ACC (B), pre-SMA (B), OMPFC (L) | Medial temporal/parahippocampal (B) | Precentral (B), postcentral (B), SMA-proper (B) | Insula (B), Thalamus (B), Cerebellum (B), Brainstem (B) |
| Associative | LG (B), IOG and MOG (B), Cuneus (B) | FG (R) | STG (R), IFG (B) | ACC (B), pre-SMA (B), OMPFC (B) | Medial temporal/parahippocampal (B) | Precentral (B) | Insula (B), Thalamus (B), Cerebellum (B), Brainstem (B) |
| Categorical | LG (L), MOG (B), Cuneus (B) | - | STG (B) | ACC (L) | - | Precentral (B), postcentral (B), SMA-proper (B), Parietal oper. (B) | Insula (B), Thalamus (B) |
The involvement of brain regions was assessed according to neuro-anatomical landmarks complemented by information from MNISpace (http://www.ihb.spb.ru/~pet_lab/MSU/MSUMain.html) (see also Fig. 3).
ACC, anterior cingulate gyrus; FG, fusiform gyrus; IFG, inferior frontal gyrus; IOG, inferior occipital gyrus; LG, lingual gyrus; MOG, middle occipital gyrus; OMPFC, orbitomedial prefrontal cortex; Parietal oper., parietal operculum; SMA, supplementary-motor area; STG, superior temporal gyrus; L, left hemisphere; R, right hemisphere; B, bilateral.
Resemblance of suppression in interference tasks to priming
We aimed to examine if suppressed brain networks resembled those previously found for priming and predicted this to be true (see Table 5; Fig. 3). Indeed, each related distractor revealed reduced brain activations in priming-related brain regions, that is, in visual areas regularly observed for perceptual/visual object priming (occipitotemporal regions; Simons et al. 2003; Wig et al. 2005; Horner and Henson 2008) and in areas related to monitoring previously found to be implicated in priming (ACC; Wible et al. 2006; Matsumoto et al. 2005; Simons et al. 2003; electrophysiological findings in Hirschfeld et al. 2008). Moreover, areas linked to word production were suppressed (bilateral precentral gyrus, insula, thalamus; Indefrey and Levelt 2004). The presence of deactivation in both hemispheres despite left-hemisphere language dominance is in accordance with our previous findings on the bilateral network of picture naming (Abel et al. 2011).
The distractors varied in the extent and plenitude of suppressed brain areas over and above these general priming effects (Table 5; Fig. 3). Phonological distractors yielded the broadest repetition suppression effects (see Table 5); they additionally placed low demands on mental imagery (precuneus; Cabeza and Nyberg 2000), conceptual processing (bilateral FG; Simons et al. 2003; Vigneau et al. 2006), cognitive control (inhibition in left orbitomedial prefrontal cortex [OMPFC]: Fuster et al. 2000), controlled processing (pre-SMA: Alario et al. 2006), memory retrieval and encoding (bilateral parahippocampal gyrus; Cabeza and Nyberg 2000), and word production (bilateral postcentral gyrus, cerebellum, brainstem; Indefrey and Levelt 2004). This pattern of deactivations most closely resembles the neural responses reported for visual object priming.
For associative distractors, we observed additional reductions for conceptual processing (right FG), cognitive control (bilateral OMPFC), memory (bilateral parahippocampal gyrus), and word production (bilateral cerebellum and brainstem). Thus, neural priming (suppression) was low in brain areas related to phonetic/articulatory processing but high in areas related to conflict processes (Table 5). Moreover, the right STG and bilateral IFG (BA 47) were suppressed. Both bilateral STG and left IFG were demonstrated for semantic priming (Wible et al. 2006). The right-hemisphere homolog of left IFG also has shown to be involved in neural priming (Maccotta and Buckner 2004; Wig et al. 2005; Schacter et al. 2007). Left dominant STG has been related to lexical-phonological processing (Indefrey and Levelt 2004) and BA 47 to semantic memory retrieval (Vigneau et al. 2006).
Finally, for categorical distractors we found additional reductions in sensory-motor areas (bilateral postcentral gyrus; SMA-proper: Alario et al. 2006; insula/parietal operculum: Kurth et al. 2010; Eickhoff et al. 2006) and areas related to lexical-phonological processing (bilateral STG). Thus, the pattern of deactivations for both semantic distractors corporates perceptual and conceptual aspects of priming. At large, for categorical distractors the extent and plenitude of primed areas were lowest (see Table 5, Fig. 6). They placed high demands on conceptual processing, semantic retrieval, cognitive control, and memory processes just as unrelated distractors, and they equally recruited areas previously shown to be implicated in erroneous and effortful word production (cerebellum, brainstem; Abel et al. 2009b; Christoffels et al. 2007). Left ACC related to monitoring was primed/suppressed; nevertheless, left ACC previously has shown to be strongly engaged at least in comparison to phonological distractors (Abel et al. 2009a: voxel P uncorrected = 0.004, Z = 2.62; Talairach, x = –9, y = 38, z = 6; see also left rostral ACC in Fig. 6) (see De Zubicaray et al. 2001). The engagement of other conflict processes is similar to the one for unrelated distractors which place high demands, since only a part of left ACC is primed in comparison to the unrelated distractor (see Table 5). There was also prominent suppression of sensory-motor regions. Even though in priming paradigms activity reductions often appear to spare motor areas (Maccotta and Buckner 2004), suppression in premotor areas has been reported (e.g., Rissman et al. 2003).
(Joint) Repetition enhancement for distractor types
Moreover, we aimed to investigate if enhanced brain activations were distractor unspecific at a lowered threshold. We also performed conjunction analyses to reveal if combinations of distractor types yielded overlapping enhanced activations. As a result, there was no brain area commonly increased for all distractor types. However, each facilitatory distractor (phonologically or associatively related) enhanced activation in left MTG and inferior parietal lobule (BA 40), with the latter being jointly activated (Fig. 4). Left MTG has been associated with semantic processing (Indefrey and Levelt 2004) and previously demonstrated to be enhanced in a lexical decision task after associative primes at a relatively short SOA (–350 msec, Sass et al. 2009; see also Mechelli et al. 2007). BA 40 has been linked to the phonological store (Vigneau et al. 2006). The shared enhancement therefore may be attributed to the dual activation of lexical access, which includes semantic and phonological processing. Furthermore, each distractor type with feature overlap to the target picture (phonologically or categorically related) revealed some activation in left middle frontal gyrus (BA 11). The orbitofrontal cortex, comprising BA 11 and 47, has been linked to semantic processing (Fiez 1997). Altogether, given the shared engagement of language functions, repetition enhancements were largely distractor unspecific at our less conservative threshold. There was no area characteristic for one distractor type; distractors revealed activations in inferior parietal gyrus, MTG, and/or middle frontal gyrus instead.
Joint repetition suppression for distractor types
Finally, we used conjunction analyses to investigate joint suppressions for distractor types. As a result, only a minor cluster in right medial temporo-occipital gyrus associated with visual processing (Cabeza and Nyberg 2000) was commonly suppressed for all distractor types as derived from conjunction analysis (legend of Table 4). Of course, there is no central “priming device” for interference in the brain. Moreover, both facilitatory distractors shared areas related to vision (bilateral occipitotemporal regions), semantic memory retrieval (bilateral IFG), conflict processing (bilateral ACC, right pre-SMA), and to a minor extent memory processing (left parahippocampal gyrus) (Fig. 5). In cognitive terms, the impact of a facilitatory distractor has been attributed to the activation of a neighboring word that primes the target that is just being prepared in the naming network. While a phonologically related word exerts its priming effect through overlapping phonological features (De Zubicaray et al. 2002), an associatively related word forwards activation to all semantically connected words, among them the target name (Sass et al. 2009). We intend to discuss neural correlates of facilitation successively.
Several neuroimaging studies have identified left IFG as critical for the retrieval, selection, and identification of semantic information (Poldrack et al. 1999; Bookheimer 2002; Kotz et al. 2002; Vigneau et al. 2006). This area previously has been demonstrated to be commonly suppressed for categorical and phonological distractors compared to pure naming (De Zubicaray and McMahon 2009). A priming study demonstrated that the IFG is sensitive to the establishment of stimulus-response associations (Horner and Henson 2008). Moreover, the behavioral effect in conceptual priming has shown to be associated with repetition suppression in left IFG (Wig et al. 2005; Orfanidou et al. 2006; Schacter et al. 2007); an according association with right IFG has also been reported (Bergerbest et al. 2004). In line with these findings, the naming RTs in the present study were lowest (see Abel et al. 2009a) and IFG deactivations most prominent for the associative facilitators as also shown in parameter estimates (Fig. 6). Thus, the IFG appears to be a good indicator for a successful response to priming. Contrary, this brain area was even enhanced for categorical distractors (see Fig. 3, Fig. S1), which might mirror reduced semantic priming effects due to high semantic selection demands. For phonological distractors, the effort for semantic retrieval appears to be somewhat in-between, as middle frontal gyrus (BA 11) was even enhanced to a small extent.
Furthermore, regions related to conflict processes were prominently suppressed for facilitatory distractors (Table 5). Conflict processes can be split into distinct components. The detection of conflict/competition was located in ACC (Carter et al. 1998; Botvinick et al. 2004) and inhibitory control in OMPFC (Fuster et al. 2000; for monitoring and control in prefrontal areas, see Badre and Wagner 2004; Amodio and Frith 2006). The SMA can be divided into an anterior part responsible for higher level planning, including the selection and encoding of words to be produced (pre-SMA), and a posterior part implicated in overt articulation and motor execution (SMA-proper) (Alario et al. 2006). Pre-SMA also has shown to be engaged in error-related processing (Ullsperger and von Cramon 2004; Abel et al. 2009b), altogether indicating its role in conflict processing. Notably, in the present study SMA-proper was suppressed for the phonological but not associative distractors. Therefore, speech execution in the associative condition appears to be equally demanding as in the unrelated condition, while speech planning and conflict processes were primed. Instead of high demands (effort), dual activation may be the reason for the missing priming effect: SMA-proper (with peak in left paracentral lobule, BA 6) was bilaterally enhanced for the associative condition when compared to the categorical condition, which was attributed to high facilitatory forward activations to phonetic processing for the former (Abel et al. 2009a).
Remarkably, several regions involved in the present priming effect have previously shown to be engaged in error-related and effortful processing (e.g., Ullsperger and von Cramon 2004; Christoffels et al. 2007; Abel et al. 2009b). Abel et al. (2009b) reported that spontaneously occurring errors in overt naming yielded activations in bilateral pre-SMA/ACC, IFG/insula, prefrontal and premotor regions, OMPFC, thalamus, as well as right parahippocampal gyrus. Moreover, right STG and cerebellum were implicated. As most of these areas were also involved in correct naming, the monitoring of one's own speech was taken to be part of the naming process in general. The present study reveals that these areas were also implicated in the processing of naming when impeded by interference. Naming interfered by unrelated words strongly engaged these areas, while they were suppressed for target-related, especially facilitatory, distractors due to lower demands.
Moreover, the caudal part of ACC has been associated with controlled priming and controlled attentional processes, while the rostral part of ACC has been related to automatic priming and might reflect an automatic attentional system and monitors the automatic lexical access to semantic relations (Rossell et al. 2001). The joint suppression for facilitatory distractors in rostral ACC reveals low demands on automated processing. Priming of controlled processing in caudal ACC can be found for all three distractor types (see Fig. 6 for parameter estimates; Table 5).
Medial temporal/parahippocampal gyrus has shown to be implicated in memory retrieval and encoding (Cabeza and Nyberg 2000). This brain region formerly has been found for priming (Rossell et al. 2003; Raposo et al. 2006). Thus, repetition suppression of this area for the facilitatory distractors may be attributed to the beneficial impact of relatedness on memory processing. We may speculate that the retrieval from memory is easier for words that have been preactivated by their connection to neighboring words. Alternatively, if the learning of new associations (Horner and Henson 2008) between distractor and target picture is considered, it may be less demanding to store two semantically or phonologically related words than to store two arbitrary word combinations.
For both distractor types with feature overlap, there were commonly suppressed brain areas related to visual and conceptual processing (bilateral occipitotemporal regions), phonetic/articulatory processing (mainly left precentral gyrus, BA 4, and parietal operculum/insula), and to a minor extent monitoring (left ACC). Cognitively speaking, an overlap of features contains a facilitatory, but also a concurring, potential. The phonological distractor is not especially competitive as it does not meet the semantic properties of the target, while it primes its phonetic features, phonemes, and syllable slots. Thus, despite partly or full activation of the concurrent word form, further conflict processing is not especially important. The overlap of semantic features in the categorical distractor also primes the target. But at the same time, this distractor type covers a large portion of target semantics and thereby, its motor preparation may occur effortless and unnoticed, until its false selection is detected by monitoring processes and inhibited by cognitive control processes (see also below for a discussion on Finkbeiner and Caramazza 2006). The facilitatory aspects of feature overlap become evident by the primed visual, conceptual, and motor brain regions. For the categorical distractor type, there is prominent priming/suppression of sensory-motor areas indicating immense forward spreading, while there is relatively low priming of areas related to conflict processes and erroneous/effortful word production. Given this conclusion, the activation spreading may require low activation amplitudes if not directly affected by the activation increase caused by “dual activation.” This effortless and efficient type of spreading may conform to automatic spreading of activation as suggested by Neely (1991). This is corroborated by the restriction of significant neural priming to a rostral part of ACC for categorical distractors (see Figs. 3, 6), which can be attributed to lower demands on controlled but not on automated processing.
The right medial temporooccipital gyrus was reduced to a minor extent for both distractors sharing semantic relationships, and to the same degree for the combination of all distractors. This area has been associated with visual processing (see above). In general, an overlap of semantic networks may be difficult to observe, as meaning is more widely distributed in the brain (Wible et al. 2006). Nevertheless, for associative and categorical distractors there was nonoverlapping deactivation of the middle section of right STG. For associative distractors, middle and posterior sections of left STG were also suppressed. In turn, STG has previously been shown to receive dual activation for phonological distractors (Abel et al. 2009a; see Fig. S1). Suppression of STG due to semantic priming (Rissman et al. 2003; Matsumoto et al. 2005; Wible et al. 2006) and categorical/phonological interference (De Zubicaray and McMahon 2009), as well as a correlation between behavioral priming in a semantic task and suppression in right STG (Bergerbest et al. 2004) previously have been reported. We assume that STG deactivation may reveal efficient activation spreading from (lexical-) semantics to lexical-phonological entries. Thus, lower activation is required to access semantically related word pairs from the phonological lexicon, than there is for a pair of unrelated entries (high demands) with separate meanings.
These results are in accordance with assumptions about two divergent cognitive mechanisms in semantic interference: The spreading of activation and the selection of the target (e.g., Finkbeiner and Caramazza 2006). We conclude that the relation between cognitive and neural processing may be as follows: For associative distractors, the selection of the target (IFG deactivation in the present study) requires low effort while there is spreading of activation (to STG), leading to fast RTs in picture naming. Categorical distractors share the spreading of activation, but there are strong demands on the selection process, leading to slower RTs. Moreover, brain areas related to conflict processing are strongly involved, including portions of the ACC that has been associated with monitoring and slowing of responses (Botvinick et al. 2004). Notably, Finkbeiner and Caramazza (2006) reported that if semantically competing distractor words (primes) were masked, they turned into facilitators. In their response selection account, they concluded that individuals automatically formulate a (covert) response to the distractor, so a response selection process is required to block the false response. The mask prevents this formulation of a phonologically well-formed response and consecutively the time-consuming selection process from being engaged. Considering task demands (here: picture naming), the selection process is able to decide which answer is correct. Thus, the semantic distractor reveals its facilitatory aspect, which is caused by beneficial activation of the target's semantics. The present study reveals that this spreading of activation appears to be associated with low neural activation amplitudes if it is not directly affected by the processing stage (i.e., semantic stages for the semantic distractors) that has been boosted by dual activation. Contrary, effortful semantic retrieval requires high amplitudes, as do processes implicated in the detection and inhibition of the competitor.
Previous findings that associative words may turn into inhibitors when presented in context (Abdel Rahman and Melinger 2007; Sass et al. 2010) underline that lexical competition alone cannot explain inhibitory effects. Abdel Rahman and Melinger (2009) proposed a swinging lexical network model that explains inhibition and facilitation in both associative and categorical distractor types through variations of the opposing effects of priming at the conceptual level and competition at the lexical level. In the present manuscript, the prominent suppression of motor-sensory areas for categorical distractors speaks in favor of the response exclusion account of Finkbeiner and Caramazza (2006): The production of the already prepared distractor needs to be inhibited. The collection of further neurofunctional evidence to adjudicate on the two cognitive accounts on interference would be fruitful.
Methodological considerations
Our findings on enhanced and suppressed brain activations partly deviate from previous findings, which may be attributed to various methodological differences. (1) We integrated four different distractor types into our paradigm, which for the first time allowed precise comparisons of distractor conditions. We only varied the linguistic relation between distractor and target while keeping other factors constant (e.g., basic task difficulty, SOA). Therefore, we were able to reveal that brain areas associated with conflict processing were suppressed, which is hardly detectable using lower baselines (e.g., De Zubicaray et al. 2001). Moreover, we chose a relatively early SOA of –200 msec to gain appropriate RT effects for all distractor types. As a result, each type elicited differential RTs as predicted (with decreasing RTs, C > U > P > A; differential effects P < 0.05 without correction). Only the comparison of U > P missed significance after Bonferroni–Holm correction (Holm 1979) (P = .056). Nevertheless, neural repetition enhancement for phonological distractors was reasonable and considerable. Naming latency differences between conditions did not systematically affect hemodynamic responses. (2) Although familiarization is a standard procedure in interference paradigms, our participants were not asked to practice target picture names. Meyer and Damian (2007) investigated possible effects of practice on naming RTs. They revealed that presence or absence of familiarization did not alter behavioral interference effects. We assumed that it might nonetheless impede the investigation of priming effects in the brain, because practice/familiarization have shown to result in reduced brain activations (compared to unpracticed/unfamiliar items) (e.g., van Turennout et al. 2000; Schacter and Badgaiyan 2001). Particularly, we suspect that familiarization of picture names might impede the detection of (a) enhancement (dual activation) due to relieved demands on word production after practice, and (b) of decreases for conflict processes because at the same time the interference task itself remains unfamiliar. (3) For similar purposes, each picture/distractor pair was presented only once, and picture or distractor did not occur in any other combination. Over and above repetition effects, associative learning of each distractor/picture pair might occur as previously reported for priming (Horner and Henson 2008), and therefore an earlier presentation might interfere with processing of later combinations. (4) Contrasts were inclusively masked by the minuend with P = 0.05 uncorrected (e.g., Vohn et al. 2007) to prevent that deactivations of the subtrahend become significant because of the subtraction. Therefore, we further reduced false positives. An investigation of these and other factors that might influence neural interference effects would be beneficial.
Moreover, in fMRI studies of overt word productions, various challenges need to be addressed properly. Our results were based on most favorable equipment and analyses. Motion and distortion correction was directly performed by a scanner software (see Zaitsev et al. 2004), and estimated realignment parameters were applied as multiple regressors in SPM 5. Therefore, continuous scanning was feasible and we were able to gain large datasets in a short time. Moreover, the headphones featured efficient noise suppression to minimize interference with auditory stimulus presentation, and the sound recording system featured reductions of scanner noise and yielded sound files with high signal-to-noise ratio (see Methods section, Apparatus). Finally, we extracted RTs manually from the resulting sound files and found high interrater reliability. Automated RT extraction has shown to yield RTs similar to those extracted manually (Nelles et al. 2003), leading to the conclusion that both methods may be appropriate. We preferred the manual extraction, because automated extraction of RTs is vulnerable to missing responses (e.g., when softly spoken) and to false positives (e.g., when breathing directly into the microphone). Recent studies critically mentioned that the detection of the acoustic onset depends on the initial phoneme. “Soft” phonemes may not reach the threshold; therefore, words beginning with a soft phoneme may be recorded with a delay compared to words starting with a plosive (Rastle and Davis 2002; Nelles et al. 2003). Based on these considerations, we took care that onsets were sufficiently balanced across conditions.
Conclusion
In the present study, we investigated the mechanisms of enhancement and suppression in a lexical interference fMRI-paradigm, following up on earlier analyses (Abel et al. 2009a). We examined changes in brain responses for target-related distractor types (phonological, associative, or categorical relation) compared to an unrelated distractor condition. The signal reductions (repetition suppression) largely resembled neural priming effects. Each related distractor yielded suppressions at least in areas related to vision (temporooccipital regions) and conflict/competition monitoring (ACC). All further brain regions suppressed for distractor types have been predescribed for priming. Enhancements were found in language-related regions involving left IFG and inferior parietal lobule as well as left and/or right MTG; however, these few activations were largely distractor unspecific because the unrelated distractor already placed high demands on the complete naming process. Moreover, overlapping areas associated with conceptual priming (bilateral IFG) were involved for both facilitatory distractors. Regions related to phonetic/articulatory processing were suppressed for distractors sharing feature overlap (mainly left precentral gyrus, parietal operculum/insula). Each distractor with semantic relatedness revealed nonoverlapping suppressions in lexical-phonological areas (STG). The IFG suppression may be linked to the low demands on semantic selection for facilitation (especially for associative distractors) resulting in speeded naming responses. Automated, effortless, and efficient spreading of activation to phonetic/articulatory processing may assist word production for distractors with overlap in semantic or phonological features; at the same time, semantic feature overlap (categorical distractors) may place high demands on semantic retrieval and on conflict processes to detect and inhibit the distractor, resulting in slowed naming responses. The nonoverlapping suppression of STG for distractors with semantic relationships may be attributed to automatic activation spreading to the phonological lexicon.
Thus, interference involves enhancement of language-related areas, which can be attributed to the simultaneous processing of distractor and target, as well as suppression of areas well known from neural priming effects. In priming paradigms, enhanced activations usually are taken to represent additional processes operating on the target, and suppression occurs in areas common to primed and unprimed stimuli (Henson 2003). However, for paradigms with short SOAs (masked priming, interference), enhancement can occur due to dual activation by prime/distractor and target picture in areas responsible for prime/distractor and target, that is, in the naming network (see also Abel et al. 2009a). Our lexical interference fMRI-paradigm has the prominent advantage to engage both inhibitory and facilitatory distractors and to present enhanced and suppressed brain regions at the same time.
Future investigations to tear apart the enhanced and suppressed components would be of benefit. In the present study, dual activation might have offset possible priming effects in language-related brain areas. Thus, further enhanced language-related brain regions sensitive to priming might have remained undetected. For example, left MTG was enhanced due to dual activation for the associative and the phonological distractor type; nevertheless, this area has previously been shown to be implicated in semantic priming effects (Giesbrecht et al. 2004; Wible et al. 2006).
The lexical fMRI interference paradigm at the same time enables an assessment of neural correlates of word-processing stages and executive processes (see Fig. 1). In healthy subjects, the separation of word-processing components in the brain may be performed in a comparison of specific linguistic distractors through an analysis of enhanced brain activations (dual activations). The neural correlates of conflict processes (including the detection and inhibition of the target) and priming effects may be determined in the comparison of the unrelated distractor to each related distractor type (repetition suppression). The short-time fMRI-paradigm has been applied successfully to three subjects with aphasic disorders of word processing (Dressel et al. 2011). Behaviorally, the procedure revealed their responsiveness to primed lexical access and their ability to inhibit distracting words. Anatomically, the functioning of lexical access stages, the performance of conflict processes, and the sensitivity to priming was determined in the brain.
Acknowledgments
The project was supported by the German Research Foundation (DFG). We thank Klaus Willmes for his advice on the manuscript revision, and the three reviewers for their many helpful comments.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Figure S1. Areas of significant brain activation when subtracting the related distractor conditions from the phonological (A), associative (B), or categorical (C) distractor condition, rendered onto the lateral and medial surface of a standard brain.
Please note: Wiley-Blackwell is not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Abdel Rahman R, Melinger A. When bees hamper the production of honey: lexical interference from associates in speech production. J. Exp. Psychol. Learn. Mem. Cogn. 2007;33:604–614. doi: 10.1037/0278-7393.33.3.604. [DOI] [PubMed] [Google Scholar]
- Abdel Rahman R, Melinger A. Semantic context effects in language production: a swinging lexical network proposal and a review. Lang. Cogn. Process. 2009;24:713–734. [Google Scholar]
- Abel S, Dressel K, Bitzer R, Kümmerer D, Mader I, Weiller C, Huber W. The separation of processing stages in a lexical interference fMRI-paradigm. NeuroImage. 2009a;44:1113–1124. doi: 10.1016/j.neuroimage.2008.10.018. [DOI] [PubMed] [Google Scholar]
- Abel S, Dressel K, Kümmerer D, Saur D, Mader I, Weiller C, Huber W. Correct and erroneous picture naming responses in healthy subjects. Neurosci. Lett. 2009b;463:167–171. doi: 10.1016/j.neulet.2009.07.077. [DOI] [PubMed] [Google Scholar]
- Abel S, Huber W, Weiller C, Amunts K, Eickhoff S, Heim S. The influence of handedness on hemispheric interaction during word production: insights from effective connectivity. Brain Connect. 2011;1:219–231. doi: 10.1089/brain.2011.0024. [DOI] [PubMed] [Google Scholar]
- Alario FX, Segui J, Ferrand L. Semantic and associative priming in picture naming. Q. J. Exp. Psychol. 2000;53A:741–764. doi: 10.1080/713755907. [DOI] [PubMed] [Google Scholar]
- Alario FX, Chainay H, Lehericy S, Cohen L. The role of the supplementary motor area (SMA) in word production. Brain Res. 2006;1076:129–143. doi: 10.1016/j.brainres.2005.11.104. [DOI] [PubMed] [Google Scholar]
- Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nat. Rev. Neurosci. 2006;7:268–277. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Badre D, Wagner AD. Selection, integration, and conflict monitoring; assessing the nature and generality of prefrontal cognitive control mechanisms. Neuron. 2004;41:473–487. doi: 10.1016/s0896-6273(03)00851-1. [DOI] [PubMed] [Google Scholar]
- Bergerbest D, Ghahremani DG, Gabrieli JD. Neural correlates of auditory repetition priming: reduced fMRI activation in the auditory cortex. J. Cogn. Neurosci. 2004;16:966–977. doi: 10.1162/0898929041502760. [DOI] [PubMed] [Google Scholar]
- Bookheimer S. Functional MRI of language: new approaches to understanding the cortical organization of semantic processing. Annu. Rev. Neurosci. 2002;25:151–188. doi: 10.1146/annurev.neuro.25.112701.142946. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Cohen J, Carter CS. Conflict monitoring and anterior cingulate cortex: an update. Trends Cogn. Sci. 2004;8:539–546. doi: 10.1016/j.tics.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Nyberg L. Imaging cognition II: an empirical review of 275 PET and fMRI studies. J. Cogn. Neurosci. 2000;12:1–47. doi: 10.1162/08989290051137585. [DOI] [PubMed] [Google Scholar]
- Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen J. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science. 1998;80:747–749. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- CELEX German database [On-line] 2001. Available: Nijmegen: centre for lexical information [Producer and Distributor]. Available at: http://www.ru.nl/celex. Accessed February, 2007.
- Christoffels IK, Formisano E, Schiller NO. Neural correlates of verbal feedback processing: an fMRI study employing overt speech. Hum. Brain Mapp. 2007;28:868–879. doi: 10.1002/hbm.20315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damian MF, Martin RC. Semantic and phonological codes interact in single word production. J. Exp. Psychol. Learn. Mem. Cogn. 1999;25:345–361. doi: 10.1037//0278-7393.25.2.345. [DOI] [PubMed] [Google Scholar]
- De Zubicaray GI, McMahon KL. Auditory context effects in picture naming investigated with event-related fMRI. Cogn Affect. Behav. Neurosci. 2009;9:260–269. doi: 10.3758/CABN.9.3.260. [DOI] [PubMed] [Google Scholar]
- De Zubicaray GI, Wilson SJ, McMahon KL, Muthiah S. The semantic interference effect in the picture-word paradigm: an event-related fMRI study employing overt responses. Hum. Brain Mapp. 2001;14:218–227. doi: 10.1002/hbm.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Zubicaray GI, McMahon KL, Eastburn MM, Wilson SJ. Orthographic/phonological facilitation of overt responses in the picture-word task: an event-related fMRI study using overt vocal responding. NeuroImage. 2002;16:1084–1093. doi: 10.1006/nimg.2002.1135. [DOI] [PubMed] [Google Scholar]
- Dressel K, Weiller C, Huber W, Abel S. Impaired word retrieval in a cognitive model and in the brain. A therapy study including three single cases. (Gestörter Wortabruf im Modell und im Gehirn. Eine Therapiestudie mit drei Einzelfällen) Sprache, Stimme, Gehör. 2011;35:19–25. [Google Scholar]
- Eickhoff SB, Amunts K, Mohlberg H, Zilles K. The human parietal operculum. II. Stereotaxic maps and correlation with functional imaging results. Cereb. Cortex. 2006;16:268–279. doi: 10.1093/cercor/bhi106. [DOI] [PubMed] [Google Scholar]
- Fiez JA. Phonology, semantics, and the role of the left inferior prefrontal cortex. Hum. Brain Mapp. 1997;5:79–83. [PubMed] [Google Scholar]
- Finkbeiner M, Caramazza A. Now you see it, now you don't: on turning semantic interference into facilitation in a Stroop-like task. Cortex. 2006;42:790–796. doi: 10.1016/s0010-9452(08)70419-2. [DOI] [PubMed] [Google Scholar]
- Fuster JM, Van Hoesen GW, Morecraft RJ, Semendeferi K. Executive systems. In: Fogel BS, Schiffer RB, Rao SM, editors. Synapsis of neuropsychiatry. Philadelphia: Lippinicott, Williams and Wilkins; 2000. pp. 229–242. [Google Scholar]
- Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. NeuroImage. 2002;15:870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- Genzel S, Kerkhoff G, Scheffter S. PC-gestützte Standardisierung des Bildmaterials von Snodgrass & Vanderwart (1980). I. Deutschsprachige Normierung. Neurolinguistik. 1995;9:41–53. [Google Scholar]
- Giesbrecht B, Camblin CC, Swaab TY. Separable effects of semantic priming and imageability on word processing in human cortex. Cereb. Cortex. 2004;14:521–529. doi: 10.1093/cercor/bhh014. [DOI] [PubMed] [Google Scholar]
- Glaser WR, Düngelhoff FJ. The time course of picture-word interference. J. Exp. Psychol. Hum. Percept. Perform. 1984;10:640–654. doi: 10.1037//0096-1523.10.5.640. [DOI] [PubMed] [Google Scholar]
- Grill-Spector K, Henson R, Martin A. Repetition and the brain: neural models of stimulus-specific effects. Trends Cogn. Sci. 2006;10:14–23. doi: 10.1016/j.tics.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Heim S, Eickhoff SB, Friederici AD, Amunts K. Left cytoarchitectonic area 44 supports selection in the mental lexicon during language production. Brain Struct. Funct. 2009;213:441–456. doi: 10.1007/s00429-009-0213-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson RNA. Neuroimaging studies of priming. Progress Neurobiol. 2003;70:53–81. doi: 10.1016/s0301-0082(03)00086-8. [DOI] [PubMed] [Google Scholar]
- Hirschfeld G, Jansma B, Bolte J, Zwitserlood P. Interference and facilitation in overt speech production investigated with event-related potentials. Neuroreport. 2008;19:1227–1230. doi: 10.1097/WNR.0b013e328309ecd1. [DOI] [PubMed] [Google Scholar]
- Holm S. A simple sequentially rejective multiple test procedure. Scand. J. Stat. 1979;6:65–70. [Google Scholar]
- Horner AJ, Henson RN. Priming, response learning and repetition suppression. Neuropsychologia. 2008;46:1979–1991. doi: 10.1016/j.neuropsychologia.2008.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indefrey P, Levelt WJM. The spatial and temporal signatures of word production components. Cognition. 2004;92:101–144. doi: 10.1016/j.cognition.2002.06.001. [DOI] [PubMed] [Google Scholar]
- Jescheniak JD, Schriefers H. Priming effects from phonologically related distractors in picture-word interference. Q. J. Exp. Psychol. 2001;54:371–382. doi: 10.1080/713755981. [DOI] [PubMed] [Google Scholar]
- Koester D, Schiller NO. The functional neuroanatomy of morphology in language production. NeuroImage. 2011;55:732–741. doi: 10.1016/j.neuroimage.2010.11.044. [DOI] [PubMed] [Google Scholar]
- Kotz SA, Cappa SF, von Cramon DY, Friederici AD. Modulation of the lexical-semantic network by auditory semantic priming: an event-related functional MRI study. NeuroImage. 2002;17:1761–1772. doi: 10.1006/nimg.2002.1316. [DOI] [PubMed] [Google Scholar]
- Kuperberg GR, Lakshmanan BM, Greve DN, West WC. Task and semantic relationship influence both the polarity and localization of hemodynamic modulation during lexico-semantic processing. Hum. Brain Mapp. 2008;29:544–561. doi: 10.1002/hbm.20419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurth F, Zilles K, Fox PT, Laird AR, Eickhoff SB. A link between the systems: functional differentiation and integration within the human insula revealed by meta-analysis. Brain Struct. Funct. 2010;214:519–534. doi: 10.1007/s00429-010-0255-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccotta L, Buckner RL. Evidence for neural effects of repetition that directly correlate with behavioral priming. J.Cogn. Neurosci. 2004;16:1625–1632. doi: 10.1162/0898929042568451. [DOI] [PubMed] [Google Scholar]
- Mahon BZ, Costa A, Peterson R, Vargas KA, Caramazza A. Lexical selection is not by competition: a reinterpretation of semantic interference and facilitation effects in the picture-word interference paradigm. J. Exp. Psychol. Learn. Mem. Cogn. 2007;33:503–535. doi: 10.1037/0278-7393.33.3.503. [DOI] [PubMed] [Google Scholar]
- Marinkovic K, Dhond RP, Dale AM, Glessner M, Carr V, Halgren E. Spatiotemporal dynamics of modality-specific and supramodal word processing. Neuron. 2003;38:487–497. doi: 10.1016/s0896-6273(03)00197-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto A, Iidaka T, Haneda K, Okada T, Sadato N. Linking semantic priming effects in functional MRI and event-related potentials. NeuroImage. 2005;24:624–634. doi: 10.1016/j.neuroimage.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Mechelli A, Josephs O, Lambon Ralph MA, McClelland JL, Price CJ. Dissociating stimulus-driven semantic and phonological effect during reading and naming. Hum. Brain Mapp. 2007;28:205–217. doi: 10.1002/hbm.20272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister IG, Buelte D, Sparing R, Boroojerdi B. A repetition suppression effect lasting several days within the semantic network. Exp. Brain Res. 2007;183:371–376. doi: 10.1007/s00221-007-1051-8. [DOI] [PubMed] [Google Scholar]
- Meyer AS, Damian MF. Activation of distractor names in the picture-picture interference paradigm. Mem. Cogn. 2007;35:494–503. doi: 10.3758/bf03193289. [DOI] [PubMed] [Google Scholar]
- Neely JH. Semantic priming effects in visual word recognition: a selective review of current findings and theories. In: Besner D, editor. Basic processes in reading. Hillsdale, NJ: Erlbaum; 1991. pp. 264–337. [Google Scholar]
- Nelles JL, Lugar HM, Coalson RS, Miezin FM, Petersen SE, Schlaggar BL. Automated method for extracting response latencies of subject vocalizations in event-related fMRI experiments. NeuroImage. 2003;20:1865–1871. doi: 10.1016/j.neuroimage.2003.07.020. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness. The Edinburgh Inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Orfanidou E, Marslen-Wilson WD, Davis MH. Neural response suppression predicts repetition priming of spoken words and pseudowords. J. Cogn. Neurosci. 2006;18:1237–1252. doi: 10.1162/jocn.2006.18.8.1237. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Wagner AD, Prull MW, Desmond JE, Glover GH, Gabrieli JD. Functional specialization for semantic and phonological processing in the left inferior prefrontal cortex. NeuroImage. 1999;10:15–35. doi: 10.1006/nimg.1999.0441. [DOI] [PubMed] [Google Scholar]
- Raposo A, Moss HE, Stamatakis EA, Tyler LK. Repetition suppression and semantic enhancement: an investigation of the neural correlates of priming. Neuropsychologia. 2006;44:2284–2295. doi: 10.1016/j.neuropsychologia.2006.05.017. [DOI] [PubMed] [Google Scholar]
- Rastle K, Davis MH. On the complexities of measuring naming. J. Exp. Psychol. Hum. Percept. Perform. 2002;28:307–314. doi: 10.1037//0096-1523.28.2.307. [DOI] [PubMed] [Google Scholar]
- Rissman J, Eliassen JC, Blumstein SE. An event-related FMRI investigation of implicit semantic priming. J. Cogn. Neurosci. 2003;15:1160–1175. doi: 10.1162/089892903322598120. [DOI] [PubMed] [Google Scholar]
- Rossell SL, Price CJ, Nobre AC. The anatomy and time course of semantic priming investigated by fMRI and ERPs. Neuropsychologia. 2003;41:550–564. doi: 10.1016/s0028-3932(02)00181-1. [DOI] [PubMed] [Google Scholar]
- Sachs O, Weis S, Zellagui N, Sass K, Huber W, Zvyagintsev M, Mathiak K, Kircher T. How different types of conceptual relations modulate brain activation during semantic priming. J. Cogn. Neurosci. 2011;23:1263–1273. doi: 10.1162/jocn.2010.21483. [DOI] [PubMed] [Google Scholar]
- Sass K, Krach S, Sachs O, Kircher T. Lion-tiger – stripes: neural correlates of indirect semantic priming across processing modalities. NeuroImage. 2009;45:224–236. doi: 10.1016/j.neuroimage.2008.10.014. [DOI] [PubMed] [Google Scholar]
- Sass K, Heim S, Sachs O, Theede K, Muehlhaus J, Krach S, Kircher T. Why the leash constrains the dog: the impact of semantic associations on sentence production. Acta Neurobiol. Exp.(Wars.) 2010;70:435–453. doi: 10.55782/ane-2010-1815. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Badgaiyan D. Neuroimaging of priming: new perspectives on implicit and explicit memory. Curr. Dir. Psychol. Sci. 2001;10:1–4. [Google Scholar]
- Schacter DL, Wig GS, Stevens WD. Reductions in cortical activity during priming. Curr. Opin. Neurobiol. 2007;17:171–176. doi: 10.1016/j.conb.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Schnyer DM, Ryan L, Trouard T, Forster K. Masked word repetition results in increased fMRI signal: a framework for understanding signal changes in priming. Neuroreport. 2002;13:281–284. doi: 10.1097/00001756-200203040-00007. [DOI] [PubMed] [Google Scholar]
- Schriefers H, Meyer AS, Levelt WJM. Exploring the time course of lexical access in language production: picture-word interference studies. J. Mem. Lang. 1990;29:86–102. [Google Scholar]
- Simons JS, Koutstaal W, Prince S, Wagner AD, Schacter DL. Neural mechanisms of visual object priming: evidence for perceptual and semantic distinctions in fusiform cortex. NeuroImage. 2003;19:613–626. doi: 10.1016/s1053-8119(03)00096-x. [DOI] [PubMed] [Google Scholar]
- Snodgrass JG, Vanderwart M. A standardized set of 260 pictures: norms for name agreement, image agreement, familiarity, and visual complexity. J. Exp. Psychol. Hum. Learn. Mem. 1980;6:174–215. doi: 10.1037//0278-7393.6.2.174. [DOI] [PubMed] [Google Scholar]
- Starreveld PA. On the interpretation of onsets of auditory context effects in word production. J. Mem. Lang. 2000;42:497–525. [Google Scholar]
- Tulving E, Schacter DL. Priming and human-memory systems. Science. 1990;247:301–306. doi: 10.1126/science.2296719. [DOI] [PubMed] [Google Scholar]
- Ullsperger M, von Cramon DY. Neuroimaging of performance monitoring: error detection and beyond. Cortex. 2004;40:593–604. doi: 10.1016/s0010-9452(08)70155-2. [DOI] [PubMed] [Google Scholar]
- van Turennout M, Ellmore T, Martin A. Long-lasting cortical plasticity in the object naming system. Nat. Neurosci. 2000;3:1329–1334. doi: 10.1038/81873. [DOI] [PubMed] [Google Scholar]
- Vigneau M, Beaucousin V, Hervé PY, Duffau H, Crivello F, Houdé O, Mazoyer B, Tsourio-Mazoyer N. Meta-analyzing left hemisphere language areas: phonology, semantics, and sentence processing. NeuroImage. 2006;30:1414–1432. doi: 10.1016/j.neuroimage.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Vohn R, Fimm B, Weber J, Schnitker R, Thron A, Spijkers W, Willmes K, Sturm W. Management of attentional resources in within-modal and cross-modal divided attention tasks: an fMRI study. Hum. Brain Mapp. 2007;28:1267–1275. doi: 10.1002/hbm.20350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuilleumier P, Schwartz S, Duhoux S, Dolan RJ, Driver J. Selective attention modulates neural substrates of repetition priming and “implicit” visual memory: suppressions and enhancements revealed by FMRI. J.Cogn. Neurosci. 2005;17:1245–1260. doi: 10.1162/0898929055002409. [DOI] [PubMed] [Google Scholar]
- Wible CG, Han SD, Spencer MH, Kubicki M, Niznikiewicz MH, Jolesz FA, Nestor P, McCarley RW. Connectivity among semantic associates: an fMRI study of semantic priming. Brain Lang. 2006;97:294–305. doi: 10.1016/j.bandl.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Wig GS, Grafton ST, Demos KE, Kelley WM. Reductions in neural activity underly behavioral components of repetition priming. Nat. Neurosci. 2005;8:1228–1233. doi: 10.1038/nn1515. [DOI] [PubMed] [Google Scholar]
- Zaitsev M, Hennig J, Speck O. Point spread function mapping with parallel imaging techniques and high acceleration factors: fast, robust and flexible method for echo planar imaging distortion correction. MRM. 2004;52:1156–1166. doi: 10.1002/mrm.20261. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotactic atlas of the human brain. Stuttgart: Thieme; 1998. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


