Abstract
Alzheimer's disease (AD), the most common form of dementia, is an age-dependent progressive neurodegenerative disorder. β-amyloid, a metabolic product of the amyloid precursor protein (APP), plays an important role in the pathogenesis of AD. The Thy1-hAPPLond/Swe+ (line 41) transgenic mouse overexpresses human APP751 and contains the London (V717I) and Swedish (K670M/N671L) mutations. Here, we used a battery of behavioral tests to evaluate general activity, cognition, and social behavior in six-month-old male Thy1-hAPPLond/Swe+ mice. We found hyperactivity in a novel environment as well as significant deficits in spontaneous alternation behavior. In fear conditioning (FC), Thy1-hAPPLond/Swe+ mice did not display deficits in acquisition or in memory retrieval in novel context of tone-cued FC, but they showed significant memory retrieval impairment during contextual testing in an identical environment. Surprisingly, in a standard hidden platform water maze, no significant deficit was detected in mutant mice. However, a delayed-matching-to-place paradigm revealed a significant deficit in Thy1-hAPPLond/Swe+ mice. Lastly, in the social novelty session of a three-chamber test, Thy1-hAPPLond/Swe+ mice exhibited a significantly decreased interest in a novel versus a familiar stranger compared to control mice. This could possibly be explained by decreased social memory or discrimination and may parallel disturbances in social functioning in human AD patients. In conclusion, the Thy1-hAPPLond/Swe+ mouse model of AD displayed a behavioral phenotype that resembles, in part, the cognitive and psychiatric symptoms experienced in AD patients.
Keywords: Alzheimer's disease, amyloid precursor protein, behavior, learning and memory, neurodegenerative disorder, social interaction
Introduction
Alzheimer's disease (AD) is the most common form of age-related dementia. The etiology of AD is still elusive, but a neuropathological hallmark is the accumulation of misfolded β-amyloid in extracellular plaques. In the “amyloid hypothesis,” an abnormal production of β-amyloid is the initial step leading to a pathophysiological cascade including hyperphosphorlylated tau protein in intracellular tangles, vascular damage, and inflammation. β-amyloid is cleaved from amyloid precursor protein (APP). The gene coding for APP is located on chromosome 21. Support for a connection between APP and AD comes from the observation that individuals with trisomy 21 show high levels of β-amyloid, early plaque formation, and early-onset AD. The importance of APP in the pathogenesis of AD is further supported by the strong relationship between familial early-onset AD and mutations in the APP gene (Bertram et al. 2010). Experimental models were developed in Caenorhabditis elegans, Drosophila, mice, and the (aged) monkey, with the latter being the most complete the most expensive (Kobayashi and Chen 2005; Rockenstein et al. 2007a). Murine models are most commonly used and often inherit a targeted overexpression of single or multiple mutant molecules associated with familial AD. These models form plaques after several months with some taking 12 months until formation starts (Rockenstein et al. 2001). We chose the Thy1-APPLond/Swe+ mouse that was shown to have an accelerated pathology with a rapid appearance of mature β-amyloid plaques in the frontal cortex as early as three months of age and in the hippocampus, thalamus, and olfactory region with five to seven months (Rockenstein et al. 2001). In addition, synaptic degeneration is apparent in the frontal cortex beginning three to four months of age and in the hippocampus starting five to seven months of age. This transgenic mouse expresses human APP751 cDNA containing the London (V717I) and Swedish (K670M/N671L) mutations under the regulatory control of the murine Thy1 gene. The different mouse models were shown to have a variety of behavioral deficits, including impairments in learning and memory in some tasks (Kobayashi and Chen 2005; Rockenstein et al. 2007b; Havas et al. 2011). In this study, we aimed to identify a range of behavioral paradigms to detect important aspects of the behavioral phenotype of Thy1-APPLond/Swe+ mice. These paradigms could then be used to test hypotheses on the pathophysiology of AD and to screen for drugs to treat the condition. We focused on the cardinal trait of altered cognition but also covered social behavior, an area that has not been previously explored in these mice as well as sensorimotor function.
Materials and Methods
Animals
Five- to six-month-old male Thy1-hAPPLond/Swe+ mice and their wild-type littermates were used in this study. Transgenic lines were maintained by crossing heterozygous Thy1-hAPPLond/Swe+ mice with C57BL/6J breeders. Littermate cage-mates were used as control mice. Five cohorts of mice with an n between nine and 19 were used. The total number of mice used was 68 controls and 65 mutants. The genotype of all animals was determined by PCR before experiments. All transgenic mice were heterozygous with respect to the hAPPLond/Swe gene. Twelve-week-old male C57BL/6J mice (from Jackson Laboratory, Bar Harbor, ME) were used for the validation of the delayed-matching-to-place (DMP) dry maze task. All animals were housed in a 12-h dark/light cycle, temperature- and humidity-controlled environment with unlimited access to water and food. The same group of animals was tested in the activity chamber, open field, and fear conditioning (FC). Different groups of mice were used for the social interaction tests, Morris water maze (MWM), DMP water maze, DMP dry maze, and hot plate test. Experimenters were blind to the genotype of the mice throughout testing. All tests were conducted in the light cycle. In all experiments, animals were habituated to the testing room 2 h before the tests and were handled by the experimenter for five days before all the behavioral tests. All experiments were in accordance with protocols approved by the Institutional Animal Care and Use Committee of Stanford University and were performed based on the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All actions were considered for reducing discomfort of animals during all experiments.
Behavioral Tests
Activity chamber
An activity chamber (Med Associates Inc., St. Albans, VT) was used to evaluate general locomotor activity and exploratory behavior in a novel environment. It consisted of a square arena (43 × 43 cm2) located in a sound-attenuated chamber (66 × 55.9 × 55.9 cm3). Mice were placed in the center of the arena and tracked by an automated tracking system with three planes of infrared detectors during a 10-min trial. Before each trial, the surface of the arena was cleaned with 10% ethanol. For analysis, the arena was divided into a central (30.4 × 30.4 cm2) and a peripheral zone. Distance moved, velocity, and rearing activity were measured and n = 12 for control mice and n = 11 for Thy1-hAPPLond/Swe+ mice.
Open-field activity
The open-field test was used to assess locomotor activity and exploration habits in a relatively large novel environment. Assessment took place in a square arena (76 × 76 cm2) with opaque white walls, surrounded with privacy blinds to eliminate external room cues. Mice were placed in the center of the open-field arena and allowed to freely move for 10 min while being tracked by Ethovision (Noldus Information Technology, Wageningen, the Netherlands) automated tracking system. Before each trial, the surface of the arena was cleaned with 10% ethanol. For analysis, the arena was divided into a central (53.5 × 53.5 cm2) and a peripheral zone (11.25-cm wide). Distance moved, velocity, and time spent in each predefined zone were recorded and n = 12 for control mice and n = 11 for Thy1-hAPPLond/Swe+ mice.
Three-Chamber sociability and social novelty test
The three-chamber test (Nadler et al. 2004) was used to assess sociability and interest in social novelty or social discrimination. The testing arena consisted of three adjacent chambers (each 20 cm length × 40.5 cm width × 22 cm height) separated by two clear plastic dividers and connected by open doorways (10.2 cm width × 5.4 cm height). The test consisted of three 10-min sessions without intertrial intervals (ITIs). In the first session, subject mice were allowed to habituate to the arena and freely investigate the three chambers. In the subsequent sociability session, a never-before-met C57BL/6J male mouse (stranger 1) was placed under an inverted stainless steel pencil cup (11 cm height × 10 cm diameter solid bottom; with stainless steel bars spaced 1 cm apart) in one of the side chambers and another identical inverted empty cup was placed in the other side chamber. The position of the object mouse was altered between left and right chambers between subjects testing. In the social novelty session, the empty pencil cup was removed and replaced by stranger 1 in a new pencil cup. A second never-before-met C57BL/6J male mouse (stranger 2) was placed at the previous position of stranger 1 under a new pencil cup. A total of 20 C57BL/6J male object mice were used, age four month, housed under similar conditions as subject mice but held in a different rack. Each mouse was used just once a day. The subject mouse was restrained in the middle chamber during the introduction of object mice. The box and pencil cups were cleaned with 10% ethanol between animals and before the first animal. Time spent sniffing each cup was scored by a blinded investigator using video recordings and n = 11 for control mice and n = 9 for Thy1-hAPPLond/Swe+ mice.
Social memory test
Before the test, singly housed subject mice were habituated to never-before-met ovariectomized (OEF) female mice (C57BL/6J) in their home-cages for five consecutive days for a total of 120 h, OEFs were changed every 24 h. A total of 40 four-month-old OEFs were purchased from The Jackson Laboratory (ovariectomy performed by The Jackson Laboratory). The OEFs were housed under similar conditions as subject mice but in different racks. For testing, subject mice encountered OEFs in a total of six meetings, OEFs were introduced by an experimenter and testing took place in the home-cage. Each meeting lasted 1 min with ITIs of 10 min. In meetings 1–4, subject mice were exposed to the same, never-before-met OEF (SAME OEF). In the fifth meeting, subject mice were exposed to a novel, never-before-met OEF (NOVEL OEF). In the sixth and final meeting, subject mice were reexposed to the first (SAME) OEF. Meetings were videotaped for subsequent scoring using Annotation (Saysosoft, http://www.saysosoft.com/). Investigation was defined as contact of the test animal toward the intruder and n = 14 for control mice and n = 13 for Thy1-hAPPLond/Swe+ mice.
Spontaneous alternation in the T-maze and Y-maze
The T-maze and Y-maze were used to test spontaneous alternation behavior. These tests are based on the innate interest of rodents to explore a new environment (Gerlai 1998). The T-maze consisted of one start arm and two identical goal arms (each arm 30 cm length × 10 cm width × 20 cm height) with guillotine doors. The guillotine doors were located in the middle of the start arm and in the entrance of each side arm. In each trial, after placing the mouse in the start arm, mice were allowed to enter either one of the goal arms. Subsequently, the guillotine door of the unchosen goal arm was closed. Arm entry was defined as having all four limbs inside the arm. Due to the explorative nature of rodents, mice returned to the start arm, after which the next trial began. This basic procedure was repeated 11 times per day, for three consecutive days, for a total of 33 trials. The T-maze was cleaned with 10% ethanol between animals and before the first animal to eliminate odor. Percent of alternations (alternated arm entry on two consecutive trials) was recorded for analysis. This protocol has been previously described by Belichenko et al. (2009) and was modified from a Deacon and Rawlins protocol (Deacon and Rawlins 2006; Belichenko et al. 2009).
The Y-maze was made of solid white plastic and consisted of two symmetrical arms and one longer arm at 120° angles (longer arm, 20.7 cm length × 12.7 cm height × 7.62 cm width; equal arms, 15.24 cm length × 12.7 cm height × 7.62 cm width). At the beginning of trials, mice were placed in the center of the maze and allowed to freely explore the three arms for 5 min. Arm entry was defined as having all four limbs inside an arm. The maze was cleaned with 10% ethanol between animals and before the first animal to eliminate traces of odor. The number of arm entries and the number of triads were recorded in order to calculate the alternation percentage, which was calculated by dividing the number of triads by the number of possible alternations multiplied by 100. A triad was defined as a set of consecutive arm entries (Drew et al. 1973; Hughes 2004). For both T-maze and Y-maze spontaneous alternation test, n = 12 for both control and Thy1-hAPPLond/Swe+ mice.
Morris water maze
The MWM was originally designed to test spatial reference memory in rats by observing and recording escape latency, distance moved, and velocity during the search of a hidden escape platform in a large pool (Morris 1984). For our test, we used a large water tank (178 cm in diameter) filled with water at a temperature of 22.0 ± 1.5°C with a circular platform (17 cm in diameter) placed about 1 cm below the water surface and approximately 50 cm away from the wall. Nontoxic tempera paints (Elmers, Westerville, OH) were used to make the water opaque. The water tank was completely surrounded by privacy blinds with at least four visual cues attached to the blinds. Four different shapes including a star shape, circle, rectangle, and diamond each with approximately 6 square feet in surface area were used as visual cues. The visual cues were located approximately 150 cm from the center of the tank. The water tank arena was monitored by an overhead video system that allowed Ethovision to track the mice. During hidden platform training, a platform was positioned in one quadrant of the tank. Mice were released from pseudorandomized drop locations and given 90 sec to find the platform. The distance to the platform was generally the same within a day. The trial either ended when the mice rested on the platform for 10 sec or until the trial duration expired. If mice failed to find the submerged hidden platform during that time, they were guided to it. Mice underwent four trials of training each day (30-min ITIs) for four consecutive days. Upon completion of the hidden platform training, the platform was removed and a 30-sec probe trial was conducted. Successful learning of MWM was determined by the gradual decrease in escape latency and discriminative quadrant exploration during the probe trial. For analysis, data was averaged per day. After the probe trial, mice were given visible platform training to ensure that no gross sensorimotor or visual deficits were present. During the visible platform training, the platform was marked with a black-and-white ping-pong ball attached to a 10-cm wooden stick. No mice were excluded based on our standard exclusion criteria in this task: excessive thigmotaxis, obvious visual impairment, excessive corkscrew swimming pattern, and obvious sensorimotor dysfunction. The water was frequently changed and the tank disinfected. Twelve control mice and 11 Thy1-hAPPLond/Swe+ mice were used.
DMP dry maze
The DMP water maze was originally designed to assess spatial working/episodic-like learning and memory in rats by Steele and Morris (Steele and Morris 1999). We designed a DMP dry maze test based on this DMP protocol, but excluding the water and swimming factors. The DMP dry maze is thought to measure similar learning abilities as the DMP water maze. It was conducted using a novel, modified Barnes maze (dry maze) apparatus (Barnes 1979). The apparatus consists of a 122-cm diameter circular platform with 40 escape holes, each with a diameter of 5 cm placed along three rings of varying distances from the center of the platform. The outer ring has 16 holes and 50 cm from the center, middle ring has 16 holes and 35 cm from the center, and the inner ring has eight holes and 20 cm from the center. An escape box was attached to one of these holes and all holes were left uncovered. High overhead lighting (1200 lux) and noise (2 KHz, 85 dB) were used to create aversive conditions that would encourage the mice to seek out the target hole to escape the light and noise. Visual cues were placed on all four sides of the maze. Mice were given a series of four trials with ITIs of 10 min; the maximum duration of each trial was 90 sec. For each trial, mice were placed in different locations at the edge of the maze and held under a dark cover to prevent a directional bias. After 10 sec, the cover was removed and the trial started. The distance from the releasing point and the escape box was generally the same within a day. The trial ended if a mouse found and entered the escape box before the end of the 90 sec. Mice that could not find the escape box were led to it by the experimenter and allowed to enter. As soon as the mouse entered the escape hole, the noise was turned off. After entering the box, the mouse was given 10 sec to remain in it before being returned to its home cage. The experiment was run for four consecutive days for the scopolamine experiment, and five consecutive days for the mutant mice experiment. On days 2–5, the location of the target escape hole was moved while all other parameters remained unchanged. All data was recorded using Ethovision. Parameters measured were escape latency, distance moved, and velocity. A total of 20 mice, control (n = 10) and Thy1-hAPPLond/Swe+ (n = 10), were used for this experiment. Before testing the Thy1-hAPPLond/Swe+ and their control littermates, a validation experiment was conducted using C57BL/6J mice and scopolamine. Scopolamine, a competitive antagonist for muscarinic acetylcholine receptors, specifically M1 receptors, is known to induce memory impairment. In this four-day experiment, scopolamine and vehicle (1 mg/kg ip) was injected daily 20 min prior to the first trial. A total of 20 mice, (n = 10) vehicle and (n = 10) scopolamine, were used for this experiment.
Fear conditioning
Coulbourn Instruments (Whitehall, PA) FC chambers were used for the assessment of conditional learning and memory. A trace FC protocol was used for the training day followed by tone-cued and contextual memory retrieval tests. On the first day (training day), mice were placed in the chamber for a 3-min baseline recording followed by five tone-shock pairings with ITIs of 100 sec. The shocks (0.5 mA, 2 sec) were delivered 18 sec following the tone (70 dB, 2 kHz, 20 sec). On the second day, a novel context (new olfactory environment, different shape of the chamber, new texture of the floor, blue plastic inserts for walls, extra source of blue light, and visual cues) was used for tone-cued testing. After 3 min of baseline recording, three tones without shocks with ITIs of 100 sec were presented to the mice. On the third day of the experiment, mice were placed in the same context as the first day for 5 min with no shocks or tones to test contextual memory retrieval (modified from the method described by Saxe et al. [2006]). The chambers were cleaned by 10% ethanol on days 1 and 3. On day 2, chambers were first cleaned by Alcide and then wiped with wet paper towels. Freezing was defined as the complete lack of motion for a minimum of 0.75 sec, as assessed by FreezeFrame software (Actimetrics, Evanston, IL). A total of 23 mice, control (n = 12) and Thy1-hAPPLond/Swe+ (n = 11), were used for this experiment.
Hot plate test
Each mouse was handled for 2 min and habituated to the testing environment 24 h before testing. The hot plate apparatus (IITC Inc., Woodland Hills, CA) was set to a temperature of 55 ± 0.1°C. On the testing day, mice were placed on the surface of the hot plate and covered with a transparent glass cylinder (height 25 cm, diameter 12 cm). A 30-sec cut-off time was assigned and a remote foot-switch pad was used to control the start/stop function. The latency to the first hind paw lick or jump was recorded. A total of 18 mice, control (n = 9) and Thy1-hAPPLond/Swe+ (n = 9), were used for this experiment.
Statistical analysis
All data were presented as mean ± SEM and P < 0.05 was considered statistically significant. Repeated measures two-way ANOVA was used for evaluation of the parameters in activity chamber, open field, water maze, DMP dry maze, training day of FC, and social tests. The Bonferroni test was used for post-hoc analysis. The Student's t-test was used where appropriate. In all statistical analysis, the normal distribution of the data was tested using the D’Agostino and Pearson omnibus normality test.
Results
A condensed summary of the outcomes of the behavioral experiments and the brain structures believed to be involved in each task is shown in Table 1.
Table 1.
Summary of behavioral experiments, statistical data, interpretation, and involved brain regions
 |
*Brain region, which is involved in each task, might not be limited to the reported one.
Activity chamber
Exploratory behavior in a novel environment and general locomotor activity were assessed in automated activity chambers for 10 min (Fig. 1a). Median tracks of Thy1-hAPPLond/Swe+ and control littermates are shown in Figure 1a. A minute-to-minute analysis revealed that Thy1-hAPPLond/Swe+ mice consistently moved a longer distance than their control littermates (Fig. 1b; effect of genotype, F1, 21 = 17.54, P = 0.0004; genotype × time interaction, F9, 189 = 0.93, P = 0.50). Accordingly, the total (cumulative) distance moved in the novel environment was significantly higher in Thy1-hAPPLond/Swe+ than in control mice (Fig. 1c; P = 0.0016). Both groups of mice showed higher activity in the perhipheral zone than in the central zone both in terms of the distance moved (Fig. 1d; effect of zone, F1, 21 = 59.25, P < 0.0001) and the time spent in the two zones (Fig. 1e; effect of zone, F1, 21 = 140.3, P < 0.0001). Thy1-hAPPLond/Swe+ mice tended to show more activity in the peripheral zone than the control mice; however, the genotype × zone interaction did not achieve statistical significance for either distance moved (genotype × zone interaction, F1, 21 = 2.33, P = 0.14) or time spent in zones (genotype × zone interaction: F1, 21 = 2.82, P = 0.11). Thy1-hAPPLond/Swe+ mice engaged in significantly more rearing behavior than their control littermates (Fig. 1f, 1g; effect of genotype, F1, 21 = 4.68, P = 0.042).
Figure 1.

Activity chamber. (a) Activity was monitored for 10 min in the activity chamber (upper panel). Display of tracks of median Thy1-hAPPLond/Swe+ and control mouse (lower panels). (b) Thy1-hAPPLond/Swe+ traveled a longer distance than control mice (P = 0.0004, repeated measures ANOVA). (c) Correspondingly, Thy1-hAPPLond/Swe+ traveled a longer cumulative distance than control mice (P < 0.01, t-test). (d) Both groups of mice traveled a longer distance in the periphery, but this effect did not differ significantly by genotype (genotype × zone interaction: F1, 21 = 2.33, P = 0.14). (e) Mutant mice spent proportionally more time in the periphery than the center compared to control mice, but this difference was not statistically significant different between genotypes (genotype × zone interaction: F1, 21 = 2.82, P = 0.11). (f) Mutant mice reared significantly more than control mice in a minute-to-minute comparison (P < 0.05, repeated measures ANOVA) (g) and correspondingly exhibited more total rearings (P < 0.05, t-test). n(control) = 12, n(Thy1-hAPPLond/Swe+) = 11.
Open-field activity
The open-field test was used for assessment of gross locomotor activity and exploration behavior in a relatively large novel environment as compared to the activity chamber (Fig. 2a). Thy1-hAPPLond/Swe+ mice moved a longer distance in the open field compared with control animals (Fig. 2b and 2c; effect of genotype, F1, 21 = 9.10, P = 0.007; genotype × time interaction, F9, 189 = 0.80, P = 0.61) and showed a significantly increased velocity (control: 9.26 ± 0.24 cm/s; mutant: 11.03 ± 0.35 cm/s; P = 0.006). Both genotypes moved a longer distance in the periphery zone than the center zone (Fig. 2d; effect of zone, F1, 21 = 934.6, P < 0.0001), but the effect of zone was more pronounced in the Thy1-hAPPLond/Swe+ mice (genotype × zone interaction, F1, 21 = 10.62, P = 0.004). Mice spent more time in the center zone (Fig. 2e; effect of zone, F1, 21 = 3064.92, P < 0.0001) but this effect was not different between genotypes (genotype × zone interaction, F1, 21 = 1.21, P = 0.28).
Figure 2.

Open field. (a) Activity levels were measured in a 10-min open field test (upper panel). Tracks of median Thy1-hAPPLond/Swe+ and control mice are displayed in the lower panels. (b) Thy1-hAPPLond/Swe+ mice traveled a significantly longer distance than control mice (P < 0.01, repeated-measures ANOVA) (c) and, correspondingly a longer total distance (P < 0.01, t-test). (d). Both genotypes traveled a longer distance in the periphery than the center and the effect of zone was more pronounced in mutant mice (genotype x zone interaction, P = 0.004; Bonferroni post-hoc tests: P < 0.001 and P > 0.05, respectively). (e) Similarly, mice spent significantly more time in the center zone but in this case the effect was not different between genotypes (genotype x zone interaction, P = 0.28). n(control) = 12, n(Thy1-hAPPLond/Swe+) = 11.
Social tests
Social behavior was assessed with the three-chamber and six-trial social memory tests (Fig. 3). In the three-chamber test, a subject mouse was first habituated to the test environment in a habituation session, then tested for sociability in a sociability session, and finally tested for preference for social novelty in a social novelty session (Fig. 3a). No side preference was detected during the habituation session (data not shown). During the sociability test (Fig. 3b), both Thy1-hAPPLond/Swe+ and control mice preferred to sniff at a cage containing a stranger mouse versus sniffing at an empty cage (Fig. 3b; effect of object, F1, 16 = 34.64, P < 0.0001), and this preference did not differ by genotype (genotype × object interaction, F1, 16 = 0.31, P = 0.58). Calculating a preference index (ratio of time sniffing stranger 1 vs. empty cage) showed no difference between genotypes (P = 0.1). During the subsequent social novelty test, control mice seemed to spend more time sniffing the novel stranger's cage than the now-familiar mouse's cage whereas Thy1-hAPPLond/Swe+ mice did not demonstrate such a preference (Fig. 3c). A two-way ANOVA showed a trend close to significance for the object effect (F1, 18 = 4.01, P = 0.06) and genotype × object interaction (F1, 18 = 4.20, P = 0.055). However, the preference index (ratio of time sniffing stranger 2 vs. stranger 1) revealed a significantly decreased preference of mutant mice for the novel stranger's cage (Fig. 3c; P = 0.031). Significance level was also reached when two outliers (33 for control mice and 3.5 for mutant mice) were excluded (P = 0.009). In the six-trial social memory test, we found a significant habituation to the SAME intruder (Fig. 3d; trial 1–4: effect of object, F3, 75 = 5.69, P = 0.0014) and this effect did not differ by genotype (genotype × object interaction, F3, 75 = 0.33, P = 0.81). Furthermore, we found a significant dishabituation with the presentation of a NOVEL intruder (trial 4–5: effect of object, F1, 25 = 49.73, P < 0.0001, genotype × object interaction, F1, 25 = 0.09, P = 0.77) and a significant effect of an additional presentation of the SAME intruder in trial 6 (trial 5–6: effect of object, F1, 25 = 71.75, P < 0.0001, genotype × object interaction, F1, 25 = 1.22, P = 0.28). No significant differences in genotype × object interactions were detected.
Figure 3.

Social behavior. (a) Three-chamber test. After a 10-min habituation to a three-chambered box, an empty cup and a cup containing stranger 1 were introduced in the side chambers for a 10-min sociability session. Thereafter, stranger 2 was added under the empty cup for a 10-min social novelty session. n(control) = 11, n(Thy1-hAPPLond/Swe+) = 9. (b) Sociability session. Left, Thy1-hAPPLond/Swe+ and control mice both sniffed significantly longer at the cage containing stranger 1 than at the empty cup (effect of object, P < 0.0001; genotype × object interaction, P = 0.58). Right, both genotypes showed the same preference (ratio time sniffing stranger 1 vs. empty cup) for the occupied versus the empty cup (P = 0.1, t-test). (c) Social novelty session. Left, control mice appeared to prefer stranger 2 whereas Thy1-hAPPLond/Swe+ mice did not seem to show this preference. However, both the object effect (P = 0.06) and object × genotype interaction (P = 0.055) failed to reach significance level. Right, the preference index (ratio time sniffing stranger 2 vs. stranger 1), however, revealed a significantly decreased preference of Thy1-hAPPLond/Swe+ mice for stranger 2 compared to control mice (P = 0.04, t-test). (d) Six-trial social memory test. Four repeated presentations of the SAME intruder showed a significant effect (effect of object, P = 0.0014) and this effect did not differ by genotype (genotype × object interaction, P = 0.77). Introduction of a NOVEL intruder in trial 5 followed by the SAME intruder in trial 6 revealed a significant object effect in both cases (effect of object P < 0.0001 in both cases). Both effects did not differ by genotype (genotype × object interaction, P = 0.77 and P = 0.28, respectively). n(control) = 14, n(Thy1-hAPPLond/Swe+) = 13.
Spontaneous alternation
The T-maze and Y-maze were used to assess spontaneous alternation and spatial working memory. Thy1-hAPPLond/Swe+ mice showed a deficit in spontaneous alternation both in the T-maze (Fig. 4a; P = 0.026) and the Y-maze (Fig. 4b; P = 0.04). However, no difference was revealed between genotypes in the number of entries made in the Y-maze (Fig. 4c; P = 0.95).
Figure 4.

T-maze and Y-maze. (a) T-maze: Thy1-hAPPLond/Swe+ showed significantly (P < 0.05, t-test) less spontaneous alterations than control mice. n(each genotype) = 12. (b) Y-maze: Thy1-hAPPLond/Swe+ showed significantly (P < 0.05, t-test) less spontaneous alterations than control mice. n(each genotype) = 12. (c) The number of total entries in the arms of the Y-maze was not significantly different. n(each genotype) = 12.
Morris water maze
During the training phase of the MWM, both Thy1-hAPPLond/Swe+ and control mice acquired the location of the hidden platform equally well, as indicated by a significant effect of training day on escape latency (Fig. 5a and b; effect of day, F3, 63 = 44.92, P < 0.0001; genotype × day interaction, F3, 63 = 1.21, P = 0.32) and distance moved to find the hidden platform (Fig. 5c; effect of day, F3, 63 = 26.62, P < 0.0001; genotype × day interaction, F3, 63 = 0.85, P = 0.47) and a lack of a genotype × day interaction in both cases. For velocity, we did not find a genotype difference (Fig. 5d; effect of genotype, P = 0.34). During the probe trial, Thy1-hAPPLond/Swe+ mice and control littermates spent significantly more time in the target quadrant, indicating normal memory retrieval (Fig. 5e; effect of genotype, F1, 21 = 0.56, P = 0.462; effect of quadrant, F3, 63 = 19.05, P < 0.0001; genotype × quadrant interaction, F3, 63 = 0.19, P = 0.90). No genotype difference was revealed in the visible platform test (Fig. 5f; effect of genotype, F1, 21 = 1.99, P = 0.173; effect of trial, F3, 63 = 5.65, P = 0.0017; genotype × trial interaction, F3, 63 = 0.71, P = 0.55).
Figure 5.

Morris water maze. (a) Display of median tracks of Thy1-hAPPLond/Swe+ and control mice during trial 16. Acquisition of the hidden platform location did not differ between genotypes as shown by escape latency (b), and distance moved (c). (d) For velocity, no significant genotype difference was detectable (P > 0.05, repeated measures ANOVA) while the day effect and interaction were significantly different between genotypes (P < 0.05, repeated measures ANOVA). (e) During the probe trial, with the escape platform missing, both genotypes preferred the target quadrant (TQ). (f) There were no significant differences between genotypes during visible platform learning. n(control) = 12, n(Thy1-hAPPLond/Swe+) = 11.
DMP dry maze
Since Thy1-hAPPLond/Swe+ mice did not display a deficit in the water maze tests, we developed a new DMP task using a dry maze (modified Barnes maze) in an attempt to enhance detection of deficits by eliminating the water and swimming factor (Fig. 6a). Before testing Thy1-hAPPLond/Swe+ and their control littermates, a validation experiment was conducted using C57BL/6J mice and scopolamine to induce experimental memory impairment. Scopolamine-injected mice exhibited decreased learning as indicated by a significant trial effect for escape latency in combination with a significant treatment × trial interaction (Fig. 6b; effect of treatment, F1, 18 = 51.58, P < 0.0001; effect of trial, F15, 270 = 10.00, P < 0.0001; treatment × trial interaction, F15, 270 = 3.15, P < 0.0001). Calculation of the averages of escape latencies per trial confirmed this finding (Fig. 6c; effect of treatment, F1, 18 = 51.58, P < 0.0001; effect of trial, F3, 54 = 31.30, P < 0.0001; treatment × trial interaction, F3, 45 = 9.9, P < 0.0001). Both the trial effect and treatment × trial interaction of the escape distance were significant (data not shown). The savings between the first and second trials (T1-T2 savings) and the first and fourth trials (T1-T4 savings) were significantly lower in scopolamine-treated versus vehicle-treated mice (Fig. 6; P = 0.0019 for T1-T2 savings and P = 0.0003 for T1-T4 savings).
Figure 6.
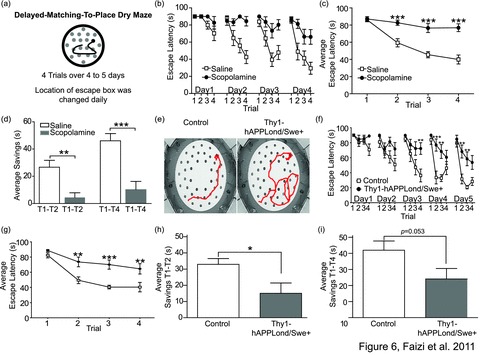
Delayed-matching-to-place dry maze. (a) On a circular platform, mice were given four trials over four to five days to find an escape box along three rings of escape holes. (b) Scopolamine-treated mice showed a significantly altered escape latency to find the escape box (P < 0.0001 both for trial effect and treatment × trial interaction). (c) Trial average comparison also shows a significantly increased escape latency of treated versus control mice for trials 2–4 (P < 0.001, Bonferroni posthoc test). Day 1 was considered as habituation to the experimental setup and therefore excluded from this analysis. (d) The time differences between escape latencies of the first and the second trials (T1-T2 savings) and the first and the fourth trials (T1-T4 savings) was significantly lower in treated mice than in controls (T1-T2 P < 0.01, T1-T4 P < 0.001). n(control) = 10; n(scopolamine-treated) = 10. (e) Median tracks of control (Left) and Thy1-hAPPLond/Swe+ mice (Right) during trial 4 of day 5. Mutant mice showed a significantly altered learning compared to control mice both in a trial-to-trial comparison (P < 0.01 and P < 0.001, Bonferroni posthoc test) (f) as well as in a trial average comparison with day 1 excluded (P < 0.01 and P < 0.001, Bonferroni posthoc test) (g). (h) The T1-T2 savings were significantly (P < 0.05) decreased in Thy1-hAPPLond/Swe+ mice compared to control littermates. (i) However, the T1-T4 savings failed to reach significance level. n(each genotype) = 10.
Thy1-hAPPLond/Swe+ mice exhibited a deficit in acquisition of the DMP dry maze task compared to control mice as supported by a significant trial effect on escape latency in combination with significant genotype × trial interaction (Fig. 6e and f; effect of genotype, F1, 18 = 15.72, P = 0.0009; effect of trial, F19, 342 = 14.08, P < 0.0001; genotype × trial interaction, F19, 342 = 2.49, P = 0.0006). Calculation of the trial average of escape latencies revealed the same overall effect (Fig. 6g; effect of genotype, F1, 18 = 14.57, P = 0.0013; effect of trial, F3, 54 = 34.06, P < 0.0001; genotype × trial interaction, F3, 45 = 3.93, P = 0.01). A similar trend (but not statistically significant) was detected in both the trial effect and genotype × trial interaction of the escape distances (data not shown). The T1-T2 savings was significantly lower in the Thy1-hAPPLond/Swe+ mice than in their control littermates (Fig. 6h; P = 0.037). A trend in the same direction was found for the T1-T4 savings (Fig. 6i; P = 0.053).
Fear conditioning
Tone-cued and contextual FC was used for evaluation of conditional learning and memory. Both genotypes acquired the task equally well as shown by a significant time effect on freezing and a lack of a genotype × time interaction (Fig. 7a; effect of genotype, F1, 21 = 3.73, P = 0.067; effect of time F5, 105 = 54.76, P < 0.0001; genotype × time is interaction, F5, 105 = 1.00, P = 0.42). For tone freezing, we found a significant time effect but no significant genotype × time effect (Fig. 7b; effect of genotype, F1, 21 = 4.92, P = 0.038; effect of ITIs F4, 84 = 28.13, P < 0.0001; genotype × ITIs is interaction, F4, 84 = 1.64, P = 0.17). Still, a significant overall genotype effect has to be accounted for. In the tone-cued FC test in a novel context, no differences were revealed between genotypes (Fig. 7c; P = 0.735). Importantly, freezing during the tone presentation on day 2 was not lower in mutant mice than control mice (data not shown). However, Thy1-hAPPLond/Swe+ mice showed a significant deficit in the contextual memory retrieval test as shown by a significantly decreased freezing behavior (Fig. 7b; P = 0.006).
Figure 7.

Fear conditioning (FC). (a) Both genotypes acquired the FC task without significant statistical difference in freezing. (b) Mutant mice, however, froze less in response to the tone alone. (c) No freezing differences between genotypes were apparent for the tone memory in FC, however, Thy1-hAPPLond/Swe+ mice showed a significantly (P < 0.01) impaired context memory compared to control littermates. n(control) = 12; n(Thy1-hAPPLond/Swe+) = 11.
Hot plate test
Sensitivity to a painful stimulus (nociception) was assessed using the hot plate test. No difference in latency of reaction to the hot surface was found between Thy1-hAPPLond/Swe+ and control littermates, suggesting no difference in responsiveness to aversive stimuli between the transgenic and control animals (P = 0.068, data not shown).
Discussion
The transgenic mouse model of AD, line 41 of Thy1-hAPPLond/Swe+ (mThy1-hAPPLond/Swe+), was introduced by Masliah and colleagues (Rockenstein et al. 2001). This transgenic strain contains the London (V717I) and Swedish (K670M/N671L) mutations and expresses a high level of human APP751 cDNA. The advantage of this line is that it shows mature β-amyloid plaques, a pathological hallmark of AD, in the frontal cortex as early as three months of age and develops plaques in other brain regions at five to seven months of age without requiring expression of mutant presenilin (Rockenstein et al. 2001, 2002). In this present study, general locomotor activity, social interaction, and learning and memory were evaluated in a broad range of behavioral paradigms.
It has been reported that most AD patients display agitation and higher motor activity (motor restlessness) (Frisoni et al. 1999; Chung and Cummings 2000). Thy1-hAPPLond/Swe+ mice also showed hyperactivity in both the activity chamber and the open-field tests. Activity-dependent abnormalities have been reported in other APP-based transgenic mouse models of AD (Holcomb et al. 1999; Lalonde et al. 2003; Morgan 2003). The prefrontal cortex and hippocampus are regions of the brain that have been previously suggested to be involved in hyperactivity (Kolb 1974; Tani et al. 2001; Katsuta et al. 2003; Viggiano 2008). Pathological abnormalities observed in the hippocampus of Thy1-hAPPLond/Swe+ mice (Rockenstein et al. 2001) may be responsible for this observed hyperactivity. Hyperactivity could partially be due to a loss of working memory and therefore, an inability to recall areas previously explored in novel testing arenas. This hyperactivity could be due to an inability of hippocampal-lesioned mice to form a contextual map of the novel arena and their continuous exploration of the arena to compensate for this deficit. Mutant mice traveled a significantly longer distance in the periphery of the open field than control mice, which suggests anxiety-like behavior. However, the velocity of mutant mice in the open field was significantly increased. Furthermore, no genotype effect was revealed in the time spent in the periphery versus the center zones of the arenas of the activity chamber and open field. These findings suggest that the increase in locomotion in Thy1-hAPPLond/Swe+ mice is primarily caused by hyperactivity rather than anxiety-like behavior.
People with AD exhibit social withdrawal (Chung and Cummings 2000), which is thought to be due to depression. Interestingly, it has also been shown that children with severe autistic behavior and aggression have higher plasma levels of APP (Sokol et al. 2006). We performed a three-chamber test and found that Thy1-hAPPLond/Swe+ mice displayed unaltered sociability. Interestingly, in the subsequent social novelty session, Thy1-hAPPLond/Swe+ mice showed a decreased preference for the newly introduced mouse. This might be caused by generally altered cognition or a lack of interest in social novelty. However, one prerequisite to develop a preference for stranger 2 is the ability to remember the identity of strangers (social memory) when alternating between the side chambers. Social memory is often tested with the five-trial social memory test (Ferguson et al. 2000). We used an extended version of this test (six-trial social memory test, Bader, 2011), which did not reveal a deficit in mutant mice. The outcomes in three-chamber and six-trial tests might differ because in the former, male object mice were used, whereas in the latter subject mice were exposed to OEF object mice. Furthermore, in the case of the three-chamber test, intruders were restrained in a cup and therefore direct contact was limited, whereas in the case of the six-trial test a direct body-to-body interaction was possible and identity cues might have been more easily collected and more easily remembered. Also, in the three-chamber test object mice were presented simultaneously whereas in the six-trial test object mice were presented with ITIs of 10 min. More research is needed to disentangle the different outcomes in the three-chamber and six-trial tests.
Memory loss is the most common problem in AD patients. The hippocampus is an important brain region involved in memory and is affected in AD (West 1993). In our study, the Thy1-hAPPLond/Swe+ mice displayed behavioral deficits in hippocampus-dependent learning paradigms such as spontaneous alternation in the T-maze and Y-maze, the DMP dry maze, and contextual FC. However, a significant deficit could not be detected in spatial reference memory using the MWM.
A significant deficit in spontaneous alternation in the Y-maze and T-maze has been reported in other APP-based mouse models of AD (Kobayashi and Chen 2005). Spontaneous alternation is highly dependent on hippocampus function (Johnson et al. 1977; Devenport et al. 1988; Gerlai 1998) and reveals the hippocampus-dependent deficits in learning and memory observed in Thy1-hAPPLond/Swe+ mice. The total number of arm entries was not significantly different between genotypes in the Y-maze, which indicates that the deficit in spontaneous alternation is not due to hyperactivity in Thy1-hAPPLond/Swe+ mice.
Spatial reference memory was assessed in the hidden platform training and the probe trial retention test in the MWM. We did not detect a significant deficit in spatial reference memory in Thy1-hAPPLond/Swe+ mice, and mutant mice appeared to have normal motivation and motor function in this task. However, other studies found a spatial reference memory deficit in mice with the same mutation (Rockenstein et al. 2003; Havas et al. 2011). Apparently, detection/presence of a special memory deficit is influenced by factors other than the mutation. These factors might be the background of mice tested (C57Bl/6J in our study vs. C57BL/6 × Swiss Webster in Havas et al.), the protocol used (no pretraining in our study vs. three-day pretraining in Rockenstein et al.; single testing in our study vs. repeated testing in Havas et al.), or gender of mice tested (male mice in our study vs. male and female mice in Havas et al.).
Thy1-hAPPLond/Swe+ mice showed a deficit in spatial working/episodic-like memory in the DMP dry maze. As previously discussed, this mouse model of AD has deficits in spatial working memory in the Y-maze and the T-maze tests. However, some factors might affect the spontaneous alternation including perseveration, lack of motivation, and loss in spatial orientation (Lalonde 2002). The results of the DMP dry maze, which is more difficult to obtain but perhaps more reliable than spontaneous alternation tests, confirmed the impaired spatial working memory in Thy1-hAPPLond/Swe+ mice. Scopolamine was used for validation of the novel DMP dry maze. Scopolamine is a muscarinic antagonist and impairs a variety of learning and memory tests in rodents (Kuc et al. 2006; Chen et al. 2008; Post et al. 2011). These data show that the novel DMP dry maze is sensitive enough for testing the spatial memory in mice.
Lastly, although Thy1-hAPPLond/Swe+ mice show a normal learning pattern during the acquisition phase of the FC test, they demonstrate a significant deficit in contextual memory retrieval. This effect is not caused by an altered thermo-sensitive reflex or a general hyperactivity in Thy1-hAPPLond/Swe+ mice, as freezing in mutant mice were not different during the training phase of this task. Moreover, mutant mice did not show decreased freezing during tone testing and therefore probably have no decreased tone memory. Decreased freezing of mutant mice during tone presentations on day 1 suggests that Thy1-hAPPLond/Swe+ mice are hearing impaired. However, this is unlikely since freezing during tone presentations was not decreased in mutant mice on day 2. Contextual memory retrieval is believed to be hippocampus-dependent (Selden et al. 1991; Kim and Fanselow 1992; Phillips and Ledoux 1992) while the response to the tone stimulus is believed to mostly rely on amygdala function (Kim and Fanselow 1992; Phillips and Ledoux 1992; Anagnostaras et al. 1999). These results highlight the hippocampus-dependent deficits in learning and memory observed in other tasks performed in this study.
In conclusion, the Thy1-hAPPLond/Swe+ mouse model of AD displays a strong behavioral phenotype that resembles, in part, the cognitive and psychiatric symptoms experienced by AD patients. In addition, we have identified robust behavioral assays (Y-maze and contextual FC) and developed new behavioral assays (DMP dry maze) for behavioral phenotyping and pharmacological screening of compounds in transgenic mouse models of AD.
Acknowledgments
We would like to thank K. L. Sainani for support in the statistical analysis. This study was supported by NINDS P30 center core grant (NS069375-01A1), grants from Mathers Foundation and the Burnett Family Fund (to R.W. Tsien), NIA (U01 AG032225), the Jean Perkins Foundation, and the Horngren Family Alzheimer's Research Fund to FML.
References
- Anagnostaras SG, Maren S, Fanselow MS. Temporally graded retrograde amnesia of contextual fear after hippocampal damage in rats: within-subjects examination. J. Neurosci. 1999;19:1106–1114. doi: 10.1523/JNEUROSCI.19-03-01106.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader PL, Faizi M, Lim LH, Owen SF, Tadross MR, Alfa RW, Bett GCL, Tsien RW, Rasmusson RL, Shamloo M. Mouse model of Timothy syndrome recapitulates triad of autisitc traits. Proc. Natl. Acad. Sci. USA. 2011;108:15432–15437. doi: 10.1073/pnas.1112667108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes CA. Memory deficits associated with senescence: a neurophysiological and behavioral study in the rat. J. Comp. Physiol. Psychol. 1979;93:74–104. doi: 10.1037/h0077579. [DOI] [PubMed] [Google Scholar]
- Belichenko NP, Belichenko PV, Kleschevnikov AM, Salehi A, Reeves RH, Mobley WC. The “Down syndrome critical region” is sufficient in the mouse model to confer behavioral, neurophysiological, and synaptic phenotypes characteristic of Down syndrome. J. Neurosci. 2009;29:5938–5948. doi: 10.1523/JNEUROSCI.1547-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertram L, Lill CM, Tanzi RE. The genetics of Alzheimer disease: back to the future. Neuron. 2010;68:270–281. doi: 10.1016/j.neuron.2010.10.013. [DOI] [PubMed] [Google Scholar]
- Chen J, Long Y, Han M, Wang T, Chen Q, Wang R. Water-soluble derivative of propolis mitigates scopolamine-induced learning and memory impairment in mice. Pharmacol. Biochem. Behav. 2008;90:441–446. doi: 10.1016/j.pbb.2008.03.029. [DOI] [PubMed] [Google Scholar]
- Chung JA, Cummings JL. Neurobehavioral and neuropsychiatric symptoms in Alzheimer's disease: characteristics and treatment. Neurol. Clin. 2000;18:829–846. doi: 10.1016/s0733-8619(05)70228-0. [DOI] [PubMed] [Google Scholar]
- Deacon RM, Rawlins JN. T-maze alternation in the rodent. Nat. Protoc. 2006;1:7–12. doi: 10.1038/nprot.2006.2. [DOI] [PubMed] [Google Scholar]
- Deacon RM, Bannerman DM, Kirby BP, Croucher A, Rawlins JN. Effects of cytotoxic hippocampal lesions in mice on a cognitive test battery. Behav. Brain Res. 2002;133:75–68. doi: 10.1016/s0166-4328(01)00451-x. [DOI] [PubMed] [Google Scholar]
- Devenport LD, Hale RL, Stidham JA. Sampling behavior in the radial maze and operant chamber: role of the hippocampus and prefrontal area. Behav. Neurosci. 1988;102:489–498. doi: 10.1037//0735-7044.102.4.489. [DOI] [PubMed] [Google Scholar]
- Drew WG, Miller LL, Baugh EL. Effects of delta9-THC, LSD-25 and scopolamine on continuous, spontaneous alternation in the Y-maze. Psychopharmacologia. 1973;32:171–182. doi: 10.1007/BF00428688. [DOI] [PubMed] [Google Scholar]
- Ferguson JN, Young LJ, Hearn EF, Matzuk MM, Insel TR, Winslow JT. Social amnesia in mice lacking the oxytocin gene. Nat. Genet. 2000;25:284–288. doi: 10.1038/77040. [DOI] [PubMed] [Google Scholar]
- Frisoni GB, Rozzini L, Gozzetti A, Binetti G, Zanetti O, Bianchetti A, Trabucchi M, Cummings JL. Behavioral syndromes in Alzheimer's disease: description and correlates. Dement. Geriatr. Cogn. Disord. 1999;10:130–138. doi: 10.1159/000017113. [DOI] [PubMed] [Google Scholar]
- Gerlai R. A new continuous alternation task in T-maze detects hippocampal dysfunction in mice. A strain comparison and lesion study. Behav. Brain Res. 1998;95:91–101. doi: 10.1016/s0166-4328(97)00214-3. [DOI] [PubMed] [Google Scholar]
- Havas D, Hutter-Paier B, Ubhi K, Rockenstein E, Crailsheim K, Masliah E, Windisch M. A longitudinal study of behavioral deficits in an AbetaPP transgenic mouse model of Alzheimer's disease. J. Alzheimers Dis. 2011;25:231–243. doi: 10.3233/JAD-2011-101866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holcomb LA, Gordon MN, Jantzen P, Hsiao K, Duff K, Morgan D. Behavioral changes in transgenic mice expressing both amyloid precursor protein and presenilin-1 mutations: lack of association with amyloid deposits. Behav. Genet. 1999;29:177–185. doi: 10.1023/a:1021691918517. [DOI] [PubMed] [Google Scholar]
- Hughes RN. The value of spontaneous alternation behavior (SAB) as a test of retention in pharmacological investigations of memory. Neurosci. Biobehav. Rev. 2004;28:497–505. doi: 10.1016/j.neubiorev.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Johnson CT, Olton DS, Gage FH, 3rd, Jenko PG. Damage to hippocampus and hippocampal connections: effects on DRL and spontaneous alternation. J. Comp. Physiol. Psychol. 1977;91:508–522. doi: 10.1037/h0077346. [DOI] [PubMed] [Google Scholar]
- Katsuta K, Umemura K, Ueyama N, Matsuoka N. Pharmacological evidence for a correlation between hippocampal CA1 cell damage and hyperlocomotion following global cerebral ischemia in gerbils. Eur. J. Pharmacol. 2003;467:103–109. doi: 10.1016/s0014-2999(03)01573-5. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Fanselow MS. Modality-specific retrograde amnesia of fear. Science. 1992;256:675–677. doi: 10.1126/science.1585183. [DOI] [PubMed] [Google Scholar]
- Kobayashi DT, Chen KS. Behavioral phenotypes of amyloid-based genetically modified mouse models of Alzheimer's disease. Genes Brain Behav. 2005;4:173–196. doi: 10.1111/j.1601-183X.2005.00124.x. [DOI] [PubMed] [Google Scholar]
- Kolb B. Dissociation of the effects of lesions of the orbital or medial aspect of the prefrontal cortex of the rat with respect to activity. Behav. Biol. 1974;10:329–343. doi: 10.1016/s0091-6773(74)91924-5. [DOI] [PubMed] [Google Scholar]
- Kuc KA, Gregersen BM, Gannon KS, Dodart JC. Holeboard discrimination learning in mice. Genes Brain Behav. 2006;5:355–363. doi: 10.1111/j.1601-183X.2005.00168.x. [DOI] [PubMed] [Google Scholar]
- Lalonde R. The neurobiological basis of spontaneous alternation. Neurosci. Biobehav. Rev. 2002;26:91–104. doi: 10.1016/s0149-7634(01)00041-0. [DOI] [PubMed] [Google Scholar]
- Lalonde R, Lewis TL, Strazielle C, Kim H, Fukuchi K. Transgenic mice expressing the betaAPP695SWE mutation: effects on exploratory activity, anxiety, and motor coordination. Brain Res. 2003;977:38–45. doi: 10.1016/s0006-8993(03)02694-5. [DOI] [PubMed] [Google Scholar]
- Lim MM, Young LJ. Neuropeptidergic regulation of affiliative behavior and social bonding in animals. Horm. Behav. 2006;50:506–517. doi: 10.1016/j.yhbeh.2006.06.028. [DOI] [PubMed] [Google Scholar]
- Morgan D. Learning and memory deficits in APP transgenic mouse models of amyloid deposition. Neurochem. Res. 2003;28:1029–1034. doi: 10.1023/a:1023255106106. [DOI] [PubMed] [Google Scholar]
- Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J. Neurosci. Methods. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- Nadler JJ, Moy SS, Dold G, Trang D, Simmons N, Perez A, Young NB, Barbaro RP, Piven J, Magnuson TR, et al. Automated apparatus for quantitation of social approach behaviors in mice. Genes Brain Behav. 2004;3:303–314. doi: 10.1111/j.1601-183X.2004.00071.x. [DOI] [PubMed] [Google Scholar]
- Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav. Neurosci. 1992;106:274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- Phillips RG, LeDoux JE. Lesions of the dorsal hippocampal formation interfere with background but not foreground contextual fear conditioning. Learn Mem. 1994;1:34–44. [PubMed] [Google Scholar]
- Post AM, Wultsch T, Popp S, Painsipp E, Wetzstein H, Kittel-Schneider S, Sontag TA, Lesch KP, Reif A. The COGITAT holeboard system as a valuable tool to assess learning, memory and activity in mice. Behav. Brain Res. 2011;220:152–158. doi: 10.1016/j.bbr.2011.01.054. [DOI] [PubMed] [Google Scholar]
- Rockenstein E, Mallory M, Mante M, Sisk A, Masliaha E. Early formation of mature amyloid-beta protein deposits in a mutant APP transgenic model depends on levels of Abeta(1–42) J. Neurosci. Res. 2001;66:573–582. doi: 10.1002/jnr.1247. [DOI] [PubMed] [Google Scholar]
- Rockenstein E, Mallory M, Mante M, Alford M, Windisch M, Moessler H, Masliah E. Effects of Cerebrolysin on amyloid-beta deposition in a transgenic model of Alzheimer's disease. J. Neural. Transm. Suppl. 2002;62:327–336. [PubMed] [Google Scholar]
- Rockenstein E, Adame A, Mante M, Moessler H, Windisch M, Masliah E. The neuroprotective effects of Cerebrolysin in a transgenic model of Alzheimer's disease are associated with improved behavioral performance. J. Neural. Transm. 2003;110:1313–1327. doi: 10.1007/s00702-003-0025-7. [DOI] [PubMed] [Google Scholar]
- Rockenstein E, Crews L, Masliah E. Transgenic animal models of neurodegenerative diseases and their application to treatment development. Adv. Drug Deliv. Rev. 2007a;59:1093–1102. doi: 10.1016/j.addr.2007.08.013. [DOI] [PubMed] [Google Scholar]
- Rockenstein E, Torrance M, Adame A, Mante M, Bar-on P, Rose JB, Crews L, Masliah E. Neuroprotective effects of regulators of the glycogen synthase kinase-3beta signaling pathway in a transgenic model of Alzheimer's disease are associated with reduced amyloid precursor protein phosphorylation. J. Neurosci. 2007b;27:1981–1991. doi: 10.1523/JNEUROSCI.4321-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxe MD, Battaglia F, Wang JW, Malleret G, David DJ, Monckton JE, Garcia AD, Sofroniew MV, Kandel ER, Santarelli L, et al. Ablation of hippocampal neurogenesis impairs contextual fear conditioning and synaptic plasticity in the dentate gyrus. Proc. Natl. Acad. Sci. USA. 2006;103:17501–17506. doi: 10.1073/pnas.0607207103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selden NR, Everitt BJ, Jarrard LE, Robbins TW. Complementary roles for the amygdala and hippocampus in aversive conditioning to explicit and contextual cues. Neuroscience. 1991;42:335–350. doi: 10.1016/0306-4522(91)90379-3. [DOI] [PubMed] [Google Scholar]
- Sokol DK, Chen D, Farlow MR, Dunn DW, Maloney B, Zimmer JA, Lahiri DK. High levels of Alzheimer beta-amyloid precursor protein (APP) in children with severely autistic behavior and aggression. J. Child Neurol. 2006;21:444–449. doi: 10.1177/08830738060210062201. [DOI] [PubMed] [Google Scholar]
- Steele RJ, Morris RG. Delay-dependent impairment of a matching-to-place task with chronic and intrahippocampal infusion of the NMDA-antagonist D-AP5. Hippocampus. 1999;9:118–136. doi: 10.1002/(SICI)1098-1063(1999)9:2<118::AID-HIPO4>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Tani K, Iyo M, Matsumoto H, Kawai M, Suzuki K, Iwata Y, Won T, Tsukamoto T, Sekine Y, Sakanoue M, et al. The effects of dentate granule cell destruction on behavioural activity and Fos protein expression induced by systemic methamphetamine in rats. Br. J. Pharmacol. 2001;134:1411–1418. doi: 10.1038/sj.bjp.0704370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viggiano D. The hyperactive syndrome: metanalysis of genetic alterations, pharmacological treatments and brain lesions which increase locomotor activity. Behav. Brain Res. 2008;194:1–14. doi: 10.1016/j.bbr.2008.06.033. [DOI] [PubMed] [Google Scholar]
- West MJ. Regionally specific loss of neurons in the aging human hippocampus. Neurobiol. Aging. 1993;14:287–293. doi: 10.1016/0197-4580(93)90113-p. [DOI] [PubMed] [Google Scholar]
- Young LM, Murphy Young AZ, Hammock EA. Anatomy and neurochemistry of the pair bond. J. Comp. Neurol. 2005;493:51–57. doi: 10.1002/cne.20771. [DOI] [PubMed] [Google Scholar]


