Abstract
Background
Physicians often experience work-related stress that may lead to personal harm and impaired professional performance. Biofeedback has been used to manage stress in various populations.
Objective
To determine whether a biofeedback-based stress management tool, consisting of rhythmic breathing, actively self-generated positive emotions and a portable biofeedback device, reduces physician stress.
Design
Randomized controlled trial measuring efficacy of a stress-reduction intervention over 28 days, with a 28-day open-label trial extension to assess effectiveness.
Setting
Urban tertiary care hospital.
Participants
Forty staff physicians (23 men and 17 women) from various medical practices (1 from primary care, 30 from a medical specialty and 9 from a surgical specialty) were recruited by means of electronic mail, regular mail and posters placed in the physicians’ lounge and throughout the hospital.
Intervention
Physicians in the intervention group were instructed to use a biofeedback-based stress management tool three times daily. Participants in both the control and intervention groups received twice-weekly support visits from the research team over 28 days, with the intervention group also receiving re-inforcement in the use of the stress management tool during these support visits. During the 28-day extension period, both the control and the intervention groups received the intervention, but without intensive support from the research team.
Main outcome measure
Stress was measured with a scale developed to capture short-term changes in global perceptions of stress for physicians (maximum score 200).
Results
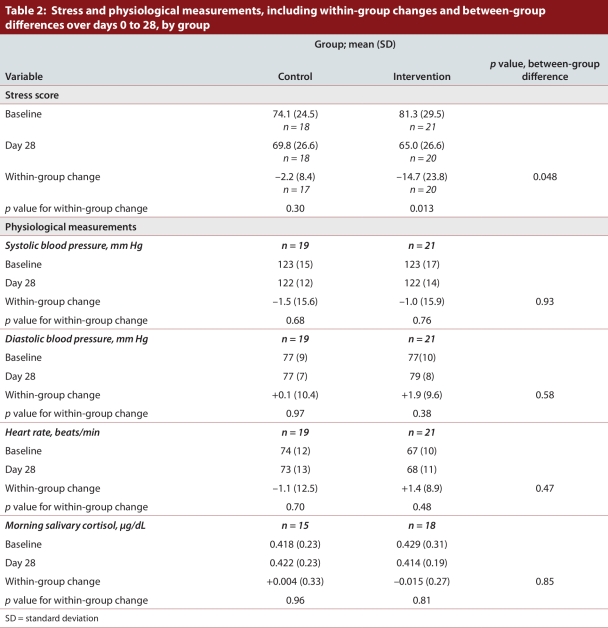
During the randomized controlled trial (days 0 to 28), the mean stress score declined significantly for the intervention group (change –14.7, standard deviation [SD] 23.8; p = 0.013) but not for the control group (change –2.2, SD 8.4; p = 0.30). The difference in mean score change between the groups was 12.5 (p = 0.048). The lower mean stress scores in the intervention group were maintained during the trial extension to day 56. The mean stress score for the control group changed significantly during the 28-day extension period (change –8.5, SD 7.6; p < 0.001).
Conclusion
A biofeedback-based stress management tool may be a simple and effective stress-reduction strategy for physicians.
Given the nature of their occupational duties and environment, physicians often experience work-related stress,1 which may lead to personal harm such as burnout, depression and substance abuse, as well as impaired professional performance, indicated by medication errors and reduced attentiveness or caring behaviour toward their patients.2 Stress management refers to a range of processes that are intended to mitigate aspects of the psychobiology of stress. Biofeedback, an intervention that involves measuring a person’s quantifiable bodily functions (e.g., blood pressure, heart rate, muscle tension) and conveying the information to the person in real time, is a useful way of providing guidance and reinforcement for successful management of the physiological response to stress. Various biofeedback techniques have been used in different populations for the treatment of, and to help deal with the stress caused by, disorders such as hypertension, migraine headaches, tinnitus, irritable bowel syndrome and fibromyalgia.3-6 Stress and emotion have also been linked to heart rate variability (HRV), a measure of naturally occurring beat-to-beat changes in the heart rate reflecting vagal antagonism of sympathetic influences.7,8 Biofeedback tools that incorporate measures of HRV enable study of the linkage between HRV and both the psychology and the biology of stress.9-12 For example, a biofeedback device that measures and communicates the beneficial HRV resulting from rhythmic breathing coupled with actively self-generated positive emotions provides reinforcement to the user that their efforts to reduce stress are effective.7
Physician wellness has been increasingly linked to the quality of patient care, yet the attention that physicians pay to self-wellness is suboptimal, because of individual, professional and health care organizational factors.2 It is therefore important to explore practical and credible means of helping physicians to contend with work-related stress, with the ultimate goal of improving their overall performance. A stress management tool that incorporates a biofeedback device provides the physician with direct evidence of positive physiological change.
The aim of this study was to determine whether the use of a biofeedback-based stress management tool (consisting of rhythmic breathing, actively self-generated positive emotions and a portable biofeedback device) helps to reduce physician stress.
Methods
Design
During the first 28 days of this study, we conducted an open-label, randomized controlled clinical trial with concealed allocation to assess the efficacy of the stress management tool for reducing physician stress. In an open-label trial extension (days 28 to 56) applied to both arms, we assessed the effectiveness of the intervention by measuring 1) any sustained stress-reduction effects in the intervention cohort, who were instructed to continue using the stress management tool until day 56; and 2) the stress-reduction effects of the tool in a real-life setting, without intensive support from the research team, by having the physicians enrolled in the control arm over days 0 to 28 participate in a training session on or about day 28 with instructions to use the stress-reduction tool until day 56. We measured outcomes at days 0, 28 and 56.
Setting, participants and randomization
Eligible participants were staff physicians practising in an urban tertiary care centre. We recruited participants in March 2009, by electronic mail, regular mail and posters placed in the physicians’ lounge and throughout the hospital. We performed the study from April to June 2009. We excluded potential participants who screened positive for major depression with the 9-item Patient Health Questionnaire (PHQ-9) depression scale 13 and referred them to the provincial physician wellness support program. We used a computer program to generate a random allocation sequence for assigning participants to either the control or the intervention group, with stratification by sex to ensure parity within groups. Participants’ allocation to the control or intervention group was concealed until after the research assistant and/or the co-investigators had confirmed eligibility criteria and received informed consent. Given the nature of the intervention and the outcome measures, the study was not blinded. We collected data primarily at the hospital, with participants occasionally completing the stress questionnaire by accessing the study website from an off-site location.
Intervention
The biofeedback-based stress management tool used in our study consisted of a combination of rhythmic breathing, self-generated positive emotion and a biofeedback device to reinforce positive physiological change when dealing with stress. The biofeedback device, the emWavePSR (Personal Stress Reliever) (HeartMath, LLC, Boulder Creek, California), is a lightweight, battery-operated device about the size of a small deck of cards that can be carried in a pocket or purse. It calculates beat-to-beat changes in heart rate (i.e., HRV) to produce a measure of physiological coherence. This state may be achieved through the quick coherence technique, which comprises rhythmic breathing coupled with actively self-generated positive emotions such as appreciation for something or someone or remembering a special place in nature. The device reads heart rate through a digit or ear lobe sensor and provides reinforcement of successful implementation of the quick coherence technique through visual cues (e.g., a light that transitions from red to blue to green with increasing coherence and another that pulses with the captured heart beat) and auditory cues (e.g., beeps signalling that coherence has been achieved). An accompanying software application (emWavePC) performs these tasks within a Microsoft Windows environment for use on a laptop or desktop computer, providing a more detailed real-time quantitative and graphical display of heart rhythm pattern. Participants received an emWavePSR to use throughout the pertinent study period (intervention group, days 0 to 56; control group, days 28 to 56) and to keep thereafter. They also attended a 30-minute standardized training session provided by personnel employed by the health region who had undergone formal training to become qualified as instructors. The study participants were taught the quick coherence technique, the principles of the biofeedback device (through demonstrations of both the emWavePSR and the emWavePC software) and how to use their personal emWavePSR, and were given contact information should questions arise. The research assistants also used the emWavePC version during their twice-weekly encounters with participants in the intervention group during days 0 to 28, to reinforce the visual display of coherence.
Randomized controlled trial (days 0 to 28)
Participants allocated to the intervention group received a brochure describing the provincial physician wellness support program; were given a biofeedback device and participated in an individual training session to learn the quick coherence technique and how to use the device, with an offer of optional follow-up instruction; and were given a prescription to use the stress management tool during study days 0 to 28 for 5 minutes at least three times daily. A research assistant contacted each participant in the intervention group twice weekly to measure stress and well-being, heart rate and blood pressure; to document their adherence to using the stress management tool; and to record a 3-minute biofeedback session using the emWavePC software. Each of these encounters enabled the research assistant to reinforce use of the stress management tool and to further visually display participants’ ability to achieve coherence levels over the course of the study.
Participants allocated to the control group received the same brochure describing the provincial physician wellness support program and were contacted twice weekly by a research assistant to measure stress and well-being, heart rate and blood pressure.
Trial extension (days 28 to 56)
Participants allocated to the intervention group were told to continue using the stress management tool at their discretion and were offered the opportunity to request and receive additional training and support. A research assistant contacted each participant in the intervention group on or about day 56 to gather outcome measures data.
Each participant allocated to the control group received a biofeedback device and participated in a personal training session on or about day 28. Each participant was given a prescription to use the stress management tool during study days 28 to 56 for 5 minutes at least three times daily and was offered the opportunity to request and receive additional training and support; however, these participants were not otherwise supported by the research team. A research assistant contacted each participant on or about day 56 to gather outcome measures data.
Outcome measures
We measured the primary outcome, stress, with a multiple-item scale developed by the research team and intended to measure global perceptions of stress and also to capture occupation-specific stress that is particularly relevant to physicians (Appendix A). The survey included 15 items from the Perceived Stress Scale, a reliable and valid psychological instrument designed to measure perceptions of stress (defined as how unpredictable, uncontrollable and overloaded respondents find their lives) over a short period (i.e., 1 to 2 months).14,15 The questions in the Perceived Stress Scale are of a general nature, not specific to any subpopulation, and are considered valid across sex and age categories. The survey also included 25 selected items from the Personal and Organizational Quality Assessment–Revised (POQA-R) questionnaire,16 an 85-item self-report inventory designed to reflect key psychological and workplace elements indicating the overall quality of one’s experiences within an organization. We chose specific items from this questionnaire on the basis of the results of a pilot study of 10 hospital-based physicians, who were asked to provide a written response describing in their own words how they felt when they were busy or stressed at work. The 25 selected items represented three themes: anxiety or anger, physical symptoms of stress and work-related time pressures. The final 40-item instrument was validated through confirmatory common factor analysis 17 with varimax rotation showing that all 40 items loaded onto a single factor with an eigenvalue of 18.8 (results available upon request). The response set for all items consisted of never (coded 0), almost never (coded 1), sometimes (coded 2), often (coded 3), very often (coded 4) and always (coded 5). Values for all 40 items were summed for a maximum possible stress score of 200, where a higher score indicated greater feelings of stress. The inter-item reliability for the summated scale was 0.97 based on Cronbach’s α, which was calculated for all participants at day 0.
The secondary outcome measures included adherence, heart rate, blood pressure, and salivary cortisol levels. We defined good adherence as at least 15 minutes per day of self-reported use of the stress management tool (based on the prescribed instructions for use), as calculated by the daily mean for days 0 to 28. We collected baseline demographic data at enrolment. Heart rate and blood pressure were measured using Physiologic Auto Blood Pressure Monitor model 106-925 (AMG Medical Inc, Montréal, Quebec). Salivary cortisol level was analyzed by enzyme immunoassay (Salimetrics LLC, State College) according to the manufacturer’s instructions, with samples collected upon awakening (fasting) and at midday, suppertime and before bedtime on or about days 0 and 28.
Statistical analysis
We performed a sample-size calculation and determined that we needed 17 participants per study arm to detect a between-group difference in stress score of 15 (with 80% power and an estimated common standard deviation [SD] of 15). In conducting the study, we targeted final enrolment of about 20 participants per group to account for possible loss to follow-up. We expressed measurements of participants’ baseline characteristics as means with SD for continuous variables (age, years in practice, heart rate, systolic and diastolic blood pressure, salivary cortisol) and as proportions for categorical variables (sex, marital status, medical practice, smoking status and exercise pattern). Stress scores at days 0, 28 and 56 were expressed as a group mean and SD. Because of the small sample size, we used nonparametric methods to compare changes in stress scores. We performed within-group comparisons using the Wilcoxon signed-rank test and between-group comparisons using the Wilcoxon rank-sum test. We limited calculation of mean change in stress score to participants for whom data were complete, as this value was calculated by subtracting, for each participant, the score on day 0 from the score on day 28, and then reporting the mean of these differences. The same analysis was used for calculating the mean changes in stress score over days 0 to 56 and days 28 to 56. We used a χ2 test to compare dichotomized (reduced v. not reduced) stress scores. We calculated daily mean adherence to prescribed use of the stress management tool by summing daily total minutes of use divided by 28 for each participant in the intervention group for days 0 to 28. All statistical analyses were performed with Stata 10 software (StataCorp LP, College Station, Texas).
Ethics approval
We obtained ethics approval from the Conjoint Health Ethics Review Board of the University of Calgary, and we obtained written informed consent from all participants.
Results
Participant characteristics
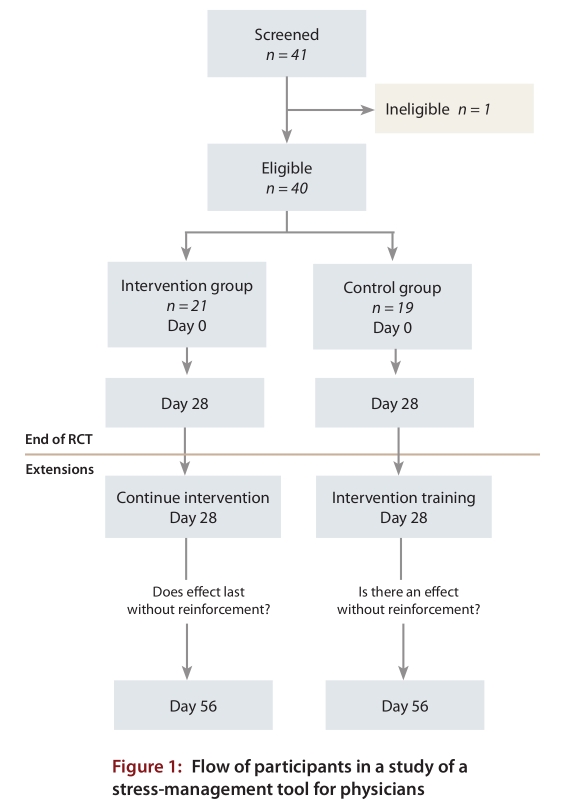
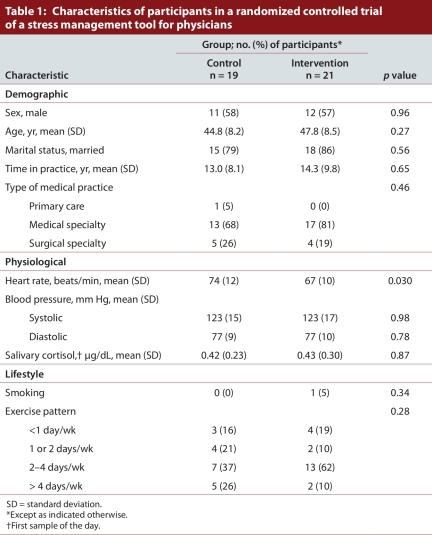
Forty-one potential participants were screened, and 40 physicians from various medical practices participated (19 in the control group and 21 in the intervention group), all of whom completed the study protocol (Figure 1). No adverse effects of the intervention were reported. Complete primary outcome data were available for all but one participant in the control group at day 0 and for all but one person in each group at day 28. The eligible participants consisted of 23 men (11 control and 12 intervention) and 17 women (8 control and 9 intervention) (Table 1). The mean age was 44.8 years in the control group and 47.8 years in the intervention group. Fifteen participants in the control group and 18 in the intervention group were married. Participants in the control and intervention groups had been practising medicine for a mean of 13.0 and 14.3 years, respectively. The mean age of starting practice (32 years) likely reflected the makeup of the cohort, which consisted almost exclusively of specialist physicians, who spend up to 5 years in residency before completing their training. Participants were from a variety of medical practices (1 [2%] from primary care, 30 [76%] from a medical specialty and 9 [22%] from a surgical specialty). With the exception of mean baseline heart rate (67 beats/minute v. 74 beats/minute in the intervention and control groups; p = 0.030), there were no statistically significant differences between the two groups (Table 1).
Figure 1.
Flow of participants in a study of a stress-management tool for physicians
Table 1.
Characteristics of participants in a randomized controlled trial of a stress management tool for physicians
Stress
The following results were obtained with respect to stress.
Randomized controlled trial (days 0–28)
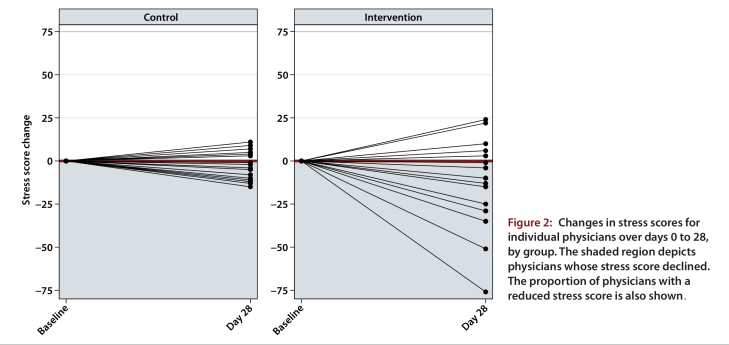
The baseline mean stress score of 81.3 (SD 29.5) for the intervention group dropped to 65.0 (SD 26.6) at day 28, corresponding to a statistically significant mean change of –14.7 (SD 23.8; p = 0.013) (Table 2). The baseline mean stress score of 74.1 (SD 24.5) for the control group dropped to 69.8 (SD 26.6) at day 28, corresponding to a mean change of –2.2 (SD 8.4), but this change was not statistically significant (p = 0.30). The difference in score change between the groups was significant (12.5; p = 0.048). A sensitivity analysis of the individual components of the stress score (Perceived Stress Scale and items derived from the POQA-R) showed a statistically significant decrease in mean stress scores for the intervention group but not for the control group, which mirrored the main study results (results available upon request). Fifteen (75%) of 20 physicians in the intervention group but only 10 (59%) of 17 in the control group had reduced stress scores at day 28 relative to day 0 (χ2 p value = 0.30) (Figure 2).
Table 2.
Stress and physiological measurements, including within-group changes and between-group differences over days 0 to 28, by group
Figure 2.
Changes in stress scores for individual physicians over days 0 to 28, by group
Trial extension (days 28–56)
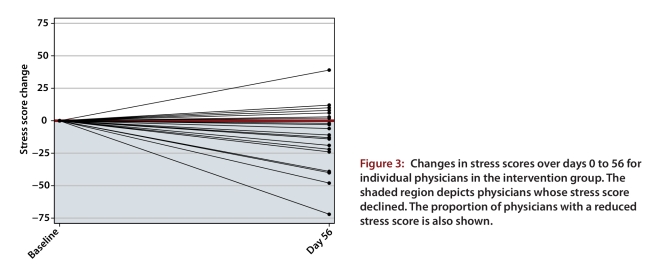
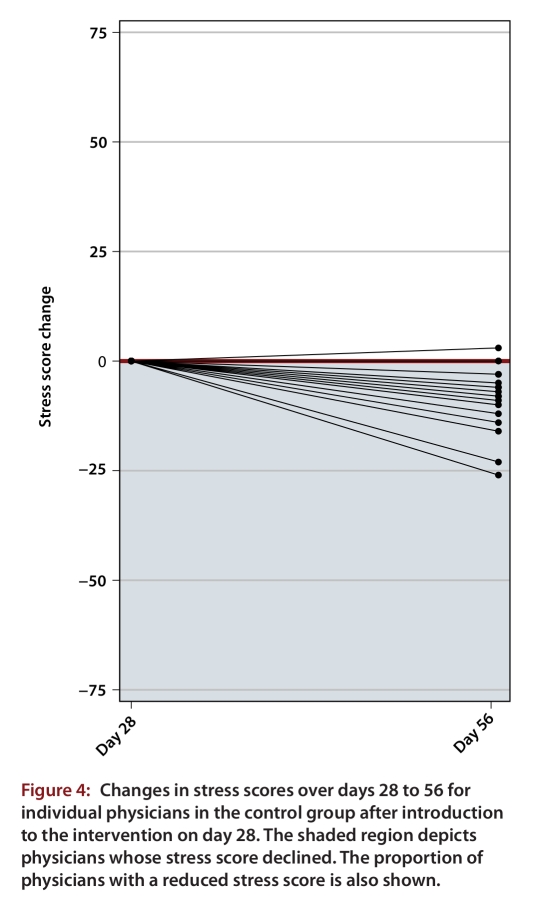
For the intervention group, a reduction in the mean stress score was maintained through to day 56. The day 0 mean score of 81.3 (SD 29.5) dropped to 68.3 (SD 29.1) at day 56, corresponding to a statistically significant change of –13.0 (SD 25.0; p = 0.027). Fourteen (67%) of 21 physicians in the intervention group had a reduced stress score at day 56 relative to day 0 (Figure 3; p = 0.12). For the control group, whose participants underwent training for use of the stress management tool without intensive reinforcement and support during days 28 to 56, mean stress scores also dropped, from 69.8 (SD 26.6) at day 28 to 61.3 (SD 25.1) at day 56, corresponding to a statistically significant change of –8.5 (SD 7.6; p < 0.001). Fifteen (83%) of 18 physicians in the control group had a reduced stress score at day 56 relative to day 28 (Figure 4; p = 0.005).
Figure 3.
Changes in stress scores over days 0 to 56 for individual physicians in the intervention group
Figure 4.
Changes in stress scores over days 28 to 56 for individual physicians in the control group after introduction to the intervention on day 28
The mean changes in stress score experienced by the intervention group in the randomized controlled trial (–14.7, 95% confidence interval [CI] –25.8 to –3.6) and by the control group exposed to the intervention during the trial extension (–8.5, 95% CI –12.3 to –4.7) were not significantly different (p = 0.30). The mean change in stress score experienced by the control group during the randomized controlled trial (–2.2, 95% CI –6.5 to 2.1) was significantly different from the mean change in stress score when the same group was exposed to the intervention during the trial extension (–8.5, 95% CI –12.3 to –4.7) (p = 0.026).
Adherence
In terms of adherence, 6 participants in the intervention group met the criteria for good adherence (an average of 15 or more minutes per day during the intervention period), and 14 had poor adherence (data were missing for one participant). Those with good adherence all showed a decrease in their stress scores during the intervention period. Of the 14 with poor adherence, 9 had decreased stress scores and 5 had increased stress scores.
In terms of stress score change in the intervention group and during the intervention period, the 15 participants with decreased stress scores used the stress management tool over a range of 2 to 40 minutes per day (mean 14, SD 10), with a total 28-day cumulative range of 47 to 1120 minutes (mean 386, SD 292). The 5 participants with increased stress scores used the stress management tool over a range of 5 to 12 minutes per day (mean 9, SD 3), with a total 28-day cumulative range of 151 to 347 minutes (mean 260, SD 76).
Physiological measurements
Over days 0 to 28, there were no statistically significant within-group changes in blood pressure, heart rate or salivary cortisol, nor any significant differences between the control and intervention groups (Table 2). Across days 0 to 56, the intervention group had no significant changes in blood pressure or heart rate, nor did the control group when using the stress management tool during days 28 to 56. Notably, only 2 people in the control group and 3 in the intervention group had systolic blood pressure above 140 mm Hg on day 0.
Discussion
An intervention with a stress management tool consisting of rhythmic breathing, actively self-generated positive emotion and a portable biofeedback device to reinforce positive physiological change in terms of HRV was associated with a significant decline in measured stress for physicians. The benefit of this decline to the intervention group was sustained over a prolonged period (i.e., during an open-label trial extension of 28 days). Furthermore, the control group, when exposed to the intervention during the trial extension period without intensive reinforcement and support, also showed a significant decrease in measured stress. Adherence data suggested that an average of 15 minutes per day of use of the stress management tool over a 1-month period may be sufficient to provide benefit. The study did not reveal changes in any of the secondary outcome measures, most likely because of the relatively short trial period. For heart rate and blood pressure, the starting values were in the normal range, which may also have contributed to the finding of no change over the study period. Although it is difficult to quantify what is a meaningful or clinically significant difference when using health-related instruments, the literature supports a threshold of change of approximately half a standard deviation as being a “minimally important difference.”18 The change observed in the intervention group met this criterion. In addition, the results of a related qualitative study in which the physicians were interviewed at the end of this study suggested that nearly all intended to continue using the stress management tool because of the positive effects it had had on how they dealt with work-related stress (unpublished data).
The practice of medicine is stressful for physicians, involving increasing workloads, emotionally charged situations, excessive cognitive requirements and frequent organizational changes. The potential psychological and physical effects of stress upon physicians were well documented in a previous review.2 In related research, Cohen and colleagues explored the basic physiology of stress facing medical students when having to deliver bad news versus good news using a simulated patient encounter. The delivery of bad news was associated with increased cardiovascular responses, self-reported distress and an increase in natural killer cell function.19 Physicians may use a variety of strategies to cope with stress, including problem-focused coping, which facilitates completion of work tasks (e.g., making a plan of action); emotion-focused coping, which assists people in managing their emotional reaction to stressors (e.g., using humour to lighten the situation); and seeking support from colleagues, family and friends; or they may use maladaptive coping strategies (e.g., alcohol abuse or drug use).20-23 A literature search (MEDLINE, 1985 to 2010) showed that health systems’ efforts to reduce occupational distress for physicians have included interventions such as alterations of the physical work schedule and environment, establishment of support groups, programs that teach coping strategies, face-to-face counselling sessions and mail-out self-help interventions.24-28 Although biofeedback of various types, in particular those based on HRV, have been used to enhance patient care, we found no evidence of research exploring biofeedback techniques as a stress management tool for physicians.
There may have been several reasons why the intervention that we used in this study was associated with reduced stress scores. First, the concrete visualization of achieving “coherence” provided by the biofeedback device and by the emWavePC sessions with the research assistant may have enhanced both physicians’ skill and their belief in their ability to manipulate psychobiologic responses to stress, thus strengthening compliance and effort. Second, public admission or acknowledgement, prevention and/or treatment of stress are sometimes stigmatized within the medical profession and may be perceived as a sign of weakness and incompetence,2 but the technology- and physiology-based stress management tool may have helped to overcome these challenges by legitimizing the psychobiology of stress and by providing a quantifiable and dynamic measure of stress. Third, the ease of portability and use of the stress management tool were likely contributing factors. Fourth, as competency in achieving coherence progressed, physicians may sometimes have recruited the breathing technique and self-generating positive emotions without enlisting biofeedback from the device. This may have further facilitated stress management even under conditions where using the biofeedback device would have been difficult (e.g., during surgical procedures).
Our study had some caveats and limitations. First, although the study results provide evidence supporting use of a biofeedback-based stress management tool as an effective stress-reduction strategy for physicians, an alternate explanation exists. It is possible that the higher baseline mean stress score in the intervention group compared with the control group, although not statistically significant, allowed the possibility of a greater decline in stress scores in the intervention group, particularly if there is a “floor effect” whereby physician stress can go only so low. However, the suggestion of a true benefit from the intervention was supported by the significant decrease in stress scores for the control group when they used the stress management tool during the open-label trial extension. Second, our measure of stress, constructed from several sources, has not been validated. A single question from the Cohen scale (How often have you felt nervous and stressed?) was split into two separate questions, which may have altered the validity of that part of the instrument used to measure stress in our study. We made this change because the physicians who described their stress in their own words during our pilot study seemed to attach a distinct meaning to each of these descriptors. In addition, our stress measure was constructed to incorporate both a general stress scale and items chosen to tap into the medical profession’s dimensions of stress, based on physicians’ descriptors of their stress experience. The subscale factor and sensitivity analysis suggested that the questionnaire was a robust measure of stress. This was reassuring, but this validation has not been replicated in an independent sample. Third, the study was not designed to identify whether the stress management tool was more effective for helping physicians cope with certain types of stress rather than other types. Fourth, the twice-weekly support from the research team may have contributed to stress reduction. However, the controlled study design should have attenuated this effect. It is also possible that these twice weekly visits increased stress because of the time commitment required from the physicians, such that the stress-reduction benefits might have been underestimated. Fifth, given that participants in the intervention group were not blinded, they may have been subject to the demand characteristics of the study and may have responded to the stress questionnaire in a manner that reduced their stress scores over time, simply because they knew such a reduction was anticipated. Finally, given that the study involved hospital-based physicians at a single centre, the results may have limited generalizability. Future research could be undertaken to confirm that the effect of the stress management tool on stress reduction is maintained over an even longer time period and whether or not it is more effective for certain stressors than others.
Although it is likely that a variety of stress management tools are needed to meet the various needs of physicians as individuals, the results of this study suggest that a biofeedback-based stress management tool is both efficacious and effective for helping hospital-based physicians to manage their feelings of stress. From these findings, further discussion about models of implementation should ensue. One approach would be to leave it to physicians themselves to initiate personal stress-reduction strategies such as using a stress management tool. Alternatively, health care organizations could proactively offer stress-reduction tools for health care providers or even assume greater accountability by introducing system-wide interventions to promote and evaluate physician wellness. Given the growing body of evidence supporting the association between physician wellness and quality of patient care, a simple biofeedback-based stress management tool may present an additional strategy to manage physician stress.
Trial registration
Clinicaltrials.gov identifier: E-22185
Acknowledgments
We thank Debbie Gray for her assistance in coordinating the HeartMath instruction sessions, the Alberta Health Services instructors in HeartMath for their valuable assistance, and W.A. Ghali at the University of Calgary for his critical review of the manuscript. No compensation related to the study was provided for these services. We are also indebted to the Ward of the 21st Century Research and Innovation Centre and the Foothills Medical Staff Association, Foothills Medical Centre, Calgary, Alberta, for providing ongoing support of physician wellness research.
Biographies
Jane B. Lemaire is a Clinical Professor in the Department of Medicine, Faculty of Medicine, University of Calgary, Calgary, Alberta, Canada.
Jean E Wallace is a Professor in the Department of Sociology, Faculty of Social Sciences, University of Calgary, Calgary, Alberta, Canada.
Adriane M Lewin is an Epidemiologist in the Department of Community Health Sciences, Faculty of Medicine, University of Calgary, Calgary, Alberta, Canada.
Jill de Grood is a Sociologist with the Ward of the 21st Century Research and Innovation Centre, University of Calgary, Calgary, Alberta, Canada.
Jeffrey P Schaefer is a Clinical Associate Professor in the Department of Medicine, Faculty of Medicine, University of Calgary, Calgary, Alberta, Canada.
Appendix
Appendix A.
Stress scale to measure global perceptions of stress
Footnotes
Competing interests: None declared.
Funding source: This research was supported by a grant from the Alberta Health Services Wellness and Citizen Engagement portfolio and grants from the Department of Medicine and the Faculty of Kinesiology University of Calgary. The researchers had total independence from the funders. The HeartMath trainers were employees of Alberta Health Services. Otherwise, the funding sources had no role in the design and conduct of the study; the collection, management, analysis and interpretation of the data; of the preparation, review or approval of the manuscript.
Contributors: JBL contributed to study conception and design, analysis and interpretation of the data (including statistical analysis), drafting of the manuscript, critical revision of the manuscript for important intellectual content and obtaining funding. JEW contributed to study conception and design, critical revision of the manuscript for important intellectual content and obtaining funding. AML contributed to study conception and design, analysis and interpretation of the data (including statistical analysis) and critical revision of the manuscript for important intellectual content. JdG contributed to study conception and design, acquisition of data, revision of the manuscript for important intellectual content and administrative support. JPS contributed to study conception and design, acquisition of data, critical revision of the manuscript for important intellectual content and technical support. JBL had full access to all of the data in the study and, as guarantor for the study, takes responsibility for the integrity of the data and the accuracy of the data analysis. All of the other authors also had full access to the data and can take responsibility for the integrity of the data and the accuracy of the data analysis. Data sharing: no additional data are available.
References
- 1.Arnetz Bengt B. Psychosocial challenges facing physicians of today. Soc Sci Med. 2001;52(2):203–213. doi: 10.1016/S0277-9536(00)00220-3. http://linkinghub.elsevier.com/retrieve/pii/S0277953600002203. [DOI] [PubMed] [Google Scholar]
- 2.Wallace Jean E, Lemaire Jane B, Ghali William A. Physician wellness: a missing quality indicator. Lancet. 2009;374(9702):1714–1721. doi: 10.1016/S0140-6736(09)61424-0. http://linkinghub.elsevier.com/retrieve/pii/S0140673609614240. [DOI] [PubMed] [Google Scholar]
- 3.Holroyd Kenneth A, Penzien Donald B. Pharmacological versus non-pharmacological prophylaxis of recurrent migraine headache: a meta-analytic review of clinical trials. Pain. 1990;42(1):1–13. doi: 10.1016/0304-3959(90)91085-W. http://linkinghub.elsevier.com/retrieve/pii/030439599091085W. [DOI] [PubMed] [Google Scholar]
- 4.Kirsch Cynthia A, Blanchard Edward B, Parnes Steven M. A multiple-baseline evaluation of the treatment of subjective tinnitus with relaxation training and biofeedback. Biofeedback Self Regul. 1987;12(4):295–312. doi: 10.1007/BF00998721. http://www.springerlink.com/index/10.1007/BF00998721. [DOI] [PubMed] [Google Scholar]
- 5.Goldstein I B, Shapiro D, Thananopavaran C. Home relaxation techniques for essential hypertension. Psychosom Med. 1984;46(5):398–414. doi: 10.1097/00006842-198409000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Ryan M, Gevirtz R. Biofeedback-based psychophysiological treatment in a primary care setting: an initial feasibility study. Appl Psychophysiol Biofeedback. 2004;29(2):79–93. doi: 10.1023/B:APBI.0000026635.03016.ef. http://www.springerlink.com/openurl.asp?id=doi:10.1023/B:APBI.0000026635.03016.ef. [DOI] [PubMed] [Google Scholar]
- 7.McCraty R, Atkinson M, Tiller W A, Rein G, Watkins A D. The effects of emotions on short-term power spectrum analysis of heart rate variability. Am J Cardiol. 1995 Nov 15;76(14):1089–1093. doi: 10.1016/S0002-9149(99)80309-9. http://linkinghub.elsevier.com/retrieve/pii/S0002914999803099. [DOI] [PubMed] [Google Scholar]
- 8.McCraty Rollin, Atkinson Mike, Tomasino Dana, Stuppy William P. Analysis of twenty-four hour heart rate variability in patients with panic disorder. Biol Psychol. 2001;56(2):131–150. doi: 10.1016/S0301-0511(01)00074-6. http://linkinghub.elsevier.com/retrieve/pii/S0301051101000746. [DOI] [PubMed] [Google Scholar]
- 9.Rozman D, Whitaker R, Becman T, Jones D. A pilot intervention program which reduces psychological symptomatology in individuals with human immunodeficiency virus. Complement Ther Med. 1996;4(4):226–232. doi: 10.1016/S0965-2299(96)80075-6. http://linkinghub.elsevier.com/retrieve/pii/S0965229996800756. [DOI] [Google Scholar]
- 10.Luskin Frederic, Reitz Megan, Newell Kathryn, Quinn Thomas G, Haskell William. A controlled pilot study of stress management training of elderly patients with congestive heart failure. Prev Cardiol. 2002;5(4):168–172. doi: 10.1111/j.1520.037X.2002.01029.x. [DOI] [PubMed] [Google Scholar]
- 11.McCraty R, Lanson S, Atkinson M. Assessment of autonomic function and balance in chronic fatigue patients using 24-hour heart rate variability analysis (Abstract. Clin Autonom Res. 1997;7(5):237. [Google Scholar]
- 12.McCraty Rollin, Atkinson Mike, Tomasino Dana. Impact of a workplace stress reduction program on blood pressure and emotional health in hypertensive employees. J Altern Complement Med. 2003;9(3):355–369. doi: 10.1089/107555303765551589. [DOI] [PubMed] [Google Scholar]
- 13.Spitzer R L, Kroenke K, Williams J B. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary Care Evaluation of Mental Disorders. Patient Health Questionnaire. JAMA. 1999 Nov 10;282(18):1737–1744. doi: 10.1001/jama.282.18.1737. [DOI] [PubMed] [Google Scholar]
- 14.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24(4):386–396. doi: 10.2307/2136404. [DOI] [PubMed] [Google Scholar]
- 15.Cohen S, Williamson G. Perceived stress in a probability sample of the United States. In: Spacapan S, Oskamp S, editors. The social psychology of health. Newbury Park (CA): Sage Publications; 1988. pp. 31–67. [Google Scholar]
- 16.POQA-R Personal and Organizational Quality Assessment–Revised. Boulder Creek (CA): Institute of HeartMath and Caring Management Consulting; 1999-2002. [Google Scholar]
- 17.Carmines E G, Zeller R A. Reliability and validity assessment. Sage University series on quantitative applications in the social sciences, No. 07-017. Newbury Park (CA): Sage Publications; 1979. [Google Scholar]
- 18.Norman Geoffrey R, Sloan Jeff A, Wyrwich Kathleen W. Interpretation of changes in health-related quality of life: the remarkable universality of half a standard deviation. Med Care. 2003;41(5):582–592. doi: 10.1097/01.MLR.0000062554.74615.4C. [DOI] [PubMed] [Google Scholar]
- 19.Cohen Lorenzo, Baile Walter F, Henninger Evelyn, Agarwal Sandeep K, Kudelka Andrzej P, Lenzi Renato, Sterner Janet, Marshall Gailen D. Physiological and psychological effects of delivering medical news using a simulated physician-patient scenario. J Behav Med. 2003;26(5):459–471. doi: 10.1023/a:1025724118504. [DOI] [PubMed] [Google Scholar]
- 20.Wallace Jean E, Lemaire Jane. On physician well being-you'll get by with a little help from your friends. Soc Sci Med. 2007;64(12):2565–2577. doi: 10.1016/j.socscimed.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 21.Graham Jill, Albery Ian P, Ramirez Amanda J, Richards Michael A. How hospital consultants cope with stress at work: implications for their mental health. Stress and Health. 2001;17(2):85–89. doi: 10.1002/smi.884. http://doi.wiley.com/10.1002/smi.884. [DOI] [Google Scholar]
- 22.King M B, Cockcroft A, Gooch C. Emotional distress in doctors: sources, effects and help sought. J R Soc Med. 1992;85(10):605–608. doi: 10.1177/014107689208501006. http://www.ncbi.nlm.nih.gov/pmc/articles/pmid/1433036/?tool=pubmed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weiner E L, Swain G R, Wolf B, Gottlieb M, Spickard A. A qualitative study of physicians’ own wellness-promotion practices. West J Med. 2001;174(1):19–23. doi: 10.1136/ewjm.174.1.19. http://www.ncbi.nlm.nih.gov/pmc/articles/pmid/11154656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dunn Patrick M, Arnetz Bengt B, Christensen John F, Homer Louis. Meeting the imperative to improve physician well-being: assessment of an innovative program. J Gen Intern Med. 2007 Sep 22;22(11):1544–1552. doi: 10.1007/s11606-007-0363-5. http://www.ncbi.nlm.nih.gov/pmc/articles/pmid/17891503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gardiner Maria, Lovell Greg, Williamson Paul. Physician you can heal yourself! Cognitive behavioural training reduces stress in GPs. Fam Pract. 2004;21(5):545–551. doi: 10.1093/fampra/cmh511. [DOI] [PubMed] [Google Scholar]
- 26.Le Blanc Pascale M, Hox Joop J, Schaufeli Wilmar B, Taris Toon W, Peeters Maria C W. Take care! The evaluation of a team-based burnout intervention program for oncology care providers. J Appl Psychol. 2007;92(1):213–227. doi: 10.1037/0021-9010.92.1.213. http://doi.apa.org/getdoi.cfm?doi=10.1037/0021-9010.92.1.213. [DOI] [PubMed] [Google Scholar]
- 27.Rowe M M. Teaching health-care providers coping: results of a two-year study. J Behav Med. 1999;22(5):511–527. doi: 10.1023/a:1018661508593. http://www.scholaruniverse.com/ncbi-linkout?id=10586384. [DOI] [PubMed] [Google Scholar]
- 28.Rø Karin E I, Gude Tore, Tyssen Reidar, Aasland Olaf G. Counselling for burnout in Norwegian doctors: one year cohort study. BMJ. 2008 Nov 11;337:a2004. doi: 10.1136/bmj.a2004. http://www.ncbi.nlm.nih.gov/pmc/articles/pmid/19001492. [DOI] [PMC free article] [PubMed] [Google Scholar]