Abstract
Rapid urbanisation and poor town planning in Malawi has been associated with poor environmental hygiene and sanitation. The aim of the present study was to investigate the prevalence, intensity and some potential risk factors of intestinal helminth infections among children aged 3 – 14 years in an urban and rural community in Southern Malawi. A randomised cross-sectional survey was conducted in July, 1998. Data were collected through questionnaire interview regarding socio-demographic and environmental conditions from households in both areas. Stool samples were collected from 273 children in the urban community and 280 in the rural. There was a significant difference (p<0.001) in the prevalence of helminth infections between the urban and rural communities, 16.5% and 3.6% respectively. Most of the infections were light (93.2% for Ascaris lumbricodes, 85.7% for hookworm). Large variance to mean ratios of egg intensity within age groups and the total study population suggested a high degree of aggregation of the parasites in the communities. Multiple logistic regression analysis showed that certain groups of children in the urban community were much more likely to develop helminth infection. They included children who had pools of water/sewage around houses (OR = 3.0, 95% CI = 1.4 ñ 6.5), did not wear shoes (OR a 7.1, 95% CI = 2.7 – 19.2), did not attend school (OR = 2.8, 95% CI = 1.2 ñ 6.5), had mothers who had 4 to 8 years of education (OR = 5.2, 95% CI = 2.0 – 14.0), had mothers below 35 years of age (OR = 4.09, 95% CI = 1.39 – 16.28) and living in an urban community (OR = 5.3, 95% CI = 2.6 – 12.1). Efforts to reduce helminth infections should focus on reducing exposures.
Introduction
Soil transmitted helminths are of great importance in the health of many populations in developing countries where the frequency of infection is a general indication of the local level of hygiene and sanitation. These helminths include hookworm (Ancylostoma duodenale and Necatoramericanus), roundworm (Ascaris lumbricodes), Trichuris trichiura and Strongyloides stercoralis. These infections are important because they are common, cause a significant burden of disease and yet can be easily treated and prevented. Children are most vulnerable as they are at risk of skin penetration by larvae or ingestion of eggs while playing in soil contaminated with human faeces.
Over 1,000 million people world-wide are estimated to be infected with A.lumbricodes and at least 20,000 die annually.1 Roundworm infestation can lead to some serious pathological conditions which include intestinal obstruction, volvulus, intussusception, liver abscesses, peritoneal lesions and biliary obstruction and inflammation.2 Ascaris has also been associated with protein-energy malnutrition, vitamin A deficiency and vitamin C malabsorption.3 Over 900 million people are infected by hookworm.1 The main sequelae of hookworm infection is the development of hypochromic microcytic anaemia which has been shown to have detrimental effect on cognitive function and school performance.4 A study in Zanzibar, Tanzania, found that 73% of anaemia in school children was attributable to hookworm infection. S. stercoralis and T. trichiura are said to infect 100 and 500 million people respectively.5
Urbanisation plays a very important role in many diseases that are associated with public health and sanitation. Stephenson6 identified socio-economic status and urban or rural environment as two of the most important factors influencing the prevalence of A. lumbricodes in Africa. One would expect that A. lumbricodes and other geohelminth infections would be more common in rural areas, since urban dwellers should have access to better education and housing facilities, and since it is more difficult to defecate indiscriminately in urban areas where vegetation is scarce. Studies in Kenya have disputed this school of thought.7 The overcrowding in urban areas may increase both the risk and intensity of infection.
In Malawi, many children attending out patient clinics are almost routinely dewormed on regular basis. Is this practice justifiable? The few studies that have been carried out in Malawi, have been concentrated on hookworm infection. Luhanga8 found a prevalence of hookworm in Chikwawa district of 46.1% and Morgan et al9 of 49% in Salima. The objective of my study was to determine the prevalence and intensity of helminth infection among children aged between 3 and 14 years in an urban and rural area in Southern Malawi and to determine risk factors for infection.
Methods
To study the effect of urbanisation, two communities on either extreme of the spectrum i.e. rural and urban areas, were chosen. These were Ndirande in Blantyre City and Namitambo in Chiradzulu district. Ndirande is one of the most densely populated townships of Blantyre and depicts a true picture of a rapid, poorly planned urbanisation. Most of the inhabitants can be grouped into the lower socio-economic classes where common occupations are non-skilled or partially skilled and require lower primary school education or none at all. Houses which are mostly metal roofed, built from unburned bricks, are closely clustered together. Drinking water is mostly obtained from public taps that are well distributed. There are no proper sewage systems and latrines are constructed very close to the house. Therefore stagnant water/sewage pools are a common sight.
In contrast, Namitambo is a quiet rural community where most people are subsistence farmers and lead typical African village lifestyle. Houses are also built from unburned bricks, are mostly grass thatched and widely spaced. There are no proper sewage systems but latrines are farther from the house. Drinking water is usually collected from a bore-hole. These two communities are geographically situated approximately 30 kilometres apart, on the Shire Highlands in Southern Malawi.
Children between the age of 3 and 14 years were identified for inclusion in the study. The sample sizes were calculated by EPI-INFO 6 (version 6.04a) computer software package using the formula described by Wayne10 which requires variables like total population in the area, standard normal deviate, degree of desired precision, estimated proportion with disease and the design effect.
The area sampling frame used for this study consisted of villages within the catchment areas of Ndirande and Namitambo health centres. Villages in a 2 km radius around the Ndirande health centre were randomly chosen. Within each chosen village each fifth house starting from the chiefs was sampled. In Namitambo, villages in a 5 km radius around the health centre were chosen and similarly every fifth house was chosen.
Community meetings were held before the study to explain it and at which oral and written consent was obtained from village authorities. Participation in the study was on a voluntary basis. Oral consent in Chichewa was obtained from participants and from parents or guardians on behalf of children. A pre-test was carried out to test the research instruments and procedures, before starting the full-scale study. In Ndirande, ten randomly selected households around the health centre were selected to take part in the pre-test. For these pre-tests the procedures planned for the full-scale study were employed.
Data collection and analysis
Any child between 3 and 14 years in the households visited were recruited into the study. Their personal information e.g. age, sex, wearing of shoes, attending school, history of geophagy, and their household information e.g. sex, age and occupation of head of household, level of education of mother and father, availability and use of latrines, source and storage of drinking water, environmental hygiene, were obtained by interview using questionnaires.
Stool samples were collected from the children at each household visited. For this purpose, labelled plastic containers were distributed and children were advised to collect the first stool sample of the day, preferably in the morning. Parents were instructed to assist in collection in the younger children. The samples were examined within 12 hours of collection using the Formalin-ether concentration technique.11 Ova of Ascaris lumbricodes, hookworm, Trichuris trichiura and larvae of Strongyloides stercoralis were sought. The intensity of the helminth infection was estimated using Stoll's egg count technique. Infected children were treated with Albendazole.
Each questionnaire was edited by the principal investigator at the end of each day. Consistency in the data was regularly checked. On very few occasions when extensive information was inconsistent or missing, repeat interviews were done. On stool microscopy, randomly chosen positive samples were re-examined by a different laboratory technician for consistency. Also during data entry and analysis, the information collected was checked again for completeness.
Laboratory results of stool examination and questionnaire information were compiled, sorted and coded. Worm loads of A. lumbricodes infection were estimated from their stool egg counts according to Sinniah12 where one female worm lays an average of 3540 eggs per gram. The infections were then classified into light (less than 4 worms/person), moderate (between 4 and 10 worms/person) and heavy infections (more than 10 worms/person).13 Similarly hookworm infections were graded into light (less than 500 e.p.g.), moderate (500 to 5000 e.p.g.) and heavy infections (greater than 5000 e.p.g.).14 The degree of overdispersion of the worms amongst their hosts was estimated by calculating the variance to mean ratio.
Analysis was done using EPI-INFO 6 (version 6.04a) and SPSS for Windows (release 6.0) software packages. One way analysis of variance was employed to compare the intensity of helminth infections between the urban and rural communities.
Multivariate analysis was performed to study the association between the prevalence of helminth infection and some potential risk factors. Maximum likelihood estimates of odds ratio and their attendant 95 per cent confidence intervals for each factor after adjusting for other factors were obtained by using logistic regression.
The study protocol was approved by the College of Medicine Research Committee.
Results
A total of 273 children were recruited in Ndirande while 280 were recruited in Namitambo after obtaining the guardianís verbal consent. Sex ratios of male to female were 1:1 in Ndirande and 1:0.9 in Namitambo. The mean age in Ndirande was 7.2 years SD 3.2 (median =7.0) and in Namitambo 7.7 years SD 3.4 (median =7.0). There were similar proportions of children attending school in the urban community (60.2%) as was in the rural community (59.9%). In both areas the proportion of children wearing shoes were low (Ndirande 37%, Namitambo 11.4%). Attributes of households from which the subjects were recruited are compared in tables 1 and 2.
Table 1.
Proportion of different attributes of households
| Urban n(%) |
Rural n(%) |
|
| Male headed of household | 249 (91.2) | 182 (65.0) |
| Mother able to read/write | 213 (78.6) | 131 (48.0) |
| Father able to read/write | 229 (91.2) | 141 (77.5) |
| Mothers years of education | ||
| 1 to 4 years | 85 (31.1) | 192 (68.6) |
| 5 to 8 years | 167(61.2) | 82 (29.3) |
| 9 to 12 years | 21 (7.7) | 6(2.1) |
| Fathers years of education | ||
| 1 to 4 years | 54 (19.8) | 173 (61.8) |
| 5 to 8 years | 150 (54.9) | 86 (30.7) |
| 9 to 12 years | 69 (25.3) | 21 (7.5) |
| Use a latrine | 273 (100) | 270 (96.4) |
| Share a latrine with other households | 195(73.0) | 13(4.8) |
| Source of waterPiped water | 27 (9.9) | - |
| Public tap | 246(90.1) | - |
| Protected well | - | 103(36.9) |
| River | - | 4(1.4) |
| Bore-hole | - | 172(61.6) |
| Pools of water/sewage surrounding house | 159 (59.1) | 2 (0.7) |
Table 2.
Means of different attributes of households
| Urban Mean (± SD) |
Rural Mean (± SD) |
F statistic | P-value | |
| Age of mother | 30.8 ± 8.4 | 34.7 ± 8.5 | 29.44 | <0.001 |
| Age of father | 37.1 ± 7.2 | 47.0 ± 8.5 | 217.90 | <0.001 |
| Number of people per household |
6.3 ± 2.1 | 5.1 ± 1.6 | 57.39 | <0.001 |
| Number of rooms per household |
1.8 ± 0.9 | 2.2 ± 0.7 | 34.13 | <0.001 |
| Crowding index (persons/rooms) |
3.9 ± 1.7 | 2.5 ± 1.1 | 138.85 | <0.001 |
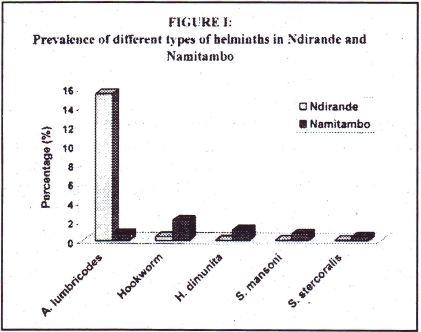
The overall helminth prevalence was 9.9%. The urban population had a higher prevalence of 16.5% as compared to 3.6% in the rural population (p<0.001). The prevalent intestinal helminths were Ascaris lumbricodes, hookworm species, Schistosoma mansoni, Strongyloides stercoralis and Hymenolepis dimunita. The distribution of the different types of intestinal helminths was different in the two communities (table 3).
Table 3.
Frequency of helminth infections in Ndirande and Namitambo
| Ndirande n(%) |
Namitambo n(%) |
Total n(%) |
|
| A. lumbricodes | 42(97.8) | 2 (4.8) | 44 (77.2) |
| Hookworm | 1 (2.3) | 6 (14.3) | 7(12.3) |
| H. dimunita | - | 3 (4.8) | 3 (3.5) |
| S. mansoni | - | 2(2.4) | 2(1.8) |
| S. stercoralis | - | 1(7.1) | 1(5.3) |
| Total | 43 (100) | 14 (100) | 57 (100) |
Double infection was observed in two children (0.4%). One had an infection of hookworm and S. mansoni while the other had A. lumbricodes and S. mansoni. There were no significant associations between the prevalence of helminth infection and host sex or age.
Egg count data, as expected15, were found to be highly variable, and as a consequence they were subjected to logarithmic transformation [log (x + 1)] before analysts of variance was undertaken. Analysis of variance revealed no significant differences in intensity between the two communities with regard to any of the types of helminth. It was also found that the helminth intensities were independent of host age and sex.
The mean worm load for Ascaris was 1.17 SD 1.88 worms. It was observed that 93.2% of A. lumbricodes were light infections. The mean egg count for hookworm infection was 241.7 SD 172.2 epg. It was also observed that a large proportion of the hookworm infections (85.7%) were light and the remaining were moderate (14.3%). Measurement of the average worm burden was not particularly helpful since the frequency distribution of numbers of A. lumbricodes and hookworm was aggregated or overdispersed. In practice, this means that for a given population or sample of hosts a few individuals will harbour most of the worms in the community.
In univariate analysis, there were some significant differences in the prevalence of intestinal helminth infection by sociodemographic and environmental factors. Some of the non-significant variables i.e. did not meet the p<0.1 criteria for inclusion in the multivariate analysis model, were sex and age of the child, parental occupation, sharing a latrine, source and storage containers of drinking water and geophagy.
In multivariate analysis, certain groups of children were found to be more likely to develop the infections after controlling for confounding variables (table 4).
Table 4.
Multivariate Logistic Regression Model: Adjusted Odds Ratios (OR) of potential risk factors in acquiring helminth infection in Ndirande
| Variables | Odds Ratio | 95% Confidence Intervals |
p value |
| Having pools of sewage/water around houses |
3.0 | 1.4 – 6.5 | 0.004 |
| Not wearing of shoes | 7.1 | 2.1 – 19.1 | <0.001 |
| Not attending school | 2.8 | 1.2 – 6.5 | 0.02 |
| Mother having 4 to 8 years of education |
5.2 | 2.0–14.0 | 0.001 |
| Mothers age being <35 years |
3.1 | 1.0 – 9.3 | 0.05 |
| Living in an urban community |
5.3 | 2.6 – 12 | <0.001 |
Living in the urban itself carried a significantly increased risk (OR = 5.3, 95% CI = 2.6–12.1). Other factors in the urban community included having pools of sewage and/or water surrounding houses, not wearing shoes, not attending school and the mothers age being less than 35 years. There was an increase in risk of helminth infection if the mother had 4 to 8 had years of education. In the rural population there was no group had of children that were significantly at risk of infection. This may Cant be due to the low prevalence, impairing significance testing.
Discussion
This study has shown that the most prevalent helminth in the urban community, Ndirande, was Ascaris lumbricodes and in the rural community, Namitambo, was hookworm, most probably Necator americanus which is dominant in this part of Africa.13 This distribution was expected. A. lumbricodes is acquired from the ingestion of eggs from contaminated soil and objects in houses and public places. Hookworm is acquired when the infective larvae in the soil contaminated by human faeces penetrates the skin. For hookworm transmission, it is required that the potential host spends a considerable amount of time in contact with the contaminated soil.
Although the prevalence Of helminth infection in the urban community is significantly greater than that observed in the rural community, they are lower than those reported in other studies that have been carried out in other parts of Malawi. Luhanga8 reported prevalence of hookworm of 46.1% among school children in Chikwawa district and Morgan et al9 found a prevalence of 49% among school children in Salima district. This difference may be attributed to the different climatic and soil conditions that are common to Chikwawa and Salima.
There are some well-documented cases of seasonal fluctuations in the prevalence of A. lumbricodes17. Seasons influence either directly on the environment, and therefore on transmission rates, or indirectly on the activities of the people. Rain plays an important role in the transmission ecology of the parasite. As a result of heavy rain, splashing may cause the eggs to be lifted above the surface of the soil and become firmly attached to leaves hence aiding transmission. The present study was undertaken during the dry season which may have had an impact on the findings.
In the present study, the intensity of the infection was measured as egg output. However, more accurate information on the morbidity, transmission intensity and other parameters of the population ecology of the parasites would have been obtained from expulsion studies.18 The limitations of quantifying intensity using the method in this study which include misdiagnosis at low intensities of infection were recognised and weighed against disadvantages of carrying out chemotherapy expulsion methods. Worm expulsion is logistically difficult to carry out, particularly when large samples are involved. Furthermore, because it is unpleasant and tedious, the use of this technique may lead to decreased is compliance from the population being screened.
The frequency distribution of both hookworm and A. lumbricodes egg counts in the study population were highly n overdispersed (data not shown). Large variance:mean ratios is were found for both hookworm and A. lumbricodes infection. The reasons why only a minority of hosts is heavily infected are not known. A. lumbricodes has been observed to stimulate partial resistance to subsequent infection. Characteristics of the individual host are likely to govern the degree of exposure to infective stages, and/or the ability of individuals to mount an immune response
Some of the most important factors determining the degree of transmission of helminths within a community are the prevailing socio-economic and environmental conditions and behavioural patterns within that community. If a community is viewed as an ecosystem that is characterised as economically depressed like Ndirande, several implications may follow; overcrowding, poverty, and poor sanitation, including indiscriminate defecation or inappropriate facilities for the disposal of human excreta may be expected to prevail in the ecosystem.
In the present study, having pools of water and/or sewage surrounding the house and not wearing shoes have been shown to be very important risk factors in the acquisition of helminth infections. The former suggests a faecal-oral mode of transmission. Good sanitation protects children by creating series of barriers to keep helminths out of their environment. Lack of wearing shoes increases the chance of skin penetration by infective larvae of hookworm while playing in contaminated soils. Among the non-school attendees, they are presumably exposed to a higher risk of infection while playing more around the house which are surrounded by pools of water and/or sewage than at school. The high risk of infection associated with crowding tallies in with the earlier observation of an economically depressed ecosystem which leads to inappropriate disposal of human excreta and consequently to helminth disease.
Nevertheless, efforts to reduce helminth infections should focus on reducing exposures. An integrated approach to the control of helminth infection is important. Playing a crucial and central role in this, is sanitary control. Studies have shown that availability of superior water and sanitation facilities help in controlling the infections21. The present study supports improved personal and environmental hygiene and sanitation in the control of helminth infection. This is evidenced by the increased risks associated with having pools of water and/or sewage surrounding the house, not wearing of shoes among children and crowding.
Another control method is the use of targeted chemotherapy as in most communities in which helminth infection is endemic the majority of worms are harboured within a relatively small fraction of the population. Common groups to target mass chemotherapy are school children. If chemotherapy was given to all school children in the present study, only half of the infections would be treated. Other variables of the population need to be known for effective control of the infection. These include the reproductive rate of the parasite, rate of re-infection and efficacy of the antihelmintic agent.20
Recommendations
The concept of an integrated control strategy including the physical, biological and cultural components of an environment is becoming increasingly relevant in attempting to decrease any public health problem. Although in this study, helminth infection is not depicted to be a major problem in the Blantyre area, this may not be the case. There is need for more studies in Blantyre area and other parts of Malawi using different sampling methods and different study designs.
For the formulation of an effective helminth prevention and control program for Malawi, there is a need for more studies to gather information on prevalence and intensity of helminth infection. This needs to be done according to age, sex and socio-economic status, re-infection rates after chemotherapy, seasonal variation, morbidity and mortality and transmission dynamics of the infection. From such data, an integrated approach for the control of helminth infection can be made which must place greater emphasis on local responsibility as compared to outside responsibility. The outsider must only act as a catalyst with the local community playing the major role. Control strategies in Malawi must be aimed at the free-living stages (eggs and larvae) of the helminths which are largely dependent upon safe disposal of faeces. Education programmes should target the habits, customs and/or beliefs concerned with cleanliness and personal hygiene as they are major obstacles to the regular use of latrines, even if cost is not a prohibitive factor. Mass chemotherapy should only be a last resort because in endemic areas, re-infection is rapid and repeated treatment is not cost-effective.
Figure 1.
Prevalence of different types of helminths in Ndirande and Namitambo
Acknowledgements
I would like to thank the World Health Organisation for funding the project. Special thanks must go to my supervisor Prof G Ssembtya-Lule and to Dr Chris Whitty and Dr Steve Graham for their guidance with project design and data analysis. I am also grateful to Nellie Banda, Dave Mwale for data collection, Rhoda Sinkani for laboratory analysis and Maria Chinjoka for typing.
This paper has been published in modified form in the Annals of Tropical Medicine and Parasitology and is reproduced with kind permission of the editors.
The following paper is the product of a Year 4 elective medical student research project by Kamija Samuel Phiri and was awarded an international prize for best Medical Student Elective Project by the Royal Society of Tropical Medicine and Hygiene, London, UK, in 2000.
References
- 1.WHO model preserving information, author. Drugs used in parasitic diseases. Geneva: World Health Organisation; 1990. pp. 82pp. 84–86. [Google Scholar]
- 2.Manson-Bahr PEC, Bell DR. Mansonis Tropical Diseases. 19th edition. London: Baillrere Tindall; 1987. [Google Scholar]
- 3.Forsum E, Nesheim MC, Crompton DWT. Nutritional aspects of Ascaris infection in young protein deficiency pigs. Parasitology. 1981;83:497–512. [Google Scholar]
- 4.Soewondo S, Husaini M, Pollitt E. Effects of iron deficiency on attention and learning processes in pre-school children: Bandung, Indonesia. Am J Clin Nutr. 1989;50(Suppl 3):667–673. doi: 10.1093/ajcn/50.3.667. [DOI] [PubMed] [Google Scholar]
- 5.Stoltzfus RJ, Chiwaya HM, Tielsch JM, Schulze KJ, Albonico M, Savioli L. Epidemiology of iron deficiency anaemia in Zanzibar school children: the importance of hookworms. Am J Clin Nutr. 1997;65:153–159. doi: 10.1093/ajcn/65.1.153. [DOI] [PubMed] [Google Scholar]
- 6.Stephenson LS. Factors affecting the prevalence and control of Ascaris lumbricodes infection in Kenya. In: Crompton DWT, Nesheim MC, Pawlowski ZS, editors. Ascaris and its Public Health Significance. London: Taylor & Francis; 1985. pp. 113–127. [Google Scholar]
- 7.Danee K, Cross A. Prevalence of Ascaris lumbricodes infection in standard 1 children in 16 Nairobi schools. 1974 – 1975. In: Crompton DWT, Nesheim MC, Pawlowski ZS, editors. Ascaris and its Public Health Significance. London: Taylor & Francis; 1985. pp. 118–119. [Google Scholar]
- 8.Luhanga HM. Report on Bilharzia and hookworm survey results. Lilongwe: Ministry of Health; 1993. [Google Scholar]
- 9.Morgan PRF, Yamamoto CH, Teesdale CH, Pugh RNH. Albendazole: a new treatment for hookworm. Malawi Med J. 1985;3:4–5. [Google Scholar]
- 10.Wayne W D. Biostatistics: a foundation for analysis in the Health Sciences. 5th edition. John Wiley & Sons; 1987. p. 157. [Google Scholar]
- 11.Cheesbrough M. Medical laboratory manual for tropical countries. 2nd edition. Vol. 1. Cambridge: Tropical Health Technology and Butterworth-Heinemann; 1987. pp. 184–186. 188–189, 192–193. [Google Scholar]
- 12.Sinniah B. Daily egg production of Ascaris lumbricodes: the distribution of eggs in faeces and the variability of egg counts. Parasitology. 1982;84:167–175. doi: 10.1017/s0031182000051763. [DOI] [PubMed] [Google Scholar]
- 13.Seo BS. Ascariasis and its control problems in Korea. In: Warren KS, Mahmoud AAF, editors. Tropical and Geographical ' Medicine. 2nd edition. New York: McGraw-Hill; 1981. p. 377. [Google Scholar]
- 14.Schad GA, Banwell JG. Hookworms. In: Warren KS, Mahmoud AAF, editors. Tropical and Geographical Medicine. 2nd edition. New York: McGraw-Hill; pp. 359–372. [Google Scholar]
- 15.Asaolu SO, Holland CV, Jegede JO, Fraser NR, Stoddard RC, Crompton DWT. The prevalence and intensity of soil-transmitted helminthiases in rural communities in Southern Nigeria. Ann Trop Med Parasitol. 1992;86:279–287. doi: 10.1080/00034983.1992.11812665. [DOI] [PubMed] [Google Scholar]
- 16.Macpherson CN, Craig PS. Parasitic helminths and zoonoses in Africa. London: Unwin Hyman; 1991. [Google Scholar]
- 17.Gelphi AP, Mustafa A. Seasonal pneumonitis and eosinophilia: a study of larval ascariasis in Saudi Arabs. Am J Trop Med Hyg. 1967;92:7–11. [PubMed] [Google Scholar]
- 18.Bundy DAP, Medley GF. Immuno-epidemiology of the human geo-helmimhiasis: ecological and immunological determinants of worm burden. Parasitology. 1992;104:105. doi: 10.1017/s0031182000075284. [DOI] [PubMed] [Google Scholar]
- 19.Sorensen E, Ismail M, Amarasinghe DKC, Hettiarachchi I, Dassenaieke TSDe C. The effect of the availability of latrines on soil-transmitted nematode infections in the plantation sector in Sri Lanka. Am J Trop Med Hyg. 1994;51:36–39. doi: 10.4269/ajtmh.1994.51.36. [DOI] [PubMed] [Google Scholar]
- 20.Anderson RM. Transmission dynamics of Ascaris lumbricodes and the impact of chemotherapy. In: Crompton DWT, Nesheim MC, Pawlowski ZS, editors. Ascaris and its Prevention and Control. London: Taylor & Francis; 1989. pp. 253–273. [Google Scholar]