Abstract
The orchid Dactylorhiza sambucina shows a stable and dramatic flower-color polymorphism, with both yellow- and purple-flowered individuals present in natural populations throughout the range of the species in Europe. The evolutionary significance of flower-color polymorphisms found in many rewardless orchid species has been discussed at length, but the mechanisms responsible for their maintenance remain unclear. Laboratory experiments have suggested that behavioral responses by pollinators to lack of reward availability might result in a reproductive advantage for rare-color morphs. Consequently, we performed an experiment varying the relative frequency of the two color morphs of D. sambucina to test whether rare morph advantage acted in the natural habitat of the species. We show here clear evidence from this manipulative experiment that rare-color morphs have reproductive advantage through male and female components. This is the first demonstration, to our knowledge, that negative frequency-dependent selection through pollinator preference for rare morphs can cause the maintenance of a flower-color polymorphism.
The evolutionary significance of floral polymorphisms has excited biologists since Darwin (1). The advertisement provided by the corollas of angiosperms is vital in enabling pollinators to locate the source of food reward (2, 3). Although flower color is diverse throughout the flowering plants, variation within species is uncommon. Most reported cases of flower-color polymorphisms cover a small color range, or the colors concerned are not distinguished by pollinators and may simply represent phenotypic plasticity or balance between mutation and selection (4, 5). Species that show a true, stable, genetically based polymorphism are comparatively rare (4).
Orchid species are unusual in that high levels of corolla-color variability have been recorded within many species (6–9). These variable species also are known not to produce a reward for their pollinators, either through nectar or pollen (10). Does the absence of reward play a role in the maintenance of these corolla-color polymorphisms? It has been suggested that both the rewardless characteristic of the plant and the naivete of pollinators may contribute to their maintenance (7, 11, 12). Rewardless orchids are usually pollinated by newly emerged naive insects, typically bumblebees in Europe (6). When encountering a novel polymorphic rewardless food source, behavioral experiments showed that bumblebees tend to sample different color morphs in alternation, because visiting an empty flower increases the probability of switching to a different color morph (12). This behavior results in rare morphs being proportionately over-visited (12). If the number of visits to a given morph is correlated positively with male and female reproductive success in rewardless orchid populations, the relative fitness of a morph is predicted to decrease as its relative frequency increases. Such negative frequency-dependent selection could maintain stable corolla-color polymorphisms in these species. To maintain a polymorphism, the relative advantage for each morph should reverse at some particular frequency. This frequency, where each morph has an equal fitness, is the predicted equilibrium morph frequency. This frequency will be reached regardless of initial gene frequencies or the system of genetic control of the color polymorphism, assuming that selection acts only on the phenotype (13, 14).
The Elderflower orchid Dactylorhiza sambucina is a rewardless species that has a striking purple-yellow flower-color polymorphism (Fig. 1). This polymorphism is present throughout the European range of the species (15) and pollinators are able to discriminate between the two distinct morphs (ref. 6; Fig. 2). To test the negative frequency-dependent selection hypothesis, we performed a manipulative experiment that used synthetic arrays to vary the relative frequency of the two color morphs while maintaining constant density.
Figure 1.
Two individuals of D. sambucina are shown.
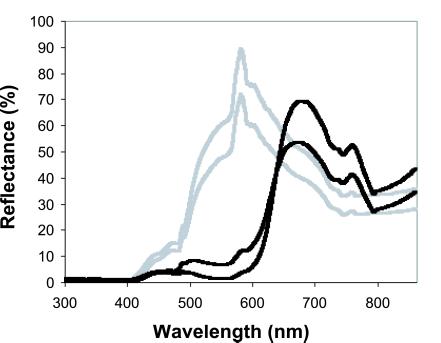
Figure 2.
The reflectance spectrum of the two flower colors is given as a function of the wavelength (light gray for the yellow morph and black for the purple morph). Two spectra are given for each color representing the range of the spectrum found over 10 individuals. Spectra were recorded by using a portable reflectance spectrophotometer (High Sensitivity Spectrophotometer S2000, New Electro-Optical Concepts, Beaufays, Belgium) that gave a reflectance spectrum across all of the wavelengths (including UV) to which insect pollinators are sensitive (16).
Materials and Methods
Biological Model.
D. sambucina is a widespread European orchid with a boreal-alpine distribution (15). The mean frequency of yellow-flowered D. sambucina across natural populations in Europe is 53% ± 2.6 (n = 174). We did not find any differences between the two morphs for other traits such as plant height or the number of leaves and flower size, and the two morphs are identical for floral scent (6).
Experimental Design.
We constructed 10 synthetic arrays of D. sambucina, varying the morph frequency within the arrays to test the influence of morph frequency on relative reproductive success. The experiment was performed in France during May and June 2000 on the central part of the Causse Noir (N 44° 10′, E 03° 20′, Altitude: 1,000 ± 11 m) in the Massif des Cévennes where D. sambucina does not occur naturally, although populations are recorded frequently elsewhere in the Massif and at the edge of the Causse (minimum 5 km from the experimental areas). We chose such isolation to ensure naivete of the pollinators to D. sambucina. The predominant pollinators of D. sambucina are bumblebees, principally Bombus lapidarius (6) and Bombus terrestris (L.G. and A.S., personal observation), which were abundant in the experimental areas. We removed 600 individual plants from a population ≈12 km from the Causse and potted them. Experimental arrays then were constructed in 10 locations on the Causse. Each location was chosen for habitat similarity both to each other and to that of natural populations. A minimum distance of 2 km separated the locations to ensure isolation of the pollinator fauna. Arrays consisted of 50 plants dispersed on a square grid. Grid size was 9.5 by 9.5 m, with grid spacing of 0.5 m, giving 400 potential positions for plants. Plants were allocated randomly to arrays after we controlled for flower number, reallocating plants among arrays where necessary. Plants then were allocated randomly to the 400 potential positions within arrays. Two replicates each of 0.1, 0.3, 0.5, 0.7, and 0.9 yellow morphs were used, with treatments randomly allocated to arrays. We commenced experiments on May 6th, 2000, the peak flowering time for D. sambucina in surrounding natural plant populations. After 5 days, the number of pollinia removed and the number of stigmas with pollen depositions were quantified (all pollinator visitation had occurred by this point). Six weeks later, fruit set was assessed by counting the total number of fruits produced per plant.
Reproductive Traits.
We calculated male reproductive success per plant by dividing the total number of pollinia removed by those available and the total number of pollen deposition recorded and fruit set by the number of available flowers. These values then were averaged separately for the two morphs within each array. We found no significant differences in flower number between morphs across arrays (F10,499 = 0.393, P > 0.05), nor between morphs for fruit length (F10,723 = 1.519, P > 0.05), fruit width (F10,723 = 0.630, P > 0.05), or dried weight of seeds per fruit (F10,723 = 0.352, P > 0.05). The average reproductive success of the arrays was very similar to those recorded in natural populations, which are limited by access to pollinators. The relative reproductive success of the yellow morph for each array was calculated as RRSy = 2.RSy/(RSy + RSp). RRSy is the relative reproductive success of the yellow morph, RSy is the average reproductive success of the yellow morph, and RSp is the average reproductive success of the purple morph. We then tested the relationship between relative reproductive success of the yellow morph and its frequency among arrays by correlation and regression. After experiments were completed, all plants were returned to their precise positions in the source population.
Results and Discussion
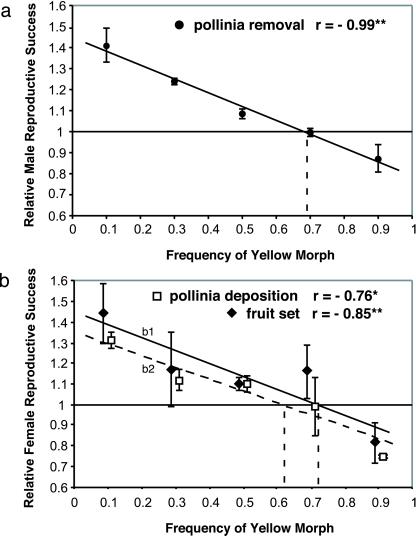
We found that relative male and female reproductive success of the morphs, as measured by relative pollinia removal, relative pollinia deposition, and fruit set, were all correlated significantly negatively with morph frequency across experimental arrays (Fig. 3). For all estimates of relative reproductive success, the identity of the morph that has the relative advantage reverses as the frequency of yellow plants increases. At both extreme frequencies, the rare morph has a significantly higher value for all indices of reproductive success considered, compared with the common morph. To our knowledge, these results are the first to clearly show that negative frequency-dependent selection, mediated through pollinator foraging preference, has the potential to maintain a color polymorphism in natural plant populations. Assuming that pollinator-induced selection acts alone on this polymorphism, we can use the relationship we found between reproductive success and morph frequency to predict the equilibrium morph frequency expected in nature. The predicted morph frequencies (Fig. 3) were 69% for yellow morphs, based on pollinia removal, and 72% and 61% for pollinia deposition and fruit set, respectively (Fig. 3). In the region of France where these array experiments were performed, the mean morph frequency is 69% ± 3% yellow morphs (n = 20 populations), which is entirely consistent with these predictions. In populations elsewhere in South Europe, we have found similar morph frequencies but higher levels of variation in morph frequency among populations at high altitude and at the northern edge of the species' range in Scandinavia. Genetic drift may result in such deviations from predicted equilibrium morph frequencies. In natural populations, the relative reproductive success of a given morph of D. sambucina also was found to correlate negatively with morph frequency (17).
Figure 3.
Relative male (a) and female (b) reproductive success of the yellow morph as a function of the relative frequency of the yellow morph in each array. Male reproductive success for a morph was quantified as the average proportion of pollinia removed from plants within each array (n = 5 to 45 plants) (●). Female reproductive success was measured as the average proportion of stigmas receiving pollen (⧫) and setting fruits (□) for plants within each array. Relative reproductive success was calculated as described in Materials and Methods. Each data point represents the mean of two arrays per frequency, with bars showing one SEM. Statistics were calculated by using all values. r is the Spearman correlation coefficient (Pearson correlation coefficients were identical). *, P < 0.05; **, P < 0.01. The slopes given are the lines fitted in regression analysis: (a) y = −0.66x + 1.452; (b1, solid line for pollinia deposition) y = −0.63x + 1.454, and (b2, broken line for fruit set) y = −0.59x + 1.354. The horizontal line corresponds to equal reproductive success between the two morphs. The intersection between regression lines and the horizontal line gives the value of predicted morph frequencies at equilibrium (represented by vertical dotted lines).
It can be seen that predicted morph frequencies are biased constantly to yellow. This bias toward yellow morphs in terms of the relative reproductive success and equilibrium morph frequency is not unexpected. Pollinator-behavior experiments frequently record innate pollinator bias to particular colors (12, 18), and the bias could reflect foraging behavior that alternates between morphs but favors one over the other. Alternatively, it has been suggested that the predominant colors of coflowering plants in the plant community surrounding rewardless orchid populations may influence the color preferences of pollinators (6). This behavior in turn could modify the predicted equilibrium morph frequency, although not the prediction that negative frequency dependence can maintain a polymorphism.
Negative frequency-dependent selection is often cited as a central mechanism in maintaining phenotypic variability in natural populations (19), but convincing demonstrations of negative frequency-dependent selection acting in natural animal or plant populations are, however, scarce (20–22). Frequency-dependent selection caused by behavioral preferences is best known from predator–prey systems, where predator behavior leads to preference for common prey morphs (23–25). This preference would be expected to lead to rare morph advantage through greater mortality of common morphs in prey populations. In contrast, such preferences for common morphs among pollinators foraging on rewarding plants would tend to lead to an advantage for the common morph. Such positive frequency-dependent selection is predicted to lead to monomorphism (26–29). This study strongly suggests a reversal in pollinator behavior for rewardless plants, giving negative frequency-dependent selection that can lead to stable polymorphism. This study is a demonstration that pollinator behavior could cause negative frequency-dependent selection. The role of such a selection pattern deserves further attention, particularly when considering its role in maintaining the exceptionally high levels of variability recorded for flower color and other traits such as scent and floral form among rewardless orchid species (7).
Acknowledgments
We thank the Parc National des Cévennes for permission to work in the periphery of the park; C. Lee, C. Jelensperger, and R. Gala for their help in the field; Mr. Rouzier and Mr. and Ms. Arjailles for allowing access to the source population; and Mr. and Ms. Baraille, Mr. and Ms. Tritz, the Passet family, and all the inhabitants of Meyrueis and the Causse Noir for their participation in locating arrays. We also thank C. Lee for the photograph of D. sambucina. L.G. was supported by National Environment Research Council (London) Grant GR3/12106 (to A.S. and M.R.M.). A.S. was supported by National Environment Research Council (London) Research Fellowship GT5/98/12/TS.
References
- 1.Darwin C. The Effects of Cross and Self Fertilisation in the Vegetable Kingdom. London: Murray; 1876. [Google Scholar]
- 2.Waser N M. In: Pollination Biology. Real L-A, editor. New York: Academic; 1983. pp. 242–285. [Google Scholar]
- 3.Chittka L, Menzel R. J Comp Physiol A. 1992;171:171–181. doi: 10.1007/BF00199332. [DOI] [PubMed] [Google Scholar]
- 4.Kay Q O N. The Pollination of Flowers by Insects. 1978. , Linnean Society Symposium Series No. 6, ed. Richards, A.-J. (Academic, London), pp. 175–190. [Google Scholar]
- 5.Waser N M, Price M-V. Evolution (Lawrence, Kans) 1981;35:376–390. doi: 10.1111/j.1558-5646.1981.tb04896.x. [DOI] [PubMed] [Google Scholar]
- 6.Nilsson A L. Bot Not. 1980;133:367–385. [Google Scholar]
- 7.Ackerman J D, Galarza-Perez M. Syst Bot. 1991;16:182–194. [Google Scholar]
- 8.Dafni A. J Ecol. 1983;71:467–474. [Google Scholar]
- 9.Cropper S C, Calder D M. Plant Syst Evol. 1990;170:11–27. [Google Scholar]
- 10.Nilsson L A. Trends Ecol Evol. 1992;7:255–259. doi: 10.1016/0169-5347(92)90170-G. [DOI] [PubMed] [Google Scholar]
- 11.Heinrich B. Evolution (Lawrence, Kans) 1975;29:325–344. [Google Scholar]
- 12.Smithson A, Macnair M R. Evolution (Lawrence, Kans) 1997;51:715–723. doi: 10.1111/j.1558-5646.1997.tb03655.x. [DOI] [PubMed] [Google Scholar]
- 13.Clarke B, O'Donald P. Heredity. 1964;19:201–206. [Google Scholar]
- 14.Clarke B C, Shelton P R, Mant G S. Philos Trans R Soc London B. 1988;319:631–640. doi: 10.1098/rstb.1988.0070. [DOI] [PubMed] [Google Scholar]
- 15.Tutin T G, Heywood V H, Burges N A, Moore D M, Valentine D H, Walters S M, Webb D A, editors. Flora Europaea. Cambridge, U.K.: Cambridge Univ. Press; 1980. [Google Scholar]
- 16.Peitsch D, Fietz A, Hertel H, de Souza J, Ventura D F, Menzel R. J Comp Physiol A. 1992;170:23–40. doi: 10.1007/BF00190398. [DOI] [PubMed] [Google Scholar]
- 17.Smithson A. In: Cognitive Ecology of Pollination: Animal Behaviour and Evolution. Chittka L, Thomson J-D, editors. Cambridge, U.K.: Cambridge Univ. Press; 2001. pp. 237–257. [Google Scholar]
- 18.Heinrich B, Mudge P R, Deringis P G. Behav Ecol Sociobiol. 1977;2:247–265. [Google Scholar]
- 19.Ayala F J, Campbell D. Annu Rev Ecol Syst. 1974;5:115–138. [Google Scholar]
- 20.Allen J A. Philos Trans R Soc London B. 1988;319:485–503. doi: 10.1098/rstb.1988.0061. [DOI] [PubMed] [Google Scholar]
- 21.Sinervo B, Lively C M. Nature (London) 1996;380:240–243. [Google Scholar]
- 22.Eckert C G, Manicacci D, Barrett S C H. Heredity. 1996;77:581–588. [Google Scholar]
- 23.Allen J A, Clarke B. Nature (London) 1968;220:501–502. doi: 10.1038/220501a0. [DOI] [PubMed] [Google Scholar]
- 24.Allen J A. Nature (London) 1972;237:348–349. doi: 10.1038/237348a0. [DOI] [PubMed] [Google Scholar]
- 25.Gordon I J. Biol J Linn Soc. 1987;31:1–23. [Google Scholar]
- 26.Levin D A. Am Nat. 1972;106:453–460. [Google Scholar]
- 27.Smithson A, Macnair M R. J Evol Biol. 1996;9:571–588. [Google Scholar]
- 28.Thompson V. Heredity. 1984;53:677–686. [Google Scholar]
- 29.Cresswell J E, Galen C. Am Nat. 1991;138:1342–1353. [Google Scholar]