Abstract
The aim of this study was to characterise breeding sites and climatic factors that influence the abundance of malaria vectors in the Lower Shire valley, Malawi. We regularly sampled adult and larval mosquitoes over the transition periods between the wet and dry seasons during 2000 and 2001. Three potential malaria vectors, An. arabiensis, An. gambine sensu stricto and An. funestus, and a fourth non-vector species An. quadriannulatus, were identified. (This is the first record of An. quadriannulatus in Malawi). These four species bred predominately in larger water bodies, particularly rice paddies, and to a lesser extent in boreholes and puddles. Smaller temporary pools and puddles evaporated too quickly to permit the completion of larval development. Abundance of An. gambiae s.l. was closely associated with minimum temperatures. We discuss the relevance of the findings to malaria vector control in Malawi.
Introduction
Malaria remains one of the most significant causes of morbidity and mortality in Malawi. Despite the recent advances in chemotherapy and in our understanding of the pathology and immunology of this disease, knowledge of the ecology and behaviour of the vectors in Malawi remains sparse. Understanding the natural history of Malawian malaria vectors is essential to understanding malaria epidemiology and to planning a rational control programme. We have initiated a series of studies that aim to characterise the malaria vector populations in Malawi, in terms of their relative abundance, breeding site preferences, biting behaviour and vectorial capacity.
The most important vectors of malaria in sub-Saharan Africa are mosquito species belonging to the Anopheles gambiae complex (An. gambiae sensu lato), two of which, An. gambiae sensu stricto and An. arabiensis are common across the continent. Because it feeds almost entirely on humans in East Africa, Anopheles gambiae s.s. is the more efficient malaria vector of the two; An. arabiensis will more readily feed on cattle and other animals as well as humans and so is less likely to transmit the malaria parasite (Coetzee, et al., 2000). An. gambiae s.s. is more common in more humid areas of Africa, whereas An. arabiensis prefers more arid zones (Lindsay, et al., 1998). However, the two species are sympatric (ie they occupy the same territories) throughout much of their range where aquatic stages of both species can be found in temporary or permanent puddles, borrow pits, irrigation ditches, vehicle ruts and rice paddies. The third major vector of malaria in Africa, Anopheles funestus, breeds in larval habitats that are somewhat different from those of the An. gambiae complex. This species prefers permanent collections of clean water with vegetation, such as marshes, ponds and the weedy edges of ditches or ricefields. These three vector species have previously been recorded in Malawi and shown to be malaria vectors in a number of districts (Tambala et al., 1992, Donnelly & Townson, 2000). Anopheline species can also transmit Wuchereria bancrofti, the causative agent of lymphatic filariasis or elephantiasis.
Because the aquatic breeding sites expand and proliferate following rainfall, malaria transmission typically increases during wet seasons. Defining changes in the breeding habits and relative abundance of each species throughout the year are important first steps towards understanding their importance as malaria vectors. We report here on a study of the breeding site preferences and changes in the species composition of anopheline populations in the Lower Shire valley during the wet and dry seasons.
Materials and methods
Description of the study area
The study was conducted in the rural village of Seseo (16o 05'S, 34o 50E), located 5 km South East of Chikwawa town centre in the Lower Shire Valley in southern Malawi. The population (approximately 400) lives in thatched adobe houses and subsists by growing rice and maize and herding small numbers of cattle, goats and sheep. Seseo village is located on the southern bank of the Shire River, with rice gardens between the village and the river bank and the large Kasinthula irrigation project 250m to the South, across a main road, where bananas, sugar cane and rice are grown. The climate in Chikwawa is hot and humid throughout the year, with daytime temperatures ranging from 25–37°C and relative humidity usually above 15%. Transmission of malaria is perennial, but reaches a peak during the wet season from January to March (Chunga, personal communication).
Mosquito collection and identification
Adult mosquito collections were carried out during the transition periods between the seasons, from April to June 2000 (12 weeks, wet to dry season) and November 2000 to January 2001 (10 weeks dry to wet season). Adults were sampled by pyrethroid knock down (PKD) collections from three randomly selected huts each week. Collections were conducted early in the morning (occupants were previously requested to create as little disturbance within the house as possible until the collection had been completed). All persons, animals, foodstuffs and cooking materials were removed to a safe distance from the house during spraying. Floors and horizontal surfaces were covered with white sheets, and a proprietary household insecticide, Doom® (Dichlorovos, Tetramethrin and d'Phenothrin); (Robertsons Homecare Ltd, South Africa), was used to spray inside and outside at the eaves, windows and doors. Mosquitoes were harvested from the sheets and transferred to a petri dish containing damp filter paper for transportation to the laboratory.
Larval collections were carried out on the same day. Permanent breeding sites, identified during an initial inspection, were sampled weekly. Known and newly detected ephemeral sites were also checked weekly. Five rice paddies were sampled per week from the Kasinthula irrigation project. Larvae were collected using a standard (350 ml) dipper with the number of dips dependent on the surface area of the breeding site: sites of <1m2, 1–5m2 and >5m2 were dipped 5, 10 and 20 times, respectively.
Where possible, all adult and larval Anopheles spp. were identified to species level using taxonomic keys (Gillies & Coetzee, 1987). Reliable species identification of early larval instars is not always possible and identification was limited to the third and fourth instars. All males, larvae and a sample of 60 adult females (20 from each house) of An. gambiae s.l. were identified to species level by polymerase chain reaction (PCR) (Scott et al., 1993). A sample of culicine mosquitoes was identified to species level (Hopkins, 1952).
Climatic data were provided by the Malawi Meteorological Department from daily records collected within the Kasinthula irrigation project. Three climatic factors, maximum temperature (Tmax), minimum temperature (Tmin) and rainfall, were measured to examine whether they affected mosquito abundance. For the purpose of analysis the mean of each parameter was calculated seven days prior to collection.
Results
Relative abundance of malaria vector species
A total of 3,643 adult and 1,169 larval Anopheles sp. was collected during the study, of which the majority (73%) were members of the Anopheles gambiae complex. PCR identification of these samples showed that An. arabiensis was the predominant species (85%), with An. gambiae s.s. and An. quadriannulatus present at lower frequencies (12.5% and 2.5% respectively) (table 1). A significant number of An. funestus were also identified in adult catches. No An. funestus larvae were identified.
Table 1.
Relative abundance (%) of adult and larval Anopheles species collected from within houses and breeding sites, respectively, in Seseo village (n = 3643 and 1169 adults and larvae respectively).
| An. Arabiensis | An. gambiae s.s. | An. quadriannulatus | An. Funestus | |
| April – June 2000 | ||||
| Larvae | 89 | 6 | 5 | 0 |
| Adults | 69 | 10 | 0 | 21 |
| November 2000 – January 2001 | ||||
| Larvae | 82 | 10 | 8 | 0 |
| Adults | 62 | 16 | 1 | 21 |
Although variation in the total numbers collected per week varied markedly, the relative abundance of each species did not change. Weekly catches of An. gambiae s.l. ranged from 34 to 266 adults per three houses, with the highest numbers collected during the late wet season, in the period from April to June 2000. Numbers of An. funestus peaked during the period of highest rainfall from January to February.
Large numbers of Culex sp. adults were also collected during the study with numbers peaking through the late wet season. Culex sp. larvae were also collected in the same breeding sites as Anopheles sp.. A sample of thirty larvae was identified as Cx. quinquefasciatus.
Description of breeding sites
Anopheles gambiae s.l. larvae were found almost exclusively in permanent water bodies, typified by the rice gardens and large irrigation pools nearby (Figure 1).
Figure 1.
- the Kasinthula irrigation project, with semi-mature rice plants; breeding also occurred within the irrigation ditch on the left of the photograph;
- a rice garden close to the Shire River; most larvae were found in the shallow areas of open pools on the left of the photograph.
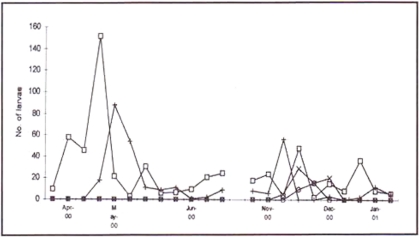
The majority (59%) was found in the village rice gardens (Figure 2). The remainder was found in irrigation project pools (31%), muddy puddles left when flooding receded (3%) and other sites (7% e.g. bore holes).
Figure 2.
Figure showing the changes in numbers of An. gambiae s.l. larvae sampled at different breeding site types: rice gardens (_), irrigation project (+), puddle (_), other (x).
Except during the peak rains, puddles and hoof prints, sites that are often associated with An. gambiae s.s. breeding, usually evaporated too quickly to permit larvae to complete development. The only puddles with larvae were those created after the floodwater receded from the banks of the Shire. These puddles often remained for up to two weeks. Breeding was also occasionally found in shaded boreholes (approximately 1.5m deep x 0.75m in diameter) and the static edges of a stream.
Effect of environmental change on breeding
The suitability of water bodies as potential breeding sites appeared to be greatly affected by changing conditions at each site. For example, larvae were never found in the irrigation project during periods when rice was mature. These habitats also became unsuitable if they were allowed to dry out when rice was not cultivated or if the pump from the river broke down. Marked change occurred when heavy rain caused the Shire River to flood and flush out the nearby rice gardens. It was during these periods that the normally less favoured sites (puddles and bore-holes) were used (Figure 2).
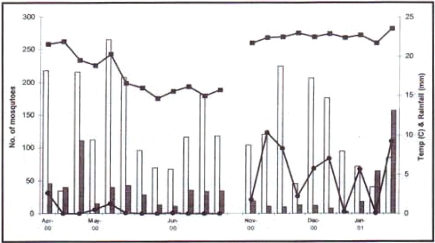
Examination of climatic data showed that abundance of An. gambiae s.l. appeared to be most closely related to Tmin. A fall in minimum temperature towards the end of the wet season, rather than the lack of water, corresponded with a drop in mosquito numbers. During the early wet season a period of heavy rains during January and February was followed by a fall in the numbers of An. gambiae, although numbers of An. funestus dramatically increased during this period (Figure 3).
Figure 3.
Figure showing the changes in abundance of An. gambiae s.l. (open bars) and An.
Discussion
These data are the first to describe the natural history of malaria vectors in Malawi. Comprising over 80% of both larval and 60% of adult samples, clearly An. arabiensis was the most abundant malaria vector species in the area. Two other known vector species, An. gambiae s.s and An. funestus were also present in large numbers. All three species are known to transmit malaria in the Shire Valley (Tambala et al. 1992) but their relative importance as vectors remains to be investigated. An. quadriannulatus is recorded here for the first time in Malawi. This species feeds predominantly on animals and so is not an important vector of human malaria (Coetzee, et al., 2000). This is apparent from the data (Table 1) where An. quadriannulatus comprised 5% of the An. gambiae s.l. larval catch but only O.11% (4 individuals) of the adult catch.
Interestingly, the predominance of An. arabiensis did not alter significantly throughout the seasons, despite the knowledge that warm, humid conditions typically favour An. gambiae s.s. (Lindsay, et al., 1998) This might be explained by weather patterns. Although the heaviest rains fell during January and February, they were intermittent; typically a short period (2 days) of heavy rain would be followed by a period of light or no rain when small pools of standing water evaporated quickly. Consequently, the only viable breeding sites were those that were permanently maintained either by the water table (the rice gardens) or by human intervention (the irrigation project), sites that are known to favour An. arabiensis (Ijumba & Lindsay, 2001). The interesting question of how these two sibling species compete in such an environment (Schneider et al., 1999) is currently under investigation in the field in Malawi.
Since breeding sites were available to mosquitoes throughout the year in either the irrigation project or rice gardens near the village, rainfall did not significantly affect mosquito abundance during the dry season. Abundance was found to be most strongly associated with minimum temperature. This may have been the result of colder drier nights causing higher mosquito mortality, or because reduced temperatures might have slowed the rate of egg production. Whether this affects transmission of malaria remains to be thoroughly investigated.
A complete understanding of the biology of any vector is essential prior to beginning any strategies aimed at controlling the disease it transmits (Chavasse, this volume). The provision of such data for Malawi will assist the planning of control strategies. Our data suggest that the populations of malaria vectors in Malawi are not markedly different in any way to populations of the same species in other parts of East or Southern Africa. Thus effective strategies applied elsewhere may also be appropriate for Malawi. The data derive from samples collected from the two environments where malaria mosquitoes can be found at different stages of their life cycle: water bodies where the aquatic larvae and pupae occur and from the insides of houses, where the adult female mosquitoes rest after feeding. These are the two areas where control might be directed through the application of insecticides. Of the two options, larval control is likely to be the more difficult. Malaria vectors in Chikwawa breed in large permanent water bodies where access for routine insecticide treatment is not always easy, where the likelihood of reaching and treating every potential site is low and where the cost of applying insecticide would be great. Moreover, the insecticide in these large pools would not persist for long periods and would be diluted or flushed out by heavy rain, requiring replenished on a frequent and regular basis to achieve any level of control. The adverse environmental effects of such large-scale insecticidal spraying are well known and undesirable. However, there are strategies for the management of rice cultivation to reduce mosquito breeding without affecting rice yields (van der Hoek, 2001), that could potentially be suitable for Malawi.
Strategies aimed at adult mosquito control are known to reduce malaria transmission very effectively and might be more cost-effective. Insecticide may be applied to the internal walls of the house, where malaria vectors rest, or to the surfaces of a bednet to kill mosquitoes when they arrive to feed on the sleepers beneath the net. Use of an ordinary untreated bednet reduces the chances of a mosquito biting anyone sleeping underneath but does not achieve total protection. Application of insecticide to the net ensures that all mosquitoes that come into contact with it will receive a dose that may eventually kill them. In communities where many households use insecticide-treated nets, it is not only the people sleeping under the nets that are protected; the number of mosquitoes biting those persons who do not use nets is also reduced. Regular re-impregnation with insecticide is essential to ensure that the nets retain their ability to kill any mosquitoes that land on them.
Simple appropriate technology already exists to reduce or prevent illness and deaths from malaria. By continuing our studies on the ecology, behaviour and vectorial capacity of the vectors of malaria, we hope to be able to define more precisely the epidemiology of malaria and utilise this data to inform and improve malaria control strategy in Malawi in the future.
Acknowledgements
We thank the people of Seseo for their co-operation and support throughout the study, particularly Maxwell Medison who assisted in sampling. The College of Medicine, Blantyre, granted ethical permission. We are indebted to the College of Medicine and the Wellcome Trust Research Laboratories Blantyre for their support throughout the study. We thank the Malawi Meteorological Department for providing data. We also thank the UK Department International Development (DfID)-funded Malaria Knowledge Programme at the Liverpool School of Tropical Medicine, the WHO Special Programme for Research and Training in Tropical Disease (TDR) and The Sir Halley Stewart Trust for financial support. The DfID can accept no responsibility for any information provided or views expressed.
References
- Coetzee M, Craig M, le Sueur D. “Distribution of the African malaria mosquitoes belonging to the Anopheles gambiae complex.”. Prasitol Today. 2000;16:74–77. doi: 10.1016/s0169-4758(99)01563-x. [DOI] [PubMed] [Google Scholar]
- Donnelly MJ, Townson H. “Evidence for extensive genetic differentiation among populations of the malaria vector Anopheles arabiensis in East Africa.”. Insect Mol Biol. 2000;9:357–367. doi: 10.1046/j.1365-2583.2000.00197.x. [DOI] [PubMed] [Google Scholar]
- Gillies MT, Coetzee M. “A supplement to the Anophelinae of Africa South of the Sahara (Afrotropical Region).”. Publ S Afr Inst Med Res. 1987;(55) [Google Scholar]
- Hopkins GHE. British Museum (Natural History) 2nd edition 1952. “Mosquitoes of the Ethiopian region: 1- Larval bionomics of mosquitoes and taxonomy of culicine larvae.”. [Google Scholar]
- Ijumba J N, Lindsay S W. “Impact of irrigation on malaria in Africa: paddies puadox.”. Med Vet Entomol. 2001;15:1–11. doi: 10.1046/j.1365-2915.2001.00279.x. [DOI] [PubMed] [Google Scholar]
- Lindsay S W, Parsons L, Thomas CJ. “Mapping the ranges and relative abundance of the two principal African malaria vectors, Anopheles gambiae sensu stricto and Anopheles arabiensis, using climate data.”. Proc Roy Soc Lond B. 1998;265:847–854. doi: 10.1098/rspb.1998.0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider P, Takken W, McCall PJ. “Interspecific competition between sibling species larvae of Anopheles arabiensis and An. gambiae.”. Med Vet Entomol. 2000;14:165–170. doi: 10.1046/j.1365-2915.2000.00204.x. [DOI] [PubMed] [Google Scholar]
- Scott J A, Brogdon WG, Collins FH. “Identification of single species specimens of the Anopheles gambiae complex by the polymerase chain reaction.”. Am J Trop Med Hyg. 1993;49:520–529. doi: 10.4269/ajtmh.1993.49.520. [DOI] [PubMed] [Google Scholar]
- Tambala P, Macheso A, Ziba C, Chitsulo L, Nyanwanlu O, Nyasulu Y, Franco C, Kazembe P, Wirima J, Hawley W, Sexton J, Steketee R. “Malaria vector assessment. Malawi, Oct 1991–Sept 1992. 1992 Unpublished Research Report. [Google Scholar]
- Van der Hoek W, Sakthivadivel R, Renshaw M, Silver JB, Birley MH. IWMI Reserch Report 47. 2001. “Alternate wet/dry Irrigation in rice cultivation: to save water and control malaria and JE?”. [Google Scholar]