Abstract
Bacteraemia is a recognised complication of severe malaria and may increase mortality. We determined 1) the rate and pattern of bacteraemia in children with severe malaria; 2) the impact of bacteraemia on case-fatality rate; and 3) the rate and pattern of bacteraemia in following blood transfusion for severe malarial anaemia. For the first two objectives, a prospective study was undertaken involving children admitted consecutively to the Malaria Research Project ward between February 1996 and June 1999. Blood culture was performed on admission. Independent associations with bacteraemia and mortality were determined by logistic regression. Of 701 children with a final diagnosis of severe malaria, 36 (5.1%) had bacteraemia. A wide range of bacteria was isolated and the commonest was non-typhoidal Salmonella (NTS: n=18 or 50% of all isolates). The rate of bacteraemia was significantly higher in children with severe malarial anaemia without coma (11.2%) than in children with cerebral malaria without anaemia (3.2%) and this was due to the significant association of NTS bacteraemia with severe malarial anaemia (p<0.001). The overall case-fatality rate was 15% and was higher in children with bacteraemia (22%) but this difference was not significant. For the third objective, data were collected retrospectively of all children who received a blood transfusion in the paediatric department from March 1996 until May 1997 inclusive. A total of 1712 children received a blood transfusion. Of these, 243 (14.2%) had a blood culture taken for the investigation of fever following transfusion; a pathogen was grown from 60 (24.7%). NTS bacteraemia accounted for 76.3% of all bacteraemia cases. NTS bacteraemia is a common complication of severe malarial anaemia.
Introduction
It has been recognised for a long time that malaria may be complicated by bacteraemia due to Salmonella, and it was also noted thirty years ago in Nigerian children that “the development of fever after transfusion is a useful diagnostic pointer to the enteric fever” due to Salmonella species.1,2 However, only recently have prospective studies reported the incidence and aetiology of bacteraemia in large numbers of children with severe malaria.3,4 Bacteraemia rates in these two studies were 5% and 7.8%, infections were due to a diversity of organisms and, in the study of Kenyan children,4 bacteraemia was associated with a threefold risk of death. There has been no study of the causes of bacteraemia in African children who develop fever following transfusion.
Non-typhoidal Salmonella (NTS) is the commonest cause of childhood bacteraemia in studies from tropical Africa, including Malawi.5–8 Malaria parasitaemia or severe anaemia are significantly more common in children with NTS bacteraemia compared to other causes of bacteraemia such as Streptococcus pneumoniae.5,6,9 It was surprising therefore that NTS bacteraemia was uncommon in the prospective studies of children with severe malaria from the Gambia and Kenya. However, these studies mostly included children with cerebral malaria.3,4 We conducted a prospective study to determine the rate, pattern and impact on outcome of bacteraemia in children with severe malaria including children with severe malarial anaemia and children with cerebral malaria. We also collected data retrospectively of bacteraemia in children who became febrile following blood transfusion.
Methods
Prospective study of bacteraemia in children with severe malaria
Blood cultures were performed on admission in consecutive children admitted to the Malaria Project research ward for treatment of severe malaria between February 1996 and June 1999. Children were admitted for ongoing studies of pathogenesis and pharmacokinetics using the criteria outlined below. Laboratory techniques for blood culture and determination of antimicrobial susceptibility have been described in earlier reports.8,9 All children had demographic and clinical information recorded. Blantyre coma score (BCS) was used to estimate severity of coma.10 Other investigations on admission included thin and thick film microscopy for malaria parasites (MPS), estimation of parasite density, packed cell volume (PCV) and blood glucose and white blood cell count (WCC; Coulter Electronics Limited, Luton, UK). Lumbar punctures were performed on all children with altered consciousness(BCS <5) unless there was a contraindication.
Following diagnosis, children were managed according to standard protocols. Parenteral quinine was used for all children with malaria parasites present on peripheral smear. A child was given a blood transfusion if PCV was <10% or if the child was in respiratory distress or coma associated with severe anaemia. Anti-convulsants were used only as clinically indicated and were not routinely administered. When septicaemia was suspected on clinical grounds, chloramphenicol (25 mg/kg four times daily) was given parenterally, and the choice and duration of antibiotic were reconsidered when the blood culture results were known. Only children with malarial parasitaemia and a final diagnosis of severe malaria are included in this analysis. Severe malaria is defined as follows: severe malarial anaemia (SMA) if PCV£15% (equivalent to haemoglobin value of £ 5g/dl) and BCS >2; severe malarial anaemia and cerebral malaria (SMA/CM) if PCV£15% and BCS£2; moderate malarial anaemia and cerebral malaria (MMA/CM) if PCV 16–25% (haemoglobin 5–8 g/dl) and BCS£2; cerebral malaria (CM) if PCV>25% and BCS£2.
Retrospective study of bacteraemia following blood transfusion
Data were collected retrospectively of all children who received a blood transfusion in the paediatric department of QECH from March 1996 until May 1997 inclusive. All admitted children routinely have a finger-prick sample taken for thick film examination for malaria parasites and packed cell volume (PCV). The indications for blood transfusion were not recorded but severe malarial anaemia is the indication for over 90% of all paediatric transfusions. All children with PCV≤12% (equivalent to a haemoglobin ≤ 4g/dl) receive a blood transfusion. If the PCV is greater than 12%, then clinical features (acidosis, altered consciousness, heart failure) and degree of parasitaemia are taken into account. Donated blood is screened for HIV hepatitis B, malaria and VDRL reactivity. The amount transfused is 20 mls/kg of whole blood because packed cells are not available. Most transfusions occur within 12 hours of admission but some children are transfused later when severe anaemia develops following admission. A blood culture was taken in children who became febrile and symptomatic following transfusion.
Analysis
The Epi-Info 6.0 statistical package was used for data analysis. Categorical proportions were compared with the c2 test. Mann Whitney U test was used for continuous non-parametric variables. Logistic regression analysis was used to determine independent associations.
Results
Prospective study of bacteraemia in children with severe malaria
A total of 701 children (median age 32 months, range 3–148 months) admitted to the Malaria Project Research ward over the study period had a final diagnosis of severe malaria. Of these, 36 (5.1%) had a positive blood culture. Two children with MMA/CM admitted consecutively grew Enterobacter cloacae from blood culture and the liquid preparation of quinine for parenteral use was later identified as the probable source of infection. Two children with malarial parasitaemia and bacteraemia were excluded owing to other final diagnoses. One, with moderate anaemia, coma and Haemophilus influenzae type b bacteraemia, was not included as cerebrospinal fluid also grew H. influenzae type b. The other child had clinical AIDS, severe anaemia, severe malnutrition and no coma and had separate episodes of bacteraemia due to Escherichia vulneris and Proteus mirabilis.
The clinical groups differed in their mean age and weight, and in frequency and pattern of bacteraemia (Table 1).
Table 1.
Pattern of bacteraemia in different clinical groups of severe malaria.
| SMA n=98 |
SMA/CM n=105 |
MMA/CM n=245 |
CM n=253 |
test of significance |
|
| Age: Mean age (mths) | 26 | 27 | 34 | 37 | F stat 13.11 |
| Median | 28.85 | 33.14 | 38.94 | 45.35 | p<0.0001 |
| WAZ: Mean WAZ score | −2.25 | −2.20 | −1.83 | −1.81 | F stat 10.36 |
| Median | −2.21 | −2.21 | −1.73 | −1.54 | p<0.0001 |
| Total blood isolates: | 11 | 6 (5.7%) | 11 (4.5%) | 8 (3.2%) | X2 for trend: |
| (11.2%) | 8.44 | ||||
| p=0.004 | |||||
| NTS | 5 (4.8%) | 5 (2.0%) | 0 | ||
| S. typhimurium | 8 (8.2%) | 1 | 3 | 0 | X2 for trend: |
| S. enteritidis | 6 | 3 | 0 | 0 | 20.9 |
| Non-typeable | 2 | 1 | 2 | 0 | p<0.0001 |
| Salmonellae | 0 | ||||
| Other organisms | 3 (3.0%) | 1 (0.9%) | 6 (2.5%) | 8 (3.2%) | X2 for trend: |
| S. pneumoniae | 2 | 0 | 0 | 3 | 0.22 |
| Other streptococci# | 0 | 0 | 2 | 1 | p=0.6 |
| Enterobacteriaceae* | 1 | 0 | 4 | 2 | |
| Others§ | 0 | 1 | 0 | 2 |
Other streptococci were Group D streptococcus (1), a-haemolytic streptococcus (1) and Enterococcus (1)
Enterobacteriacere were Enterobacter cloacae (2), Acinetobacter (2), Escherichia coli (1), Salmonella typhi (1), and Klebsiella (1)
Others were Campylobacter sp (1), Haemophilus influenzae (1) and an undentifiable Gram negative rod (1).
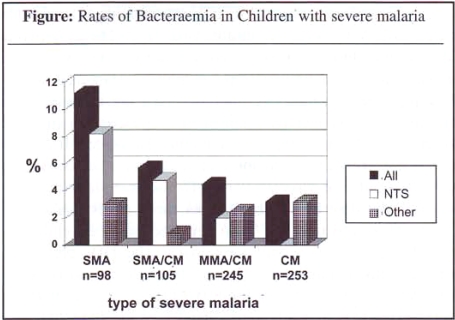
The rate of bacteraemia was significantly higher in SMA compared to CM. This difference was due to the significant association of NTS bacteraemia with SMA (see Figure next page).
Figure: Rates of Bacteraemia in Children with severe malaria
A wide range of bacteria was isolated from children with CM whereas 13 of 17 isolates in those with SMA (with or without CM) were NTS. Children with SMA were younger and more malnourished than those with CM. Independent associations with NTS bacteraemia were age of less than 2 years, severe anaemia and hypoglycaemia but not malnutrition (Table 2).
Table 2.
Independent associations with TNS bacteraemia
| Clinical association |
Group | No. | NTS (%) bacteraemia |
Crude OR (95%CI) |
Adjusted OR (95%CI) |
| Age | < 2 yrs | 201 | 13 (6.5%) | 6.85 (2.2–22.5) | 4.98 (1.69–14.7) |
| (years) | ≥ 2 yrs | 500 | 5 (1.0%) | 1 | p=0.002# |
| Anaemia | ≤15% | 203 | 13 (6.4%) | 6.75 (2.2–22.2) | 3.86 (1.29–11.5) |
| (PCV) | >15% | 498 | 5 (1.0%) | 1 | p=0.01 |
| Blantyre | > 2 | 98 | 8 (8.2%) | 5.27 (1.8–15.1) | 2.4 (0.7–1.8) |
| Coma Score | ≤ 2 | 603 | 10 (1.7%) | 1 | p=0.16 |
| Blood glucose | ≤ 2.2 | 122 | 10 (8.2%) | 6.37 (2.2–18.3) | 4.7 (1.7–12.7) |
| (mmol/l) | >2.2 | 579 | 8 (1.4%) | 1 | p=0.002 |
| Weight-for | ≤ −2 | 322 | 11 (3.4%) | 1.88 (0.7–5.5) | 1.49 (0.6–3.9) |
| -age Z score | > −2 | 379 | 7 (1.8%) | 1 | p=0.4 |
p values refer to the likelihood ratio test
The peripheral white blood cell count was not significantly higher in children with bacteraemia (median WCC 16.1 × 106 per mm3) than in those without bacteraemia (median WCC: 11.5 × 106).
The overall case-fatality rate was 15%. The case-fatality rate was the same in children with NTS bacteraemia and other bacteraemia (22%) and was higher in comparison to children without bacteraemia, but this difference was not significant. Severe anaemia, low coma score, hypoglycaemia, deep breathing or chest retractions and an admission temperature of less than 38°C were associated with a higher case-fatality rate. A history of prior aspirin ingestion was associated with deep breathing (X2=3.86; p=0.049) but not with a poor outcome or with bacteraemia. Deep breathing was associated with bacteraemia (X2=5.54; p=0.02). Although seizures prior to or after admission were not associated with a poorer outcome, not receiving anti-convulsants was associated with a significantly higher case-fatality rate.
For all study children, case-fatality rate was similar in those who received antibiotics compared to those who did not (14.7% versus 14.8% respectively). In children with bacteraemia, the case-fatality rate was lower in the 24 who received antibiotics (12.5%) compared to that in the 12 who did not (41.7%) but this difference was not statistically significant (X2=3.16; p=0.076). Two of the three deaths in the former group and four of the five deaths in the latter group occurred within 3 hours of admission to hospital. Table 3 shows independence of risk factors associated with a higher case-fatality rate. Young age and malnutrition were not associated with a significantly poorer outcome.
Table 3.
Independent associations with mortality
| Clinical factor | Adjusted OR (95% CI) |
| Young age | 1.07 (0.66–1.72) |
| <2 years | p=0.77# |
| Severe anaemia | 1.87 (1.1–3.2) |
| Pcv ≤ 15% | p=0.02 |
| Coma | 2.63 (1.2–5.16) |
| BCS≤2 | p=0.01 |
| Bacteraemia | 1.45 (0.6–3.44) |
| p=0.4 | |
| Malnutrition | 1.25 (0.8–1.92) |
| WAZ ≤−2 | p=0.3 |
| Hypoglycaemia | 3.5 (2.2‘5.6) |
| BSL≤2.2 mmol/l | p<0.0001 |
p values refer to the likelihood ratio test
Retrospective study of bacteraemia following blood transfusion
There were 11,527 admissions to the paediatric department over the 15-month study period, of whom 1009 (8.8%) had severe anaemia and 2583 (22.4%) had moderate anaemia on admission. The proportions of anaemic children who also had malaria parasitaemia were 64% (646 of 1009) with severe anaemia and 54% (1398 of 2583) with moderate anaemia respectively. A total of 1712 children received a blood transfusion. Of these, 243 (14.2%) had a blood culture taken for the investigation of fever following transfusion; a pathogen was grown from 60 (24.7%). The overall rate of bacteraemia identified following blood transfusion was 3.5%. The range of isolates is listed in table 4.
Table 4.
Range of isolates from blood culture taken after transfusion
| NTS species | 46 (76.6%)a |
| Salmonella typhimurium | 33 (55.0%) |
| Salmonella enteritidis | 8 (13.3%) |
| Non-typable Salmonella spp | 5 ( 8.3%) |
| Other bacteria | 14 (23.4%) |
| Escherichia coli | 3 ( 5.0%) |
| Gram negative rods | 3 ( 5.0%) |
| Escherichia vulneris | 1 ( 1.7%) |
| Klebsiella | 1 ( 1.7%) |
| _haemolytic streptococcus | 1 ( 1.7%) |
| Group A streptococcus | 1 ( 1.7%) |
| Staphylococcus aureus | 1 ( 1.7%) |
| Streptococcus pneumoniae | 1 ( 1.7%) |
| Haemophilus influenzae type b | 1 ( 1.7%) |
| Neisseria meningitidis group B | 1 ( 1.7%) |
| Total | 60 (100%) |
number of isolates (proportion of total isolates)
NTS bacteraemia accounted for 76.3% of all bacteraemia cases. The median age for children who had NTS bacteraemia following blood transfusion was 18 months (range 4–144 months), the mean PCV on admission was 14.6% (median 14%, range 6–26%) and 31 (66%) also had malaria parasitaemia. The mean percentage weight-for-age was 72.2. Outcome was recorded for 41 children with NTS bacteraemia and 7 (17%) died in hospital. There were a further 108 cases of NTS bacteraemia identified in the paediatric department during this 15 month study period who had not received a transfusion. There were no significant differences in age, percent weight-for-age or temperature on admission between children with NTS bacteraemia who were transfused and not transfused. Those with NTS bacteraemia that were transfused were more anaemic (mean PCV of 14.9% compared to 27.5% for NTS bacteraemia cases who were not transfused: p<0.001) and more likely to be parasitaemic (66% compared to 25.6%: p<0.001). However, the case-fatality rate (17%) was not significantly different compared to children with NTS bacteraemia who were not transfused (24.7%; p=0.35).
Discussion
The rate of bacteraemia was higher in children with severe malarial anaemia(11%) than in those with malaria and coma (4%) (p=0.003). The excess of bacteraemia among patients with severe anaemia was almost wholly due to NTS. Previous prospective studies of bacteraemia in children with severe malaria mainly included patients with cerebral malaria, when it was found that bacteraemia was due to a wide range of Gram positive and Gram negative organisms.3,4,11 In a report from The Gambia of 276 consecutive cases of cerebral malaria, the only child with NTS bacteraemia was also severely anaemic at presentation and the child had blood culture taken after blood transfusion on day 3 following admission.3
We found a high rate of bacteraemia in Malawian children who develop fever following a blood transfusion and that NTS was the cause in the majority (76.3%). This compares to an overall rate of 38.4% for NTS bacteraemia in the paediatric department during the same year.8 The prospective data from our study suggests that the predominance of NTS bacteraemia following blood transfusion is a reflection of the association of NTS bacteraemia with severe malarial anaemia which is the indication for transfusion rather than an association with transfusion per se. This means that the child is likely to have had NTS bacteraemia on admission but the diagnosis was delayed because symptoms are assumed to be malaria-related for the first 48 hours following admission.
Potential confounding factors of an association between NTS bacteraemia and severe malarial anaemia in Malawian children include young age and malnutrition. Severe malarial anaemia typically presents at a younger age than cerebral malaria. Severely malnourished children from a non-malarious area of South Africa had a significantly higher frequency of bacteraemia and a higher case-fatality rate than those with normal nutrition.12 NTS was the commonest isolate. We found that severe anaemia remained a significant risk for NTS bacteraemia independent of age, malnutrition and level of coma.
An important limitation of this study is that the potential impact of HIV infection on rate of bacteraemia or outcome was not examined. NTS septicaemia is common and often fatal in HIV-infected African adults.13,14 The relationship in African children is not as well documented and available data are conflicting. HIV-seropositive Rwandan children had higher rates of bacteraemia than HIV-seronegative children overall but HIV seropositivity was not associated with a higher proportion of NTS bacteraemia.15 In a cross-sectional study of sick, febrile Zimbabwean children, bacteraemia due to S. enteriditis was significantly more common in HIV-seropositive (5.4%) compared to HIV-seronegative children (0.7%).16 The majority of Malawian children with HIV infection are infected by vertical transmission and mortality is high in the first few years of life.17 HIV prevalence is therefore likely to be higher in the younger age group and in our study young age was associated with severe anaemia and higher rates of bacteraemia. However, HIV infection is not likely to be a major confounder for a number of reasons. A significant association of NTS bacteraemia with severe anaemia was described in the Gambia, Zaire and Rwanda either before HIV infection had been reported or when childhood prevalence rates were still very low.5–7 The HIV prevalence among children admitted to Queen Elizabeth Central Hospital, Blantyre, with severe malaria (including severe malarial anaemia) is 13% which is lower than for any of the other common childhood illnesses.18 This is consistent with data from similar studies performed in Zambia and Cote d'Ivoire.19,20
We found that the case-fatality rate was higher in children with bacteraemia (8/36 = 22%) that in those without (98/665= 15%), but the difference was not significant. In a series of 540 children with severe malaria, Berkley et al reported a case-fatality rate of 33.3% in the 42 patients with bacteraemia, which was significantly higher than the case-fatality rate in those without bacteraemia (10.4%).4 In contrast to the Kenyan study, mortality was 7% in Gambian children with cerebral malaria and bacteraemia.3 However, the authors conceded that some of the isolates represented contaminants.
Should parenteral antibiotics be used routinely for children with severe malaria? This requires careful consideration. It would demand a substantial increase in resources and facilitate emergence of antibiotic resistance but may not improve outcome among children with severe malaria if such children are dying with bacteraemia rather than of bacteraemia. It was noted in the earlier studies of children with severe malaria that many children with bacteraemia improved without antibiotics.3,4 In our study, poor outcome was strongly associated with severity of coma and severity of anaemia, and not significantly with bacteraemia. Those children with bacteraemia that received antibiotics did have a lower case-fatality rate but this was not statistically significant. Our study was not designed to assess the benefit of antibiotics on outcome. Further, 6 of the 8 deaths in children with bacteraemia occurred within 3 hours of admission which makes it even more difficult to determine the possible effectiveness of antibiotics in this study. Our data highlight the contributions of a number of important clinical variables to the occurrence of bacteraemia and to malaria-related mortality but would not support routine use of antibiotics for children with severe malaria.
However, children with severe malarial anaemia require further consideration. We have shown that the rate and pattern of bacteraemia is different in Malawian children with severe malarial anaemia compared to those with cerebral malaria. There is a strong association of NTS bacteraemia with severe malarial anaemia and young age. NTS bacteraemia is also common following transfusion for severe malarial anaemia. Particularly in infants bacteraemia may be complicated by focal sepsis, such as meningitis.21–24 NTS is a common cause of meningitis in Malawian children under 2 years of age and there is a peak incidence after the end of the malaria season.25 In a recent large study of bacterial meningitis in this department, 9 (31%) of 29 cases with NTS meningitis had a history of recent blood transfusion compared to 10 (1.8%) of 568 cases with other causes of bacterial meningitis: _2 test 68.8, p<0.0001 (E.M. Molyneux, unpublished data). It is therefore important to identify and treat episodes of NTS bacteraemia. We already recommend parenteral antibiotics aimed at NTS in children who develop fever following transfusion for severe malarial anaemia and would consider routine use in all children with severe malarial anaemia, depending on available resources. Chloramphenicol is an appropriate choice8,9 but the recent and rapid emergence in Blantyre of chloramphenicol-resistant Salmonella is a major concern.
References
- 1.Hayasaka C. Im verlauf einer malarikur durch Bacillus enteritidis Gartner enstandene meningitis and sepsis. Tohoku J Exp Med. 1933;21:466–503. (Fre). [Google Scholar]
- 2.Duggan MB, Beyer L. Enteric fever in young Yoruba children. Arch Dis Child. 1975;50:67–70. doi: 10.1136/adc.50.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Enwere G, Van Hensbroek MB, Adegbola R, Palmer A, Onyiora E, Weber M. Bacteraemia in cerebral malaria. Ann Trop Paediatr. 1998;18:275–278. doi: 10.1080/02724936.1998.11747959. [DOI] [PubMed] [Google Scholar]
- 4.Berkley J, Mwarumba S, Bramham K, Lowe B, Marsh K. Bacteraemia complicating severe malaria in children. Trans R Soc Trop Med Hyg. 1999;93:283–286. doi: 10.1016/s0035-9203(99)90024-x. [DOI] [PubMed] [Google Scholar]
- 5.Mabey DCW, Brown A, Greenwood BM. Plasmodium falciparum malaria and Salmonella infections in Gambian children. J Infect Dis. 1987;155:1319–1321. doi: 10.1093/infdis/155.6.1319. [DOI] [PubMed] [Google Scholar]
- 6.Lepage P, Bogaerts J, Van Goethem C, Ntahorutaba M, Nsengumuremyi F, Hitimana DG, et al. Community-acquired bacteraemia in African children. Lancet. 1987;1:1458–1461. doi: 10.1016/s0140-6736(87)92207-0. [DOI] [PubMed] [Google Scholar]
- 7.Green SDR, Cheesbrough JS. Salmonella bacteraemia among young children at a rural hospital in western Zaire. Ann Trop Paediatr. 1993;13:45–54. doi: 10.1080/02724936.1993.11747624. [DOI] [PubMed] [Google Scholar]
- 8.Walsh AL, Phiri AJ, Graham SM, Molyneux EM, Molyneux ME. Bacteremia in febrile Malawian children: clinical and microbiological features. Pediatr Infect Dis J. 2000;19:312–318. doi: 10.1097/00006454-200004000-00010. [DOI] [PubMed] [Google Scholar]
- 9.Graham SM, Walsh AL, Molyneux EM, Phiri A, Molyneux ME. The clinical presentation of non-typhoidal Salmonella bacteraemia in Malawian children. Trans R Soc Trop Med Hyg. 2000;94:310–314. doi: 10.1016/s0035-9203(00)90337-7. [DOI] [PubMed] [Google Scholar]
- 10.Molyneux ME, Taylor TE, Wirima JJ, Borgstein A. Clinical features and prognostic indicators in paediatric cerebral malaria: a study of 131 comatose Malawian children. Q J Med. 1989;71:441–459. [PubMed] [Google Scholar]
- 11.World Health Organization, author. Severe and complicated malaria. Trans R Soc Trop Med Hyg. 1990;84(suppl 2):1–65. [PubMed] [Google Scholar]
- 12.Berkowitz FE. Bacteraemia in black South African children: a one year study emphasizing nosocomial bacteraemia and bacteraemia in severely malnourished children. Am J Dis Child. 1984;138:551–556. doi: 10.1001/archpedi.1984.02140440035008. [DOI] [PubMed] [Google Scholar]
- 13.Gilks CF, Brindle RJ, Ottieno LS, Bhatt SM, Newnham RS, Simani PM, et al. Life threatening bacteraemia in HIV-1 seropositive adults admitted to hospital in Nairobi, Kenya. Lancet. 1990;336:545–549. doi: 10.1016/0140-6736(90)92096-z. [DOI] [PubMed] [Google Scholar]
- 14.Gordon MA, Walsh AL, Chaponda M, Soko D, Mbvwinji M, Molyneux ME, et al. Bacteraemia and mortality among adult medical admissions in Malawi - predominance of non-typhi salmonellae and Streptococcus pneumoniae. J Infect. 2001;42:44–49. doi: 10.1053/jinf.2000.0779. [DOI] [PubMed] [Google Scholar]
- 15.Lepage P, Van de Pene P, Nsengumuremyi F, Van Goethem C, Bogaerts J, Hitimana DG. Bacteraemia as predictor of HIV infection in African children. Acta Paediatr Scand. 1989;18:763–766. doi: 10.1111/j.1651-2227.1989.tb11140.x. [DOI] [PubMed] [Google Scholar]
- 16.Nathoo KJ, Chigonde S, Nhembe M, Ali MH, Mason PR. Community-acquired bacteraemia in human imunodeficiency virus-infected children in Harare, Zimbabwe. Pediatr Infect Dis J. 1996;15:1092–1097. doi: 10.1097/00006454-199612000-00007. [DOI] [PubMed] [Google Scholar]
- 17.Taha TE, Kumwenda NI, Broadhead RL, Hoover Dr, Graham SM, Van Der Hoven L, et al. Mortality after the first year of life among HIV-1 infected and uninfected children. Pediatr Infect Dis J. 1999;18:689–694. doi: 10.1097/00006454-199908000-00007. [DOI] [PubMed] [Google Scholar]
- 18.Gladstone MJ, Callaghan M, Rogerson SJ, Borgstein E, Rogerson SRK. HIV prevalence in paediatric admissions in Malawi. Arch Dis Child. 2001;84(suppl 1):A43. [Google Scholar]
- 19.Chintu C, Luo C, Bhat G, DuPont HL, Mwansa-Salamu P, Kabika M, et al. Impact of human immunodeficiency virus type-1 on common pediatric illnesses in Zambia. J Trop Pediatr. 1995;41:348–353. doi: 10.1093/tropej/41.6.348. [DOI] [PubMed] [Google Scholar]
- 20.Vetter KM, Djomand G, Zadi F, Diaby L, Brattegaard K, Timite M, et al. Clinical spectrum of human immunodeficiency virus disease in children in a West African city. Pediatr Inf Dis J. 1996;15:438–442. doi: 10.1097/00006454-199605000-00011. [DOI] [PubMed] [Google Scholar]
- 21.Sirinavin S, Chiemchanya S, Vorachit M. Systemic nontyphoidal Salmonella infection in normal infants in Thailand. Pediatr Infect Dis J. 2001;20:581–587. doi: 10.1097/00006454-200106000-00007. [DOI] [PubMed] [Google Scholar]
- 22.Zaidi E, Bachur R, Harper M. Non-typhi Salmonella bacteremia in children. Pediatr Infect Dis J. 1999;18:1073–1077. doi: 10.1097/00006454-199912000-00009. [DOI] [PubMed] [Google Scholar]
- 23.Dube SD, Bhagwat AG. Non-typhoidal Salmonella infection in Zambian infants. Trans R Soc Trop Med Hyg. 1983;77:336–337. doi: 10.1016/0035-9203(83)90157-8. [DOI] [PubMed] [Google Scholar]
- 24.Davis RC. Salmonella sepsis in infancy. Am J Dis Child. 1981;135:1096–1099. doi: 10.1001/archpedi.1981.02130360004003. [DOI] [PubMed] [Google Scholar]
- 25.Molyneux EM, Walsh A, Phiri A, Molyneux M. Acute bacterial meningitis in children admitted to the Queen Elizabeth Central Hospital, Malawi in 1996–97. Trop Med Int Health. 1998;3:610–618. doi: 10.1046/j.1365-3156.1998.00278.x. [DOI] [PubMed] [Google Scholar]