Abstract
Non-typhoidal salmonella (NTS) bacteraemia is a common, recurrent illness in HIV-infected African adults. We aimed to describe the presentation and outcome of NTS bacteraemia, the pattern of recurrence, and to determine whether recurrence results from re-infection or recrudescence. 100 consecutive adult inpatients with NTS bacteraemia in Blantyre, Malawi were treated with chloramphenicol (500mg qid for 14 days). Survivors were prospectively followed to detect bacteraemic recurrence. Index and recurrent isolates were typed by antibiogram, pulsed field gel electrophoresis and plasmid analysis to distinguish recrudescence from re-infection. Inpatient mortality was 47%, and 1-year mortality was 77%. 77/78 cases were HIV positive. Anaemia was associated with inpatient death, and several features of AIDS were associated with poor outpatient survival. Among survivors, 43% (19/44) had a first recurrence of NTS bacteraemia at 23–186 days. Among these, 26% (5/19) developed multiple recurrences up to 245 days. No recurrence was seen after 245 days, despite follow-up for up to 609 days (median 214). Suppurative infections were not found at presentation, and were only seen twice at recurrence. Index and recurrent paired isolates were identical by phenotyping and genotyping, consistent with recrudescence, rather than re-infection. NTS bacteraemia has a high mortality (47%) and recurrence rate (43%) in HIV-infected African adults. Recurrence is caused by recrudescence rather than re-infection. Since focal infections were rarely found, recrudescence may often be a consequence of intracellular tissue sequestration. There is an urgent need for improved primary treatment and secondary prophylaxis in Africa.
Introduction
Non-typhoidal salmonellae (NTS) are one of the commonest invasive bacterial infections among HIV-infected adults in Malawi, and comprise 37% of adult blood culture isolates in Blantyre [1]. In immunocompetent adults, NTS cause a self-limiting diarrhoeal illness, with 0.5% mortality. By contrast, HIV-infected adults are highly susceptible to NTS bacteraemia, with a devastating inpatient mortality of up to 80% [1,2,3]. Recurrent NTS bacteraemia was recognised as a feature of AIDS in 1985 [4–7], but the pattern and outcome of recurrences have not been established prospectively in Africa.
NTS bacteraemia could be recurrent in HIV-infected adults for several reasons. Firstly, the increased susceptibility of HIV-infected adults to NTS [8] might lead to frequent re-infections. In a study of 4 Italian HIV-infected patients with 7 recurrences of NTS bacteraemia, molecular IS200 fingerprinting showed that 5/7 events were recurrence with a different organism, suggesting new re-infection [9]. If re-infections were also the commonest cause of recurrence in Malawi, then the risk of recurrent NTS infections in susceptible individuals might be reduced through behavioural strategies.
Secondly, NTS may emerge repeatedly from a suppurative focus of infection (eg. damaged urinary tract, endothelium, joints, bones [10] or more unusual sites in HIV [11–16]). Additionally, schistosomiasis, which is endemic in some parts of Malawi, can cause persistence of NTS [17], because the bacterium adheres to the adult worm, which acts as an intravascular focus [18].
Thirdly, salmonellae can survive inside human cells [19] and the immune deficit in HIV/AIDS may allow persistence of NTS in the tissue macrophages / monocytes of the reticuloendothelial system.
In this study we aimed firstly to describe the presentation of NTS bacteraemia in HIV-infected Malawian adults, and secondly to study prospectively the rate, timing and outcome of recurrent NTS bacteraemia. We then aimed to establish the degree of concordance between index and recurrent organisms, to distinguish new reinfection from recrudescent NTS infections.
Methods
All adult general medical admissions to Queen Elizabeth Central Hospital (QECH) Blantyre, who presented with fever had venous blood (5ml) taken for culture. Blood was inoculated into brain heart infusion broth, and incubated at 37∞C in air for 7 days, with routine sub-cultures at 1, 2 and 7 days. One hundred consecutive patients with community acquired NTS bacteraemia were recruited when a positive blood culture was reported. Standardised history and examination were recorded. Blood was taken for full blood count, and examined for malaria parasites. HIV testing using 2 methods (HIV Serocard, Trinity Biotech, and HIV ELISA, Ortho Clinical), and automated CD4 count Facscount, Becton Dickinson) were performed for individuals who gave specific informed consent. Clinically apparent foci of infection were investigated.
Empiric Treatment was started at admission, pending blood culture results. Following identification of NTS from blood cultures, treatment with chloramphenicol was either started or continued in all patients at a minimum prescribed dose of 500mg qid for 14 days. The maximum total prescribed dose of chloramphenicol was 42g.
Survivors were asked to attend an open access follow-up clinic at least once a month. Transport expenses and a modest allowance were paid. At the first clinic attendance, 4–6 weeks after index presentation, standardised history and examination were recorded, and compliance with treatment was assessed using inpatient drug charts, pharmacy dispensing records, and direct questioning. The minimum acceptable index treatment taken was considered to be 28g total dose, and 14 days duration. Irrespective of clinical condition, venous blood was taken for culture from all patients. Stool (formal ether concentration) and urine (centrifuged) samples were examined for ova, parasites and cysts.
At all subsequent monthly visits, blood culture was repeated only if patients were symptomatic or febrile. Monthly follow-up was continued for up to 20 months, or until death. Non-attenders were contacted, or visited at home by study staff.
“True” recurrence of NTS bacteraemia was defined as growth of NTS from blood culture, after a full treatment course of chloramphenicol. Positive NTS blood cultures in patients who had taken an incomplete chloramphenicol course were considered to be “false” recurrences. Recurrences of NTS bacteraemia were managed by a search for foci of infection, and re-treatment with chloramphenicol (250mg qid for 28 days).
Pairs or series of index and recurrent NTS blood culture isolates were tested for antibiotic susceptibility by disc diffusion. Pulsed field gel electrophoresis (PFGE) of chromosomal DNA was performed as follows: bacteria were harvested from solid media, embedded in lysozyme-containing agarose and lysed, and released nucleases were neutralised with proteinase K. Chromosomal DNA was digested with Spel, and fragments were separated by PFGE (CHEF 3D, BioRad Ltd). Relatedness of strains on PFGE was assessed according to the Tenover criteria [20]. Strains which could not be differentiated using these methods were further investigated by plasmid typing, performed by standard alkaline lysis and agarose gel electrophoresis.
Statistical analysis
Data were prospectively collected on standard proformas, entered in a MS Access database, and analysed using STATA 6.0. The association of inpatient death with clinical and laboratory presenting features was first tested in univariate analyses. X2 tests were used for binary variables, and t-tests or ranksum tests for continuous variables. Any association reaching a significance of p<0.1 in univariate analyses was then tested for interaction, and corrected multivariate Mantel-Haenszel Odds Ratios were calculated.
The association of outpatient recurrence and outpatient survival with clinical and laboratory presenting features were tested using Cox's proportional hazard regression analysis. Kaplan Meier survival estimates were plotted for time to first recurrence and death.
Ethical approval
This study was given ethical approval by the University of Malawi College of Medicine Research Committee.
Results
Recruitment and presentation
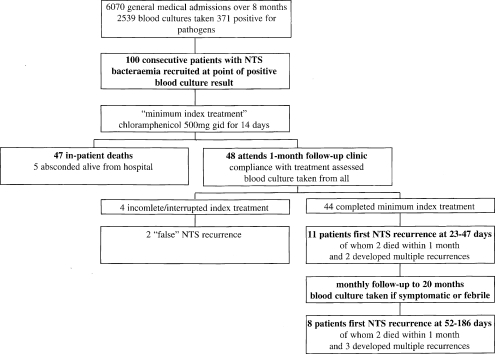
Recruitment is summarised in figure 1. One hundred patients (54 males) were recruited at median day 2 (range 1–5). Median age was 32 years (range 18–60). Clinical presentation is summarised in table 1. There was no clinical evidence of infective endocarditis, gastrointestinal bleeding or perforation in any case.
Figure 1.
Flowchart of recruitment and outcomes for 100 patients with NTS bacteraemia
Table 1.
Clinical features of NTS bacteraemia at index presentation and first recurrence
| Presenting feature | Index presentation % (n=100) |
First recurrence % (n=19) |
| HISTORY | ||
| Fever/sweats | 95 | 84 |
| Headache | 60 | 21 |
| Vomiting | 51 | 16 |
| Diarrhoea | 46 | 43 |
| Cough | 45 | 26 |
| Abdominal pain | 36 | 27 |
| Dyspnoea | 31 | 10 |
| Confusion | 28 | 2 |
| Chest pain | 27 | 10 |
| EXAMINATION | ||
| Temperature °C median (range) | 39 (35.3–41) | 37.7 (34.6–40.3) |
| GCS median (range) | 15 (15-3) | 15 (15-14) |
| Splenomegaly | 38 | 32 |
| Respiratory crackles | 35 | 16 |
| Abdominal tenderness | 17 | 21 |
| Hepatomegaly | 17 | 11 |
| Bronchial breath sounds | 11 | 0 |
| Pleural effusion | 8 | 0 |
| CLINICIANS ASSESSMENT | ||
| Gastrointestinal focus * | 44 | 37 |
| Respiratory focus † | 33 | 16 |
| Fever without apparent focus | 20 | 47 |
predominant diarrhoeal illness, or fever with abdominal pain.
respiratory symptoms with chest signs
Microbiological findings
NTS blood isolates from the 100 cases comprised S. typhimurium (75), S. enteritidis (19), a dual growth of S. typhimurium and S. enteritidis (1), and other Salmonella spp (5). Bacterial susceptibility testing showed 5% resistance to chloramphenicol, 73% to co-trimoxazole, 79% to ampicillin, 43% to gentamicin and 40% to tetracycline.
Investigation of clinically apparent foci of infection in 47 patients included 23 sputum examinations, culture of 5 transthoracic aspirates, and microscopy and culture of 8 pleural aspirates, 10 CSF samples, and 1 each of joint, skin pustule and ascitic aspirates. These investigations identified non-salmonella pathogens in 11 patients: 4 with respiratory bacterial co-infections (1 S. pneumoniae, 2 klebsiella and 1 citrobacter), 2 patients with AAFB positive pulmonary tuberculosis, 3 with cryptococcal meningitis, 2 with Staphylococcus aureus focal infections, and 2 with tuberculosis pleural effusion. Pleural effusions in 2 patients and ascites in 1 were attributed to widespread Kaposi's sarcoma. There was no evidence of localised salmonella infection in any case at presentation.
Laboratory findings and HIV testing
FBC (n=70) showed median Hb 6.8 g/dl (2.5 – 11.7), median WCC 3.8x109/1 (1 – 17), and median platelet count 104x109/1 (14 – 406). Malaria parasites were seen in 12/70 (17%) samples. Stool microscopy showed helminths in 7/40 patients, including Schistosoma mansoni in 2. 99% (77/78) patients tested were seropositive for HIV by two methods. Median CD4 count at presentation was 99 cells/ul (range 6–313, n=50), and at follow-up was 108 cells/ml (range 6–445, n=34). The highest recorded count (median 101 cells/ul, n=59) was taken as the best estimate of baseline CD4 count.
Inpatient treatment and outcome
41/100 patients died within 1 month as inpatients, at median 4 days (range 0–30). Median length of admission among survivors was 5 days (range 0–26). 93/100 patients received chloramphenicol, commenced at median day 0 (0–5). Empiric chloramphenicol was given to 49 patients empirically on day 0, and to 44 patients when blood culture results became available.
Follow-up visits
48 surviving patients attended the follow-up clinic. The median total dose of chloramphenicol taken was 32g (range 10–42), and median duration of treatment was 14 days (range 4–18). 44/48 survivors completed the full index treatment, and 4 took incomplete courses. 410 follow-up visits were made, and there were 156 intercurrent clinical episodes. The commonest were acute diarrhoea (40), acute lower respiratory infection (34), non-specific febrile illness (34), skin infections (9), painful peripheral neuropathy (8) and pulmonary tuberculosis (6).
Follow-up blood cultures and recurrences
48 patients had 145 blood cultures taken during follow-up, and 42/145 cultures (29%) grew a bacterial pathogen. 21 patients had blood cultures positive for NTS on 31 occasions. Of these 21, 19 represented “true” recurrence of NTS bacteraemia on 29 occasions, and were re-treated with chloramphenicol. 8 patients had positive blood cultures for other pathogens on 11 occasions (7 S.pneumoniae, 4 others).
Detection, timing and outcome of NTS recurrence
Follow-up, detection and outcome of recurrences are summarised in figure 1. At the first clinic attendance, 11/44 subjects had true recurrence of NTS bacteraemia; all 11 were symptomatic or febrile, despite previous response to treatment. The period since the last dose of chloramphenicol was median 18 days (range 8–35). One later developed multiple recurrences of NTS bacteraemia. As follow-up progressed, 8 more individuals developed later first recurrence of NTS bacteraemia, following negative blood culture at the first visit. Three developed multiple events of recurrent NTS bacteraemia. Only 2 of the 29 documented recurrent events showed evidence of focal NTS infection (1 urinary tract infection, 1 thoracic empyema).
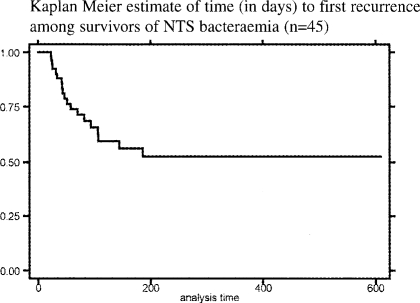
The overall timing of recurrences of NTS bacteraemia is shown in figure 2b.
Figure 2b.
Kaplan Meier estimate of time (in days) to first recurrence among survivors of NTS bacteraemia (n=45)
No first NTS recurrence was detected after 186 days, and no multiple events were detected after 245 days, despite follow-up of cases to a maximum of 609 days (median 214 days).
Clinical features of first recurrence
The clinical features of the 19 first events of recurrent NTS bacteraemia are summarised in table 1. Overall there were fewer symptoms and signs at recurrence compared to index presentation, and fever without identifiable clinical focus became the commonest presentation. 5/19 (26%) of patients died within 1 month of first recurrence, a lower immediate mortality than at the index presentation.
Features associated with inpatient death and poor survival
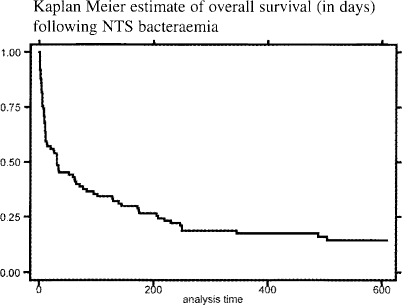
Kaplan Meier estimate of overall survival is shown in figure 2a. Mortality at 1 year was 77%. Inpatient death was associated with reduced admission Hb concentration ([Hb]) and reduced Glasgow Coma Score (GCS) on univariate analysis. [Hb] when corrected for GCS remained highly significant as a predictor of in-patient death (OR 1.6 for each fall of 1 gram of [Hb], CI 1.3–2.0, p=0.0001)(see table 2).
Figure 2a.
Kaplan Meier estimate of overall survival (in days) following NTS bacteraemia
Table 2.
Clinical and laboratory features associated with poor outcome
| Presenting Feature* |
% at presentation (n=100) |
Inpatient death (univariate) OR (CI)§ |
Inpatient death (corrected) OR (CI)§ |
Outpatient survival (univariate) HR (CI)§ |
| Clinical | ||||
| GCS med (range) |
15 (15-3) | 1.3 (1.2–1.5) p=0.0026 |
0.36 (0.06–2.3) p=0.3 |
1.0 (0.64–1.52) p=0.9 |
| Oral thrush | 32 | 1.7 (0.7–4.0) p=0.2 |
- | 2.8 (1.4–5.6) p=0.003 |
| Pruritic rash | 9 | 2.2 (0.5–9.9) p=0.3 |
- | 3.7 (1.1–12.7) p=0.03 |
| Previous sputum positive TB |
19 | 1.9 (0.5–7.5) p=0.2 |
- | 13.9 (3.9–49.2) p<0.0001 |
| Laboratory | ||||
| Hb (g/dl) med (range) n=70 |
6.8 (2.5–11.7) | 1.4 (1.1–1.8) p=0.0014 |
1.6 (1.3–2.0) p=0.0001 |
0.9 (0.8–1.1) p=0.3 |
| CD4 cells/ml med (range) n=59 |
101 (6–445) | 1.0 (0.9–1.0) p=0.5 |
- | 1.1 (1–1.1) p=0.021 |
This table includes all presenting features which were associated at a significance p<0.1 in univariate analyses
OR and HR me given per fall of GCS by 1 point, per fall of Hb by 1g/dl, and per fall of CD4 count by 10 cells/ml
Poor outpatient survival was associated with several features of AIDS on univariate Cox's regression analysis (see table 2). Recurrence of NTS bacteraemia was not associated with any clinical or laboratory feature of presentation, including NTS serovar and antibiotic resistance pattern, and recurrence did not affect survival as an outpatient in this study.
Phenotypic and genotypic analysis of recurrent isolates
15 pairs or series of index and recurrent isolates were available for analysis (summarised in table 3). Serology, antibiogram and PFGE showed 6/15 subjects to have unique index organisms. Plasmid analysis allowed the remaining 9 subjects to be further split into 3 distinct groups. All 15 pairs of isolates showed intra-individual concordance at the first recurrence. There were 3 longer series of isolates, of which only patient E showed evidence of re-infection with a non-concordant strain. occuring at the 3rd recurrence.
Table 3.
Phenotyping and genotyping of index and recurrent NTS isolates
| Subjects and isolates* |
Days (0=index) |
Serology | Antibiogram† | Genotype by PFGE |
Plasmid profile |
| A1 | 0 | O4 | RSSRSR | 1B | |
| A2 | 144 | O4 | RSSRSR | 1B | |
| B1 | 0 | O4 | RSSSSS | 1 | |
| B2 | 32 | O4 | RSSSSS | 1 | |
| C1 | 0 | O4 | RSSRSR | 1 | 81, 22, 8.7 |
| C2 | 26 | O4 | RSSRSR | 1 | 81, 22, 8.7 |
| C3 | 81 | O4 | RSSRSR | 1 | 81, 22, 8.7 |
| D1 | 0 | O4 | RSSRSR | 1 | 81, 22, 8.7, 6.3 |
| D2 | 42 | O4 | RSSRSR | 1 | 81, 22, 8.7, 6.3 |
| E1 | 0 | O4 | RSSRSR | 1 | 81,22, 8.7, 6.3 |
| E2 | 34 | O4 | RSSRSR | 1 | 81, 22, 8.7, 6.3 |
| E3 | 71 | O4 | RSSRSR | 1 | 81, 22, 8.7, 6.3 |
| E4 | 125 | O4 | RSSRSR | 1 | 81, 8.7, 6.3 |
| E5 | 205 | O4 | RSSRSR | 1 | 81, 8.7, 6.3 |
| F1‡ | 0 | O4 | RSSRSR | 1 | 81, 22, 8.7 |
| F2 | 22 | O4 | RSSRSR | 1 | not exmined |
| F3 | 106 | O4 | RSSRSR | 1 | 81, 22, 8.7 |
| F4 | 136 | O4 | RSSRSR | 1 | 81, 22, 8.7 |
| G1 | 0 | O4 | RSSRSR | 1C | |
| G2 | 107 | O4 | RSSRSR | 1C | |
| H1 | 0 | O4 | RSSRSR | 1A | |
| H2 | 94 | O4 | RSSRSR | 1A | |
| I1 | 0 | O4 | RSSRSR | 1 | 81, 22, 8.7, 6.3 |
| I2 | 186 | O4 | RSSRSR | 1 | 81, 22, 8.1, 6.3 |
| J1 | 0 | O4 | RSSRSR | 1 | 81, 8.7 |
| J2 | 70 | O4 | RSSRSR | 1 | 81, 8.7 |
| K1 | 0 | O4 | RSSRSR | 1 | 81, 8.7 |
| K2 | 47 | O4 | RSSRSR | 1 | 81, 8.7 |
| L1 | 0 | O4 | RSSSSS | 1A | |
| L2 | 52 | O4 | RSSSSS | 1A | |
| M1 | 0 | O4 | RSSRSR | 1 | 81, 22, 8.7 |
| M2 | 58 | O4 | RSSRSR | 1 | 81, 22, 8.7 |
| N1 | 0 | O4 | RSSRSR | 1 | 81, 22, 8.7, 6.3 |
| N2 | 44 | O4 | RSSRSR | 1 | 81, 22, 8.7, 6.3 |
| O1 | 0 | O9 | RSSSSR | - | |
| O2 | 82 | O9 | RSSSSR | - |
subjects A–O in order of presentation
amoxycillin, co-amoxyclav, ceftriaxone, gentamicin, ciprofloxacin, trimethoprim.
F1 isolate mixed with S. enteritidis at week 0 only.
Discussion
This is the largest description of the clinical course of this common HIV-related condition in African adults; there was a varied clinical presentation, 47% died in hospital within 1 month, 77% had died after 1 year, and 43% of outpatient survivors developed a recurrence of NTS bacteraemia on one or more occasions. Using molecular techniques, we demonstrated that these recurrences were caused by isolates that were the same as the original infecting organism. NTS bacteraemia is a severe, recrudescent disease.
Reduction in Hb concentration ([Hb]) was strongly associated with inpatient death. Anaemia is a common feature of advanced HIV disease in Malawi; all the patients in the study were anaemic, 50% being severely anaemic with [Hb] below 7g/dl. Unsurprisingly, several features of AIDS were also associated with poor outpatient survival.
We have described the importance of NTS recurrence in African HIV disease for the first time. NTS comprised 27% of positive blood cultures among medical admissions, but 72% of positive blood cultures during follow-up. At least one recurrence of NTS bacteraemia was found in 43% of survivors, and 26% of these had multiple recurrences. Disappointingly, no clinical features at presentation predicted later recurrence. All patients with recurrence were symptomatic and/or febrile, indicating that we did not merely document incidental bacteraemia. Only 5ml of blood were taken for culture, so our data may under-represent the true rate of recurrence. Recurrence presented with fewer a symptoms and signs, had a lower case fatality than the index illness, and did not affect long-term survival; recurrence may be intrinsically a milder clinical event than the index episode, but this study involved active follow-up; the course of late self-presenting recurrences might be very different.
PFGE and plasmid typing showed heterogeneity of isolates between individuals, but concordance of the index isolates with all first recurrences and most later recurrences. Taken together these data make recrudescence (rather than re-infection) the most likely explanation for the high recurrence rate, in contrast to the findings in the Italian study [9].
Focal infections could have contributed to the high rate of recrudescence; reports have described suppurative salmonella infections in the chest in HIV disease, and many of our patients had respiratory symptoms and signs. Focal NTS infections were carefully sought, but were found in no case at index presentation, and in only 2/19 patients at recurrence. In contrast, co-infections with other pathogens were identified in 11/100 patients. Focal NTS infections therefore contributed to recrudescence in only a minority of cases. Similarly, schistosomal ova were found in only 2/40 patients. HIV infection reduces egg count, and more sensitive methods might have identified more cases, but the very low numbers detected make it unlikely that schistosomal co-infection played a major role in causing NTS recurrence. It therefore seems likely that in many cases NTS have an alternative sanctuary site within the HIV-infected human host. By analogy with typhoid fever, this may be intracellular, within monocyte/macrophages in reticuloendothelial tissues.
Our findings carry implications for the management of these cases. The low rate of re-infection suggests that secondary hygiene advice to avoid re-infection after an index presentation is unlikely to be helpful. Chloramphenicol failed to effect a radical cure of NTS bacteraemia, but the strategy of active follow-up and re-treatment with chloramphenicol for recurrence was partially successful. Index treatment with fluoroquinolones might reduce inpatient mortality or recurrence, but this is untested. However, the increase of multi-drug resistant (MDR) NTS in Malawi and elsewhere in Africa [21] means that more expensive agents may become the only effective treatment. Long term secondary suppressive treatment is a treatment option, but chloramphenicol is too toxic for this purpose, and fluoroquinolones, while effective [22], are too costly in most of Africa. Co-trimoxazole may be useful for secondary suppression in areas where susceptibility is high, but where susceptibility is low, including Malawi, re-treatment may remain the only financially viable option. HAART, if available, reduces or abolishes the need for long term secondary suppression.
The scarcity of microbiological facilities, the rise of MDR strains of NTS worldwide, and the high cost of effective antimicrobial agents will continue to made this a difficult infection to diagnose and treat effectively in the immediate future in Malawi, but it is to be hoped that improved availability of treatment regimes may improve outcomes of NTS bacteraemia in Malawi.
Acknowledgements
The authors extend their grateful thanks to the staff and patients of the Department of Medicine (University of Malawi College of Medicine), and to the staff of the Main Laboratory at QECH, Blantyre, for their generous participation and co-operation. This paper is a shortened and modified version of an article that was previously published in AIDS 2002; 16: 1–9.
MEM was supported by a Research Leave Fellowship, and SBG was supported by a Training Fellowship in Clinical Tropical Medicine, both from the Wellcome Trust, UK. This work was partly supported by a grant from the DfiD HIV Knowledge Programme. DfiD take no responsibility for the views in this report.
References
- 1.Gordon MA, Walsh AL, Chaponda M, et al. Bacteraemia and Mortality Among Adult Medical Admissions in Malawi - Predominance of Non-typhi Salmonellae and Streptococcus pneumoniae. J Infect. 2001;42:44–49. doi: 10.1053/jinf.2000.0779. [DOI] [PubMed] [Google Scholar]
- 2.Arthur G, Nduba VN, Kariuki SM, Kimari J, Bhatt SM, Gilks CF. Trends in Bloodstream Infections among Human Immunodeficiency Virus-Infected Adults Admitted to a Hospital in Nairobi, Kenya, during the Last Decade. Clin Infect Dis. 2001;33:248–256. doi: 10.1086/321820. [DOI] [PubMed] [Google Scholar]
- 3.Thamlikitkul V, Dhiraputra C, Paisarnsinsup T, Chareandee C. Non-typhoidal Salmonella bacteraemia: clinical features and risk factors. Trop Med Int Health. 1996;1:443–448. doi: 10.1046/j.1365-3156.1996.d01-92.x. [DOI] [PubMed] [Google Scholar]
- 4.Smith PD, Macher AM, Bookman MA, et al. Salmonella typhimurium enteritis and bacteremia in the acquired immunodeficiency syndrome. Ann Intern Med. 1985;102:207–209. doi: 10.7326/0003-4819-102-2-207. [DOI] [PubMed] [Google Scholar]
- 5.Nadelman RB, Mathur-Wagh U, Yancovitz SR, Mildvan D. Salmonella bacteremia associated with the acquired immunodeficiency syndrome (AIDS) Arch Intern Med. 1985;145:1968–1971. [PubMed] [Google Scholar]
- 6.Casado JL, Valdezate S, Calderon C, et al. Zidovudine therapy protects against Salmonella bacteremia recurrence in human immunodeficiency virus-infected patients. J Infect Dis. 1999;179:1553–1556. doi: 10.1086/314749. [DOI] [PubMed] [Google Scholar]
- 7.Jacobs JL, Gold JW, Murray HW, Roberts RB, Armstrong D. Salmonella infections in patients with the acquired immunodeficiency syndrome. Ann Intern Med. 1985;102:186–188. doi: 10.7326/0003-4819-102-2-186. [DOI] [PubMed] [Google Scholar]
- 8.Levine WC, Buehler JW, Bean NH, Tauxe RV. Epidemiology of nontyphoidal Salmonella bacteremia during the human immunodeficiency virus epidemic. J Infect Dis. 1991;164:81–87. doi: 10.1093/infdis/164.1.81. [DOI] [PubMed] [Google Scholar]
- 9.Rubino S, Spanu L, Mannuzzu M, et al. Molecular typing of non-typhoid Salmonella strains isolated from HIV-infected patients with recurrent salmonellosis. AIDS. 1999;13:131–139. [PubMed] [Google Scholar]
- 10.Cohen JI, Bartlett JA, Corey GR. Extra-intestinal manifestations of Salmonella infections. Medicine (Baltimore) 1987;66:349–388. doi: 10.1097/00005792-198709000-00003. [DOI] [PubMed] [Google Scholar]
- 11.Aliaga L, Mediavilla JD, Lopez dlO, Lopez-Gomez M, de Cueto M, Miranda C. Nontyphoidal salmonella intracranial infections in HIV-infected patients. Clin Infect Dis. 1997;25:1118–1120. doi: 10.1086/516101. [DOI] [PubMed] [Google Scholar]
- 12.Casado JL, Navas E, Frutos B, et al. Salmonella lung involvement in patients with HIV infection. Chest. 1997;112:1197–1201. doi: 10.1378/chest.112.5.1197. [DOI] [PubMed] [Google Scholar]
- 13.Fernandez GM, Ramos JM, Nunez A, de Gorgolas M. Focal infections due to non-typhi Salmonella in patients with AIDS: report of 10 cases and review. Clin Infect Dis. 1997;25:690–697. doi: 10.1086/513747. [DOI] [PubMed] [Google Scholar]
- 14.Fernandez GM, Torres PR, Gomez RJ, Nunez GA, Jusdado JJ, Ramos RJ. Infectious endocarditis due to non-typhi Salmonella in patients infected with human immunodeficiency virus: report of two cases and review. Clin Infect Dis. 1996;22:853–855. doi: 10.1093/clinids/22.5.853. [DOI] [PubMed] [Google Scholar]
- 15.Medina F, Fuentes M, Jara LJ, Barile L, Miranda JM, Fraga A. Salmonella pyomyositis in patients with the human immunodeficiency virus. Br J Rheumatol. 1995;34:568–571. doi: 10.1093/rheumatology/34.6.568. [DOI] [PubMed] [Google Scholar]
- 16.Collazos J, Mayo J, Martinez E, Blanco MS. Muscle infections caused by Salmonella species: case report and review. Clin Infect Dis. 1999;29:673–677. doi: 10.1086/598652. [DOI] [PubMed] [Google Scholar]
- 17.Gendrel D, Kombila M, Beaudoin-Leblevec G, Richard-Lenoble D. Nontyphoidal salmonellal septicemia in Gabonese children infected with Schistosoma intercalatum. Clin Infect Dis. 1994;18:103–105. doi: 10.1093/clinids/18.1.103. [DOI] [PubMed] [Google Scholar]
- 18.Melhem RF, Lo Verde PT. Mechanism of interaction of Salmonella and Schistosoma species. Infect Immun. 1984;44:274–281. doi: 10.1128/iai.44.2.274-281.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buchmeier NA, Heffron F. Intracellular survival of wild-type Salmonella typhimurium and macrophage-sensitive mutants in diverse populations of macrophages. Infect Immun. 1989;57:1–7. doi: 10.1128/iai.57.1.1-7.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tenover FC, Arbeit RD, Goering RV, Mickelson PA, Murray BE, Persing DH. Interpreting chromosomal DNA restriction patterns produced by pulsed field gel electrophoresis: criteria for bacterial strain typing. J Clin Microbiol. 1995;33:2233–2239. doi: 10.1128/jcm.33.9.2233-2239.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hart CA, Beeching NJ, Duerden BI, et al. Infections in AIDS. J Med Microbiol. 2000;49:947–967. doi: 10.1099/0022-1317-49-11-947. [DOI] [PubMed] [Google Scholar]
- 22.Hohmann EL. Nontyphoidal salmonellosis. Clin Infect Dis. 2001;32:263–269. doi: 10.1086/318457. [DOI] [PubMed] [Google Scholar]