Summary
We herein report three cases of dural arteriovenous fistula (DAVF) in which the venous outlet immediately adjacent to the fistula was selectively embolized. Case 1: A 69-year-old man presented with a subarachnoid hemorrhage (SAH). Angiography demonstrated a DAVF in the left superior petrous sinus. Case 2: A 59-year-old woman presented with dizziness. Angiography demonstrated a DAVF adjacent to great vein of Galen. The DAVF drained through the great vein of Galen with retrograde leptomeningeal venous drainage (RLVD). The basal vein of Rosenthal was enhanced from the great vein of Galen. Case 3: A 51-year-old man presented with an occipital seizure. Angiography demonstrated a DAVF adjacent to the left side of the superior sagittal sinus with RLVD. All three cases were successfully treated by the selective embolization of the venous outlet immediately adjacent to the fistula. Therefore, selective embolization preserved normal venous return.
Key words: Dural arteriovenous fistula, selective transvenous embolization
Introduction
Endovascular treatment can stabilize the dural arteriovenous fistula (DAVF) and it has thus been considered the "gold standard". Transvenous embolization has been considered the most effective therapy for obtaining complete cure of DAVF. But TVE without any results in venous infarction. Owing to recent advances, we can now obtain quite detailed information regarding the fistula and the connecting vein to the fistula.
We herein report three cases of DAVF who had been selectively embolized the venous outlet immediately adjacent to the fistula.
Case1
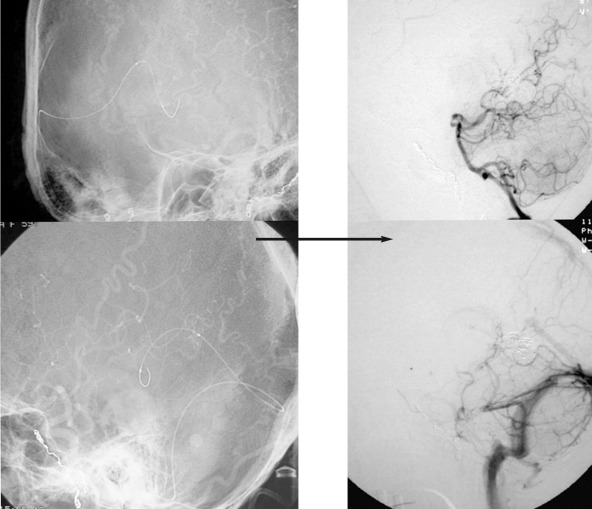
A 69-year-old man was admitted because of sudden severe headache followed by nausea, vomiting and gait disturbance. Upon admission, a CT scan revealed a subarachnoid hemorrhage (SAH) in the left ambient cistern. MRI showed SAH at the same lesion as CT revealed and an abnormal signal void suggested a distended vessel at the left cerebellopontine angle. Angiography demonstrated a DAVF at the left superior petrous sinus fed by the left occipital artery, the left middle meningeal artery, and the left meningohypophysial trunk. It drained through the left lateral mesencephalic vein and the left inferior vermian vein with varicose change (figure 1). Transvenous embolization was planned. Because 3D CT-A showed the left transverse sinus and left inferior vermian vein to be an acute angle (figure 1E), A renegade microcatheter (Terumo) was selectively advanced to the vein direct connected fistula via right jugular vein, right transverse sinus and left inferior vermian vein. The varicose vein connecting with the DAVF was selectively embolized with GDC. After embolization, angiography demonstrated an angiographycal cure without any obliteration of the normal venous return (figure 2).
Figure 1.
Case 1. A,C) Left common carotid artery angiogram (early phase). B,D) Left common carotid artery angiogram (late phase). Left common carotid artery angiogram showed a DAVF at the left superior petrous sinus fed by the left occipital artery, left middle meningeal artery and left meningohypophysial trunk. It drained through the left lateral mesencephalic vein and left inferior vermian vein with varicose change.
Figure 2.
Case 1. A microcatheter (Terumo) was selectively advanced into the vein and then it was directly connected to the fistula via right jugular vein, right transverse sinus and left inferior vermian vein. The varicose vein connecting to the DAVF was selectively embolized with GDC. After embolization, angiography demonstrated an angiographycal cure without the obliteration of the normal venous return.
Case 2
A 59-year-old woman presented with dizziness. MRI showed an abnormal signal void suggestive of a distended vessel at the quadrigeminal cistern. Angiography demonstrated a DAVF adjacent to the great vein of Galen which was fed by the bilateral superficial temporal artery, bilateral middle meningeal artery, bilateral occipital artery, bilateral ascending pharyngeal artery and bilateral tentrial branch of the posterior cerebral artery. It drained through the great vein of Galen with retrograde leptomeningeal venous drainage (figure 3). The basal vein of Rosenthal was enhanced from the great vein of Galen.
Figure 3.
Case 2. A) Left vertebral artery angiogram. B) Right common carotid artery angiogram. C) Left common carotid artery angiogram. Angiography demonstrated a DAVF adjacent to great vein of Galen fed by bilateral superficial temporal artery, bilateral middle meningeal artery, bilateral occipital artery, bilateral ascending pharyngeal artery and bilateral tentrial branch of posterior cerebral artery. It drained through the great vein of Galen with retrograde leptomeningeal venous drainage. The basal vein of Rosenthal was enhanced from the great vein of Galen.
After transarterial coil embolization of the feeding artery to reduce the blood flow to the DAVF, a renegade microcatheter (Terumo) was successfully advanced to the vein immediately adjacent to the DAVF. A Few coils (GDC) were then deposited in the vein. After embolization, angiography showed a cure of the DAVF without any disturbance of the flow of the great vein of Galen (figure 4).
Figure 4.
Case 2. A microcatheter was selectively advanced to the vein immediately adjacent to the DAVF. The vein immediately adjacent to the DAVF was embolized with GDC. After embolization, angiography demonstrated an angiographycal cure without any obliteration of the normal venous return.
Case 3
A 51-year-old man presented with an occipital seizure. MRI showed an abnormal signal void at left medial parietal lobe. Angiography demonstrated a DAVF adjacent to the left side of the superior sagittal sinus. The DAVF was fed by the bilateral superior temporal artery, the bilateral middle meningeal artery and the left occipital artery. In addition it was also drained through the distended cortical vein connecting the superior sagittal sinus and the retrograde leptomeningeal venous drainage (figure 5). Internal cerebral artery angiography revealed the superior sagittal sinus to not have a cerebral venous return.
Figure 5.
Case 3. Angiography demonstrated a DAVF adjacent to the left side of the superior sagittal sinus. The DAVF was fed by the bilateral superior temporal artery, bilateral middle meningeal artery and left occipital artery. In addition, it also drained through a distended cortical vein connecting to the superior sagittal sinus and retrograde leptomeningeal venous drainage.
First of all transarterial coil (GDC) embolization of feeding artery (left superficial temporal artery and left middle meningeal artery) was planned with the aim of reducing of the flow to the DAVF. Next, a renegade microcatheter (Terumo) was selectively advanced to the vein direct connected fistula. Thereafter, the varicose vein connecting between the DAVF and the normal cortical vein was selectively embolized with few coils (GDC) (figure 6). After embolization, angiography showed a cure of the DAVF and no retrograde leptomeningeal venous drainage. Cerebral venous return through superior sagittal sinus (not shown).
Figure 6.
Case 3. A) A microcatheter was selectively advanced to the vein immediately adjacent to the DAVF. A varicose vein connecting with DAVF was selectively embolized with GDC. B) A coil mass can be seen embolizing the vein immediately adjacent to the DAVF. C) After embolization, angiography demonstrated an angiographical cure.
Discussion
The symptoms and clinical severity of DAVFs are strongly influenced by the existing venous drainage patterns.
DAVF are classified according to their pattern of venous drainage, which determines the patient's clinical symptoms and prognosis. Each treatment strategy should be tailored to the type of DAVF1,2.
The presence of cortical venous drainage and of large variceal dilatation in particular predicts an aggressive neurological course, such as intracranial bleeding, cerebral venous infarction, brain edema and cerebrospinal malabsorption because of venous congestion 1,3,4.
Pathophysiologically a DAVF is considered to represent a venous disease and a permanent cure of a cranial DAVF with direct cortical venous reflux can be obtained by a selective intradural division of the venous outlet of the fistula, analogous to the well-known treatment of a spinal DAVF 5,6,7.
While Grisoli et Al and Thompson et Al and Collice et Al suggest that instead of attempting complete excision of DAVFs, cure can be achieved by simply ligating the draining veins as they enter the subarachnoid space6,8,9. If venous occlusive techniques are limited to the venous outlet immediately adjacent to the fistula while preserving the sinus itself in the same way as simply ligating the veins, then transvenous embolization with platinum coils can be used to safely and successfully treat DAVFs10. In our cases represented in this study, we were able to achieve good treatment results like this conception.
Conclusions
We herein reported three cases of DAVF which were all treated with selectively transvenous embolization.
Selective transvenous embolization is thus considered to be useful for treating DAVF without sacrifice the normal venous flow. However, careful catheterization is needed to obtain good treatment results.
References
- 1.Angiographic predictors of intracranial hemorrhage and clinical outcome in nonsurgical patients. J Neurosurg. 1994;81:531–538. doi: 10.3171/jns.1994.81.4.0531. [DOI] [PubMed] [Google Scholar]
- 2.Davis MA, terBrugge K, et al. The validity of classification for the clinical presentation of intracranial arteriovenous fistulas. J Neurosurg. 1996;85:830–837. doi: 10.3171/jns.1996.85.5.0830. [DOI] [PubMed] [Google Scholar]
- 3.Lasjaunias P, Chiu M, et al. Neurosurgical manifestations of intracranial arteriovenous malformations. J Neurosurg. 1986;64:724–730. doi: 10.3171/jns.1986.64.5.0724. [DOI] [PubMed] [Google Scholar]
- 4.Malik GM, Pearce JE, et al. Dural arteriovenous malformations and intracranial hemorrhage. Neurosurgery. 1984;15:332–339. doi: 10.1227/00006123-198409000-00007. [DOI] [PubMed] [Google Scholar]
- 5.Colloce M, Dí Aliberti G, et al. Surgical treatment of intracranial dural arteriovenous fistulae: role of venous drainage. Neurosurgery. 2000;47:56–67. doi: 10.1097/00006123-200007000-00012. [DOI] [PubMed] [Google Scholar]
- 6.Colloce M, Dí Aliberti G, et al. Surgical interruption of leptomeningeal drainage as treatment for intracranial dural arteriovenous fistulas without dural sainus drainage. J Neurosurg. 1996;84:810–817. doi: 10.3171/jns.1996.84.5.0810. [DOI] [PubMed] [Google Scholar]
- 7.Hor BL, Choudhri TF, et al. Surgical management of high-grade intracranial arteriovenous fistulas: leptomeningeal venous disruption without nidus excision. Neurosurgery. 1998;42:796–805. doi: 10.1097/00006123-199804000-00066. [DOI] [PubMed] [Google Scholar]
- 8.Grisoli F, Vincentelli F, et al. Surgical treatment of tentorial arteriovenous malformations draining into the subarachnoid space: report of four cases. J Neurosurg. 1984;60:1059–1066. doi: 10.3171/jns.1984.60.5.1059. [DOI] [PubMed] [Google Scholar]
- 9.Thompson BG, Doppman JL, et al. Treatment of cranial dural arteriovenous fistulae by interruption of leptomeningeal venous drainage. J Neurosurg. 1994;80:617–623. doi: 10.3171/jns.1994.80.4.0617. [DOI] [PubMed] [Google Scholar]
- 10.Kallmes DF, Jensen ME, et al. Percutaneous transvenous coil embolization of a Djindjian Type 4 tentrial dural arteriovenous malformation: Report of a cese. Am J Neuroradiol. 1997;18:673–676. [PMC free article] [PubMed] [Google Scholar]