Summary
After coil embolization for an aneurysm, edema surrounding the aneurysm revealed by magnetic resonance imaging (MRI) is rarely seen and is usually associated with neurological symptoms. Perianeurysmal edema was found by postoperative MRI in three out of 182 patients with cerebral aneurysm, which was treated with Guglielmi Detachable Coil (GDC), and neurological symptoms developed simultaneously.
In cases where neurological symptoms improved with conservative medical treatment, a temporary increase in the volume of an aneurysm, due to coil and thrombus formation, may result in edema. In cases where symptoms were not alleviated with conservative medical treatment, persistent water-hammer effect against the residual lumen of the aneurysm as well as an increase in the volume of aneurysm by hemorrhage in the aneurysmal wall may contribute to the development of perianeurysmal edema. Consideration of the mechanism of edema development by neurological symptoms, MRI findings, and angiographic findings is needed in order to select appropriate treatment.
Key words: cerebral aneurysm, endovascular embolization, perianeurysmal edema
Introduction
Because GDC embolization, unlike a direct surgical procedure, does not require direct mechanical movement in cerebral tissue around an aneurysm, the adjacent brain tissue is less affected. However, it is recognized that perianeurysmal edema appears in rare situations after embolization and leads to the development and deterioration of neurological symptoms1,2. There are few studies on the cause of perianeurysmal edema.
We studied the mechanism in the development of perianeurysmal edema and its course of treatment in our three cases.
Methods
182 cases of cerebral aneurysms treated with GDC (Boston Scientific/Target, MA) from 1997 to 2005 and their MRI records, taken before and after the surgery, were examined.
Diagnosis was based on MRI findings where a high signal on diffusion weighted image (DWI) was not seen but high signal on T2 weighted image (T2WI) or fluid-attenuated inversion recovery (FLAIR) image was seen, and the region was defined as perianeurysmal edema. Edema associated with postoperative cerebral infarction was excluded. The primary course of treatment for all three patients at the outset of perianeurysmal edema development was the use of steroid therapy. Additional embolization was performed when neurological symptoms did not improve through medical treatment.
Characteristics of the aneurysm, angiographic findings before and after surgery, development of an edema and neurological symptoms seen by MRI, response to medical treatment, and response to additional embolization, were carefully compared and the mechanism in the development of perianeurysmal edema and its course of treatment were examined.
Results
A summary of three cases is shown in table 1. The maximum diameter of the aneurysms at the initial treatment was 15 to 22 mm (mean 17 mm). Intraluminal thrombus and perianeurysmal edema were not detected by preoperative MRI. The aneurysms of the patients were imbedded in brain parenchyma. The length of time to develop perianeurysmal edema after embolization was between two days and two months (mean 0.71 months). MRI findings in all three cases were vasogenic edema. Cerebral angiography after the development of neurological symptoms did not show significant change when compared with that performed just after the operation. No recanalization was evident by angiography at the time of perianeurysmal edema development in all three cases. The length of time required for neurological symptoms to improve was ten days to two months (mean 1.1 months). Edema detected by MRI remained even after improvement of neurological symptoms but later disappeared (mean 4.3 months). The presentation of three cases is shown in figures 1 to 3.
Table 1.
Summary of three patients presenting with perianeurysmal edema following GDC embolization.
| Case N. | 1 | 2 | 3 |
|---|---|---|---|
| Age/Gender | 60/M | 39/M | 67/M |
| Clinical presentation | incidental | subarachnoid hemorrhage | incidental |
| Aneurysm location | AcomA | BA-Rt SCA | Lt VA-PICA |
| Aneurysm size (mm) | 15 | 22 | 15 |
| Preoperative MRI | |||
| Embedded portion of aneurysm | subfrontal lobe | cerebral peduncle | cerebellar peduncle |
| Intraluminal thrombus | no | no | no |
| Perianeurysmal edema | no | no | no |
| Initial embolization | |||
| Angiographic outcome | neck remnant | dome filling | neck remnant |
| Neurological symptoms | cognitive dysfunction | progressive cons disturbance left hemiparesis |
limb ataxia dysarthria, dysphagia |
| Onset of symptom and Perianeurysmal edema |
2 days | 2 days | 2 months |
| Postoperative angiography | |||
| Recanalization | no | no | no |
| Response to steroid therapy | yes | no | no |
| Additional intervention | no | additional embolization | parent artery occlusion |
| Duration of symptoms | 10 days | 1 month | 2 months |
| Degree of perianeurysmal edema | disappearance | disappearance | disappearance |
| Duration of perianeurysmal edema | 4 months | 4 months | 5 months |
| Outcome in GOS | GR | GR | GR |
|
M: male, F: female, Rt: right, Lt: left AcomA: anterior communicating artery, BA: basilar artery, SCA: superior cerebellar artery, VA: vertebral artery PICA: posterior inferior cerebellar artery, cons: consciousness, GOS: Glasgow outcome scale, GR: Good Recovery | |||
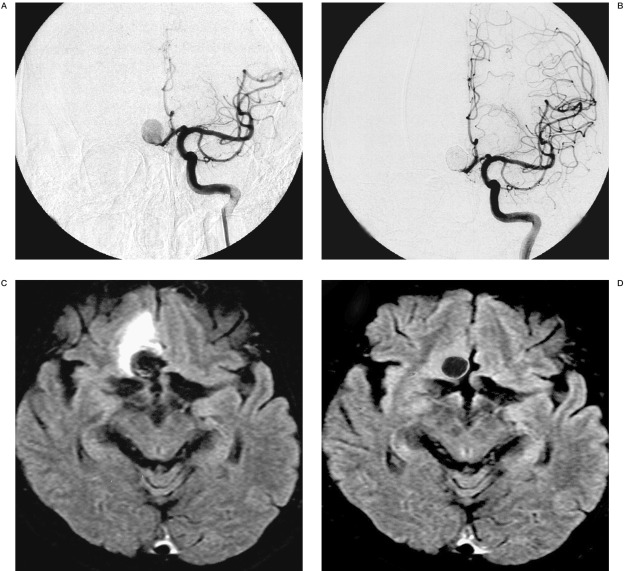
Figure 1.
60-year-old male displaying an incidental anterior communicating artery (AcomA) aneurysm. Pre-embolization left internal cerebral angiogram, antero-posterior projection (A), shows a large AcomA aneurysm. Post-embolization internal cerebral angiogram, antero-posterior projection (B), shows residual neck remnant. Axial FLAIR image after aggravation of the clinical symptoms shows the perianeurysmal edema (C). Axial FLAIR image at four months after the treatment shows the disappearance of the perianeurysmal edema (D).
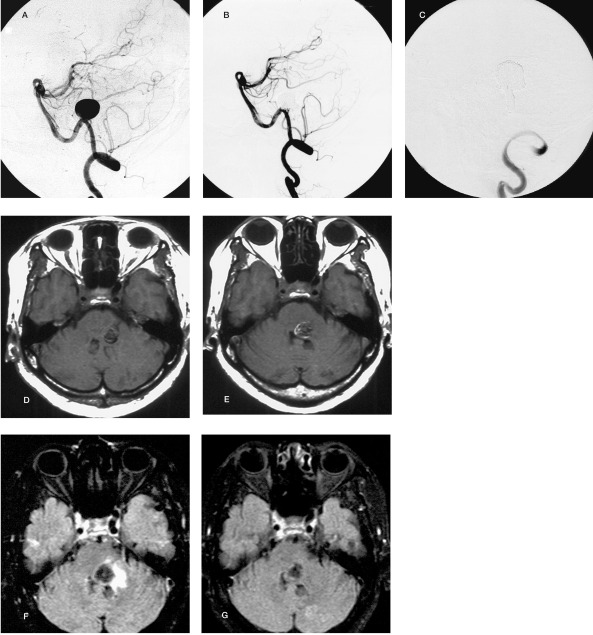
Figure 2.
39-year-old male displaying a ruptured basilar artery-superior cerebellar artery (BA-SCA) aneurysm (Hunt & Hess grade 1, Fisher group 3). Pre-embolization left vertebral angiogram, antero-posterior projection (A), shows a large BA-right SCA aneurysm. Post-embolization left vertebral angiogram, antero-posterior projection (B), shows small dome filling. Left vertebral angiogram after the additional embolization, antero-posterior projection (C), shows tiny neck remnant. Axial T2-weighted image after aggravation of the clinical symptoms showing perianeurysmal edema (D). Axial T2-weighted image at four months after the treatment shows the disappearance of the perianeurysmal edema (E).
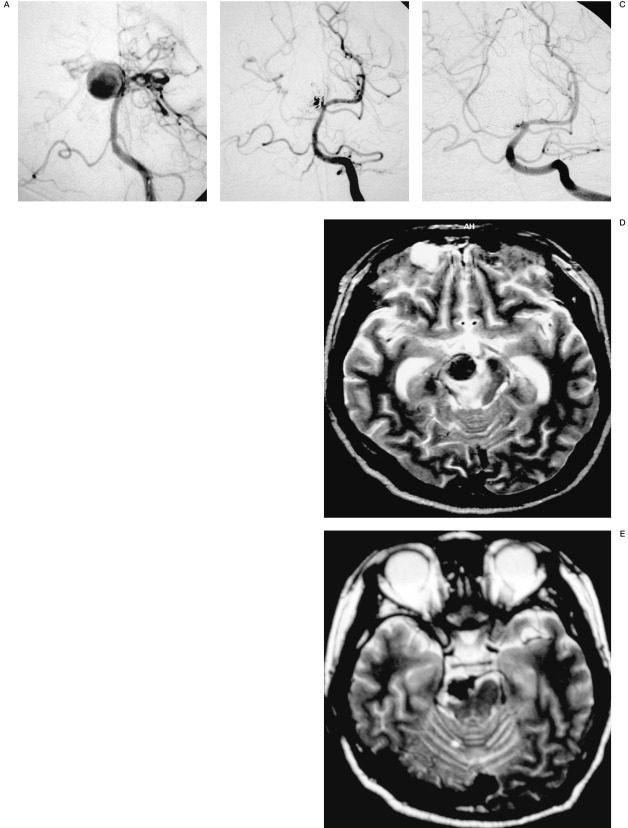
Figure 3.
67-year-old female displaying an incidental vertebral artery-posterior inferior cerebellar artery (VA-PICA) aneurysm. Pre-embolization left vertebral angiogram, lateral projection (A), shows a large VA-PICA aneurysm. Post-embolization left vertebral angiogram, lateral projection (B), shows residual neck remnant. Vertebral angiogram after additional embolization, lateral projection (C), shows the parent artery occlusion involving the origin of the PICA. Axial T1-weighted image just after the treatment showing no high intensity rim surrounding the aneurismal sac (D). Axial T1 weighted image (E) and axial FLAIR image (F), after aggravation of the clinical symptoms, show a high intensity rim (arrowheads) surrounding the aneurismal sac and the perianeurysmal edema with aneurysmal wall thickness (arrows). Axial FLAIR image at five months after the treatment shows the disappearing of the perianeurysmal edema (G).
Discussion
After GDC embolization, GDC-thrombus complex is formed by thrombus formation in the aneurysmal sac. Increase in the volume of aneurysm, which may be possibly induced by the GDC-thrombus complex, is presumed to develop and subside in a short period of time. Since the size of aneurysm is one of the major contributors in the development of perianeurysmal edema 3, the size of GDC-thrombus complex increases in proportion to the increase in size of the aneurysm. When the aneurysm itself grows quickly, its mass effect may possibly lead to the development of a perianeurysmal edema. Steroid therapy is the first-line drug for the perianeurysmal edema 1,2,3,4,5. In case 1, edema and neurological symptoms, developed two days after surgery, and improved in response to steroid therapy for ten days. No additional embolization was required for treatment of the edema and no recurrence was experienced even after completing steroid therapy, suggesting that the temporary increase in the volume of aneurysm by GDC-thrombus complex might be a major cause for the development of perianeurysmal edema in this patient.
It is pointed out that the water-hammer effect has a large effect when there is strong parental vessel homodynamic stress on the aneurysm1,6. Blockage of blood flow to the aneurysm is considered absolutely necessary to improve perianeurysmal edema when water-hammer effect is developed after embolization. If the water-hammer effect was the primary reason for the development of edema, complete occlusion inside the aneurysm is presumed sufficient to stop progression of edema. In case 2, the initial treatment was performed by dome filling to prevent re-rupture in the acute phase. However, the patient developed perianeurysmal edema and neurological symptoms two days after the surgery, which were resistant to steroid therapy, and the neurological symptoms progressed very quickly. In addition to increase in the volume of the aneurysm due to GDC-thrombus complex, continuous water-hammer effect against arterial wall may have had a large influence. Development of neurological symptoms subsided, followed by gradual improvement of edema and symptoms, by additional embolization of the residual lumen. The major mechanism to develop perianeurysmal edema in this patient was the water-hammer effect and additional embolization could neutralize this effect to the lumen of the aneurysm. Giant aneurysms and thrombosed giant aneurysms are often associated with perianeurysmal edema. Recurrent hemorrhage to the aneurysmal wall by abundant vascular channels, derived from parental artery and in the aneurysmal wall, may progressively develop into an aneurysm 1,7. The mechanism in the development of a perianeurysmal edema by the progressive enlargement of an aneurysm after embolization may be similar with that in thrombotic giant aneurysm. Reconstruction of the parental artery by parent artery occlusion and/or aneurysmectomy may be required to improve edema in such patients 2,7. In case 3, initial treatment was completed leaving only tiny neck remnant and the size of the neck remnant did not show change by an angiogram taken at the appearance of edema and neurological symptoms. Steroid therapy in this case did not improve neurological symptoms. Since MRI images suggested not only perianeurysmal edema but also increase in the aneurysmal wall by hemorrhage to the wall, additional embolization in the aneurysmal lumen was not possible. Edema and the symptoms were gradually improved by occlusion of vertebral artery and the aneurysm itself. Enlargement of aneurysmal wall due to breakdown of vascular channels to the wall, which were supplied from vertebral artery, may have contributed to the development of perianeurysmal edema in this patient.
The finding that each aneurysm was imbedded in cerebral parenchyma in our patients may suggest that mass effect of GDC-thrombus complex affects cerebral parenchyma, and results in the development of edema. On the other hand, some patients did not develop a perianeurysmal edema even after embolization of a similar form of aneurysm, suggesting that additional factors, such as disturbance of cerebral autoregulation, incomplete embolization of aneurysm, occlusion or compression of veins surrounding aneurysm, may also be involved in the development of perianeurysmal edema1,3,4,8.
Conclusions
Edema developed after embolization was restricted to the area surrounding aneurysm and reversible postoperative change occurred. Factors, such as the size of the aneurysm being large or giant, localization of aneurysm being imbedded in cerebral parenchyma, increase in the volume of aneurysm by GDC-thrombus complex, influence of hemodynamic stress, and progressive enlargement of aneurysm due to hemorrhage from vaso vasorum into the aneurysmal wall, are thought to be involved in the development of perianeurysmal edema.
Examination of intravascular therapy strategies for aneurysms that elucidate the mechanism involved in the development of edema is important.
References
- 1.Russell SM, Nelson PK, Jafar JJ. Neurological deterioration after coil embolization of a giant basilar apex aneurysm with resolution following parent artery clip ligation. J Neurosurg. 2002;97:705–708. doi: 10.3171/jns.2002.97.3.0705. [DOI] [PubMed] [Google Scholar]
- 2.Iihara K, Murao K, et al. Continued growth of and increased symptoms from a thrombosed giant aneurysm of the vertebral artery after complete endovascular occlusion and trapping: the role of vasa vasorum. J neurosurg. 2003;98:407–413. doi: 10.3171/jns.2003.98.2.0407. [DOI] [PubMed] [Google Scholar]
- 3.Heros RC, Kolluri S. Giant intracranial aneurysms presenting with massive cerebral edema. Neurosurgery. 1984;15:572–577. doi: 10.1227/00006123-198410000-00020. [DOI] [PubMed] [Google Scholar]
- 4.Hammoud D, Gailloud P, et al. Acute vasogenic edema induced by thrombosis of a giant intracranial aneurysm: A cause of pseudostroke after therapeutic occlusion of the parent vessel. Am J Neuroradiol. 2003;24:1237–1239. [PMC free article] [PubMed] [Google Scholar]
- 5.Halbach VV, Higashida RT, et al. The efficacy of endosaccular aneurysm occlusion in alleviating neurological deficits produced by mass effect. J Neurosurg. 1994;80:659–666. doi: 10.3171/jns.1994.80.4.0659. [DOI] [PubMed] [Google Scholar]
- 6.Mericle RA, Wakhloo AK, et al. Delayed aneurysm regrowth and recanalization after Guglielmi detachable coil treatment. J Neurosurg. 1998;89:142–145. doi: 10.3171/jns.1998.89.1.0142. [DOI] [PubMed] [Google Scholar]
- 7.Choi IS, David C. Giant intracranial aneurysms: development, clinical presentation and treatment. European Journal of Radiology. 2003;46:178–194. doi: 10.1016/s0720-048x(03)00090-1. [DOI] [PubMed] [Google Scholar]
- 8.Yamada K, Shrier DA, et al. Cerebral giant aneurysm with extensive vasogenic edema. Radiation medicine. 1998;16:305–307. [PubMed] [Google Scholar]