Summary
Within the group of giant and large aneurysms the subgroup of the so-called "partially thrombosed" aneurysms can be differentiated according to clinical and neuroimaging findings. The present study was carried out to determine the site of bleeding oof these aneurysms and what implications concerning their pathomechanism can be drawn from these findings.
Twenty patients aged two to 77 (mean 44) years who exhibited a partially thrombosed aneurysm that had recently bled were included. Images (MRI including T1 pre- and postcontrast and T2 weighted images in multiple planes, CT and digital subtraction angiography) and patients' charts were reviewed.
MRI showed an onion-skin appearance of the thrombus in 19 patients, rim enhancement of the aneurysm wall (either partial or complete) in 17, and a perifocal edema in 16 patients. The acute hemorrhage was typically crescent-shaped and located at the periphery of the aneurysm, distant from the perfused lumen of the aneurysm within the thrombosed part of the aneurysm.
The current denomination "partially thrombosed" intracranial arterial aneurysms leads to the presumption that thrombus is present endoluminal whereas in fact the site of hemorrhage is within the vessel wall. A more accurate nomination would, therefore, be "aneurysms with intramural hemorrhage". The enhancing wall and the edematous reaction of the adjacent brain parenchyma might be a sign for an inflammatory pathomechanism which is reinforced by histological and pathophysiological studies. This disease should be regarded as a clinical entity separate from saccular or non-thrombosed giant or large aneurysms.
Key Words: intracranial partially thrombosed aneurysm, treatment, hemorrhage, mass effect
Introduction
On cross-sectional imaging studies large or giant intracranial arterial aneurysms can mimic mass lesions. Their symptomatology is typically related to their size producing mass effect with subsequent neurological deficits. There has been a debate about the rate of subarachnoid hemorrhage of these aneurysms. While in some studies the opinion prevails that giant aneurysms seldom rupture 1, the ISUIA study observed that aneurysms with a diameter of more than 24mm have a 50% chance of presenting with a subarachnoid hemorrhage in the posterior circulation within a period of five years2.
This contradiction might be in part be due to the fact that the term "large" or "giant aneurysm" is only related to a specific size of the aneurysm but fails to convey further information concerning the presence or absence of thrombosis or intramural hematoma, the underlying vessel wall disease and the thus expected clinical course and the response of the surrounding tissues3. Moreover, the term "giant" aneurysm is often used in a clinical context to describe a specific subgroup of aneurysms, i.e. the partially thrombosed aneurysms. Thus, different clinical and pathological entities might be subsumed under the same term. Therefore, it is unknown whether the high risk of subarachnoid hemorrhage observed in ISUIA is less in partially thrombosed aneurysms, since this subcategory was not reported out in this trial.
Using neuroimaging criteria, one can differentiate within the groups of large and giant aneurysms those that are completely perfused from the subgroup of the so-called "partially thrombosed" aneurysms, in which the diameter of the aneurysm as detected by cross-sectional studies is larger than the diameter of the perfused aneurysm detected by digital subtraction angiography. These partially thrombosed aneurysms exhibit particular features and must therefore be categorized as a separate entity, regardless of their size: they often continue to grow4, sometimes even after parent vessel occlusion5, they typically present with mass effect6 and show classical features of thrombus of different ages in an onion-skin type fashion with a smaller patent lumen7.
Given these peculiarities of large and giant partially thrombosed aneurysms, it may be a too simplistic approach to think that their pathogenesis resembles those of the classical saccular aneurysms that are supposed to be related (at least in part) to shear stresses (flow, turbulences, jet effects). If a different pathomechanism was present, the treatment strategies would also be likely to differ between the partially thrombosed aneurysms and the nonthrombosed aneurysms or the classical saccular aneurysms. Although it has been proposed, that partially thrombosed aneurysms grow due to recurrence of intramural hemorrhages in the aneurysmal wall8-10, their exact pathogenesis is not known, and the aforementioned hypothesis has not been tested yet in a larger series of patients.
The present study was carried out to determine where so-called "partially thrombosed" aneurysms bleed, to understand their growth mechanism and what implications concerning the patho-etiology of this subgroup of arterial intracranial aneurysms can be drawn from these findings.
Methods
Patients were selected after a retrospective search through the databanks of our hospitals from 1989 to 2005. From all patients with cerebral arterial aneurysms those patients in who the search term "partially thrombosed aneurysm" was found were further evaluated, patients with transmural dissecting aneurysms without partial thrombosis, patients with nonthrombosed giant or large aneurysms and patients with fusiform or serpentine aneurysms without signs of thrombosis were not included in the following analysis. Using this method, a total of 41 patients were found whose files and images were subsequently reviewed. Inclusion criteria based on patients' charts were history of an acute hemorrhagic event or a progress of pre-existing symptoms prior to imaging, in six patients, no imaging data in this acute context were present (as deducted from the clinical history and as visible on non-enhanced CT and T1 weighted sequences). In 12 patients, MR or CT imaging data were not complete to allow for the retrospective confirmation of the diagnosis of a partially thrombosed aneurysm, and in two patients the imaging quality was too bad to be retrospectively interpreted. To evaluate the site of bleeding in partially thrombosed giant aneurysms, the aforementioned 20 patients were excluded from any further analysis. These strict inclusion parameters were chosen to make sure, that only those partially thrombosed aneurysms were included that had recently bled, i.e. where recent acute symptoms suggesting acute enlargement and image evidence of recent thrombus (methemoglobin signal - hyperintensity on T1, crescent shaped hyperdensity on non-enhanced CT), were present.
Twenty-one patients were thus included whose age ranged from two to 77 with a mean age of 47 years. There was a slight male preponderance (13/8). Eight patients had a history of smoking, and 11 patients had a history of hypertension (which was sufficiently treated in four). The images (MRI including T1 pre- and postcontrast and T2 weighted images in multiple planes, CT and digital subtraction angiogra-phy) were reviewed according to the following criteria: number and location of the aneurysm, size (width by height) of the perfused part of the aneurysm and total size, presence of associated anatomical variations of the circle of willis, presence of rim enhancement, perifocal edema, or onion-skin shaped layers of thrombis of different ages. Charts of the patients were subsequently reviewed for age and gender, clinical presentation (subarachnoid hemorrhage, intracerebral hemorrhage, neurological deficits due to mass effect, seizure, or headaches) associated diseases (hypertension, vessel wall disease such as fibromuscular dysplasia, Ehlers Danlos IVb disease, etc.)
Results
Ten partially thrombosed aneurysms were located in the posterior circulation (six intradural vertebral artery, two lateral basilar artery, one basilar tip and one posterior communicating artery aneurysm), 11 aneurysms were in the anterior circulation including four supraophthalmic ICA, three MCA bifurcation, two iCA bifurcation, one cavernous ICA aneurysm and one aneurysm of the petrous portion of the ICA. Width of the aneurysms ranged from 11 to 44mm with a mean of 22 mm while the height of the aneurysms ranged from 14 to 36 mm with a mean of 27 mm. The perfused part of the aneurysms ranged in width from 3 to 21 mm with a mean of 10 mm, and demonstrated a height of 4-28 mm with a mean of 14 mm. In 19 patients no second aneurysm was found, two patients with ICA aneurysm showed a second, unruptured aneurysm (interestingly both were of the "mirror" or "twin"- type) 3, that was not thrombosed and therefore not included in the further evaluation. In two patients angiographic criteria pointed to an underlying vessel wall disease (fibromuscular dysplasia). Anatomical variations of the circle of Willis were encountered in only two patients (hypoplasia of the A1 segment and an AICA-PICA, respectively). A history of hypertension was obtained in seven patients.
Neurological presentation was due to mass effect with neurological deficits in the majority of patients (14 of 21). These deficits were either due to brain stem compression, or direct compression of the cranial nerves depending on the localization of the aneurysm. Four patients presented with neurological deficits related to intraparenchymal hemorrhage. In four patients, headaches were present, only two patients presented with subarachnoid hemorrhage (Hunt and Hess grade 2 and 3, respectively) and two patients had seizures. Diffusion weighted images (available in four patients) and FLAIR images (available in 16 patients) did not show signs of recent embolic events in the territory distal to the aneurysm.
MRI showed an onion-skin appearance of the thrombus in 20 patients, rim enhancement of the aneurysm wall (either partial or complete) in 18, and a perifocal edema in 17 patients. A signal void (which was best discerned on T2 weighted images) matched the configuration of the radiographically demonstrated patent lumen. The acute hemorrhage was typically crescent-shaped and located at the periphery of the aneurysm. In all cases, the hemorrhage was distant from the perfused lumen of the aneurysm within the thrombosed part of the aneurysm (figures 1 to 4).
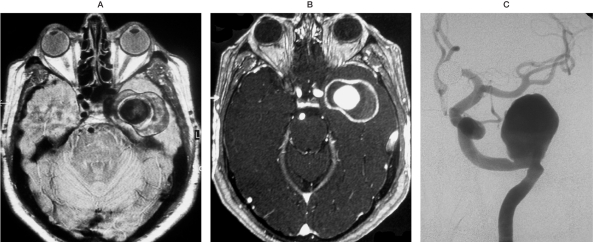
Figure 1.
MRI (Proton Density in Frame A,T1 post contrast enhancement in Frame B) in a patient with a giant partially thrombosed aneurysm demonstrates onion-skin appearance of the thrombus and dense rim enhancement of the aneurysm wall.
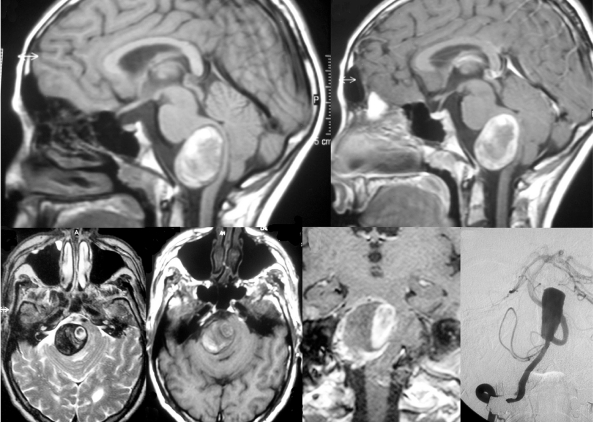
Figure 2.
This patient became symptomatic due to brain stem compression, MRI (T1 pre contrast enhancement in frames A and D, post-contrast in frames B and E and T2-weighted images in frame C) shows the typical findings of a partially thrombosed aneurysm of the V4 segment. Methemoglobin (T1 hyperintense on pre-contrast images) as a sign for an acute bleeding is present at the rim of the aneurysm far from the perfused part that is in this case located medially. T2-weighted images show perifocal edema and the onion-skin layer of mural thrombus of different ages.
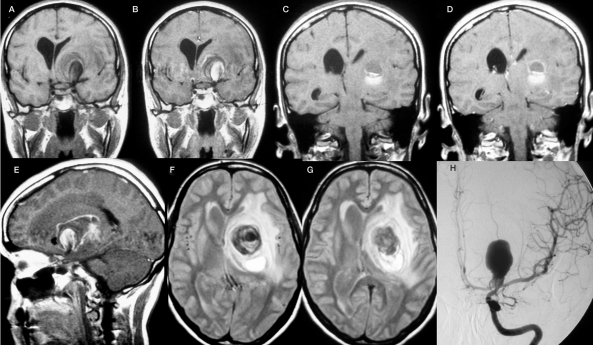
Figure 3.
This patient became symptomatic with an intraparenchymal bleeding that was observed posterior and superior to a mass lesion in the left basalganglia. The mass lesion extended to the basal cisterns and could be identified as a partially thrombosed carotid bifurcation aneurysm. T1-weighted images pre-contrast (A,C) and post contrast (B,D,E) demonstrate that the site of the bleeding is distant from the perfused part of the aneurysm that is located more anterior and inferior. There is partial rim enhancement, and T2-weighted images (F,G) demonstrate an extensive perifocal edema.
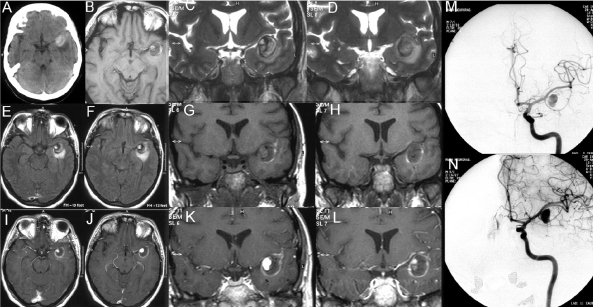
Figure 4.
Non-enhanced CT (frame A) shows a hyperdense mass lesion in the MCA cistern with perifocal edema. On T1-weighted non-enhanced images (frame B, G and H) a crescent shaped hyperintensity on the lateral wall of the lesion suiting intramural hemorrhage can be seen. The source of the bleeding is not close but distant to the perfused aneurysm (that is demonstrated on T1 weighted images post contrast (frames I-L). There is clot/intramural hematoma inbetween the site of the bleeding and the aneurysm lumen. After contrast administration, a contrast enhancing ring surrounding the aneurysm can be perceived.
Discussion
The salient points of this retrospective review of partially thrombosed aneurysms are: hemorrhage occurring at the periphery of the aneurysm, within the "thrombosed part" of the aneurysm, far from the patent lumen and in a high percentage of aneurysms, a strongly enhancing rim and an edematous reaction (or abluminal inflammatory activity) of the adjacent brain parenchyma were visible. In the following we will discuss our results in the light of clinical and neuroimaging characteristics reviewed by other groups, surgical, histological and pathophysiological findings that might shed light on the mechanism of growth and rupture of this particular subgroup of aneurysms and, which, therefore, might alter current treatment strategies.
Clinical Features
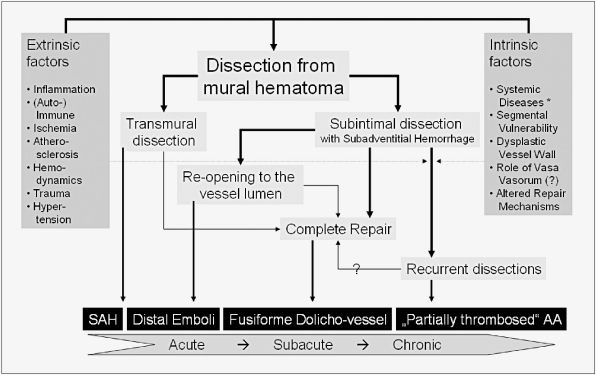
Unlike classical saccular aneurysms, partially thrombosed aneurysms presented typically with progressive symptoms due to mass effect and only seldom presented with subarachnoid hemorrhage (SAH). Mizutani et Al. explained the subacute and chronic changes leading to this symptomatology by a dissecting process 11. Whereas in "classic" dissecting aneurysms a transmural dissection leads to an acute SAH, partially thrombosed aneurysms were characterized by recurrent subacute and non-transmural dissections that resulted in repeated subadventitial bleeding and progressive enlargement of the aneurysm. If SAH in these types of aneurysms was present, the neck of the aneurysm is most often involved, presumably due to a transmural dissection at this site. Arterial dissection might therefore generate a wide range of intracranial arterial aneurysms depending on the impact of extrinsic factors (inflammation, hemodynamic forces, etc.), the vessel wall integrity and the intrinsic repair systems as depicted in figure 5.
Figure 5.
Schematic representation of aneurysms resulting from dissecting processes
Neuroimaging
Evidence for intramural hemorrhage of partially thrombosed aneurysms were first put forward in 1980 when Schubiger et Al suggested that the formation of intracranial giant artery aneurysms could be due to a subacute recurrent dissection process associated to repeated subadventitial hemorrhages from the vasa vasorum 8,9. They found fresh clot at the periphery of the thrombosed portion of the aneurysm that had no communication with the patent lumen. Typically a sickle-shaped zone or a complete ring of contrast enhancement was present in the periphery of the aneurysm. These authors argued that the aneurysm wall was vascularized by a dense network of vasa vasorum that behave like the membrane of a chronic subdural hematoma and that intramural hemorrhages are the main factor for the observed growth.
Further neuroimaging support of this hypothesis can be derived from a recent review of MR imaging characteristics of partially thrombosed aneurysms by Teng et Al 7. In nine of ten patients with partially thrombosed aneurysms, a hyperintensity on non-enhanced T1-weighted images indicative of fresh hemorrhage was present within the thrombus along the aneurismal wall and away from the patent lumen. In three of five patients in whom contrast was given, an enhancing rim was perceived. The typical onion-skin appearance of a multilayered thrombus wall was present in eight of ten cases.
In their series of giant aneurysms, Vorkapic et Al performed MRI in four patients with partially thrombosed aneurysms and found perianeurysmal bleeding in one, rim enhancement in all four patients and an onion-skin appearance in all patients that demonstrated layers of fresh thrombus within the periphery, whereas older thrombus as detected by MRI criteria was present in the central parts. Surrounding edema was present in nearly all cases 12.
Kaneko et Al report on a serial neuroimaging findings of a patient with a thrombosed giant anterior cerebral artery aneurysm that demonstrated a stable size regarding the aneurysm lumen but whose thrombosed portion grows due to hemorrhage into the thrombus and/or the aneurismal wall4. Whenever new hemorrhage was encountered it was distal to the patent lumen, close to the periphery of the aneurysm.
In a case report by Koyama et Al an intramural hemorrhage (that presented as a convexshaped high-density lesion on CT) suggestive of dissection between the layers of the aneurysm wall or the periphery of the thrombus could be appreciated13.
Katayama et Al report on a case of growth of a completely thrombosed giant aneurysm, that demonstrated no patent lumen on angiography 14. Despite this, the patient gradually deteriorated and imaging studies demonstrated an ever increasing mass with the typical onion skin appearance of intramural thrombus of different ages and a strong peripheral contrast enhancement that the authors attributed to the highly vascularized arterial wall. Although there is no mention of imaging signs of fresh intramural hemorrhage, the reported growth despite the lack of luminal filling points towards a different source of clotted blood that lead to subsequent aneurysm growth.
These studies are in line with our findings, both concerning the onion-skin appearance, the location of the fresh hemorrhage in the periphery of the aneurysm, the vessel wall enhancement and the perifocal edema that will be discussed in further detail later.
It is known, that following aneurismal wall rupture, a protective layer of collagen is formed and capillaries begin to proliferate to form a neomembrane 3. A vicious circle might start, in which minimal trauma (or other triggering events such as inflammatory responses that will be discussed later) might induce bleeding into the neomembrane and this hemorrhage might then induce further proliferation of neomembranes 15,16. The more peripheral layers of the thrombus therefore represent the more recent clots leading to progressive growth of the aneurysm.
Surgical Findings
Macroscopic inspection during surgery further underlines the proposed pathomechanism: Yasargil reported that on surgical exploration of partially thrombosed intracranial aneurysms a tremendous network of fine vessels covering the aneurysm can be seen, most likely representing the vasa vasorum or angiogenesis induced by the hematoma17.
Iihara et Al noted growth of a giant partially thrombosed aneurysm after complete occlusion of the parent vessel in a patient. Although there was no angiographically demonstrated evidence of filling of a lumen, the aneurysm continued to enlarge, requiring surgery5. During the operation, the presence of markedly developed vasa vasorum was recognized on the parent artery and neck of the aneurysm. During surgery oozing of blood from the thrombus persisted even after the parent artery was clipped and only stopped after removing the clot and coagulating the aneurysm wall. This case report therefore demonstrated that the area of bleeding in fact were vasa vasorum of the aneurysm wall rather than the parent lumen.
Histological and Pathophysiological Studies
Histological examinations of a thrombosed giant aneurysm reported by Yasui demonstrated more recent hemorrhage between old thrombus and the aneurysm wall with clefts of fresh blood present, indicative of a dissection of the aneurismal wall by blood flow18.
In the same line, Nagahiro et Al demonstrated on three histological specimen of partially thrombosed aneurysms intrathrombotic vascular channels some of which had endothelial lining and demonstrated proliferating smooth muscle cells19. They argued that not vasa vasorum but instead a proliferation of newly formed capillaries within the thrombus of the aneurysm might be the cause of the intramural hemorrhage that lead to subsequent aneurysm growth.
The study of Atlas et Al focussed on a second important observation in partially thrombosed aneurysms 6. They found in two histological specimens that macrophages were present in the aneurysm wall, especially near the periphery of the thrombus. The role of inflammation in the formation of giant aneurysms has recently been further underlined by a study of Zhao et Al20. They found that macrophages positive for a key enzyme in the inflammatory pathway, i.e. the 5-lipoxygenase (5-LO), localized to the adventitia of diseased mouse and human arteries in areas of neoangiogenesis and that these cells constitute a main component of aneurysms. 5-LO generates different forms of leukotrienes that are potent mediators of inflammation by further activating macrophages and recruiting monocytes and T-cells21. One of the leukotrienes, LTD4 binds to endothelial cells of the newly formed vascular channels which leads to an increase of leukocyte extravasation. This adventitial inflammation leads to a weakening of the media by release of proinflammatory factors that invade the media and lead to a dilation that subsequently results in aneurysm formation10. Inflammation of the vessel wall by a chemokine intermediary route was thereby linked to weakening of the vessel wall due to degradation of the extracellular matrix and the elastic lamina with subsequent aneurysm formation22.
A possible inflammatory component in our series of patients might be deduced from the extensive rim enhancement of the aneurysm wall seen in some patients and from the concomitant edema that was present, although the role of vasa vasorum concerning the rim enhancement and mass effect with subsequent venous congestion might also account for these findings. MRI contrast agents marking macrophages could be used to further differentiate these findings 10.
Therapeutic Implications
Given the considerations discussed above, we think that the term "partially thrombosed aneurysm" might be misleading since it might imply that thrombus is located within the aneurysm lumen. Since neuroimaging, surgery and histology point toward clots of different ages within the vessel wall we prefer the term of "aneurysms with intramural hematoma". In addition, the inflammatory component that is likely to be present in these pathologies and the role of growth factors as also pointed out by Nagahiro et Al should be kept in mind19.
Aneurysms with intramural hematoma might therefore be regarded as a proliferative disease of the vessel wall with growth induced by extravascular activity3. Mural thrombosis might act as a chronic triggering factor for perivascular growth factors which might stimulate the further proliferation both of vessels within the clot and of the vessel wall itself, thereby differing grossly from the presumed pathomechanism in non-thrombosed or saccular aneurysms even of a size larger than 2 cm, and should be regarded as a separate clinical and pathological entity. It seems obvious that, since different kinds of aneurysms do not share a common pathophysiology, their respective treatment strategies will also likely to differ.
Given the specific histological considerations discussed above, we do not think that endovascular repair of these aneurysms by coils is an apt treatment option. In fact, we have found in those patients treated with coils, that the aneurysm regrew over time, maybe due to compaction maybe due to the fact that the pathological process is likely to be maintained in the vessel wall employing a purely luminal therapeutic approach.
Parent vessel occlusion might be a treatment option, since especially in the ICA territory, the vasa vasorum which might be involved in the disease process arise form the ICA itself and are thus occluded. However, for these kinds of aneurysms, the results of parent vessel occlusion are not predictable and it has been shown that aneurysms might continue to grow even after their parent vessels have been sacrificed 5. Again, we presume that this might be due to pertinence of vessel wall disease.
The ideal treatment should be complete surgical excision of the lesion, however, this procedure might only be possible after distal and proximal vessel wall occlusion (trapping) which might not be tolerated by the patient depending on the location of the aneurysm 3. Moreover, these surgical procedures are technically highly demanding and might pose serious risks to the patient19.
Apart from medical, i.e. anti-inflammatory treatment (such as steroids), one might also consider, in the future, a treatment regimen that is able to cross the vessel wall to reach the newly formed vascular channels and the most peripheral parts of the aneurysm wall. Drug eluting stents could for example be a possible treatment option that has to be further evaluated in the future. Recently, it has been shown that by inhibiting an enzyme that is involved in the degradation of the extracellular matrix due to chronic inflammation, regression of aortic aneurysms could be achieved 23. It would be interesting to determine whether the same mechanisms might act also in intracerebral aneurysms with intramural hematoma.
Conclusions
The current denomination "partially thrombosed" intracranial arterial aneurysms is misleading and might be changed to aneurysms "with intramural thrombus". This disease should be regarded as a clinical entity separate from saccular or non-thrombosed giant or large aneurysms. Neurological defect due to mass effect instead of subarachnoid hemorrhage is the typical presenting symptom. If present, hemorrhage typically occurs at the periphery of the aneurysm wall within the clot but far from the patent lumen. The strongly enhancing rim and an edematous reaction of the adjacent brain parenchyma might be a sign for an inflammatory pathomechanism (either causative or reactional) which is reinforced by histological and pathophysiological studies. Treatment strategies should include complete exclusion of the aneurysm, surgery, and, if not possible, anti-inflammatory treatment; an isolated endovascular coiling approach, on the other hand, might not be sufficient.
References
- 1.Sarwar M, Batnitzky S, Schechter MM. Tumorous aneurysms. Neuroradiology. 1976;12:79–97. doi: 10.1007/BF00333123. [DOI] [PubMed] [Google Scholar]
- 2.Wiebers DO, Whisnant JP, et al. Unruptured intracranial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet. 2003;362:103–110. doi: 10.1016/s0140-6736(03)13860-3. [DOI] [PubMed] [Google Scholar]
- 3.Berenstein A, Lasjaunias P, TerBrugge KG. Surgical Neuroangiography. Vol 2.1. Berlin: Springer; 2004. [Google Scholar]
- 4.Kaneko T, Nomura M, et al. Serial neuroimaging of a growing thrombosed giant aneurysm of the distal anterior cerebral artery - case report. Neurol Med Chir (Tokyo) 2001;41:33–36. doi: 10.2176/nmc.41.33. [DOI] [PubMed] [Google Scholar]
- 5.Iihara K, Murao K, et al. Continued growth of and increased symptoms from a thrombosed giant aneurysm of the vertebral artery after complete endovascular occlusion and trapping: the role of vasa vasorum. Case report. J Neurosurg. 2003;98:407–413. doi: 10.3171/jns.2003.98.2.0407. [DOI] [PubMed] [Google Scholar]
- 6.Atlas SW, Grossman RI, et al. Partially thrombosed giant intracranial aneurysms. Correlation of MR and pathologic findings. Radiology. 1987;162:111–114. doi: 10.1148/radiology.162.1.3786749. [DOI] [PubMed] [Google Scholar]
- 7.Teng MM, Qadri SMN, et al. MR imaging of giant intracranial aneurysm. J Clin Neurosci. 2003;10:460–464. doi: 10.1016/s0967-5868(03)00092-4. [DOI] [PubMed] [Google Scholar]
- 8.Schubiger O, Valavanis A, Wichmann W. Growth-mechanism of giant intracranial aneurysms; demonstration by CT and MR imaging. Neuroradiology. 1987;29:266–271. doi: 10.1007/BF00451765. [DOI] [PubMed] [Google Scholar]
- 9.Schubiger O, Valavanis A, Hayek J. Computed tomography in cerebral aneurysms with special emphasis on giant intracranial aneurysms. J Comput Assist Tomogr. 1980;4:24–32. doi: 10.1097/00004728-198002000-00005. [DOI] [PubMed] [Google Scholar]
- 10.Krings T, Piske R, Lasjaunias P. Intracranial arterial aneurysm vasculopathies: Targeting the outer vessel wall. Neuroradiology. 2005;47:931–937. doi: 10.1007/s00234-005-1438-9. [DOI] [PubMed] [Google Scholar]
- 11.Mizutani T, Miki Y, et al. Proposed classification of nonatherosclerotic cerebral fusiform and dissecting aneurysms. Neurosurgery. 1999;45:253–259. doi: 10.1097/00006123-199908000-00010. discussion 259-260. [DOI] [PubMed] [Google Scholar]
- 12.Vorkapic P, Czech T, et al. Clinico-radiological spectrum of giant intracranial aneurysms. Neurosurg Rev. 1991;14:271–274. doi: 10.1007/BF00383260. [DOI] [PubMed] [Google Scholar]
- 13.Koyama S, Kotani A, Sasaki J. Giant basilar artery aneurysm with intramural hemorrhage and then disastrous hemorrhage: Case report. Neurosurgery. 1996;39:174–177. doi: 10.1097/00006123-199607000-00039. [DOI] [PubMed] [Google Scholar]
- 14.Katayama Y, Tsubokawa T, et al. Growth of totally thrombosed giant aneurysm within the posterior cranial fossa. Diagnostic and therapeutic considerations. Neuroradiology. 1991;33:168–170. doi: 10.1007/BF00588260. [DOI] [PubMed] [Google Scholar]
- 15.Friede RL, Schachenmayr W. The origin of subdural neomembranes. II Fine structure of neomembranes. Am J Pathol. 1978;92:69–84. [PMC free article] [PubMed] [Google Scholar]
- 16.Schachenmayr W, Friede RL. The origin of subdural neomembranes. I Fine structure of the dura-arachnoid interface in man. Am J Pathol. 1978;92:53–68. [PMC free article] [PubMed] [Google Scholar]
- 17.Yasargil MG. Micorneurosurgery. Vols 1 and 2. Stuttgart: Thieme; 1984. Clinical considerations, surgery of the intracranial aneurysms and results. [Google Scholar]
- 18.Yasui T, Sakamoto H, et al. Rupture mechanism of a thrombosed slow-growing giant aneurysm of the vertebral artery - Case report. Neurol Med Chir (Tokyo) 1998;38:860–864. doi: 10.2176/nmc.38.860. [DOI] [PubMed] [Google Scholar]
- 19.Nagahiro S, Takada A, et al. Thrombosed growing giant aneurysms of the vertebral artery: growth mechanism and management. J Neurosurg. 1995;82:796–801. doi: 10.3171/jns.1995.82.5.0796. [DOI] [PubMed] [Google Scholar]
- 20.Zhao L, Moos MP, et al. The 5-lipoxygenase pathway promotes pathogenesis of hyperlipidemia-dependent aortic aneurysm. Nat Med. 2004;10:966–973. doi: 10.1038/nm1099. [DOI] [PubMed] [Google Scholar]
- 21.Spanbroek R, Grabner R, et al. Expanding expression of the 5-lipoxygenase pathway within the arterial wall during human atherogenesis. Proc Natl Acad Sci USA. 2003;100:1238–1243. doi: 10.1073/pnas.242716099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Palinski W. Aneurysms: leukotrienes weaken aorta from the outside. Nat Med. 2004;10:896–898. doi: 10.1038/nm0904-896. [DOI] [PubMed] [Google Scholar]
- 23.Yoshimura K, Aoki H, et al. Regression of abdominal aortic aneurysm by inhibtion of c-Jun N-terminal kinase. Nat Med. 2005;11:1330–1338. doi: 10.1038/nm1335. [DOI] [PubMed] [Google Scholar]