Summary
Four cases of posterior cerebral artery (PCA) aneurysms are described. The aneurysms were located at the P2 segment of PCA. All cases presented with a subarachnoid hemorrhage (SAH). Endovascular treatment was performed, with occlusion of the aneurysm and parent vessel, using platinum coils. Two patients developed a homonymus lateral hemianopia after treatment.
Key words: fusiform aneurysms, posterior cerebral artery, intracranial hemorrhage, endovascular treatment
Introduction
Aneurysms of the posterior cerebral artery (PCA) are quite rare, accounting for about 1% of all intracranial aneurysms4. Among PCA aneurysms, P2 segment aneurysms develop distal to the junction of the posterior communicating artery with the posterior cerebral artery and are located at the level of the posterior part of the midbrain, within the ambient cistern; they may be saccular, fusiform or dissecting.
Endovascular or surgical treatment consists of selective occlusion of the aneurysm, when possible, or parent artery occlusion. There are only a few reports about endovascular treatment of PCA aneurysms1,2,3,5,6,7.
Rewiewing our series of aneurysms presenting with SAH and treated by endovascular approach, we observed four P2 segment aneurysms, one saccular and three fusiform. Treatment with coil occlusion of the aneurysm and parent artery, results, complications, and follow-up studies are reported.
Material and Methods
Clinical Material and Diagnostic Examinations
Between 2001 and 2006 four patients were diagnosed having an acutely ruptured aneurysm of the PCA. The patients' age ranged between 19 and 54 years. Two patients were females and two were males. Upon admission, all four patients were in Hunt and Hess grade 1, and in GCS 15 to 13. Neurological examination did not show any visual field deficit. In all patients the CT findings demonstrated a Fisher grade 1 SAH.
Angiography demonstrated that three fusiform and one saccular aneurysm had been the cause of the hemorrhage. Three aneurysms were located at the P1-P2 junction, while one aneurysm was located at the P2-P3 junction of the PCA. Three aneurysms were large, while one was small.
Endovascular treatment of the lesions was performed in all cases using bare platinum coils (Table 1).
Table 1.
Patient's characteristics, treatment type, and follow-up studies
| Patient no. Sex, Age, (y) |
Aneurysm Type, Side and Size |
Presenting Symptom |
Test | Treatment Type |
Clinical Outcome and Follow-up findings |
|---|---|---|---|---|---|
| 1/M/19 2001 |
Fusiform P2 L |
SAH HH1 GCS 15 Large |
Angiography + balloon occlusion |
GDC | Excellent, no deficit; at 1-y Angiography, no recanalization |
| 2/F/45 2004 |
Fusiform P2 L Large |
SAH HH1 GCS 14 |
angiography | GDC | right lateral hemianopsia, at 1-y Angiography, no recanalization |
| 3/F/54 2006 |
Fusiform P2-P3 L Large |
SAH HH1 GCS 13 |
angiography | GDC | right lateral hemianopsia at 9-mo Angiography, no recanalization |
| 4/M/41 2006 |
Berry P2 L Small |
SAH HH1 GCS 13 |
angiography | GDC+ Micrus coils |
Excellent, no deficit at 8-mo Angiography, no aneurysm recanalization pca recanalization |
Endovascular Treatment and Follow-up Studies
All embolization procedures were performed via transfemoral approach, under general anesthesia and systemic heparinization. In all cases a microcatheter was positioned in the aneurysm and platinum coils were delivered until aneurysm and parent vessel occlusion were achieved.
In one case (case n° 1) a balloon test occlusion of the PCA (proximal to the aneurysm) was performed prior to the induction of general anesthesia. In this case, clinical examination during the test occlusion showed no deficits. In the remaining three cases no test occlusion was performed.
Results
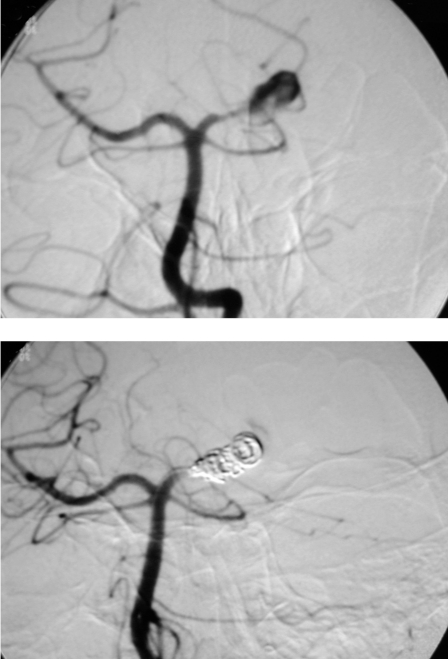
Complete occlusion of the aneurysm and parent vessel was achieved in all four cases (figure 1A,B). No technical complications were encountered.
Figure 1.
A) Angiogram showing a large fusiform aneurysm at the P2 segment of the left posterior cerebral artery. B) Aneurysm and parent vessel were occluded by depositing 9 GDC coils. The aneurysm is now excluded from the brain circulation.
The immediate post treatment clinical examinations showed no clinical sequelae in two cases (Cases 1 and 4). In the remaining two cases (Cases 2 and 3), however, a homonymous lateral hemianopia was detected which persisted at the follow-up studies (Table 1). Long term clinical follow-up studies in these four cases showed no episode of rebleeding.
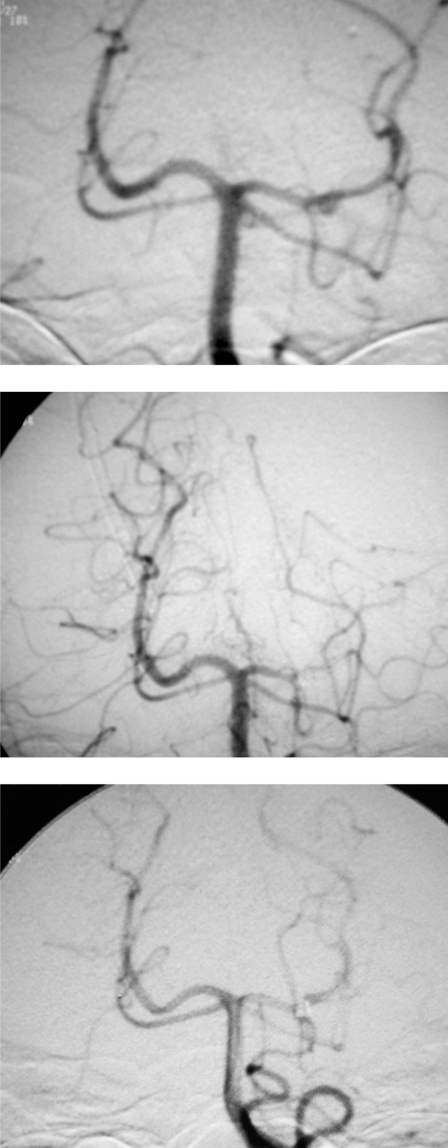
Follow-up studies were performed at one month, six months, and 12 months. The one and six month studies consisted of clinical and MRA examinations. Digital subtraction angiography was performed at the 12 month follow-up in three cases. In the remaining case (case n° 4) 30 day, three month and 12 month angiographic follow-ups were performed, which showed a recanalization of the parent artery and persistent occlusion of the aneurysm (figure 2A-C).
Figure 2.
A) Angiogram showing a small, saccular aneurysm arising from the P2 segment of the left posterior cerebral artery (arrow). B) Aneurysm and parent vessel were occluded by depositing 3 platinum coils. C) This 30-day follow-up angiogram shows persistent occlusion of the aneurysm and repermeabilization of the entire posterior cerebral artery vascular tree (arrows) (see text).
Discussion
The most common clinical presentation of PCA aneurysms is subarachnoid hemorrhage SAH 3. Clinical presentation of P2 fusiform aneurysms with SAH, however, is rare6,7. Drake and Amacher4 reported that the most common site of origin of PCA aneurysms is at the first major branch, beyond the junction with the PCoA. Aneurysms, however, can occur at any site along the course of PCA. PCA aneurysms are classified as those of the P1 segment, P1-PCoA junction, P2, P3, or P4 segments.
As to the largest published series on the endovascular treatment of PCA aneurysms, Hallacq et Al. reported ten cases of P2 segment aneurysms, of which only two with clinical history of SAH6, while Van Rooij et Al7 reported 22 PCA aneurysms, of which only two were non-saccular and presented with a clinical history of SAH.
Endovascular approach for the treatment of P2 aneurysms is an accepted form of treatment. Previous reports described the occlusion of the aneurysm and the parent artery as the most efficacious kind of endovascular treatment 1,2,3,5,6,7.
In our series we report four P2 aneurysms (three fusiform and one saccular) with a clinical history of SAH. No symptoms were reported in our cases before the subaracnoid hemorrage. All aneurysms were occluded with coils including the P2 main trunk. After the first patient (Case 1, vide supra), we did not consider preoperative test occlusion in the remaining three patients. This was because other series reported a well tolerated PCA occlusion by virtue of the rich collateral leptomeningeal arterial network6,7. We did not consider the use of intracranial stents because all cases had a clinical history of recent SAH. In addition, there have been no reports to date on the use of stents in the P2 segment of PCA.
It is the author's opinion that this is suggestive for the two following reasons:
1) Post treatment neurological deterioration was observed in two out of four cases. Previous reports, on the contrary, indicated that the occlusion of the PCA does not lead to neurologi-cal deterioration6,7; this represents a discrepancy with our clinical results. Nevertheless, in a recent report, Andreou et Al1 described six cases of PCA aneurysms treated with parent vessel occlusion: in two of these six cases, post-treatment campimetric deficits were observed. Furthermore, Ciceri et Al 3 reported that in seven similar cases two patients were affected by post-treatment campimetric deficits. Moreover, Eckard et Al5 reported post-treatment campimetric deficits in one out of three cases treated with PCA occlusion.
2) In one of our four cases (case n° 4) treated with aneurysm and PCA coil occlusion the artery resulted reopened at the 30 day follow-up angiogram. Occlusion of the aneurysm and patency of the parent vessel were confirmed at the three-month as well as at the 12-month follow-up angiograms. Recanalization of the parent vessel could possibly be due in this case to a modification of the structure of the thrombus with "de novo" re-endothelialization of channels within the thrombus, with consequent revascularization of the artery. Persistent aneurysm occlusion will have to be confirmed in this case with a longer term follow-up angiogram.
Conclusions
Our results confirm that parent vessel occlusion is a viable therapeutic option for distal posterior cerebral aneurysms. However, a homonymous lateral hemianopia may develop after treatment. All therapeutic options should be considered on a case-by-case basis.
References
- 1.Andreou A, Ioannidis I, Mitsos A. Endovascular treatment of peripheral intracranial aneurysms. Am J Neuroradiol. 2007;28:355–361. [PMC free article] [PubMed] [Google Scholar]
- 2.Arat A, Islak C, Saatci I. Endovascular parent artery occlusion in large giant fusiform distal posterior cerebral artery aneurysms. Neuroradiology. 2002;44:700–705. doi: 10.1007/s00234-002-0747-5. [DOI] [PubMed] [Google Scholar]
- 3.Ciceri E, Klucznik R, Grossman R. Aneurysms of the posterior cerebral artery: classification and endovascu-artreatment. Am J Neuroradiol. 2001;22:27–34. [PMC free article] [PubMed] [Google Scholar]
- 4.Drake C, Amacher A. Aneurysms of the posterior cerebral artery. J Neurosurg. 1969;30:468–474. doi: 10.3171/jns.1969.30.4.0468. [DOI] [PubMed] [Google Scholar]
- 5.Eckard D, O’Boynick P, McPherson C. Coil occlusion of the parent artery for treatment of symptomatic peripheral intracranial aneurysms. Am J Neuroradiol. 2000;21:137–142. [PMC free article] [PubMed] [Google Scholar]
- 6.Hallacq P, Piotin M, Moret J. Endovascular occlusion of the posterior cerebral artery for the treatment of P2 segment aneurysms: retrospective review of a 10-years series. Am J Neuroradiol. 2002;23:1128–1136. [PMC free article] [PubMed] [Google Scholar]
- 7.Van Rooij W, Sluzewski M, Beute G. Endovascular treatment of posterior cerebral artery aneurysms. Am J Neuroradiol. 2006;27:300–305. [PMC free article] [PubMed] [Google Scholar]