Summary
A case of multiple cerebral aneurysms caused by left atrial myxoma is reported. We present the details of this case and discuss the hypothetical pathogenesis, radiological aspects and treatment of these neoplastic aneurysms.
Key words: multiple cerebral aneurysms; left cardiac myxoma
Introduction
Atrial myxoma represents approximately 50% of all cardiac tumors. Systemic emboli from myxomas can lead to cerebral infarction and aneurysm formation. Cerebral aneurysms are rare, and around 40 cases have been reported to date. If histological studies have showed that these arterial lesions are neoplastic, no reports of the long-term course are available.
The pathogenesis of the aneurysmal lesions is not well-known, and there is still no definitive treatment. We report here the case of a woman who presented multiple cerebral aneurysms five years after successful resection of a left atrial myxoma.
We analysed the different published articles to draw additional information on the pathogenesis, symptomatology, the radiological aspects and management of myxomatous aneurysms.
Case Report
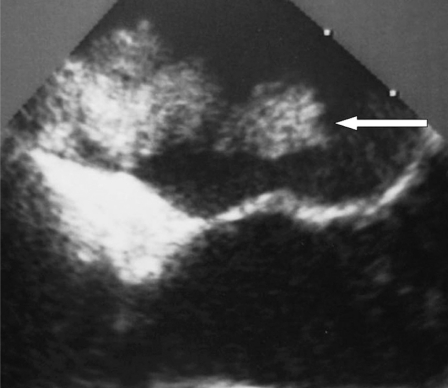
A 50-year-old woman without no medical history suddenly presented dysarthria and right-side weakness. She fully recovered within some days. The cardiac examination showed a mass in the left atrium (figure 1) which was removed successfully by surgery. Histological examination confirmed the diagnosis of myxoma. After surgery, the patient was discharged from hospital with aspirin (250 mg/d).
Figure 1.
Echocardiography showing atrial mass (white arrow).
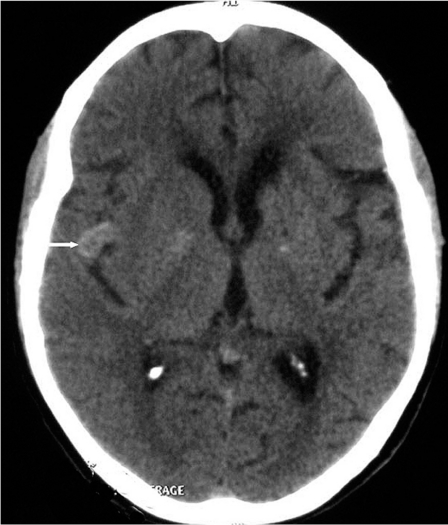
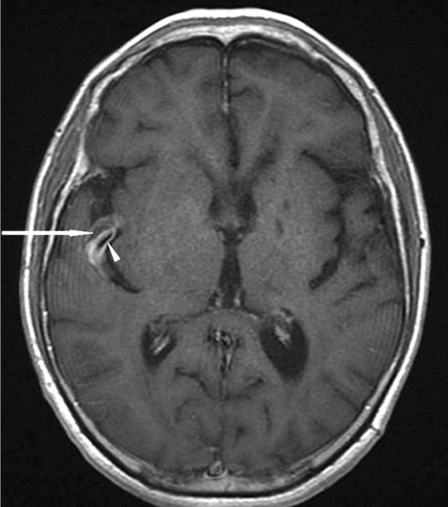
The patient presented five years later with a regressive left hemiplegia, despite the antiplatelet agent. CT scan showed lacunar defects in the left internal capsule and in the left basal ganglia and a left frontal infarct. On the right side, a nodular lesion with spontaneous hyperdense appearance was demonstrated within the sylvian fissure (figure 2). MR Imaging showed that this lesion was a fusiform aneurysm. The aneurysm presented intra-arterial clot and thick walls enhanced intensely after contrast injection (figure 3). The T2*-weighted gradient-echo sequence showed multiple small areas of signal loss, interpreted as microbleeds corresponding to small deposits of hemosiderin.
Figure 2.
CT scanner without contrast: hyperdense nodular lesion within the right sylvian fissure.
Figure 3.
MRI (T1-weighed image with contrast): Fusiform arterial aneurysm in the right sylvian fissure with intra-arterial clot (white arrow) and intense enhancing of aneurismal walls after contrast injection (white arrowhead).
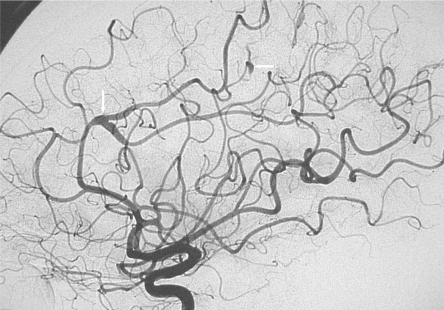
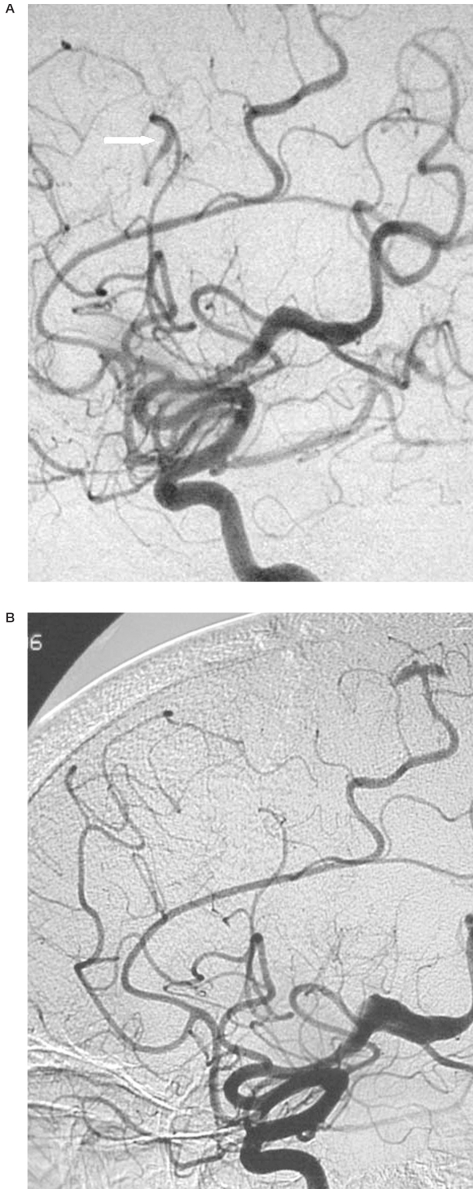
Cerebral angiography showed multiple fusiform aneurysms (figure 4) on the middle, anterior, and posterior cerebral arteries. Most of these aneurysmal lesions were distal; the most proximal of them was the largest and was sited within the right sylvian fissure (figure 5). None of these aneurysms was located on the Willis circle, carotid arteries or on the basilar artery. Some aneurysmal lesions were segmental, others involved arterial bifurcations. Several distal aneurysms presented a very slow flow and were observed at the venous phase of the an-giogram. A low-dose radiation therapy was decided and performed (46 Gy).
Figure 4.
Lateral view of the left carotid angiogram: fusiform aneurysms on the middle and anterior cerebral arteries (white arrows).
Figure 5.
Lateral view of the right carotid angiogram at the time of the diagnosis (A) and one year later (B): fusiform aneurysms on the middle and the anterior cerebral arteries; one of these aneurysms was occluded with its parent artery (white arrow) on the control angiography.
At the time of writing (one year later) the patient is still asymptomatic. The size of the lesions has not increased on a recent cerebral angiography. Furthermore, this examination disclosed occlusion of one of the aneurysms with its parent artery (figure 5).
Discussion
Cardiac myxoma is a tumor of mesenchymal origin accounting for half of all primary cardiac neoplasms1,2,3. Patients with cardiac myxoma usually present systemic emboli, both cerebral and peripheral, intracardiac obstruction, and constitutional symptoms like fever, weight loss, anemia and raised erythrocyte sedimentation rate 4,5,6. Cardiac myxomas are responsible for a variety of neurologic complications even in the absence of cardiac symptoms 1,2. Central nervous system embolization of left atrial myxoma is well documented7.
The most common neurological sequel is acute cerebral ischaemia secondary to vessel occlusion by tumour8. Delayed neurological complications are much less common and may result from tumour recurrence with embolization, progressive vascular stenosis, aneurysm formation, or parenchymal metastasis8. The true incidence of myxoma-related aneurysms is unknown; only around 40 cases could be found in the literature since the first description by Marchand9. Aneurysms secondary to cardiac myxoma may present before or after excision of the primary tumour and be responsible for delayed neurological manifestations up to 25 years after resection of the cardiac mass 10.
Because of the rarity of the disease, little is known about the natural history and nature of such aneurysms. The pathogenesis of aneurysm formation in myxoma patients is not fully understood. Several possible mechanisms have been proposed. Tumor cells may infiltrate cerebral vessels via vasa vasorum leading to destruction of arterial walls. However, vasa vasorum are quite rare in cerebral arteries of experimental animals and humans 11. Vascular occlusion by tumor material with subsequent scarring and pseudoaneurysm constitute the second explanation12. This hypothesis is, however, challenged by the fact that in the most reported cases, no history of cerebrovascular emboli was reported. Direct invasion of the tumor cells through the arterial wall constitutes another theory3,13: histological studies have demonstrated proliferation of myxoma cells in the wall of aneurysms 14,15 and interruption of the internal elastica lamina by invading cells 16.
This theory of "tumoral aneurysms" is compatible with observations that have shown rapid growth or regression of these aneurysms12,17,18. According to Stock19, myxoma cells could pro liferate in the vessel wall with or without apoptosis: without apoptosis the mass would grow progressively in the lumen leading to occlusion of the cerebral vessel and infarction. On the contrary apoptosis within the myxoma tumor located in the arterial wall would result in widening of the arterial lumen and fusiform aneurysms formation. For Wada et Al20, inflammatory reaction and production of endogenous interleukin-6 by myxoma cells may play a potential role in this invasion so that IL-6 serum level could be helpful to monitor the time course of aneurysm formation.
In a review article concerning the delayed neurological complications of left atrial myxoma, Sabolek et Al 21 did not find risk factors for the formation of cerebral aneurysms, except the age: patients with cerebral aneurysms being younger at the age of onset compared with the total population of patients suffering from cardiac myxoma.
MRI and CT scans can demonstrate multiple small infarcts, cerebral haemorrhage or cerebral infarction. Myxomatous aneurysms on MR Imaging may appear as tubular dilatations of cerebral arteries within the sulci on T1- and T2- weighted images. Vascular dilatation may be surrounded by oedema and haemorrhage22. Contrast enhancement of aneurysmal lesions may be secondary to slow flow inside the lumen of the fusiform aneurysm 23,24 but for Nucifora et Al 23 this enhancement could also be due to enhancing of the tumor tissue within the wall of the aneurysm. On CT scanner, aneurysms appear hyperdense spontaneously. For Hwang et Al this aspect could be secondary to accumulation of myxoid matrix in the wall of the aneurysm 25.
On angiography myxomatous aneurysms produce filling defects, interruption of flow and local and fusiform arterial dilatation12. Intracranial myxomatous aneurysms occur with the highest frequency in the peripheral arterial branches of the anterior and middle cerebral arteries, although central fusiform aneurysms have been reported. Saccular aneurysms are a less common feature of myxomatous emboli 26. Myxomatous aneurysms located on extracerebral arteries are very rare in comparison with cerebral ones. The reasons proposed to explain this have been the hemodynamics of the blood flow out of the heart27, and/or a specific affinity of the myxomatous emboli for the cerebral blood vessels21.
For Walker28 and in this case report, conventional angiography was more sensitive than Magnetic resonance angiography to detect pe-ripheral and small aneurysms. These lesions, which constitute the majority of aneurysms, are detectable on angiography only by delayed washout of contrast relative to the arterial phase, a finding that cannot be appreciated on Magnetic resonance angiography or CT angiography.
The natural history of myxomatous aneurysms is not well characterized. Some authors have shown stability of the lesions over many years 29,30. Spontaneous regression of aneurysms has been well documented in patients in whom the primary atrial tumor has been excised17 and spontaneous resolution due to aneurysmal thrombosis has been documented in several published cases30,31. Subarachnoid haemorrhage and intracerebral haemorrhage have been described for patients with myxomatous aneurysms 3,15,17, and for some authors, myxomatous lesions have a higher risk of bleeding than usual non myxoma-related cerebral aneurysms 7,31,32. However, in many reports, a direct link between aneurysms and haemorrhage has been difficult to prove 3,34,35 and moreover, in the literature, intracerebral haemorrhage in patients with atrial myxomas has been documented in the absence of cerebral aneurysms1,35.
In a review of 32 cases of myxomatous cerebral aneurysm with delayed neurological complications 20, cerebral haemorrhage was described in only 27% of the case reports, whereas cerebral infarction was at the origin of the symptoms in 60% of patients. Consequently, ischaemic attack seems more frequent than cerebral haemorrhage in patients with myxomatous cerebral aneurysms.
Cerebral infarction may be explained by tumor embolization from the aneurysmal lesions, or by local arterial occlusion or slowdown in the fusiform aneurysms 30.
Because of the rarity of neoplastic aneurysms due to cardiac myxoma, and the uncertainty of their natural history it is difficult to know how to best manage these patients after diagnosis. Removal of the cardiac mass when discovered after the neurological symptoms is mandatory to prevent further emboli, but cardiac surgery cannot abolish the risk of delayed cerebral aneurysm formation. The slow flow inside the aneurysmal lesions, the possibility of aneurysmal thrombosis and the occlusion of smaller caliber vessels are probably the cause of ischemic events. These are at the origin of the most frequent symptomatology associated with myxomatous aneurysms, and according to Jean et Al30 antiplatelet treatment has to be considered to prevent strokes. However, in this case report, ischemic symptoms were observed despite aspirin. Although, in some cases, endovascular coiling10 or surgical treatment16 has been reported, most of the aneurysms are fusiform without a neck and cannot be clipped or coiled.
The tumoral origin of the aneurysms suggests the possibility of using chemotherapy to prevent aneurysmal growth, but the results of doxorubicin alone were equivocal17. Low-dose radiation therapy in conjunction with chemotherapy revealed more encouraging results 36. The stability of cerebral aneurysms over several years argues for conservative management. All the authors agree that careful follow-up of patients with known aneurysms is essential. Chemotherapy and radiotherapy should be considered in patients with growing aneurysms.
Conclusions
Originating from arterial metastasis, cerebral myxomatous aneurysms may be observed even several years after removal of the primary cardiac tumour. No risk factors for the development of these aneurysms are known, but an increase in IL-6 serum level could predict their formation and/or development. Myxomatous aneurysms are associated with ischemic rather than haemorrhagic symptoms, and so neurosurgical clipping and/or endovascular coiling do not seem useful to most patients. Because fusiform aneurysms are often stable over several years, conservative management seems sensible.
Clinical and radiological follow-up including cerebral angiography and MRI are critical. For patients with growing aneurysms chemotherapy and/or radiotherapy must be considered.
References
- 1.Knepper LE, Biller J, et al. Neurologic manifestations of atrial myxom. A 12-year experience and review. Stroke. 1988;19:1435–1440. doi: 10.1161/01.str.19.11.1435. [DOI] [PubMed] [Google Scholar]
- 2.Mattle HP, Maurer D, et al. Cardiac myxomas: a long term study. J Neurol. 1995;242:689–694. doi: 10.1007/BF00866921. [DOI] [PubMed] [Google Scholar]
- 3.New PF, Price DL, et al. Cerebral angiography in cardiac myxoma. Correlation of angiographic and histopathological findings. Radiology. 1970;96:335–345. doi: 10.1148/96.2.335. [DOI] [PubMed] [Google Scholar]
- 4.Reynen K. Cardiac myxomas. N Engl J Med. 1995;333:1610–1617. doi: 10.1056/NEJM199512143332407. [DOI] [PubMed] [Google Scholar]
- 5.Blondeau P. Primary cardiac tumors French studies of 533 cases. Thorac Cardiovasc Surg. 1990;38(Sup 2):192–197. doi: 10.1055/s-2007-1014065. [DOI] [PubMed] [Google Scholar]
- 6.Goodwin JF, Stanfield CA, et al. Clinical features of left atrial myxoma. Thorax. 1962;17:91–110. doi: 10.1136/thx.17.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DeSousa AL, Muller J, et al. Atrial myxoma: a review of the neurological complications, metastases and recurrences. J Neurol Neurosurg Psychiatry. 1978;41:1119–1124. doi: 10.1136/jnnp.41.12.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sandok BA, von Estorff I, et al. Subsequent neurological events in patients with atrial myxoma. Ann Neurol. 1980;8:305–307. doi: 10.1002/ana.410080314. [DOI] [PubMed] [Google Scholar]
- 9.Marchand F. Zur Kenntnis der Embolie und Thrombose der Gerhirnarterien, Zugleich ein Beitrag zur Casuistik der primaren Herztumoren und der gekreuzten Embolie. Klin Wochenschr. 1894;31:1–5. [Google Scholar]
- 10.Yilmaz MB, Akin Y, et al. Late recurrence of left atrial myxoma with multiple intracranial aneurysms. Int J Cardiol. 2003;87:303–305. doi: 10.1016/s0167-5273(02)00348-0. [DOI] [PubMed] [Google Scholar]
- 11.Takaba M, Endo S, et al. Vasa vasorum of the intracranial arteries. Acta neurochir (Wien) 1998;140:411–416. doi: 10.1007/s007010050118. [DOI] [PubMed] [Google Scholar]
- 12.Stoane L, Allen JH, et al. Radiologic observations in cerebral embolisation from left heart myxomas. Radiology. 1966;87:262–266. doi: 10.1148/87.2.262. [DOI] [PubMed] [Google Scholar]
- 13.Budzilovich G, Aleksic S, et al. Malignant cardiac myxoma with cerebral metastases. Surg Neurol. 1979;11:461–469. [PubMed] [Google Scholar]
- 14.Burton C, Johnston J. Multiple cerebral aneurysms and cardiac myxoma. N Engl J Med. 1970;282:35–36. doi: 10.1056/NEJM197001012820109. [DOI] [PubMed] [Google Scholar]
- 15.Rodriguez FJ, Brown RD, et al. Embolic atrial myxoma with neoplastic aneurysm formation and haemorrhage: a diagnostic challenge. Neuropathology and Applied Neurobiology. 2006;32:213–216. doi: 10.1111/j.1365-2990.2006.00721.x. [DOI] [PubMed] [Google Scholar]
- 16.Furuya K, Sasaki T, et al. Histologically verified cerebral aneurysm formation secondary to embolism from cardiac myxoma: case report. J Neurosurg. 1995;83:170–173. doi: 10.3171/jns.1995.83.1.0170. [DOI] [PubMed] [Google Scholar]
- 17.Roeltgen DP, Weimer GR, et al. Delayed neurologic complications of left atrial myxoma. Neurology. 1981;31:813. doi: 10.1212/wnl.31.1.8. [DOI] [PubMed] [Google Scholar]
- 18.Suzuki T, Nagai R, et al. Rapid growth of intracranial aneurysms secondary to cardiac myxoma. Neurology. 1994;44:570–571. doi: 10.1212/wnl.44.3_part_1.570. [DOI] [PubMed] [Google Scholar]
- 19.Stock K. Multiple cerebral aneurysms in a patient with recurrent cardiac myxomas: a case report. Interventional Neuroradiology. 2004;10:335–340. doi: 10.1177/159101990401000408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wada A, Kanda T, et al. Cardiac myxoma metastasized to the brain: potential role of endogenous interleukin6. Cardiology. 1993;83:208–211. doi: 10.1159/000175971. [DOI] [PubMed] [Google Scholar]
- 21.Sabolek M, Bachus-Banaschak K, et al. Multiple cerebral aneurysms as delayed complication of left cardiac myxoma: a case report and review. Acta Neurol Scand. 2005;111:345–350. doi: 10.1111/j.1600-0404.2005.00413.x. [DOI] [PubMed] [Google Scholar]
- 22.Friedman DP, Rapoport RJ. Giant fusiform oncotic aneurysm: MR and angiographic findings. Am J Roentgenol. 1996;167:538–539. doi: 10.2214/ajr.167.2.8686653. [DOI] [PubMed] [Google Scholar]
- 23.Nucifora PGP, Dillon WP. MR Diagnosis of Myxomatous Aneurysms: Report of Two Cases. Am J Neuroradiol. 2001;22:1349–1352. [PMC free article] [PubMed] [Google Scholar]
- 24.Herbst M, Wattjes MP, et al. Cerebral Embolism from Left Atrial Myxoma leading to Cerebral and Retinal Aneurysms: A Case Report. Am J Neuroradiol. 2005;26:666–669. [PMC free article] [PubMed] [Google Scholar]
- 25.Hwang BJ, Connelly MM, et al. Distinctive MR Imaging Appearance of Hemorrhagic Cerebral Aneurysms Associated with Atrial Myxoma. Am J Roentgenol. 2001;177:925–927. doi: 10.2214/ajr.177.4.1770925. [DOI] [PubMed] [Google Scholar]
- 26.Fujita K, Yanaka K, et al. Ruptured middle cerebral artery aneurysm with intramural myxoid degeneration in a child. Pediatr Neurosurg. 2003;39:108–111. doi: 10.1159/000071323. [DOI] [PubMed] [Google Scholar]
- 27.Steegmann AT, De La Fuente J. Experimental cerebral embolism. II. Microembolism of the rabbit brain with seran polymer resin. J Neuropathol Exp Neurol. 1959;18:537–558. doi: 10.1097/00005072-195910000-00005. [DOI] [PubMed] [Google Scholar]
- 28.Walker MT, Kilani RK, et al. Central and peripheral fusiform aneurysms six years after left atrial myxoma resection. J. Neurol. Neurosurg. Psychiatry. 2003;74:281–282. doi: 10.1136/jnnp.74.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Josephson SA, Johnston SC. Multiple stable fusiform intracranial aneurysms following atrial myxoma. Neurology. 2005;64:526–528. doi: 10.1212/01.WNL.0000145838.61057.E8. [DOI] [PubMed] [Google Scholar]
- 30.Jean WC, Walski-Easton SM, et al. Multiple Intracranial Aneurysms as Delayed Complications of an Atrial Myxoma: Case Report. Neurosurgery. 2001;49:200–203. doi: 10.1097/00006123-200107000-00031. [DOI] [PubMed] [Google Scholar]
- 31.Branch CL, Jr, Laster DW, et al. Left atrial myxoma with cerebral emboli. Neurosurgery. 1985;16:675–680. doi: 10.1227/00006123-198505000-00018. [DOI] [PubMed] [Google Scholar]
- 32.Price DL, Harris JL, et al. Cardiac myxoma- A clinico-pathologic and angiographic study. Arch Neuro. 1970;23:558–567. doi: 10.1001/archneur.1970.00480300080011. [DOI] [PubMed] [Google Scholar]
- 33.Bobo H, Evans OB. Intracranial aneurysms in a child with recurrent atrial myxoma. Pediatr Neurol. 1987;3:230–232. doi: 10.1016/0887-8994(87)90024-5. [DOI] [PubMed] [Google Scholar]
- 34.Chen HJ, Liou CW, et al. Metastatic atrial myxoma presenting as intracranial aneurysms with hemorrhage: Case report. Surg Neurol. 1993;40:61–64. doi: 10.1016/0090-3019(93)90173-x. [DOI] [PubMed] [Google Scholar]
- 35.Macaulay VM, Crawford PJ, et al. Atrial myxoma presenting with cerebral haemorrhage. Postgrad Med J. 1985;61:331–332. doi: 10.1136/pgmj.61.714.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bernet F, Stulz PM, et al. Long-term remission after resection, chemotherapy, and irradiation of a metastatic myxoma. Ann Thorac Surg. 1998;66:1791–1792. doi: 10.1016/s0003-4975(98)00917-5. [DOI] [PubMed] [Google Scholar]