Abstract
Organisms producing resting stages provide unique opportunities for reconstructing the genetic history of natural populations. Diapausing seeds and eggs often are preserved in large numbers, representing entire populations captured in an evolutionary inert state for decades and even centuries. Starting from a natural resting egg bank of the waterflea Daphnia, we compare the evolutionary rates of change in an adaptive quantitative trait with those in selectively neutral DNA markers, thus effectively testing whether the observed genetic changes in the quantitative trait are driven by natural selection. The population studied experienced variable and well documented levels of fish predation over the past 30 years and shows correlated genetic changes in phototactic behavior, a predator-avoidance trait that is related to diel vertical migration. The changes mainly involve an increased plasticity response upon exposure to predator kairomone, the direction of the changes being in agreement with the hypothesis of adaptive evolution. Genetic differentiation through time was an order of magnitude higher for the studied behavioral trait than for neutral markers (DNA microsatellites), providing strong evidence that natural selection was the driving force behind the observed, rapid, evolutionary changes.
Keywords: resting egg banks, dormant propagules, adaptive evolution, neutral model, predator-induced defenses
Predation is important in structuring communities (1) and may drive evolution in prey populations, as illustrated by the ubiquitous presence of predator-induced defenses (2). Several studies have reported among-population genetic differentiation in predator-induced traits, suggestive of adaptation to predation pressure in the local habitat (3, 4). Yet, studies directly documenting evolution within a natural population are relatively rare and have involved either long-term field studies (5) or elaborate field experiments comprising transplantation (6). In organisms that produce long-lived dormant propagules, such as many plants and aquatic animals, the stratified “seed” or “egg-bank” accumulating in the soil or in lacustrine sediments provides an alternative and readily accessible record of local population history (7, 8). Long-term viability (>100 years) of the dormant eggs allows reconstruction of recent evolutionary changes in quantitative traits through a quantitative genetic analysis of “resurrected” populations (8, 9). In addition, PCR-based DNA microsatellite markers are sufficiently powerful to be used directly on minute resting eggs to assess gene frequency changes at loci that are selectively neutral (10). We capitalize on these possibilities to test the hypothesis that changes in predation pressure induce rapid and adaptive evolutionary responses in natural populations of prey species. We discriminate between changes resulting from natural selection and changes reflecting random genetic drift or gene flow by comparing patterns of evolutionary change for both neutral markers (microsatellite DNA) and ecologically relevant traits. Provided that the population of dormant propagules is sufficiently large and in the absence of tight genetic linkage, we expect that natural selection acting directly on an ecologically relevant trait does not have a strong impact on the genetic make-up of the population with respect to neutral markers. Conversely, if genetic drift or massive gene flow are the evolutionary forces driving the observed change in an ecologically relevant trait, then these changes should be paralleled by changes at neutral genetic markers. By comparing genetic differentiation among subpopulations separated in time at neutral markers and ecologically relevant traits in a given population, we thus can test directly against the neutral model as an explanation of the observed genetic changes in the quantitative trait. This approach has been applied previously in studies on genetic differentiation among geographically separated populations (11), but its interpretation is even more straightforward in studies involving genetic changes through time within a single population.
We studied genetic changes in a natural population of the waterflea Daphnia in relation to changes in fish predation pressure, because fish are known to have a strong impact on zooplankton community structure and population development (1), and earlier studies on genetic differentiation among populations with respect to predator-induced defenses (3, 4, 12) are suggestive of adaptive evolution. The quantitative trait studied by us is phototactic behavior of the Daphnia in the absence and presence of fish kairomone (defined as a chemical signal among species, beneficial to the receiver but not to the sender). Phototactic behavior is involved in predator avoidance through diel vertical migration (13–15). It is one of the traits that was found to differ strongly among Daphnia populations inhabiting ponds that are characterized by strongly different fish predation pressure in their natural habitat (4, 12). These earlier studies suggest that phototactic behavior may be important in the predator–prey arms race and thus may be a good candidate for documenting in situ local adaptation.
Methods
We reconstructed the history of population abundance, body size, and genetic variation in neutral markers and predator-avoidance behavior in the resident waterflea (Daphnia magna; Crustacea, Cladocera) population in Oud Heverlee Pond, Belgium. Oud Heverlee Pond is a small (8.7 ha), shallow, manmade pond that was constructed in 1970 for fish culture. It has a well documented history of fish stocking over the 30 years of its existence (ref. 16; Fig. 1). During the first 3 years after its creation, it was stocked annually with a limited number of benthivorous fish. From 1973 to the early 1980s, a much higher number of planktivorous fish were stocked (>250 kg/ha). The amount of stocked fish subsequently was reduced and varied from the mid-1980s until stocking ceased completely in 1993. In terms of fish predation pressure, we thus can differentiate between three main periods: a period of relatively low fish predation pressure during the early 1970s, a period of high fish predation pressure between 1973 and 1982, and a period of relaxed fish predation pressure from 1982 onward.
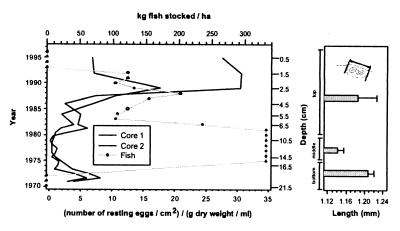
Figure 1.
(Left) Changes in resting egg flux and density of stocked fish in Oud Heverlee Pond during a period of 26 years after its creation (1970–1996). Fish stock densities for each year were obtained from the owners of the pond (16) and aligned with the sediment record of ephippia abundance assuming a constant rate of sediment accumulation through time, corrected for compaction. The right (depth) axis applies to core 1. (Right) Average length (±2 SE) of resting egg cases isolated from three successive core sections. (Inset) Our definition of dorsal rim length as an index of egg size.
Paleolimnological Analysis.
One long (1 m) and two short (30 cm) sediment cores were recovered from Oud Heverlee Pond with a standard sediment corer consisting of a Plexiglas tube (Ø 5.2 cm inner cross-section), of which the lower end was reinforced with a metal cutting edge. Sediment chronology is anchored in 1970 (the start of lacustrine sedimentation) and assumes conformable sediment deposition and a constant rate of sedimentation (g dry sediment/year) through time. Radio-isotope dating of the sediment record proved difficult because the pond is younger than the signature peak of anthropogenic 137Cs production in 1964, and recent manmade ponds have incomplete inventories of unsupported 210Pb. However, excellent replication between cores in patterns of sediment texture, water, and organic-matter content (data available from the authors) supports the assumption of conformable and spatially uniform sedimentation through time. In addition, patterns of fossil egg abundance and size structure of the egg cases (Fig. 1) as well as of hatchability of the eggs (16) proved highly repeatable among cores and substantiate the quality of the sediment record as an archive of lake history. Reconstruction of Daphnia population abundance and size structure is based on counts and measurements of fossil resting egg cases (so-called ephippia, which, in Daphnia, can contain two eggs; ref. 17) in both short cores (Fig. 1). Eggs were isolated quantitatively from 1-cm core increments by using standard techniques (16). From each core sample, 30–40 ephippia were selected randomly from the measurement of their length to the nearest 0.1 mm (Fig. 1).
Establishment of Clonal Lineages.
D. magna is a cyclical parthenogen and reproduces by amictic parthenogenesis as long as environmental conditions are favorable (17). This allows the establishment of clonal lineages in the laboratory. Resting eggs from the three core sections, each corresponding to a distinct fish-stocking period, were exposed to hatching stimuli (16). Because hatching success was age-dependent and low for sediment deeper than 9 cm downcore (16), a large number of eggs (>1,000) had to be exposed to hatching stimuli to obtain a total of 20 clones from each of the three core sections. The three core sections from which we established subpopulations were: bottom (18- to 21-cm depth range), corresponding to the first period of low fish predation pressure (1970–1972; Fig. 1); middle (11–14 cm), during the period of high fish predation pressure (1976–1979); and top (3 cm), during the period of relaxed fish predation pressure (ca. 1988). The resulting laboratory populations were used to quantify genetic changes in both DNA microsatellites and phototactic behavior.
Neutral Markers.
We first screened the Daphnia population for genetic discontinuities in allele frequencies of microsatellite markers (Dma3/2, Dma11, and Dma12) directly from fossil eggs recovered from seven depth intervals in the sediment record. Microsatellite loci information is available on GenBank (accession nos. AF291910—AF291912). Within the Oud Heverlee population, Dma3/2 showed three alleles (nucleotide lengths 207, 197, and 193), Dma11 showed six alleles (lengths 181, 175, 173, 172, 171, and 167), and Dma12 showed six alleles (lengths 158, 157, 155, 153, 151, and 149). Eggs for genotyping were transferred individually to Eppendorf tubes containing a 10% Chelex solution (35 μl). To facilitate extraction of DNA, each egg was punctured with a needle before boiling for 15 min. Optimal primer-specific PCR conditions are available from the first author. Forty eggs were analyzed from each depth interval.
Phototactic behavior cannot be quantified on the resting eggs themselves and thus needed to be studied on populations that hatched from the resting eggs. One may argue that biased hatching of the resting eggs thus may impede a direct comparison of genetic differentiation among populations as observed for phototactic behavior and microsatellite markers. To tackle this problem, we also analyzed the hatched populations for their variation at microsatellite loci. This allows a direct comparison of patterns of genetic differentiation among subpopulations separated in time for the quantitative trait and the neutral markers. Moreover, a comparison of the pattern of genetic differentiation observed for microsatellites in hatched populations vs. that determined directly on resting eggs allowed us to test whether biased hatching influenced the observed patterns of genetic differentiation among subpopulations.
Genetic differentiation among subpopulations separated in time was quantified by Nei's GST (18). GST is equivalent to Wright's FST (19) and quantifies the fraction of genetic variation that is due to population subdivision. Calculations were carried out by using the software package tfpga (20).
Phototactic Behavior.
Evolutionary changes in phototactic behavior were quantified by determining the mean phototactic response of 10 randomly chosen isolates from each of the three subpopulations (n = 3 × 10) in the presence and absence of fish kairomone. The experimental design and setup to measure phototactic behavior was as in previous work (4), but with a modified experimental column (21). In short, Daphnia clones were cultured under standard conditions (1 l bottles, 20 ± 2°C, 14-h light/10-h dark photoperiod, 20 adults liter−1; high food concentration: 2.5 105 Scenedesmus acutus cells ml−1, adjusted daily). There were two treatments: animals cultured in the absence and in the presence of fish kairomone. Medium loaded with fish kairomone was prepared by allowing two ide (Leuciscus idus; Teleostei, Cyprinidae) to condition tap water for 24 h. The fish were fed Daphnia in a separate aquarium to avoid contamination of the medium with Daphnia alarm substances. One-quarter of the Daphnia culture medium was renewed daily with aged (24-h) tap water or fish-conditioned medium depending on the treatment. All clonal lineages were cultured under experimental conditions for at least two generations before experimentation to exclude maternal effects. Four replicate experiments, each with 10 adults (second adult instar; from different cultures to eliminate common environment effects), were performed for every clone and treatment. The experimental column consists of an outer and an inner vertical column (see ref. 21 for details). The inner column is the experimental chamber (10-cm height) and is divided externally into four compartments, each 2.5 cm in height. Before the experiment, animals were adapted for 3 h to dechlorinated tap water without food or fish kairomone and subsequently given 5 min of dark adaptation before switching on the light. Each experiment lasted 10 min. During the last 5 min of the experiment, the number of animals in each of the four compartments was counted at 1-min intervals, and an index of phototactic behavior was calculated as the average number of animals in the upper compartment minus that in the lower compartment, divided by the total number of animals. The resulting phototactic index may vary between + 1 and −1 and has proven to be stable and highly reproducible, both in the absence and presence of fish kairomone (4, 21, 22).
In addition to the phototactic index in the absence (INF) and presence (IF) of fish kairomone, we also quantified the change in phototactic behavior upon exposure to fish kairomone as (IF − INF). Genetic differentiation among subpopulations separated in time was calculated as QST values (11). QST quantifies the fraction of the total amount of genetic variation in a quantitative trait that is due to differences among populations and thus is the quantitative-trait functional analogue of GST as calculated for single-locus markers. Broad-sense heritabilities were estimated for phototactic behavior in the absence and presence of fish kairomone for all three subpopulations by a clonal repeatability analysis (23).
Results
The core profile of ephippial abundances (Fig. 1) shows high densities at the bottom (18–22 cm) and near the top (0–4 cm) of the sediment record and low to very low abundances in the middle section of the record (4–18 cm). The middle section, with low ephippial densities, corresponds to the period when fish stocking was intermediate to high. The top and bottom sections, both with high abundances of ephippia, correspond to periods when fish stocking was low or absent. This relationship between ephippial densities and fish-stocking densities is strongly negative and highly significant by Pearson's product–moment correlation (r = −0.61, P = 0.003). Resting eggs produced during the period of highest fish stocking (11- to 14-cm core depth) are significantly smaller in size than those produced during the two periods when fish stocking was relaxed (Fig. 1; one-way ANOVA with averages per 1-cm core increment as input data, P < 0.01; Spearman Rank correlation between ephippium length and the densities of stocked fish in the pond is significant: r = −0.60, P = 0.007, n = 19). These results suggest that fish predation pressure mediated both the densities and body sizes of Daphnia within the studied population.
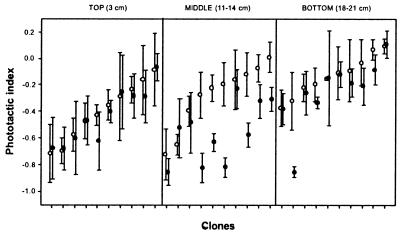
Data on mean phototactic behavior of 10 randomly chosen isolates from each of the three subpopulations separated in time indicate clear differences in behavioral responses to fish. Of the 10 clones that were hatched from ephippia recovered from sediment samples corresponding to the period of intense fish stocking (middle subpopulation), six exhibited a more negatively phototactic behavior when exposed to fish-conditioned medium. However, only one of 20 isolates from samples corresponding to periods of relaxed predation (top and bottom subpopulations) displayed such a response (Fig. 2; the difference is highly significant by Fisher's Exact test, P = 0.0021). A three-way nested ANOVA with clone (random factor) nested in depth downcore reveals a significant main effect of depth, clone, and fish kairomone treatment on the phototactic index (Table 1). There are also significant clone × fish kairomone and a depth × fish kairomone interaction effects, indicating a genotype and subpopulation-dependent response to predator kairomone, respectively.
Figure 2.
Phototactic behavior (mean ± 2 SE) of 10 clones from each of the three main periods in fish-stocking history [bottom (1970–1972), low predation; middle (1976–1979), high predation; top (ca. 1988), reduced predation] in the absence (open symbols) and presence (solid symbols) of fish kairomone. Within each subpopulation, the clones are ordered according to increasing values of phototactic index in the absence of fish kairomone.
Table 1.
Results of a three-way nested ANOVA testing for the effect of subpopulation (sediment depth), clone (random factor nested in depth), and kairomone presence on the phototactic index of D. magna isolated from Oud Heverlec Pond sediments
| Factor | df effect | MS effect | df error | MS error | F | P |
|---|---|---|---|---|---|---|
| Depth (D) | 2 | 1.418 | 27 | 0.312 | 4.55 | 0.01981 |
| Clone (C) | 27 | 0.312 | 180 | 0.039 | 7.98 | <0.0001 |
| Treatment (F) | 1 | 1.044 | 27 | 0.073 | 14.32 | 0.00078 |
| D × F | 2 | 0.310 | 27 | 0.073 | 4.26 | 0.02472 |
| C × F | 27 | 0.073 | 180 | 0.039 | 1.87 | 0.00888 |
Because we know the sediment depths from which the resting eggs were isolated that gave rise to particular clones, we also can calculate correlations between trait values and the densities of stocked fish in the pond at the time corresponding to the depth from which the clone was hatched. This correlation was highly significant for the Daphnia's change in phototactic behavior upon exposure to fish kairomone, indicating that the genetically determined amplitude of predator-induced plasticity is positively related to predation pressure in the habitat (Spearman Rank correlation, r = 0.462; P = 0.0012, n = 30).
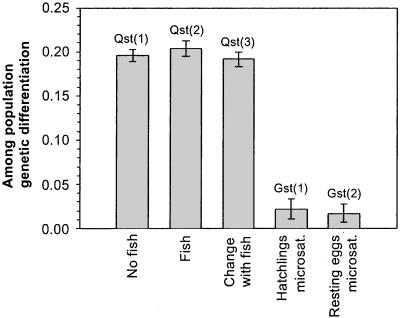
QST values range from 0.192 to 0.203 (Fig. 4), indicating strong genetic differentiation for the quantitative traits studied among the temporally separated subpopulations. Broad-sense heritability estimates for phototactic behavior in the absence and presence of fish kairomone range from 0.20 to 0.60 and are significantly different from zero in all three subpopulations (P < 0.05), suggesting that the population at all times harbored substantial genetic variation for this behavioral trait.
Figure 4.
Comparison of measures of genetic differentiation among three D. magna subpopulations separated in time. QST values refer to phototactic behavior: QST(1), phototactic behavior in absence of fish kairomone: QST(2), phototactic behavior in presence of fish kairomone; QST(3), change in behavior upon exposure to fish kairomone. GST values refer to microsatellite markers: GST(1), hatched populations; GST(2), resting eggs. Confidence intervals around QST values were obtained by bootstrapping; confidence intervals around GST values were calculated from values obtained for the different loci studied.
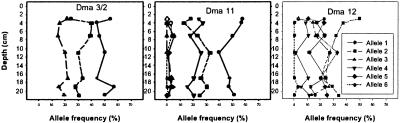
Microsatellite allele frequencies in Daphnia eggs deposited at seven different time intervals in the pond's history show little genetic differentiation among these temporally separated subpopulations (Fig. 3). Similarly little genetic change was observed for microsatellites among hatchlings from the three depth intervals used in the study of phototactic behavior. GST values were lower than 0.022 in both cases (Fig. 4). Allele frequencies among the three hatched subpopulations were not significantly different by Fisher's Exact test (ref. 20; P = 0.112). Allele frequencies determined directly on eggs (seven depth intervals) were different among intervals (Fisher's Exact test; P = 0.003). The GST value determined for eggs from 3-, 11-, and 19-cm sediment depth (corresponding to depths of hatched subpopulations; GST = 0.0222) is almost identical to the value obtained for the hatched populations (GST = 0.0219), thus showing that genetic differences among subpopulations were not inflated in the populations of hatchlings as compared with the resting egg bank. Fig. 4 shows that QST values for phototactic behavior are approximately an order of magnitude higher than GST values for neutral markers for the same set of subpopulations separated in time.
Figure 3.
Vertical profiles of allele frequencies for three microsatellite loci (Dma3/2, Dma11, and Dma12) as determined directly from resting eggs isolated from seven successive sediment depths.
Discussion
Ephippial abundance and size in the sediment record of Oud Heverlee Pond are inversely correlated with fish density, in agreement with a priori expectations based on the theory of size-selective visual predation. Planktivorous fish are highly efficient and positively size-selective predators (1), and large zooplankton species such as D. magna are very vulnerable to fish predation. Sedimentary records of large-bodied zooplankton such as Daphnia have been found to reliably reflect fish-induced historical changes in population abundance (24, 25). The strong fit between the egg-density profile and inferred fish predation pressure supports the notion that the Oud Heverlee sediment record is intact and not disturbed at either of our core sites. The observation that the average egg capsule isolated from sediments corresponding to a period of high fish predation pressure is smaller than the average egg capsule in the flanking sediment sections adds to the evidence for a strong impact of size-selective fish predation pressure on the structure of the Daphnia population. There is evidence from the literature that Daphnia egg counts indeed are proportional to population abundance, whereas egg size (length of dorsal rim) is linearly related to the body size of the sexual females producing them (26). In summary, our data clearly show that the impact of stocked planktivorous fish on population densities and size structure of the resident Daphnia population in Oud Heverlee Pond has been very pronounced.
Our data on genetic variation at neutral microsatellite markers suggest that there is essentially no genetic subdivision among subpopulations of the Oud Heverlee Daphnia separated in time. This result is not unexpected, given that the large size of the resting egg bank (>109 eggs; ref. 16) may buffer the population from genetic drift. Genetic differentiation among subpopulations for phototactic behavior was, however, very pronounced. Phototactic behavior is an ecologically important trait, because it is associated with daytime depth selection, known to be important in predator avoidance (14). The observed genetic change toward a stronger plasticity response and a more negatively phototactic behavior upon exposure to fish kairomone is in agreement with the hypothesis of adaptive evolution, because negatively phototactic animals are less vulnerable to visual predators (4, 14, 15). Considering the short time span that elapsed between deposition of the three studied core sections (≈7 years for bottom–middle and 10 years for middle–top; Fig. 1), our results clearly indicate that the resident Daphnia population evolved rapidly in response to changing predation pressure by fish. The observation that genetic differentiation is much more pronounced for the quantitative traits than for neutral markers (QST values are approximately an order of magnitude higher than GST values; Fig. 4) strongly suggests that natural selection rather than stochastic effects (genetic drift) or massive gene flow has driven the observed genetic changes in phototactic behavior.
The present study extracts and compares patterns of evolutionary change in neutral markers and quantitative traits from a natural resting egg bank. The weighing of genetic variation in neutral vs. ecologically relevant traits is important to assess the contribution of natural selection to the observed evolutionary changes in the quantitative trait. In our study, pronounced genetic changes in a behavioral trait without associated shifts in allele frequencies of neutral markers exclude an important impact of stochastic events or massive gene flow as an explanation for the observed pattern. We thus clearly documented rapid in situ evolution in a natural population in response to temporal changes in local conditions, fueled by genetic variation already present in the resident population.
Acknowledgments
Radiodating was performed by P. G. Appleby (Univ. of Liverpool, U.K.). This study benefited from critical reading by M. Boersma, D. Chiavelli, A. Gomez, E. Jeppesen, M. Lynch, M. McPeek, S. Mitchell, R. Ortells, K. Schwenk, P. Spaak, R. Stoks, and three anonymous referees. We are grateful to Miss M. Wellens for supplying the historical data on fish stocking intensity in Oud Heverlee Pond. We thank B. Hellemans for invaluable technical assistance and I. Vught for her contribution to the assessment of phototactic behavior. This research was supported financially by the K. U. Leuven Research Fund (Projects OT/96/13 and OT/00/14) and the Fund for Scientific Research–Flanders (G.0260.97). Funds for developing Daphnia microsatellite markers were provided by a Natural Sciences and Engineering Research Council (Canada) grant to P. D. N. Hebert. J.K.C. was supported by an Ontario Graduate Scholarship.
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Kerfoot W C, Sih A. Predation, Direct and Indirect Impacts on Aquatic Communities. Hanover, NH: Univ. Press of New England; 1997. [Google Scholar]
- 2.Tollrian R, Harvell C D. The Ecology and Evolution of Inducible Defences. Princeton: Princeton Univ. Press; 1999. [Google Scholar]
- 3.Parejko K, Dodson S I. Evolution. 1991;45:1665–1674. doi: 10.1111/j.1558-5646.1991.tb02671.x. [DOI] [PubMed] [Google Scholar]
- 4.De Meester L. Evolution. 1996;50:1293–1298. doi: 10.1111/j.1558-5646.1996.tb02369.x. [DOI] [PubMed] [Google Scholar]
- 5.Grant P R. Ecology and Evolution of Darwin's Finches. Princeton: Princeton Univ. Press; 1986. [Google Scholar]
- 6.Reznick D A, Bryga H, Endler J A. Nature (London) 1990;346:357–359. [Google Scholar]
- 7.Vavrek M C, McGraw J B, Bennington C C. J Ecol. 1991;79:645–662. [Google Scholar]
- 8.Hairston N G, Jr, Lampert W, Cáceres C E, Moltreier C L, Weider L J, Gaedke V, Fischer J R, Fox J A, Post D M. Nature (London) 1999;401:446. [Google Scholar]
- 9.Kerfoot W C, Robbins J A, Weider L J. Limnol Oceanogr. 1999;44:1232–1247. [Google Scholar]
- 10.Gómez A, Carvalho G R. Mol Ecol. 2000;9:203–214. doi: 10.1046/j.1365-294x.2000.00849.x. [DOI] [PubMed] [Google Scholar]
- 11.Spitze K. Genetics. 1993;135:367–374. doi: 10.1093/genetics/135.2.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boersma M, Spaak P, De Meester L. Am Nat. 1998;152:237–248. doi: 10.1086/286164. [DOI] [PubMed] [Google Scholar]
- 13.Ringelberg J. Biol Rev. 1999;74:397–423. [Google Scholar]
- 14.De Meester L, Davidowicz P, Van Gool E, Loose C. In: The Ecology and Evolution of Inducible Defences. Tollrian R, Harvell C D, editors. Princeton: Princeton Univ. Press; 1999. pp. 160–176. [Google Scholar]
- 15.De Meester L, Weider L J, Tollrian R. Nature (London) 1995;378:483–485. [Google Scholar]
- 16.Cousyn C, De Meester L. Arch Hydrobiol Beih Ergebn Limnol. 1998;52:127–139. [Google Scholar]
- 17.De Meester L. Ecoscience. 1996;3:385–390. [Google Scholar]
- 18.Nei M. Ann Hum Genet. 1977;41:225–233. doi: 10.1111/j.1469-1809.1977.tb01918.x. [DOI] [PubMed] [Google Scholar]
- 19.Wright S. Evolution and the Genetics of Populations: Variability Within and Among Natural Populations. Vol. 4. Chicago: Univ. of Chicago Press; 1978. [Google Scholar]
- 20.Miller M P. Tools for Population Genetic Analyses. Flagstaff: Northern Arizona Univ.; 1997. , Version 1.3. [Google Scholar]
- 21.Michels E, Leynen M, Cousyn C, De Meester L, Ollevier F. Water Res. 1998;33:401–408. [Google Scholar]
- 22.De Meester L. Hydrobiologia. 1991;225:217–227. [Google Scholar]
- 23.Falconer D S, Mackay T F C. Introduction to Quantitative Genetics. 4th Ed. New York: Longman; 1996. [Google Scholar]
- 24.Kitchell J A, Kitchell J F. Limnol Oceanogr. 1980;25:389–402. [Google Scholar]
- 25.Leavitt P R, Carpenter S R, Kitchell J F. Limnol Oceanogr. 1989;34:700–717. [Google Scholar]
- 26.Verschuren D, Marnell L F. Trans Am Fish Soc. 1997;126:21–34. [Google Scholar]