Abstract
Mitochondrial encephalomyopathy, lactic acidosis, and stroke-like episodes (MELAS) is a maternally inherited mitochondrial syndrome characterized by seizures, migrainous headaches, lactic acidosis, vomiting, and recurrent stroke-like episodes. Patients often suffer from cognitive dysfunction of unclear pathogenesis. In this study, we explored a possible link between cognitive dysfunction and hippocampal expression of calbindin D28KD (CB), a high affinity calcium-binding protein, in four MELAS patients, using post mortem hippocampal tissues. We found significantly reduced CB levels in all patients by immunohistochemistry, Western blot, and quantitative real-time PCR. Reduction in CB expression has been associated with aging and with neurodegenerative disorders, including Alzheimer’s disease. We postulate that the reduced CB expression may play a role in the cognitive abnormalities associated with MELAS.
Keywords: mitochondria, MELAS, cognitive dysfunction, calbindin, hippocampus, calcium-binding protein, mitochondrial DNA
INTRODUCTION
MELAS is the second most common disease due to mitochondrial DNA (mtDNA) mutations, after Leber optic hereditary neuropathy (LHON). Although at least 15 distinct mtDNA mutations have been associated with MELAS, more than 80% of patients harbor the m.3243A>G mutation in the tRNALeu (UUR) gene [1]. The prevalence of this mutation is 1 in 6000 in the general population and higher in more defined populations [2]. The clinical hallmark of MELAS is stroke-like episodes at a young age, typically before age 40. Other neurologic abnormalities include seizures, migraine, hearing loss, psychiatric features, and global cognitive impairment involving language, perception and memory function [3]. Most MELAS patients also have variable non-neurologic manifestations such as short stature, diabetes, gastrointestinal symptoms (recurrent vomiting, intestinal pseudoobstruction), and heart failure, reflecting the multisystemic nature of the disorder [4].
While many studies have focused on the vascular pathology of stroke-like episodes [5], the pathomechanism of the neuropsychologic symptoms of MELAS has not received equal attention. To gain further insight into this phenomenon, we investigated the regional expression pattern of CB, as its functional involvement in memory, learning, and long-term potentiation has been reported in many studies [6].
MATERIALS AND METHODS
Subjects
We studied human postmortem brain specimens from 4 MELAS patients (P1, P2, P3, P4) harboring the m.3423A>G mutation and obtained as part of an NICHD-supported study on natural history of the disease. Salient clinical features of the patients are listed in Table 1. The diagnosis was based on medical history, clinical examination, muscle biopsy, respiratory chain enzyme activities in muscle and brain, and molecular genetic studies revealing the m.3243A>G mutation. Cognitive function was evaluated using a global neuropsychological score (GNP), which combined a brief global mental status examination and specific tests to assess abstract reasoning, verbal memory, visual memory, language (consisting of naming and fluency), executive function, attention, and visual-spatial abilities. Each domain was evaluated using standard tests and each test was scored according to standard guidelines. Individual domain scores were expressed as percentile of normative data and a global mean score was derived by dividing the individual domain results by the number of domains. Normal score in 30 controls was 0.14±0.35 (mean±standard deviation [SD])[7]. The Karnofsky score was used to evaluate daily living functional abilities.
Table 1.
Demographic and Clinical data
| Sex | Age at autopsy (y) | First stroke (age,y) | Type of seizure (age,y) | Education level (y) | Karnofsky Performance scale (age,y) | GNP (age,y) | Memory difficulties (age,y) | Headaches (age,y) | MRI findings | Autoptic findings | Other features | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P1 | F | 37 | 31 | Complex extratemporal partial seizures: occipital (31) | 18 | 80: normal activity with effort; some signs/symptoms (33) | 0.57 (31); 0.71 (35) | Yes (31) | Migraine (after 33) | Multiple infarcts (temporal and occipital cortex) | Multiple infarcts in the temporal and the occipital cortex, subtle neuronal loss in the CA4 region of hippocampus | Hearing loss, facial paresthesia, neuropathy, muscle weakness, fatigue, myoclonus |
| P2 | M | 39 | 27 | Generalized seizures (27) | 12 | 40: disable; require special care and assistance (34) | 2.4 (34) | Yes (NA) | Non-migrainous headache (27) | Extensive anterior frontal, parietal, temporal and occipital white matter/cortical infarcts; moderate cerebral and cerebellar atrophy | Multifocal infarcts (frontal, temporal, parietal, occipital cortex) with focal microcystic formations; mild atherosclerotic vascular disease | Diabetes mellitus, muscle weakness, exercise intolerance, cramps, myoclonus, hearing loss, behavioral problems, incoordination, aphasia, alcohol and cocaine abuse in adolescence |
| P3 | F | 35 | 32 | Generalized seizures (32) | 12 | 30: severely disable (35) | 2.8 (35) | Yes (33) | Migraine (32) | Temporal, posterior frontal, parietal and occipital infarcts; cerebral and cerebellar atrophy | Cerebral atrophy, multifocal infarcts of variable ages in bilateral temporal, frontal, parietal and occipital lobes; laminar necrosis in frontal cortex | Aphasia, ataxia, behavioral problems, gestational diabetes, exercise intolerance, cramps, myoclonus, hearing loss, hirsutism, pigmentary retinopathy |
| P4 | F | 38 | 17 | Partial seizures: occipital (17) | 12 | 60: requires occasional assistance, but is able to care for most needs (33) | 2.5 (31); 3.0 (34) | Yes (17) | Migraine (17) | Pons, bilateral temporal, bilateral hippocampi, bilateral parietal, left occipital, right frontal lobe cortical infarcts | Multifocal infarcts of variable ages in bilateral temporal, bilateral parietal, left occipital, and right frontal lobes, bilateral microscopic infarcts in subiculum; mild neuronal loss in CA4 of hippocampus | Diabetes mellitus, proximal muscle weakness, exercise intolerance, myoclonus, hearing loss, peripheral neuropathy, retinopathy, agnosia, dyspraxia, ataxia, ptosis |
NA: data not available; y: years; GNP: global neuropsychological score.
Two control hippocampal tissues were obtained through the New York Brain Bank (NYBB) at Columbia University (www.nybb.hs.columbia.edu) from two individuals, 74 and 78 years old, who were deemed clinically free of dementia or any other cognitive dysfunction, and whose brain did not show significant age-related neurodegenerative changes by neuropathological examination.
DNA analysis
Total DNA from the hippocampi of MELAS patients was extracted by standard procedures. For restriction fragment length polymorphism (RFLP) analysis, a 238-bp mtDNA fragment was amplified. The mutant DNA introduces a HaeIII restriction site detectable by RFLP analysis. The digested PCR product was electrophoresed in a 12% non-denaturing acrylamide gel, which was analyzed in a phospho-imager (Molecular Analyst, BioRad, Hercules, CA) to quantitate the percentage of the mutation (Image-Quant software, Molecular Dynamics, Sunnyvale, CA).
Histology and Immunohistochemistry
Five-μm-thick sections were cut from the paraffin embedded hippocampal tissue of all patients. Sections were stained with hematoxylin-eosin after being deparaffinized in xylene and rehydrated through graded alcohol solutions for conventional histological evaluation. For immunohistochemical staining of CB, paraffin sections of both P2 and P4 were used, because sections from P2 and P4 contained similar mid-hippocampal coronal planes, and because the duration of formalin fixation was similar to that of controls. Formalin-fixed hippocampal tissue was not available in P1, and the tissue from P3 had been fixed in formalin significantly longer than the others and comprised only the anterior part of the hippocampus. Available sections were, after rehydration, incubated in 3% hydrogen peroxide solution for 5 minutes to quench endogenous peroxidase activity. Prior to immunohistochemical labeling, we retrieved the antigen by microwaving the sections for 6 min in 10 mM citrate buffer (pH 6.0). The sections were then incubated with the primary antibody against CB (www.sigmaaldrich.com/Antibodies) (1:100) overnight, followed by biotinylated anti-mouse IgG (1:200), and streptoavidin, using the ABC method [8]. In order to semi-quantitatively evaluate CB immunoreaction, we took the ratio (%) of dentate granular neurons discretely and strongly stained by the CB antibody over the total number of granular neurons in a representative high-power (×400) microscopic field of sections from P2 and P4.
Western blot analysis
To confirm the reduction of CB in the hippocampus of MELAS patients, we also performed Western blot analysis. Hippocampal formation from both patients and controls were collected on ice and homogenized in 10 mM Tris–HCl buffer (pH 7.4) containing 1M sucrose. The homogenates were centrifuged at 1,000 g for 10 minutes (min) at 4 °C, and the supernatant was collected and centrifuged at 17,000 g for 45 min. Equal amounts of brain protein (30 μg) were loaded onto a 15% acrylamide gel. The samples were electrophoresed at 150V for 90 min. The proteins were then transferred onto an immuno-blot PVDF membrane (Bio-Rad Laboratories) for 1 hour (hr) at 4 °C at 100 V. After blocking the blots in 5% nonfat dry milk in PBST (PBS, pH 7.4, containing 0.1% Tween-20) for 1 hr, they were incubated overnight with mouse monoclonal anti-calbindin D28K antibody (Sigma-Aldrich, 1:500), and mouse monoclonal anti-β-actin antibody (Sigma-Aldrich, 1:5000), followed by peroxidase-conjugated sheep anti-mouse IgG (Sigma-Aldrich, 1:5000) for 1 hr at room temperature. Immunoreactive proteins were visualized using an enhanced chemilluminescence kit (ECL; Amersham Pharmacia Biotech, Piscataway, NJ) followed by exposure of the membranes to autoradiographic films. Quantification of immunoreactive bands was performed by densitometric analysis with the Image J 1.44o software package (National Institute of Health, USA) after subtracting background intensity.
RNA isolation and quantitative real-time PCR
To determine the relative amount of RNA transcripts for the CB gene, we performed real-time RT-PCR using an ABI PRISM 7000 sequence detection system. Total RNA was extracted from hippocampi of patients and controls, using a Qiagen RNeasy® Mini kit. Reverse transcription was performed using the SuperScript III First-strand (Invitrogen, Paisley, UK) and 50μM oligo (dT)20 in a total volume of 20μl. The primers and probe for human calbindin gene and human β-actin (Applied Biosystem, Foster City, CA) were used for that purpose.
Statistical analysis
For Western blot and real time RT-PCR, we repeated each experiment four times to obtain the mean value and the standard deviation of four independent measurements. All values were normalized to those of β-actin. The data were expressed as a percentage of the controls’ mean. For each quantitative variable, statistical significance was assessed by one-way ANOVAs with post hoc Bonferroni corrections. Significance was established at P value <0.05.
RESULTS
Patients
As shown in Table 1, all four patients (P1, P2, P3, and P4) had typical features of MELAS: they all harbored the pathogenic m.3243A>G point mutation, and all had stroke at early ages (17 to 32 years), and seizures. The onset of seizures was either shortly before or shortly after the first stroke. Two of the four patients (P2, P3) had generalized seizures (grand mal), and the other two had partial seizures of occipital origin evidenced by electroencephalography in P1 and clinically (amaurosis) in P4. None of the patients had temporal epilepsy. Multiple cerebral lesions (infarcts) were detected in all patients, while hippocampal lesions were detected only in one patient (P4), who showed bilateral hippocampal signal abnormalities on MRI. All of them died in their 30’s (range 35–39 years; median 37.5 years) from complications of stroke.
As to the cognitive aspect, all patients had met normal developmental milestones in childhood before they developed cognitive impairment and memory dysfunction. The most severely affected was P3 with a Karnofsky performance scale of 30, severe enough to preclude administration of any other neuropsychological testing. The other 3 patients were followed every 4–5 years. P1 was the least severely affected, with only mild cognitive loss. P2 required special care and assistance at the age of 34 because of severe cognitive dysfunction. P4 had severe cognitive loss by global assessment, but required only occasional assistance for daily activities. Notably, two of the four patients (P2, P3) had behavioral problems in addition to cognitive dysfunction, including low frustration threshold, poor attention span, agitation, physical aggression, and crying spells. P2 developed addiction to alcohol and cocaine at age 18.
PCR/restriction fragment length polymorphism (RFLP) analysis
The proportions of mutant genomes, as determined by PCR/RFLP analysis, ranged from 79% to 92% in the hippocampal tissues of the patients. None of the patients showed any significant difference in mutation load amongst the different brain regions examined (Table 2).
Table 2.
Restriction Fragment Length Polymorphism (RFLP) of m.3243A>G mutation
| Frontal | Temporal | Parietal | Occipital | Hippocampus | |
|---|---|---|---|---|---|
| P1 | 90% | 91% | 90% | 87% | 85% |
| P2 | 78% | 78% | 81% | 77% | 88% |
| P3 | 81% | 75% | NA | 78% | 92% |
| P4 | 89% | 89% | 86% | 87% | 79% |
NA: data not available; P: patient.
Histology and Immunohistochemistry
Conventional histological examination of hippocampus from P1, P2, and P3 showed no infarction, while P4 showed acute microscopic infarctions in the bilateral subiculum. There was no significant loss of pyramidal neurons except in the CA4 subregion of P1 and P4, which showed minimal to mild neuronal loss and reactive gliosis. Sparse hypereosinophilic shrunken neurons, suggestive of acute (agonal) ischemic changes, were found in the CA1 subregion of all patients. The density of granular neurons in the dentate fascia was well preserved in P1 and P2, while mild, patchy neuronal loss was detected in P3 and P4. Focal mineralization of vascular walls was seen in P1.
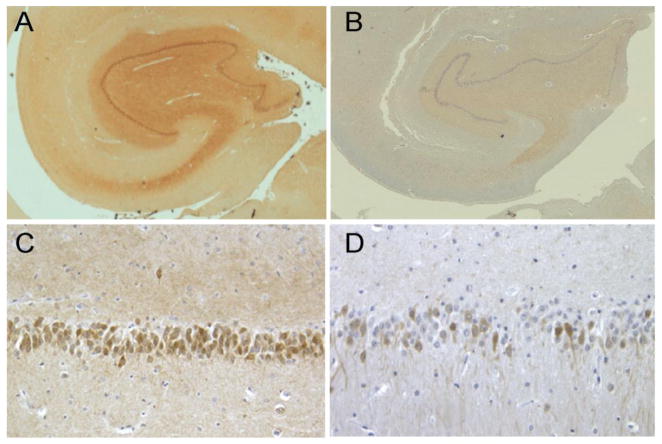
Immunohistochemically, control slides showed strong CB immunoreactivity in all dentate granular cell bodies and mossy fibers, whereas there was lesser intensity in perikarya of hippocampal pyramidal neurons and their dendrites in the CA2 subregion. In CA4, there were only sparse CB-immunopositive cells, presumably interneurons. In agreement with earlier studies [9,10], no immunoreactivity was observed in CA1. In striking contrast, in the MELAS hippocampus (P2, P4), the granular cell layer of the dentate gyrus showed diffusely reduced CB staining. Many neurons were devoid of immunoreaction, while many others had reduced staining intensities (Fig. 1). The neuropil and the pyramidal neuronal staining were also markedly reduced. Semi-quantitatively, 17.8% (P2) and 16.8% (P4) of dentate granular neurons were discretely stained, as compared to 100% in the controls.
Fig. 1. Immunohistochemical staining for calbindin D 28KD (CB).
Representative photomicrographs illustrating the distribution of CB immunoexpression neurons in hippocampal sections from a control (A,C) and a MELAS patient (P2) (B,D). A striking reduction in CB immunoexpression is demonstrated in both low (×20) and high (×400) magnifications. In the patient, the CB-immunoreaction in perikaryon of many dentate granular neurons is weak or absent. The staining intensity in the background neuropil is also markedly reduced.
Western blot analysis
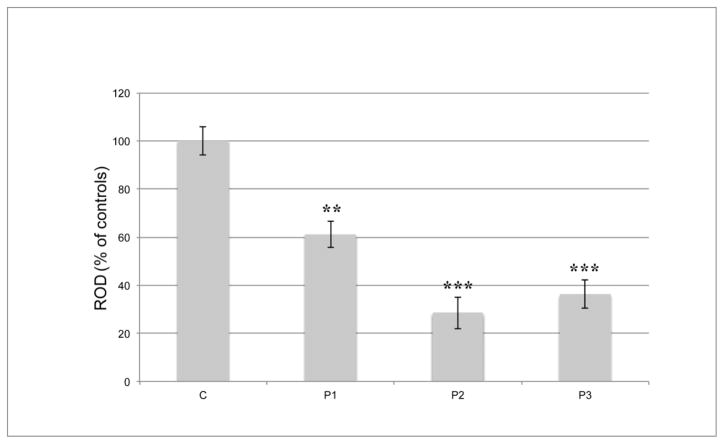
The frozen hippocampal tissues from P1, P2, and P3, along with the 2 controls, were available for Western blot analysis. When the percentage values of CB optical densities in the patients were referred to the mean value of two controls (100%), they were significantly reduced, ranging from 28.5% to 61.2% (p < 0.05) (Fig. 2). We could not obtain any reliable data from P4, due to protein degradation.
Fig. 2. CalbindinD 28KD (CB) protein expression by Western blot.
Each bar indicates the percentage value of CB protein expression by western blot, compared to the mean value of two control samples (100%). All available samples from P1, P2, and P3 show significant reduction in regional. CB at the protein level. Error bars represent the standard deviation. ROD= relative optical density; **, P < 0.01; *** P < 0.001.
Quantitative real-time PCR
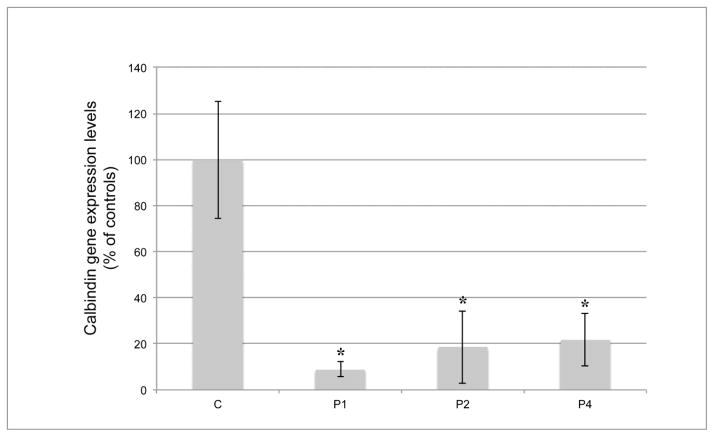
We were able to measure mRNA expression levels of CB in the hippocampi of P1, P2, and P4 (the P3 sample was used up for Western blot), and of the two controls. In all three patients, CB mRNA expression was significantly reduced (p < 0.05), ranging from 8.9% to 21.7%, when the values were expressed as percent of the mean control value (Fig. 3).
Fig. 3. Calbindin D28KD (CB) gene expression by real time RT-PCR.
Each bar indicates the percentage value of CB gene expression by real time RT-PCR, compared to the mean value of two control samples (100%). Error bars represent the standard deviation. All available samples from P1, P2, and P4 show significant reduction in regional expression at the message level. *, P < 0.05.
DISCUSSION
Considering the brain’s high dependence on oxidative metabolism, it is not surprising that various cognitive problems have been seen in patients with mitochondrial encephalomyopathies, in addition to other neurological abnormalities. A large cohort study on adults with mitochondrial disorders revealed a global pattern of cognitive dysfunction in patients, and specific studies focused on MELAS patients also showed global deficiencies in neuropsychologic performance [3,11–13]. The natural history study being conducted at Columbia University Medical Center has already demonstrated that MELAS patients manifest severe cognitive deficits including impaired reasoning, memory, language, attention, and visuo-spatial orientation [14]. Despite this epidemiologically and clinically well-recognized association between cognitive and psychiatric problems and MELAS, the biological basis of these problems is not well understood. In this study, we explored the possible role of CB in the pathogenesis of cognitive dysfunction, and demonstrated a significant reduction of CB in the MELAS hippocampus by immunohistochemistry, western blot, and real-time RT-PCR. Various previous studies in aging [15–17], Alzheimer’s disease (AD) [18], and other neurodegenerative disorders [19,20] have pointed out alterations of hippocampal CB expressions in human cognitive dysfunction. In our study, all four MELAS patients had shown memory and/or learning disability, and the reduced hippocampal CB expressions probably resulted in unbuffered increase of intracellular calcium in the region, and may account for the patients’ hippocampal dysfunctions. Notably, animal studies have related altered CB expressions to deficits of hippocampal long-term potentiation (LTP), a primary experimental model of memory formation in neuronal circuits [6,21,22]. Thus, LTP deficits could be one neurophysiologic dysfunction causing memory/learning disability in the MELAS patients, similar to what has been proposed in Alzheimer’s disease [6,23,24]. Recent studies on LTP support the notion that at least three forms of LTPs are mechanistically separated at synapses in the dentate gyrus and CA1 of the hippocampus, and each of them may play a different role in learning and memory processing [22]. It is conceivable that the significant reduction of ATP in MELAS [25] could interfere with phosphorylation of various enzymes critically involved in LTP induction, maintenance, and expression and result in clinical memory impairment appearing at a much earlier age than in patients with sporadic AD.
The precise mechanism of the CB reduction in causing hippocampal dysfunction in MELAS remains unclear. In this study, the degree of CB reduction did not correlate with regional mutation loads. Neither did it appear to correlate with regional neuronal loss: the histological examination of MELAS hippocampal sections did not reveal a neuronal loss comparable to the profound CB reduction detected at the protein and messenger levels. Considering that all of our patients had seizures, altered CB expressions might be ascribable, at least in part, to seizure activity, possibly via synaptodendritic injury in hippocampal circuits. This could be associated with axonal sprouting or altered GABA receptor expression among dentate granular neurons and hippocampal interneurons [26–29]. Interestingly, our preliminary Western blot data show that CB is variably reduced not only in the hippocampus, but also in frontal, temporal, parietal, and occipital cerebral cortices of all patients (data not shown). This allows us to further postulate that loss of CB could be a global phenomenon rather than an isolated event in the intrinsic hippocampal circuits. However, on the basis of our data in MELAS autoptic brains, it is difficult to discriminate whether this cerebral cortical CB reduction is due to a functional alteration of the protein at the cellular level, or to a selective loss of CB-bearing neurons including inhibitory GABAergic neurons. This problem is due to the fact that all our patients had multiple cerebral infarcts, tissue loss, edema, or reactive gliosis associated with infarction, all of which might have influenced the regional neuronal density and/or neurotransmitter distribution.
Nonetheless, the significant reduction of CB, once it occurs, could easily place the regional neuronal circuit in a vicious cycle of malfunction, if not cause outright cell death. Given the mitochondrial ATP starvation in the MELAS brain, the voltage-dependent calcium ion channels of inhibitory neurons may work improperly, allowing a large influx of calcium into the cells [30]. In addition, cells harboring the m.3243A>G mutation may not be able to sequester excessive calcium, due to a decrease in mitochondrial membrane potential [31]. The already abnormal cellular calcium homeostasis would be further exacerbated by the depletion of calcium binding proteins. Moreover, cell surface receptors on interneurons and astrocytes that are activated by both exogenous and endogenous ATP [32] may not be adequately activated in MELAS brains with faulty mitochondrial respiratory chain function, resulting in decreased synaptic inhibition of hippocampal circuits, and increased excitatory neuronal activities. Clinically, this could mean additional seizures in MELAS patients.
In conclusion, we demonstrated in this study a significant reduction of CB in the post mortem hippocampal tissues from MELAS patients. We believe that the depletion of CB is not merely due to the regional neuronal loss, and that it may be relevant to the compromised cognitive functions often observed in MELAS patients.
Acknowledgments
The authors thank Drs. James E Goldman and Jean Paul Vonsattel, Department of Pathology and Cell biology, Columbia University Medical Center, for critical reading. This work was supported by NIH grant P01 HD032062 and by the Marriott Mitochondrial Clinical Research Fund (MMCRF).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hirano M, Kaufmann P, De Vivo DC, Tanji K. Mitochondrial Neurology I: encephalopathies. In: DiMauro S, Hirano M, Schon EA, editors. Mitochondrial Medicine. London: Informa Healthcare; 2006. pp. 27–44. [Google Scholar]
- 2.Manwaring N, Jones MM, Wang JJ, Rochtchina E, Howard C, Mitchell P, Sue CM. Population prevalence of the MELAS A3243G mutation. Mitochondrion. 2007;7:230–3. doi: 10.1016/j.mito.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 3.Neargarder SA, Murtagh MP, Wong B, Hill EK. The neuropsychologic deficits of MELAS: evidence of global impairment. Cogn Behav Neurol. 2007;20:83–92. doi: 10.1097/WNN.0b013e3180335faf. [DOI] [PubMed] [Google Scholar]
- 4.DiMauro S, Hirano M. MELAS. In: Pagon RA, Bird TD, Dolan CR, Stephens K, editors. GeneReviews. Seattle (WA): 1993. [Google Scholar]
- 5.Tanji K, Kunimatsu T, Vu TH, Bonilla E. Neuropathological features of mitochondrial disorders. Semin Cell Dev Biol. 2001;12:429–39. doi: 10.1006/scdb.2001.0280. [DOI] [PubMed] [Google Scholar]
- 6.Molinari S, Battini R, Ferrari S, Pozzi L, Killcross AS, Robbins TW, Jouvenceau A, Billard JM, Dutar P, Lamour Y, Baker WA, Cox H, Emson PC. Deficits in memory and hippocampal long-term potentiation in mice with reduced calbindin D28K expression. Proc Natl Acad Sci U S A. 1996;93:8028–33. doi: 10.1073/pnas.93.15.8028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaufmann P, Shungu DC, Sano MC, Jhung S, Engelstad K, Mitsis E, Mao X, Shanske S, Hirano M, DiMauro S, De Vivo DC. Cerebral lactic acidosis correlates with neurological impairment in MELAS. Neurology. 2004;62:1297–302. doi: 10.1212/01.wnl.0000120557.83907.a8. [DOI] [PubMed] [Google Scholar]
- 8.Tanji K, Bonilla E. Light microscopic methods to visualize mitochondria on tissue sections. Methods. 2008;46:274–80. doi: 10.1016/j.ymeth.2008.09.027. [DOI] [PubMed] [Google Scholar]
- 9.Freund TF, Buzsaki G. Interneurons of the hippocampus. Hippocampus. 1996;6:347–470. doi: 10.1002/(SICI)1098-1063(1996)6:4<347::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 10.Sloviter RS, Sollas AL, Barbaro NM, Laxer KD. Calcium-binding protein (calbindin-D28K) and parvalbumin immunocytochemistry in the normal and epileptic human hippocampus. J Comp Neurol. 1991;308:381–96. doi: 10.1002/cne.903080306. [DOI] [PubMed] [Google Scholar]
- 11.Sartor H, Loose R, Tucha O, Klein HE, Lange KW. MELAS: a neuropsychological and radiological follow-up study. Mitochondrial encephalomyopathy, lactic acidosis and stroke. Acta Neurol Scand. 2002;106:309–13. doi: 10.1034/j.1600-0404.2002.01089.x. [DOI] [PubMed] [Google Scholar]
- 12.Pachalska M, DiMauro S, Forminska-Kapuscik M, et al. The course of vision disturbances in a patient with the MELAS syndrome. Med Sci Monit. 2002;8:CS11–20. [PubMed] [Google Scholar]
- 13.Kartsounis LD, Troung DD, Morgan-Hughes JA, Harding AE. The neuropsychological features of mitochondrial myopathies and encephalomyopathies. Arch Neurol. 1992;49:158–60. doi: 10.1001/archneur.1992.00530260058020. [DOI] [PubMed] [Google Scholar]
- 14.Kaufmann P, Engelstad K, Wei Y, Kulikova R, Oskoui M, Battista V, Koenigsberger DY, Pascual JM, Sano M, Hirano M, DiMauro S, Shungu DC, Mao X, De Vivo DC. Protean phenotypic features of the A3243G mitochondrial DNA mutation. Arch Neurol. 2009;66:85–91. doi: 10.1001/archneurol.2008.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moreno H, Burghardt NS, Vela-Duarte D, Masciotti J, Hua F, Fenton AA, Schwaller B, Small SA. The absence of the calcium-buffering protein calbindin is associated with faster age-related decline in hippocampal metabolism. Hippocampus. 2011 doi: 10.1002/hipo.20957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bu J, Sathyendra V, Nagykery N, Geula C. Age-related changes in calbindin-D28k, calretinin, and parvalbumin-immunoreactive neurons in the human cerebral cortex. Exp Neurol. 2003;182:220–31. doi: 10.1016/s0014-4886(03)00094-3. [DOI] [PubMed] [Google Scholar]
- 17.Geula C, Nagykery N, Wu CK, Bu J. Loss of calbindin-D28k from aging human cholinergic basal forebrain: relation to neuronal loss. J Comp Neurol. 2003;455:249–59. doi: 10.1002/cne.10475. [DOI] [PubMed] [Google Scholar]
- 18.Riascos D, de Leon D, Baker-Nigh A, Nicholas A, Yukhananov R, Bu J, Wu CK, Geula C. Age-related loss of calcium buffering and selective neuronal vulnerability in Alzheimer’s disease. Acta Neuropathol. 2011;122:565–76. doi: 10.1007/s00401-011-0865-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iacopino AM, Christakos S. Specific reduction of calcium-binding protein (28-kilodalton calbindin-D) gene expression in aging and neurodegenerative diseases. Proc Natl Acad Sci U S A. 1990;87:4078–82. doi: 10.1073/pnas.87.11.4078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heizmann CW, Braun K. Changes in Ca(2+)-binding proteins in human neurodegenerative disorders. Trends Neurosci. 1992;15:259–64. doi: 10.1016/0166-2236(92)90067-i. [DOI] [PubMed] [Google Scholar]
- 21.Jouvenceau A, Potier B, Poindessous-Jazat F, Dutar P, Slama A, Epelbaum J, Billard JM. Decrease in calbindin content significantly alters LTP but not NMDA receptor and calcium channel properties. Neuropharmacology. 2002;42:444–58. doi: 10.1016/s0028-3908(01)00202-7. [DOI] [PubMed] [Google Scholar]
- 22.Raymond CR. LTP forms 1, 2 and 3: different mechanisms for the “long” in long-term potentiation. Trends Neurosci. 2007;30:167–75. doi: 10.1016/j.tins.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 23.Rowan MJ, Klyubin I, Cullen WK, Anwyl R. Synaptic plasticity in animal models of early Alzheimer’s disease. Philos Trans R Soc Lond B Biol Sci. 2003;358:821–8. doi: 10.1098/rstb.2002.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Palop JJ, Jones B, Kekonius L, Chin J, Yu GQ, Raber J, Masliah E, Mucke L. Neuronal depletion of calcium-dependent proteins in the dentate gyrus is tightly linked to Alzheimer’s disease-related cognitive deficits. Proc Natl Acad Sci U S A. 2003;100:9572–7. doi: 10.1073/pnas.1133381100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shepherd RK, Checcarelli N, Naini A, De Vivo DC, DiMauro S, Sue CM. Measurement of ATP production in mitochondrial disorders. JIMD. 2006;29:86–91. doi: 10.1007/s10545-006-0148-8. [DOI] [PubMed] [Google Scholar]
- 26.Ruttimann E, Vacher CM, Gassmann M, Kaupmann K, Van der Putten H, Bettler B. Altered hippocampal expression of calbindin-D-28k and calretinin in GABA(B(1))-deficient mice. Biochem Pharmacol. 2004;68:1613–20. doi: 10.1016/j.bcp.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 27.Luscher B, Fuchs T, Kilpatrick CL. GABAA receptor trafficking-mediated plasticity of inhibitory synapses. Neuron. 2011;70:385–409. doi: 10.1016/j.neuron.2011.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Avanzini G, Franceschetti S. Cellular biology of epileptogenesis. Lancet Neurol. 2003;2:33–42. doi: 10.1016/s1474-4422(03)00265-5. [DOI] [PubMed] [Google Scholar]
- 29.Ellis R, Langford D, Masliah E. HIV and antiretroviral therapy in the brain: neuronal injury and repair. Nat Rev Neurosci. 2007;8:33–44. doi: 10.1038/nrn2040. [DOI] [PubMed] [Google Scholar]
- 30.Calabresi P, Centonze D, Bernardi G. Cellular factors controlling neuronal vulnerability in the brain: a lesson from the striatum. Neurology. 2000;55:1249–55. doi: 10.1212/wnl.55.9.1249. [DOI] [PubMed] [Google Scholar]
- 31.Moudy AM, Handran SD, Goldberg MP, Ruffin N, Karl I, Kranz-Eble P, DeVivo DC, Rothman SM. Abnormal calcium homeostasis and mitochondrial polarization in a human encephalomyopathy. Proc Natl Acad Sci U S A. 1995;92:729–33. doi: 10.1073/pnas.92.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bowser DN, Khakh BS. ATP excites interneurons and astrocytes to increase synaptic inhibition in neuronal networks. J Neurosci. 2004;24:8606–20. doi: 10.1523/JNEUROSCI.2660-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]