Abstract
Cough and swallow are airway protective behaviors. The pharyngeal phase of swallow prevents aspiration of oral material (saliva, food and liquid), by epiglottal movement, laryngeal adduction, and clearing of the mouth and pharynx. Cough is an aspiration-response behavior which removes material from the airway. Coordination of these behaviors is vital to protect the airway from further aspiration-promoting events, such as a swallow occurring during the inspiratory phase of cough. The operational characteristics, primary strategies, and peripheral inputs which coordinate cough and swallow are unknown. This lack of knowledge impedes understanding and treatment of deficits in airway protection, such as the co-occurrence of dystussia and dysphagia common in Parkinson's and Alzheimer's diseases, as well as stroke.
A variety of neuromuscular diseases result in dystussia (disordered cough) and/or dysphagia (disordered swallow) (Kalia, 2003; Pitts et al., 2010). Recent research on clinical populations (Parkinson's disease and stroke) has found that many of these patients have a disorder of airway protection consisting of both dystussia and dysphagia (Pitts et al., 2009; Smith Hammond et al., 2009; Pitts et al., 2010). Objective analysis of voluntary and reflexive cough has been shown to detect and/or predict dysphagia in stroke and Parkinson's disease (Smith Hammond et al., 2009; Pitts et al., 2010). In Parkinson's and Alzheimer's diseases, the leading cause of death is aspiration pneumonia presumably from impaired airway protection (Kalia, 2003). However, until recently cough and swallow in animal models have only been studied in isolation, and the complex brainstem networks which control these behaviors were thought to be largely separate.
Airway protection during deglutition is the prevention of and/or coordinated response to penetration/aspiration of material (Pitts et al., 2008; Pitts et al., 2009; Smith Hammond et al., 2009; Pitts et al., 2010). Swallowing is the oral preparation of a bolus and the movements required to move the bolus from the oral through the pharyngeal cavities to the esophagus (Doty & Bosma, 1956; Thexton et al., 2007). Swallowing presents unique challenges to effective airway protection, especially in humans, because the bolus is directed over and around the larynx. The pharyngeal phase of swallowing is a reflexive patterned behavior, which can be modulated by supra-pontine pathways (Miller, 1982; Jean, 2001). There are several motor tasks that take place during this phase which, if uncoordinated, would result in aspiration. First, the tongue base retracts and then moves superior and posterior, which in turn directs the bolus toward the pharynx. During tongue movement there is closure of the velopharyngeal port. Velopharyngeal closure is important because it allows for a build-up of pressure in the pharynx to help propel the bolus toward the esophagus, and the contact of the soft palate with the posterior pharyngeal wall prevents the bolus from moving into the nasopharynx (Logemann et al., 1998; Kendall & Leonard, 2001). The pharynx then undergoes two basic movements: a) elevation and b) a descending activation of various pharyngeal muscles to produce a peristaltic wave to move the bolus towards the esophagus. Pharyngeal and laryngeal elevation occurs simultaneously. The submental muscles contract to move the hyoid bone and larynx superior and anterior into a position under the tongue base (Logemann et al., 1998; Kendall & Leonard, 2001). During movement of the larynx, the vocal folds and aryepiglottic folds adduct preventing material from entering the lower airway. The epiglottis folds over the glottal space to act as another layer of protection to keep material from entering larynx. The movement of the larynx also elongates the superior portion of the esophageal sphincter. Following contraction of the inferior pharyngeal muscle the upper esophageal sphincter relaxes transiently,allowing the bolus to be passed into the esophagus. The minimal neural circuitry responsible for the production of the pharyngeal and esophageal phases of swallow is contained within the brainstem, although this behavior is subject to significant modification by suprapontine mechanisms (Jean, 2001; Widdicombe et al., 2006).
Coordination of swallow with the respiratory system can include specific changes in breathing patterns (i.e. swallow apnea). The stereotypical coordination pattern during the pharyngeal phase of swallow is created by action of a variety of muscles including, but not limited to, myohyoid, geniohyoid, thyropharyngeus, cricopharyngeus (upper esophageal sphincter), thyroarytenoid, posterior cricoarytenoid, and sternohyoid. The duration of motor activation of these muscles is approximately 500 ms, and much of their activity occurs during the opening of the upper esophageal sphincter (cricopharyngeus muscle). However, if an aspiration does occur, cough, or a series of coughs are induced. The inspiratory phase, initiated by diaphragmatic muscle activity (Bolser & Davenport, 2000; Fontana & Lavorini, 2006) together with laryngeal dilation via posterior cricoarytenoid and the cricothyroid muscle activation, expands the thoracic cavity, increasing lung volume allowing inspiratory airflow (Fontana et al., 1998; Bach et al., 2006; Fontana & Lavorini, 2006). The inspiratory phase lasts approximately 0.65 seconds with the inspiratory volume varying from 50% of tidal volume to approximately 50% of vital capacity (Yanagihara, 1965; Widdicombe & Chung, 2007). A decreased ability to inflate the lungs decreases the potential expiratory airflow for cough (Harris & Lawson, 1968; Fontana et al., 1998), by creating a suboptimal length-tension relationship for expiratory muscle(s) contraction.
Following the inspiratory phase there is rapid vocal fold adduction and contraction of the expiratory muscles, including all abdominal muscles with a majority of force production from the internal and external oblique muscles (Harris & Lawson, 1968; Fontana et al., 1998). The closing of the glottis is known as the compression phase and this phase lasts an average of 0.2 seconds in the human (Fontana et al., 1998; Fontana & Lavorini, 2006). Cough effectiveness is dependent on airflow velocity and greater airflow velocities are achieved with the narrowing of the airways and closing of the glottis (via contraction of the thyroarytenoid and interarytenoid muscles) (Harris & Jonson, 1974). The compression phase creates high intrapleural/intrathoracic pressures due to the isometric contraction of the expiratory muscles. This allows the muscles to maintain the length-tension relationship necessary to generate the high positive pressures. Vocal fold abduction marks the beginning of the expiratory phase. The rapid opening of the glottis, and the explosive release of expiratory air, generates the initial airflow acceleration and high peak flow rates (Harris & Lawson, 1968; Fontana et al., 1998; Fontana & Lavorini, 2006). Additionally, there is contraction of the smooth muscle in the airway, controlled by the vagus nerve, narrowing the bronchi, decreasing the cross-sectional area within the lungs, creating greater shear forces during the expiratory phase (Harris & Lawson, 1968; Fontana et al., 1998; Fontana & Lavorini, 2006).
Aspiration of material though the larynx into the lungs produces a series of airway protective behaviors including cough and swallow. We propose that a series of brainstem networks termed “behavior control assemblies” (BCAs) exert definable control over the cough, swallow and the breathing pattern generators (Bolser et al., 2006). To prevent further aspiration and to clear the pharynx of material removed during coughing the BCAs must (i) filter incoming information from multiple afferent beds and (ii) functionally determine which behavior(s) is/are executed and/or suppressed, and (iii) set the order in which potentially competing behaviors are executed with precise timing. A simplified representation of the BCAs regulating interactions among three relevant pattern generators for cough, swallow, and breathing is shown in Figure 1. This scenario is meant to describe our conceptualization of the brainstem organization for cough and swallow. In awake humans there are also known super-pontine control circuits which play a role in shaping both behaviors (Bolser & Davenport, 2007; Mazzone et al., 2009). Classical representations of pattern generators usually depict the minimum neural substrate required to generate a particular motor pattern that produces a known behavior (Marder & Abbott, 1995). However, BCAs are considered to be regulatory systems which control pattern generators. The elements that make up BCAs may or may not be contiguous with the critical elements of a pattern generator. Evidence exists for brainstem BCAs that control coughing, but which contain elements that are not required for breathing, even though both of these behaviors share a common central pattern generator (Shannon et al., 1996; Shannon et al., 1998; Bolser et al., 1999; Shannon et al., 2004; Poliacek et al., 2007).
Figure 1.
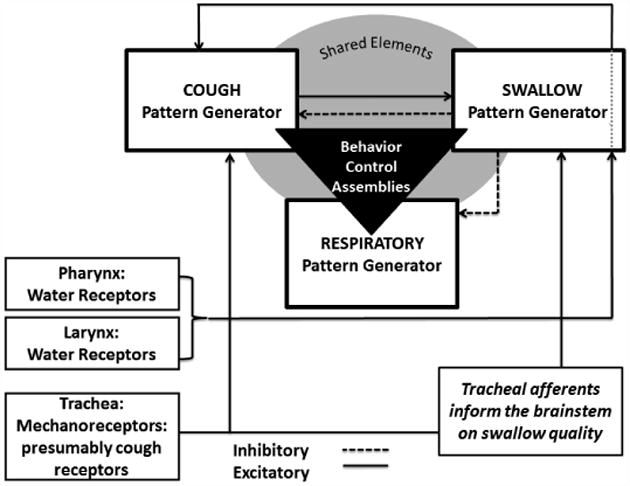
Is a schematic representation of functional organization of breathing, cough and swallow. The cough, swallow, and breathing pattern generators are controlled and coordinated by one or more populations of neurons called behavior control assemblies (triangle). Receptors in the pharynx, larynx, and trachea provide frequency dependent information to the medulla to initiate behaviors.
Central pattern generators have been modeled for a variety of behaviors, in different species (Marder & Calabrese, 1996). These models are usually depicted in the form of neuronal networks and can encompass very detailed proposed interactions between neuronal groups that are thought to generate a particular behavior. Commonly employed approaches to testing such models can include: analysis of activity patterns and/or intrinsic membrane properties of individual neurons in the network and determination of synaptic interrelationships between network elements. However, regardless of the methods employed to test a proposed network, the model must obey the organizational and operational principles generated from empiric investigation of the pattern itself. A detailed synaptic model of the medullary network for cough and swallow has been developed (Figure 2). The stick and ball model shows a subset of brainstem neurons with their inferred connectivity. This model is supported by in vitro and intracellular recordings (Shannon et al., 1996; Shannon et al., 1998; Bolser et al., 1999; Shannon et al., 2004; Poliacek et al., 2007). Figure 3 is a simulation from the current network model from Figure 2. The simulations produce trains of action potentials for each population that can be analyzed with the same tools used for the in vivo parameters which seeded the model. This simulation was able to produce repetitive coughing with a swallow occurring during the appropriate period of the cough cycle. The results of this simulation can be used to predict interactions of the control systems for cough and swallow which are not currently known.
Figure 2.
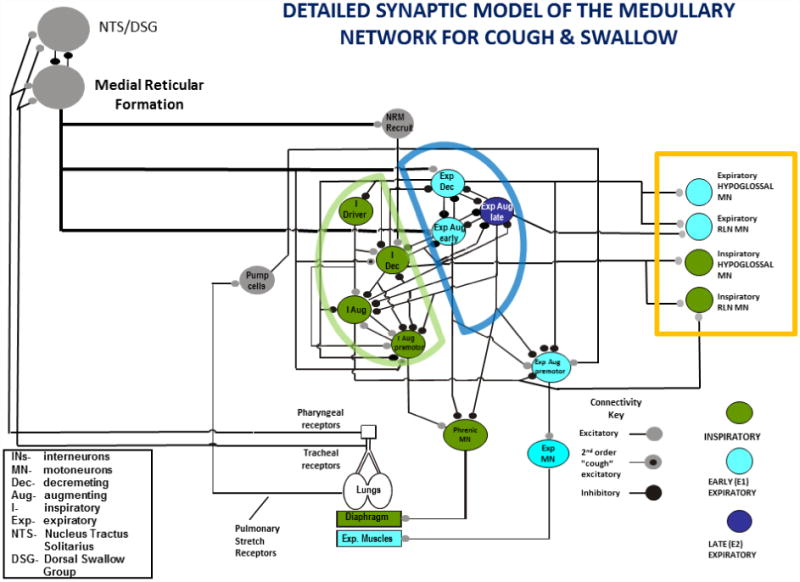
Proposed functional organization of the neurogenic model of cough and swallow. Neuronal populations are color coded for their dominant phase of activation during coughing. RLN-recurrent laryngeal nerve, NRM-non-modulated during breathing. The specific identities of neurons participating in behavioral control assemblies are not well understood, but are hypothesized to be outside of the core respiratory network.
Figure 3.
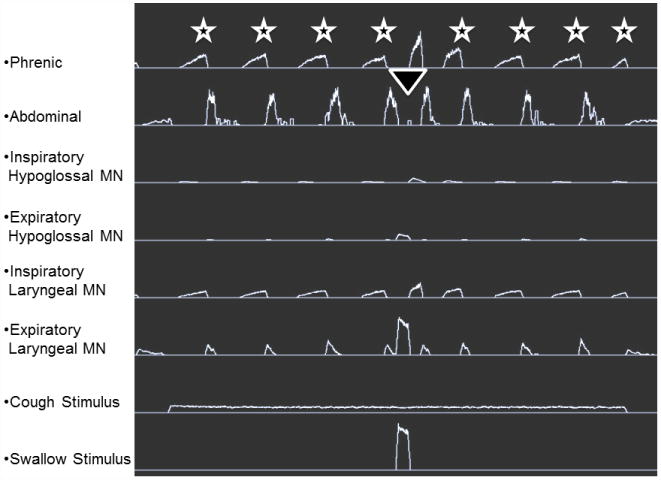
Results of a simulation from the current network model producing coughs (stars) and a swallow (triangle). The cough stimulus elicited repetitive coughing. The swallow stimulus elicited one swallow (triangle) at the appropriate phase of the cough cycle, following the cough expiratory burst and before the next cough inspiratory effort (cough E2).
The need for further understanding of cough and swallow as independent behaviors, and as a coordinated response to aspiration events, is of increasing relevance to patient populations with neurologic disease. In the past few years, clinical studies have highlighted commonalities in the behaviors. However animal models for studying the effects of neurogenic diseases on both cough and swallow are not available. The manner in which different diseases interact with behavior control assemblies and pattern generators is not known. New models and coordinated in vivo experiments may lead to novel pharmacological agents and therapies for disorders of airway protection.
References
- Bach JR, Goncalves MR, Paez S, Winck JC, Leitao S, Abreu P. Expiratory flow maneuvers in patients with neuromuscular diseases. Am J Phys Med Rehabil. 2006;85:105–111. doi: 10.1097/01.phm.0000197307.32537.40. [DOI] [PubMed] [Google Scholar]
- Bolser DC, Davenport PW. Volume-timing relationships during cough and resistive loading in the cat. J Appl Physiol. 2000;89:785–790. doi: 10.1152/jappl.2000.89.2.785. [DOI] [PubMed] [Google Scholar]
- Bolser DC, Davenport PW. Codeine and cough: an ineffective gold standard. Curr Opin Allergy Clin Immunol. 2007;7:32–36. doi: 10.1097/ACI.0b013e3280115145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolser DC, Hey JA, Chapman RW. Influence of central antitussive drugs on the cough motor pattern. J Appl Physiol. 1999;86:1017–1024. doi: 10.1152/jappl.1999.86.3.1017. [DOI] [PubMed] [Google Scholar]
- Bolser DC, Poliacek I, Jakus J, Fuller DD, Davenport PW. Neurogenesis of cough, other airway defensive behaviors and breathing: A holarchical system? Respiratory Physiology and Neurobiology. 2006;152:255–265. doi: 10.1016/j.resp.2006.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doty R, Bosma J. An electromyographic analysis of reflex deglutition. Journal of Neurophysiology. 1956;19:44–60. doi: 10.1152/jn.1956.19.1.44. [DOI] [PubMed] [Google Scholar]
- Fontana GA, Lavorini F. Cough motor mechanisms. Respir Physiol Neurobiol. 2006;152:266–281. doi: 10.1016/j.resp.2006.02.016. [DOI] [PubMed] [Google Scholar]
- Fontana GA, Pantaleo T, Lavorini F, Benvenuti F, Gangemi S. Defective motor control of coughing in Parkinson's disease. Am J Respir Crit Care Med. 1998;158:458–464. doi: 10.1164/ajrccm.158.2.9705094. [DOI] [PubMed] [Google Scholar]
- Harris RS, Lawson TV. The relative mechanical effectiveness and efficiency of successive voluntary coughs in healthy young adults. Clin Sci. 1968;34:569–577. [PubMed] [Google Scholar]
- Harris S, Jonson B. Lung function before and after laryngectomy. Acta Oto-Laryngologica. 1974;78:287–294. doi: 10.3109/00016487409126358. [DOI] [PubMed] [Google Scholar]
- Jean A. Brain stem control of swallowing: neuronal network and cellular mechanisms. Physiological Review. 2001;81:929–969. doi: 10.1152/physrev.2001.81.2.929. [DOI] [PubMed] [Google Scholar]
- Kalia M. Dysphagia and aspiration pneumonia in patients with Alzheimer's disease. Metabolism. 2003;52:36–38. doi: 10.1016/s0026-0495(03)00300-7. [DOI] [PubMed] [Google Scholar]
- Kendall KA, Leonard RJ. Hyoid movement during swallowing in older patients with dysphagia. Arch Otolaryngol Head Neck Surg. 2001;127:1224–1229. doi: 10.1001/archotol.127.10.1224. [DOI] [PubMed] [Google Scholar]
- Logemann JA, Pauloski BR, Colangelo L. Light digital occlusion of the tracheostomy tube: a pilot study of effects on aspiration and biomechanics of the swallow. Head Neck. 1998;20:52–57. doi: 10.1002/(sici)1097-0347(199801)20:1<52::aid-hed8>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Marder E, Abbott L. Theory in motion. Current opinion in neurobiology. 1995;5:832–840. doi: 10.1016/0959-4388(95)80113-8. [DOI] [PubMed] [Google Scholar]
- Marder E, Calabrese RL. Principles of rhythmic motor pattern production. Physiological Reviews. 1996;76:687–717. doi: 10.1152/physrev.1996.76.3.687. [DOI] [PubMed] [Google Scholar]
- Mazzone SB, McGovern AE, Koo K, Farrell MJ. Mapping supramedullary pathways involved in cough using functional brain imaging: Comparison with pain. Pulmonary Pharmacology & Therapeutics. 2009;22:90–96. doi: 10.1016/j.pupt.2008.08.003. [DOI] [PubMed] [Google Scholar]
- Miller AJ. Deglutition. Physiological Review. 1982;62:129–184. doi: 10.1152/physrev.1982.62.1.129. [DOI] [PubMed] [Google Scholar]
- Pitts T, Bolser D, Rosenbek J, Troche M, Okun MS, Sapienza C. Impact of expiratory muscle strength training on voluntary cough and swallow function in Parkinson disease. Chest. 2009;135:1301–1308. doi: 10.1378/chest.08-1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitts T, Bolser D, Rosenbek J, Troche M, Sapienza C. Voluntary cough production and swallow dysfunction in Parkinson's disease. Dysphagia. 2008;23:297–301. doi: 10.1007/s00455-007-9144-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitts T, Troche MS, Carnaby-Mann G, Rosenbek JC, Okun MS, Sapienza CM. Utilizing voluntary cough to detect penetration and aspiration during oropharyngeal swallowing in Parkinson's disease. Chest. 2010 doi: 10.1378/chest.10-0342. [DOI] [PubMed] [Google Scholar]
- Poliacek I, Corrie LW, Wang C, Rose MJ, Bolser DC. Microinjection of DLH into the region of the caudal ventral respiratory column in the cat: evidence for an endogenous cough-suppressant mechanism. J Appl Physiol. 2007;102:1014–1021. doi: 10.1152/japplphysiol.00616.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon R, Baekey D, Morris K, Lindsey B. Brainstem respiratory networks and cough. Pulmonary Pharmacology. 1996;9:343–347. doi: 10.1006/pulp.1996.0045. [DOI] [PubMed] [Google Scholar]
- Shannon R, Baekey D, Morris K, Nuding S, Segers L, Lindsey B. Production of reflex cough by brainstem respiratory networks. Pulmonary Pharmacology & Therapeutics. 2004;17:369–376. doi: 10.1016/j.pupt.2004.09.022. [DOI] [PubMed] [Google Scholar]
- Shannon R, Baekey DM, Morris KF, Lindsey BG. Ventrolateral medullary respiratory network and a model of cough motor pattern generation. Journal of Applied Physiology. 1998;84:2020. doi: 10.1152/jappl.1998.84.6.2020. [DOI] [PubMed] [Google Scholar]
- Smith Hammond CA, Goldstein LB, Horner RD, Ying J, Gray L, Gonzalez-Rothi L, Bolser DC. Predicting aspiration in patients with ischemic stroke: comparison of clinical signs and aerodynamic measures of voluntary cough. Chest. 2009;135:769–777. doi: 10.1378/chest.08-1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thexton AJ, Crompton AW, German RZ. Electromyographic activity during the reflex pharyngeal swallow in the pig: Doty and Bosma (1956) revisited. J Appl Physiol. 2007;102:587–600. doi: 10.1152/japplphysiol.00456.2006. [DOI] [PubMed] [Google Scholar]
- Widdicombe J, Chung KF. Cough. Pulm Pharmacol Ther. 2007;20:305–306. doi: 10.1016/j.pupt.2006.11.008. [DOI] [PubMed] [Google Scholar]
- Widdicombe J, Eccles R, Fontana G. Supramedullary influences on cough. Respir Physiol Neurobiol. 2006;152:320–328. doi: 10.1016/j.resp.2006.02.018. [DOI] [PubMed] [Google Scholar]
- Yanagihara N, von Leden H, Werner-Kukuk E. The Physical Parameters of Cough: The Larynx in a Normal Single Cough. 1965 doi: 10.3109/00016486609127088. [DOI] [PubMed] [Google Scholar]


