Abstract
Objective
The goal of this project was to determine if mechanical stimulation to the posterolateral oropharynx would elicit the urge-to-cough and/or cough.
Background
Inhaled agents, such as capsaicin and citric acid, readily produce coughing and the sensation of urge-to-cough. Areas below the glottis are thought to be the primary sensory mediators of these responses, however it is unknown if there are specific areas in the oropharynx or laryngopharynx that are important for the sensation and production of coughing.
Methods
Paired-pulse air puffs were delivered to the posterolateral oropharyngeal walls of 11 healthy adults (5 men, 6 women) between the ages of 18 and 30 years. Air puffs were delivered via custom mouthpiece in 4 trials, 50 sets per trial. Instances of cough were recorded, and a modified Borg scale was used to gauge urge-to-cough throughout each trial.
Results
Instances of cough were recorded in 12/37 trials, and the sensation of an urge-to-cough was present in 25/37 trials. No motor cough response was elicited with an urge-to-cough rating less than 2.4 on the modified Borg scale. A trend towards higher urge-to-cough was noted for later (3rd and 4th) trials.
Conclusions
Oropharyngeal mechanical stimulation elicits urge-to-cough and cough in healthy young adults. Like other methods to elicit coughing, the motor and sensory thresholds are different using the oropharyngeal air-puff stimuli. Further, it appears there is a sensitization to the air puff stimuli with later trials associated with stronger urge-to-cough and higher likelihood of coughing versus the first and second trial (Tab. 1, Fig. 5, Ref. 21). Full Text in free PDF www.bmj.sk.
Keywords: cough, urge-to-cough, oropharyngeal afferents, mechanical stimulation
The primary function of coughing is to protect the airways. There are three general types of stimuli that elicit cough; locally produced substances, such as mucus, inhaled agents, such as capsaicin, and aspirate material (including foreign bodies). The type of non-volitional cough elicited by these stimuli may be either reflexive, or preceded by a sensation of an urge-to-cough that may or may not be followed by motor expression of cough depending on the intensity of the stimulus (1–3). The latter type of cough, deemed ‘type 3 cough’ (4) essentially indicates that the cough ‘reflex’ may be subject to cortical and subcortical modulation under various conditions.
Anecdotal evidence speaks to the importance of cortical modulation of cough – particularly that related to cough suppression. Many social situations lead would-be-coughers to suppress the motor expression of their urge-to-cough until more appropriate circumstances are attained. However, cough suppression is likely important for more basic physiological reasons. For example, if one senses an urge-to-cough in the event of penetration of bolus material during swallowing, it is advantageous to suppress the motor expression of cough in favor of an expiration reflex. This will allow bolus material to be cleared from the immediate area surrounding the glottal opening, and for material that has aggregated in the oral cavity or base-of-tongue area to be expectorated and not drawn towards the airway. A cough response would otherwise likely draw material farther into the airway during the inspiratory phase. Empirically, Hutchings and colleagues have demonstrated the ability of healthy individuals (5) and individuals with upper respiratory tract infections (3) to voluntarily suppress cough induced by capsaicin. They found that cough could be suppressed at capsaicin concentrations that, in the absence of instructions not to cough, readily elicited rigorous coughing. The ability to voluntarily suppress cough is presumably dependent on the ability to first sense an urge-to-cough.
We have shown that sensation and expression of cough is readily elicited with inhalation of capsaicin, and that increasing the concentration of capsaicin in the breathing line increases both the perceived urge-to-cough, and the number of coughs produced (1, 2). Urge-to-cough and cough can also be elicited using inhaled citric acid (6) or aerosolized distilled water (fog) (7). The sensory mechanism(s) by which these inhaled agents produce cough remain ambiguous; however a combination of mechano-and chemo-sensitive receptors is most likely (8). While it has not been empirically demonstrated, researchers hypothesize that these inhaled agents induce cough via receptors located below the level of the larynx (7, 8).
Yet, irritation in the upper airways or penetration of food or liquid into the laryngeal vestibule readily results in coughing. The upper airways (cranial to the larynx) derive their sensory innervation from various branches of cranial nerves IX and X e.g. (9) including the pharyngeal branch of IX, the pharyngeal branch of X, and the internal superior laryngeal (ISLN) branch of X (10). Various locations within the oropharygneal and laryngopharyngeal muscosa have been identified with dense conglomerations of sensory nerve fibers, including the lateral pharyngeal walls, laryngeal surface of the epiglottis, and arytenoid area, and to a lesser degree the posterior pharyngeal wall of the oropharynx and base of tongue (10). These nerves are likely a combination of mechano- and chemosensors however this has not been empirically demonstrated. It remains unknown what sensory mechanism(s) regulate coughing above the level of the glottis. Further, the specific areas of the oropharynx or laryngopharynx that may produce coughing are unknown. Identification of these areas is extremely important as sensory degradation following neurologic injury, for example, plays an integral role in the development of dysphagia (disordered swallowing). Because cough is an important mechanism for clearing and protecting the lower airways, it may be that sensory areas important for timely initiation of healthy swallowing are equally important and sensitive in the generation of healthy cough.
It was the purpose of this study to determine if mechanical stimulation in the form of short-latency air-puffs directed at the lateral aspects of the posterior pharyngeal wall would (1) induce an urge-to-cough, and (2) induce cough. It was hypothesized that both and urge-to-cough and cough could be elicited by pharyngeal air-puff stimulation, and that the urge-to-couch and the motor expression of cough would increase with increases in the air-puff pressure.
METHODS
Eleven healthy adults, 5 men and 6 women between the ages of 18 and 30 years (mean age 22 years), with no history of chronic respiratory disease, neurological diseases, and no acute respiratory illness served as participants. All subjects were nonsmokers. The University of Florida Institutional Review Board reviewed and approved the study, and informed consent was obtained for all subjects.
Equipment
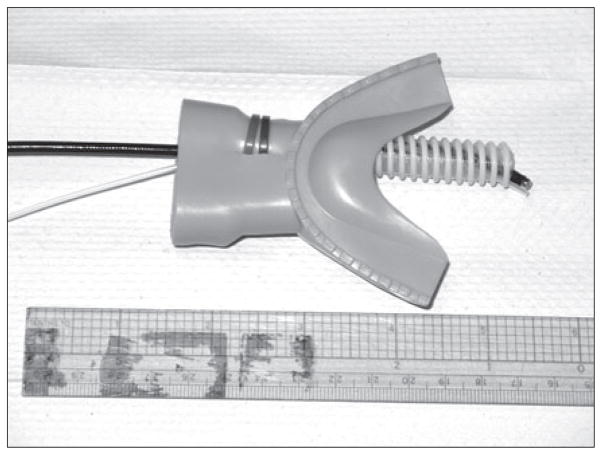
Air-puff delivery was accomplished via specially adapted rubber mouthpiece with a polyeurethane tube extending posteriorly from the front right or left side (Fig. 1). The length of the mouthpiece tube ranged from 80–95 mm, and the inner diameter (ID) was 10 mm. The length of the mouthpiece tube varied for each participant and was based on their tolerance of the mouthpiece in the oral cavity. The mouthpiece tube served as a conduit for passage of a flexible endoscopic camera. The camera was covered with a thin Slide-On® Sheath for Sensory Testing (Metronic Xomed, Inc. Jacksonville, FL) that provided both hygienic cover of the camera and a longitudinally-oriented air-puff delivery port. The Slide-On® Sheath was connected to a pressurized air-tank in series with an air-chamber connected to a PEEP valve and manometer at one end, allowing for quantification of air pressure at the tank. The other end of the chamber was connected to flexible tubing (ID 3 mm) routed to a customized double trigger system that delivered paired-pulse air puffs (pairs were separated by 500 ms) upon manual trigger by one of the researchers. The air tank, air chamber, and trigger system were screened from the participants; tubing connecting to the sheath travelled through a small hole in the dividing wall. Thus, two researchers were required to conduct the experiment, one to one to position the mouthpiece and camera/sheath (researcher 1), and the second to manually trigger air-puff delivery (researcher 2).
Fig. 1.
Custom mouthpiece and tubing with camera air puff delivery port.
A modified Borg category scale (11) was used to quantify urge-to cough (Tab. 1). Briefly, the scales ranged in value from 1–10, with one indicative of no need to cough and 10 being maximum urge-to-cough.
Tab. 1.
Modified Borg scale for urge-to-cough.
| 1 | No need to cough |
| 1.5 | Just noticeable urge to cough |
| 2 | Slight urge to cough |
| 3 | Slight-to-moderate urge to cough |
| 4 | Moderate urge to cough |
| 5 | Moderate-to-strong urge to cough |
| 6 | Strong urge to cough |
| 7 | Strong-to-severe urge to cough |
| 8 | Severe urge to cough |
| 9 | Severe-to-maximum urge to cough |
| 10 | Maximum urge to cough |
Protocol
Participants were seated comfortably in a reclining chair. They were familiarized with the Borg scale prior to initiation of the experiment, and instructed that only those sensations related to cough should be considered when providing ratings. Specifically, the urges to gag or swallow should not be reflected in the rating indicated. Participants were instructed to put the mouthpiece in place, and relax as one of the researchers passed the sheath-covered camera through the oral cavity. Camera placement was to the left or right side of midline posterior to the uvula and approximately 5 mm from the posterior pharyngeal wall. Camera placement was verified visually by the first researcher on a desktop computer screen that displayed the camera image.
Once the mouthpiece tube and camera were in place, the second researcher began air-puff delivery. The first series consisted of air-puffs increasing in intensity in a stepwise fashion, until the maximum pressure the participant could tolerate was reached. “Maximum” was defined as the highest pressure at which only one single paired-pulse puff produced coughing. Based on that maximum, a high and low pressure condition was identified. High pressure was 80 % of the maximum value, and low was 40 % of maximum. Four trials of 50 paired-pulse stimuli were then delivered, with 2–3 minutes of rest, and sips of water between trials in order to minimize a drying effect of the stimuli. Two of the 4 trials were delivered at high pressure, and 2 at low. Whether high or low was delivered first or second was randomized for each participant. All participants could detect air puff delivery for both pressure conditions. Participants were not instructed regarding coughing during the trials (they were not asked to delay or suppress cough until the trial was over).
Throughout each trial of 50 paired puffs, participants were asked to rate their urge-to-cough. Ratings were requested by the second researcher every 10th puff pair, for a total of 6 ratings per trial. Participants indicated their numerical rating value (1–10) using their fingers. The rating was then verbally relayed by researcher 1, and recorded by researcher 2.
Instances of cough were recorded for each trial in a categorical manner: Participants either (1) did not cough during or after the trial, (2) coughed after the trial, or (3) coughed during the trial. Thus, there were two primary outcome measures: (1) Borg rating, and (2) cough expression value (1, 2, or 3 as defined above).
Statistics
Descriptive statistics and analysis of variance (ANOVA) were used to analyze the data. When tests of normality failed, Kruskal-Wallis One Way ANOVA on Ranks was used. Post-hoc testing was completed using Dunn’s Method for multiple comparisons.
Results
Two of the 11 participants, one male and one female, could not tolerate study procedures. Of those two, one coughed profusely during the very first trial even when the stimulus intensity was decreased, and one could not tolerate placement of the mouthpiece and camera because of a sensitive gag reflex. Of the nine participants (4 males and 5 females) who completed the study procedures, 4 (2 males, 2 females) did not cough during the experiment. One of those 4 (a female) only completed 2 trials in the high condition secondary to increasingly aversive urge to gag. The remaining 5 subjects coughed either after or during one or more trials. In total (across all subjects), 37 trials were completed.
High and low pressure conditions
The average high pressure value for participants was 25 cmH2O, and for low pressure was 12.25 cmH2O. There was no effect for pressure category (high or low) on cough expression (H = 0.111, df = 1, p = .739) or on Borg rating (H = .010, df = 1, p = .920). When collapsed across the two pressure conditions for all trials, 25/37 resulted in no coughing, 7/37 resulted in coughing after the trial, and 5/37 resulted in coughing during the trial. Individual Borg ratings for the high and low pressure conditions ranged from 1 – 8.25 (severe urge to cough), with overall median values of 2.61 and 2.15, respectively.
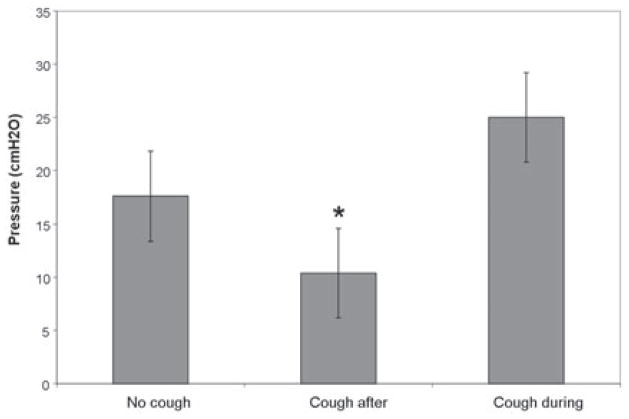
Figure 2 shows the median pressure collapsed across pressure condition, according to cough expression group. There was a significant difference in median pressure between the three cough expression categories (H = 12.679, df = 2, p = .002). Post hoc testing indicated that cough expression group 2 (coughed after the trial) had lower median pressure values versus either expression group 1 (no cough) or 3 (cough during trial) (Fig. 2).
Fig. 2.
Median pressure for each cough expression category with standard error bars. Asterisk indicates pressure was significantly lower versus the other two cough expression categories (p<.05).
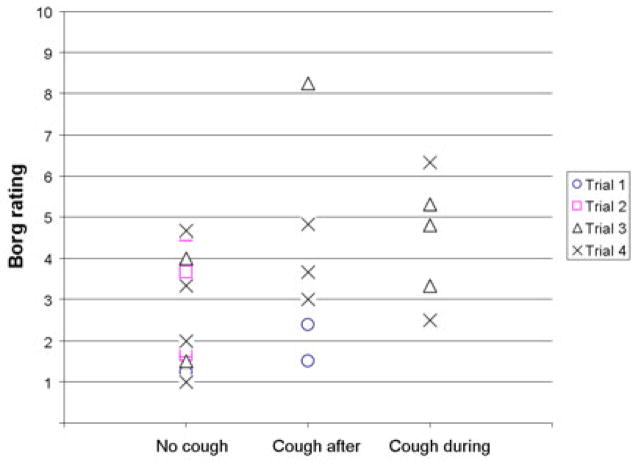
There was no significant effect of trial number on cough expression (H = .641, df = 3, p = .269) or on Borg ratings (F = 0.925, df = 3, p = 0.461). However, there was a trend indicating with increasing trial number (1 & 2 versus 3 & 4) ratings of urge to cough increased, and the likelihood of a cough response also increased (Fig. 3).
Fig. 3.
Cough expression category versus Borg rating according to trial. While no statistically significant differences were found according to trial, there was a trend for higher Borg rating, and higher likelihood of cough expression with increasing trial number.
Borg ratings and cough expression
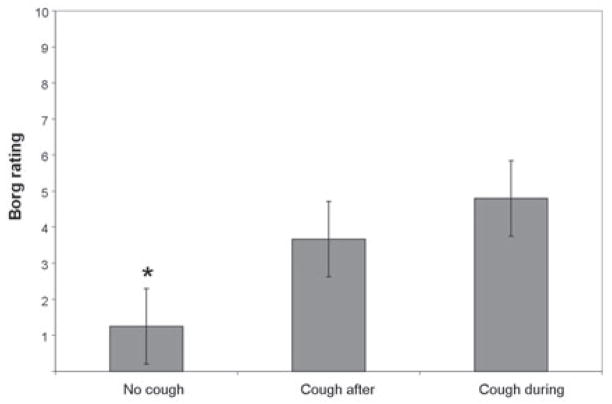
For all trials across all subjects 25/37 were associated with an urge-to-cough (Borg rating grater than or equal to 1.5 ‘just noticeable urge to cough’) and 12/37 were not (Borg rating = 1 ‘no need to cough’). Borg ratings of ‘1’ were never associated with coughing during or after the trial. There was a significant difference for Borg ratings between cough expression categories (H = 12.679, df = 2, p = .002). Post hoc testing indicated the median Borg ratings for cough expression group 1 (no cough) were lower than ratings for the groups 2 and 3, which were not significantly different from each other. This is depicted in Figure 4, showing median Borg ratings for subjects placed into the 3 cough expression categories. Individual ratings for subjects in group 1 (no cough) ranged from no need to cough (Borg=1) to moderate urge to cough (Borg=4.58). Ratings for subjects in groups 2 (cough after trial) and 3 (cough during trial) ranged from slight urge to cough (Borg=2) to severe urge to cough (Borg =8.25) or strong urge to cough (Borg=6.33), respectively.
Fig. 4.
Median Borg ratings according to cough expression category across both pressure conditions with standard error bars. Asterisk indicates Borg rating was significantly lower than the other cough expression categories (p<.05).
Discussion
Application of mechanical stimuli in the form of air-puffs to the lateral posterior oropharyngeal wall elicits a cough sensory and motor response. For 9 of 11 participants a definitive cough motor threshold existed such that a single high pressure stimulus elicited a cough response. However, sub-threshold high and low pressure stimuli inconsistently elicited a cough motor response. Hence there was no graded pressure effect determined in this study. However, regardless of cough motor expression, the majority of air puff stimulus trials (25/37) did elicit an urge-to-cough, and there was no case of cough motor response that was not preceded by an urge-to-cough of at least 2.4 on the Borg scale (slight/slight-to-moderate urge to cough). This is similar to results found for capsaicin (1, 2) and citric-acid induced cough (6) indicating a cough sensory/motor threshold differential. In those studies, no cough motor response was elicited for urge-to-cough ratings less than 3 on the Borg scale. It may be that there is a cough sensory threshold that exists as a pre-requisite for reflex cough motor response. Cough may be produced below sensory-motor threshold levels; however it would likely be volitional versus type 1 or 3 coughing (4).
While it has been posited that reflexive cough is exclusively a vagally-mediated phenomenon (12), it may be that type 3 cough (modulated reflexive cough) depends also on secondary sensory input from pharyngeal regions (13). Pharyngeal sensory branches of cranial nerves IX and X innervate the pharyngeal areas directly stimulated by the air-puffs used in this study. This area of innervation is also important in the triggering of the pharyngeal swallow, and as such has evolved to include a dense aggregation of sensory nerve endings (10). Both mechanical and chemical stimuli trigger pharyngeal swallowing (14). Therefore, we hypothesize two mechanisms by which the current protocol produced coughing: (1) repetitive mechanical stimulation of lateral posterior oropharynx, (2) pharyngeal irritation secondary to drying and evaporative cooling of the mucosa. Study participants drank water during the 2–3 minute rest period between trials, the assumption being that each trial would be initiated with similar mucosal wetness. Hence, our current prevailing hypothesis is that repetitive mechanical stimulation of the lateral posterior oropharynx induces urge-to-cough and cough in healthy individuals.
The majority (25/37) of air-puff trials were not associated with coughing during or after the trial (cough expression group 1), however 11 of those 25 were associated with a noticeable urge-to-cough. Likewise, in the 7/37 trials associated with coughing after the trial (cough expression group 2), urge-to-cough ratings during the trial reached values as high as 8.25 (severe urge-to-cough). Interestingly, participants in this study were not asked to suppress coughing; there were not given instructions regarding cough. The decision to suppress cough even when sensory levels were quite high was entirely self-monitored. This may be similar to voluntary suppression of cough during eating or drinking, where the presence of foreign material within the oral and/or pharyngeal cavities may influence the suppression, expression, or modulation of cough motor response. While the functional physiologic underpinnings of the two tasks are clearly very different, it is nonetheless an interesting model concept for cough expression/suppression modulation during swallowing.
A trend suggesting an effect for trial number on urge-to-cough rating was also found (Fig. 3). The third and fourth trials were associated with slightly higher urge-to-cough ratings than the first and second trials (median values of 3.67, 2.75 versus 1.67, 1.67, respectively). Thus, there may be a sensitization effect for the air-puff stimulus whereby urge-to-cough ratings are higher for the same stimulus intensity levels with increasing trial number. This relatively rapid sensitization may have implications with regards to patient populations that have decreased cough sensitivity. Decreased cough sensitivity in response to penetration/ aspiration of bolus material is a serious problem that is associated with high rates of aspiration pneumonia (15). As such, the re-sensitization of pharyngeal sensitivity for cough is a provocative idea that certainly deserves more consideration and research.
Several patient populations, including those with history of stroke and Parkinson’s disease, have demonstrably reduced volitional and reflexive cough characteristics (reviewed by Widdicombe & Singh, 2006) (16). Ebihara and colleagues (2003) (17) found decreased sensitivity to inhaled citric acid for patients with late stage Parkinson’s disease. Decreased ability to protect the airway with intact cough greatly increases the chance of developing aspiration pneumonia (18). A recent study investigating cough reflex sensitivity and urge-to-cough in elderly participants indicated that decreased urge-to-cough at sub-threshold citric acid concentrations for participants with aspiration pneumonia versus healthy age-matched controls (6). That study highlights the importance of type 3 coughing, which includes a motivational component (i.e., sensing and acting on the urge to cough) and may be important for maintaining adequate airway protection. It is currently unknown how the cough motivation system would be impacted by pharyngeal desensitization that is associated with swallowing disorders, however evidence suggests this is a worthwhile area of study.
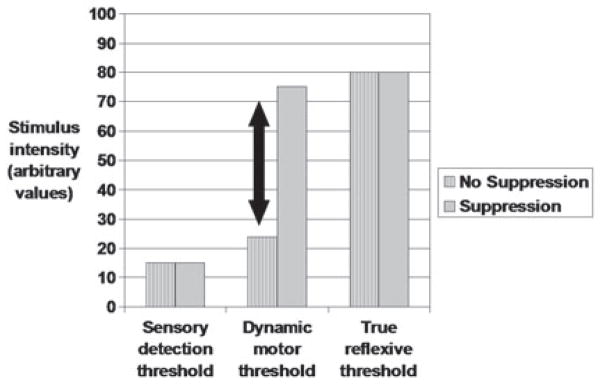
In summary, results of this data, as well as data from other cough-induction methods and studies suggests that (1) motor and sensory stimulus thresholds for cough are different; (2) sensory thresholds are generally lower than motor thresholds; (3) motor threshold may be dynamic in a task-dependent manner (i.e., cough motor response can be suppressed, effectively raising the motor threshold depending on suppression effort) (Fig. 5); and (4) rapid sensitization or desensitization (fog) (19) can occur depending on the stimulus used to induce cough. There is reduced cough motor and sensory function for various patient populations (17, 18, 20, 21). Preliminary evidence suggests decreased motivational urge-to-cough in elderly patients with aspiration pneumonia (6). Subsequent study should examine urge to cough in patients, and possibly look to re-sensitization of the urge-to-cough as a therapeutic method. Air-puff stimuli to the pharynx may be a good candidate to examine re-sensitization as there may be relatively quick sensitizing effect for air puff stimuli in the pharynx, associated with increased urge-to-cough and increased likelihood of a cough motor response.
Fig. 5.
Theoretical model for sensory and motor cough thresholds. Solid arrow indicates potential area for change in the motor threshold depending on degree of cough suppression and intensity of the stimulus.
Acknowledgments
The paper is dedicated to the memory of Professor Juraj Korpas.
References
- 1.Davenport P, Sapienza C, Bolser D. Psychophysical assessment of the urge-to-cough. Eur Respir Rev. 2002;12 (19):249–253. [Google Scholar]
- 2.Davenport PW, Bolser DC, Vickroy T, Berry RB, Martin AD, Hey JA, et al. The effect of codeine on the urge-to-cough response to inhaled capsaicin. Pulm Pharmacol Ther. 2007;20 (4):338–346. doi: 10.1016/j.pupt.2006.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hutchings HA, Eccles R, Smith AP, Jawad MS. Voluntary cough suppression as an indication of symptom severity in upper respiratory tract infections. Eur Respir J. 1993;6 (10):1449–1454. [PubMed] [Google Scholar]
- 4.Eccles R. Central mechanisms IV: Conscious control of cough and the placebo effect. Handb Exp Pharmacol. 2009;187:241–262. doi: 10.1007/978-3-540-79842-2_12. [DOI] [PubMed] [Google Scholar]
- 5.Hutchings HA, Morris S, Eccles R, Jawad MS. Voluntary suppression of cough induced by inhalation of capsaicin in healthy volunteers. Respir Med. 1993;87 (5):379–382. doi: 10.1016/0954-6111(93)90052-2. [DOI] [PubMed] [Google Scholar]
- 6.Yamanda S, Ebihara S, Ebihara T, Yamasaki M, Asamura T, Asada M, et al. Impaired urge-to-cough in elderly patients with aspiration pneumonia. Cough. 2008;4:11. doi: 10.1186/1745-9974-4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lavorini F, Pantaleo T, Geri P, Mutolo D, Pistolesi M, Fontana GA. Cough and ventilatory adjustments evoked by aerosolised capsaicin and distilled water (fog) in man. Resp Physiol Neurobiol. 2007;156 (3):331–339. doi: 10.1016/j.resp.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 8.Mazzone SB. An overview of the sensory receptors regulating cough. Cough. 2005 Aug 4;1:2. doi: 10.1186/1745-9974-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miller AJ. Deglutition. Physiol Rev. 1982;62 (1):129–184. doi: 10.1152/physrev.1982.62.1.129. [DOI] [PubMed] [Google Scholar]
- 10.Mu L, Sanders I. Sensory nerve supply of the human oro- and laryngopharynx: A preliminary study. Anat Rec. 2000;258 (4):406–420. doi: 10.1002/(SICI)1097-0185(20000401)258:4<406::AID-AR9>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 11.Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14 (5):377–381. [PubMed] [Google Scholar]
- 12.Widdicombe JG. Neurophysiology of the cough reflex. Eur Respir J. 1995;8 (7):1193–1202. doi: 10.1183/09031936.95.08071193. [DOI] [PubMed] [Google Scholar]
- 13.Hanacek J, Tatar M, Widdicombe J. Regulation of cough by secondary sensory inputs. Resp Physiol Neurobiol. 2006;152 (3):282–297. doi: 10.1016/j.resp.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 14.Jean A. Brain stem control of swallowing: Neuronal network and cellular mechanisms. Physiol Rev. 2001;81 (2):929–269. doi: 10.1152/physrev.2001.81.2.929. [DOI] [PubMed] [Google Scholar]
- 15.Bravata DM, Ho SY, Meehan TP, Brass LM, Concato J. Readmission and death after hospitalization for acute ischemic stroke: 5-year follow-up in the medicare population. Stroke. 2007;38 (6):1899. doi: 10.1161/STROKEAHA.106.481465. [DOI] [PubMed] [Google Scholar]
- 16.Widdicombe J, Singh V. Physiological and pathophysiological down-regulation of cough. Resp Physiol Neurobiol. 2006;150 (2–3):105–117. doi: 10.1016/j.resp.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 17.Ebihara S, Saito H, Kanda A, Nakajoh M, Takahashi H, Arai H, et al. Impaired efficacy of cough in patients with parkinson disease. Chest. 2003;124 (3):1009–1015. doi: 10.1378/chest.124.3.1009. [DOI] [PubMed] [Google Scholar]
- 18.Addington WR, Stephens RE, Gilliland K, Rodriguez M. Assessing the laryngeal cough reflex and the risk of developing pneumonia after stroke. Arch Phys Med Rehab. 1999;80 (2):150–154. doi: 10.1016/s0003-9993(99)90112-0. [DOI] [PubMed] [Google Scholar]
- 19.Fontana GA, Pantaleo T, Lavorini F, Maluccio NM, Mutolo D, Pistolesi M. Repeatability of cough-related variables during fog challenges at threshold and suprathreshold stimulus intensity in humans. Eur Resp J. 1999;13 (6):1447–1450. [PubMed] [Google Scholar]
- 20.Pitts T, Bolser D, Rosenbek J, Troche M, Sapienza C. Voluntary cough production and swallow dysfunction in Parkinson’s disease. Dysphagia. 2008;23 (3):297–301. doi: 10.1007/s00455-007-9144-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith Hammond CA, Goldstein LB, Horner RD, Ying J, Gray L, Gonzalez-Rothi L, et al. Predicting aspiration in patients with ischemic stroke: Comparison of clinical signs and aerodynamic measures of voluntary cough. Chest. 2009;135 (3):769–777. doi: 10.1378/chest.08-1122. [DOI] [PMC free article] [PubMed] [Google Scholar]