Abstract
Alcoholic liver disease progresses through several stages of tissue damage, from simple steatosis to alcoholic hepatitis, fibrosis, or cirrhosis. Alcohol also affects the intestine, increases intestinal permeability and changes the bacterial microflora. Liver disease severity correlates with levels of systemic bacterial products in patients, and experimental alcoholic liver disease is dependent on gut derived bacterial products in mice. Supporting evidence for the importance of bacterial translocation comes from animal studies demonstrating that intestinal decontamination is associated with decreased liver fibrogenesis. In addition, mice with a gene mutation or deletion encoding receptors for either bacterial products or signaling molecules downstream from these receptors, are resistant to alcohol-induced liver disease. Despite this strong association, the exact molecular mechanism of bacterial translocation and of how changes in the intestinal microbiome contribute to liver disease progression remains largely unknown. In this review we will summarize evidence for bacterial translocation and enteric microbial changes in response to alcoholic liver injury and chronic alcoholic liver disease. We will further describe consequences of intestinal dysbiosis on host biology. We finally discuss how therapeutic interventions may modify the gastrointestinal microflora and prevent or reduce alcoholic liver disease progression.
Keywords: Alcoholic liver disease, Microbiome, Dysbiosis, Bacterial translocation, Steatohepatitis
INTRODUCTION
Alcohol abuse is one of the leading causes of chronic liver disease. In its early stage, alcoholic liver disease is characterized by fatty infiltration of the liver, also known as steatosis. Forty-four percent of patients consuming modest amounts (40-80 g/d) of alcohol exhibit fatty liver, while it is even more common in heavy drinkers[1]. With steatosis alone, the liver continues to function well and few patients present with any clinical symptoms[2]. The most effective therapy for alcoholic steatosis is cessation of alcohol consumption. However, if this cannot be achieved, subsequent inflammation and alcoholic hepatitis can take place. This ultimately results in liver fibrosis, which is an accumulation of scar tissue in the liver parenchyma that distorts the hepatic architecture. As hepatic fibrogenesis progresses to cirrhosis, disruption of the synthetic and metabolic functions of the liver occurs. Increased resistance to portal blood flow results in portal hypertension, the clinical consequence of which includes ascites, esophageal varices, and splenomegaly through shunting of blood to portal caval anastamoses. Cirrhosis is an end-stage disease and one of the leading causes of morbidity and mortality in the world, with liver transplantation as the sole remedy for survival.
Annually, over 27 000 people die from cirrhosis in the United States[3]. Half of all cirrhotic patients die within 2 years of diagnosis. Mortality from alcoholic liver disease (ALD) has been declining in recent years, likely due to improvements in clinical management of complications of ALD including portal hypertension and bleeding from esophageal varices[4]. A significant percentage of cirrhotic patients succumb to bacterial infections with infection-attributed mortality of 30% to 50%[5-7]. Mortality as a consequence of infection is increased 20-fold in patients with cirrhosis. These infections include spontaneous bacterial peritonitis, bacteremia, pneumonia, and urinary tract infections. The most common pathogens involved are Staphylococcus aureus, Enterococcus faecalis, Streptococcus pneumonia, Klebsiella spp, and Escherichia coli[8,9]. The origin of these organisms is thought to be the enteric microflora, but may also be associated with nosocomial infections due to the higher number of invasive procedures performed in this patient population[10].
In the following article, we will review bacterial translocation and the changes in the intestinal microbiome associated with ALD. For each topic we will discuss the data from experimental and preclinical animal models of alcohol-induced liver injury followed by current evidence in patients with ALD.
BACTERIAL TRANSLOCATION
Bacterial translocation is defined as the passage of viable indigenous bacteria or their products, such as lipopolysaccharide and bacterial DNA, from the gut to extraintestinal sites, notably the mesenteric lymph nodes and the systemic circulation. In particular, lipopolysaccharide (LPS) is the cell wall molecule derived from gram-negative bacteria, and has been found to be increased in alcoholics with fatty liver disease or patients with alcoholic cirrhosis[11-13]. The presence of bacterial DNA in an animal model of cirrhosis has also been established as a surrogate marker of bacterial translocation, associated with marked inflammatory response in the host[14]. This migration of bacteria and bacterial products has been implicated in spontaneous bacterial peritonitis and sepsis in patients with end-stage liver disease[15]. In addition, this phenomenon is also considered to play a key role in the pathogenesis of liver fibrosis in experimental animal models of alcohol-induced liver injury[16-18].
Bacterial translocation and alcoholic liver disease
There have been several studies in animal models examining the association between bacterial translocation and alcohol administration. Some studies have demonstrated no significant difference in bacterial translocation to the mesenteric lymph nodes or the systemic circulation after alcohol administration for 2 wk[19,20]. However, one report provides evidence for the translocation of viable bacteria in rats fed alcohol as early as 14 d[21]. Bacterial products, including endotoxin, have also been seen translocating in experimental animal models of alcohol consumption. A positive correlation between alcohol ingestion and increased systemic levels of endotoxin have been observed[22-24]. Plasma levels of peptidoglycan, which makes up about 70% and 20% of Gram-positive and Gram-negative bacterial cell walls respectively, are also increased following acute administration of alcohol in rats[25]. Discrepancies in these studies might be explained by differences in species, treatment length, alcohol dose, and the model used to administer alcohol (drinking water or diet, gastric gavage, intragastric feeding tube).
The presence of endotoxemia in liver disease has also been seen in human studies. LPS is significantly higher in patients with alcoholic cirrhosis compared to patients with cirrhosis from other causes[11,12]. Endotoxemia has also been shown to increase with more severe liver dysfunction (Child-Pugh class) in patients with cirrhosis[26]. Furthermore, endotoxin is also present in patients with mild forms of alcoholic hepatitis that do not have evidence of fibrosis or cirrhosis[27,28]. These studies suggest that bacterial translocation occurs early in ALD, and that the degree of translocation of bacterial products may be related to the severity of liver injury present. There are many possibilities for why this occurs. As liver disease progresses, reduced hepatic clearance of toxins may result in higher systemic levels of translocated bacteria and bacteria products. Likewise, alcohol may directly injure the defensive intestinal barrier, contribute to intestinal dysmotility, result in bacterial overgrowth, and change the intestinal microflora.
Bacterial translocation contributes to the progression of alcoholic liver disease
Bacterial translocation not only results in severe infections in cirrhotic patients, but also leads to the progression of alcohol-induced liver injury and fibrosis. The enteric microflora may therefore play a role in augmenting the progression of liver disease. One mechanism by which this occurs is through the activation of the innate immune system in the liver. The innate immune system has developed phylogenetically conserved pattern recognition receptors including the Toll-like receptors (TLR), which recognize distinct microbial products, known as pathogen-associated molecular patterns (PAMPs). PAMPs not only include LPS, but also bacterial peptidoglycan, double-stranded RNA, and unmethylated DNA[29]. Ligand binding to PAMPs trigger intracellular signaling cascades which activates downstream transcription and expression of a variety of genes involved in the immune and inflammatory host response. PAMPs stimulated by commensal enteric microflora are not necessarily interpreted as disease states but rather contributes to maintenance of homeostasis. For a detailed view of the role of the innate immune system in health and disease, we would refer to recently published reviews[30].
Animal studies have suggested integral roles for LPS signaling in the pathogenesis of ALD. In particular, TLR4, the cellular LPS receptor, plays an important role in the innate immune response to bacterial translocation. Selective intestinal decontamination with antibiotics have shown to reduce plasma endotoxin levels and prevents liver injury in animal models of ALD[31,32,22]. Moreover, mice deficient in CD14, the cellular co-receptor for LPS, are also resistant to alcohol-induced liver injury[17]. Similar findings were also noted in these mice with experimental hepatic injury induced by bile duct ligation[33]. However, the strongest evidence that supports the role LPS plays in ALD are studies with TLR4 mutant C3H/Hej mice and TLR4 deficient mice. These genetically modified animals demonstrate marked reductions in hepatic steatosis, inflammation, and necrosis in models of ALD, as compared to wildtype control mice[16,34]. Similar to TLR4, TLR9 is another pattern recognition receptor that is activated by CpG motifs specific to bacterial DNA. TLR9-deficient mice have been shown to be resistant to experimentally induced liver fibrosis[35].
In patients, TLR4 is identified as one of seven genes associated with an increased risk of developing cirrhosis in patients with chronic hepatitis C[36]. TLR4 D299G and T399I single nucleotide polymorphisms are associated with protection from hepatic fibrosis by reduced TLR4-mediated inflammatory and fibrogenic signaling[37].
Taken together, these studies highlight the important roles translocated bacteria and their products play in both hepatic fibrogenesis and infections in patients with chronic liver disease. Bacterial translocation must happen early for initial liver injury and fibrogenesis, while bacterial translocation in end-stage liver disease is partly responsible for the resulting infections and mortality.
Disruption of the intestinal barrier function as mechanism for bacterial translocation
The mechanism behind bacterial translocation with alcohol ingestion is not clear. Tight junctions normally join together at the apicolateral membranes of enterocytes, providing a mucosal barrier against paracellular diffusion of intestinal contents. Damage to these protective barriers may result in structural deficiencies that enable bacterial translocation[38-41,28].
Several studies have examined oxidative stress on the intestinal mucosa as a possible etiology for barrier dysfunction. Acute ingestion of alcohol has been shown to alter the epithelial barrier in the colonic mucosa of rats via ethanol oxidation into acetaldehyde by the enteric microflora with subsequent downstream activation of mast cells[42]. There is also an increase in tissue oxidative stress in jejunal, ileal, and colonic mucosa seen as early as 4 wk after alcohol administration in rats[43]. Furthermore, LPS is known to activate macrophages resulting in their subsequent release of pro-inflammatory cytokines, which in turn induce liver damage[44]. These cytokines, such as tumor necrosis factor (TNF), interleukin 1β (IL-1β), iNOS, and IL-6, have been shown to be elevated in the distal ileum of mice administered alcohol for 14 d[20]. Cytokines such as IL-1β and TNF are known to cause a disruption of tight junctions[45].
In one study, distension of the intercellular spaces below these tight junctions was observed in duodenal biopsies of cirrhotic patients[46]. Alcoholic patients without liver cirrhosis that cease alcohol consumption demonstrated higher intestinal permeability in 3 d by way of a chromium-51-EDTA absorption test. These finding persisted beyond 2 wk in patients with evidence of liver cirrhosis, despite abstaining from alcohol[47]. Taken together, this suggests that underlying liver disease may prolong the damaging effects of alcohol on the intestinal epithelium.
Alcohol may also exert its effects on the intestinal epithelium indirectly through its oxidized metabolite, acetaldehyde. This has been shown both in cultured Caco-2 cell monolayers, as well as biopsy specimens of the intestines, where acetaldehyde disrupts tight junctions and adherens junctions with an associated rise in tyrosine phosphorylation[48,49,52].
QUANTITATIVE AND QUALITATIVE CHANGES OF THE ENTERIC MICROFLORA
In addition to the direct and indirect toxic effects of alcohol to the intestinal epithelial barrier, the quantitative and qualitative changes of the enteric microflora themselves may contribute to bacterial translocation. For example, animal studies demonstrate a correlation between bacterial overgrowth and bacterial translocation. Experimentally induced bacterial overgrowth results in bacterial translocation, liver injury, and inflammation[50]. In addition, as we have discussed above, selective intestinal decontamination improves experimental alcoholic steatohepatitis. This might be mediated by a decrease in the intestinal bacterial burden, which subsequently results in a reduction of bacterial translocation and alcoholic steatohepatitis. Furthermore, in non-cirrhotic patients that have small-intestinal bacterial overgrowth with colonic flora, increased intestinal permeability has been observed[51].
Intestinal bacterial overgrowth in alcoholic liver disease
Bacterial overgrowth and translocation have been observed in animal models of end-stage liver disease[52,53]. In cirrhotic rats with bacterial translocation, there is a higher prevalence of bacterial overgrowth. The prevalence of overgrowth in cirrhotic rats was 67%, with 47%-78% of animals also exhibiting bacterial translocation[14,54-56]. Following experimentally-induced cirrhosis via bile duct ligation, there is an increase in number of gram-negative bacteria in the cecum of animals, which includes E. Coli, Enterococci, Klebsiella, Pasteurella, Proteus, Pseudomonas, and Shigella.
Intestinal bacterial overgrowth also occurs more frequently in patients with ALD. The number of aerobic and anaerobic bacteria has been found to be higher in jejunal aspirates in patients with chronic alcohol abuse[57,58]. Small intestinal bacterial overgrowth has also been found in cirrhotic patients with increasing prevalence corresponding with the severity of liver dysfunction - as high as 58%-75% in cirrhotics classified Child-Pugh C[59-62]. Serum antibodies to microbial components are also found more frequently in patients with more advanced liver disease[63]. The cultured flora from cirrhotic patients include Bacteroides spp., E. Coli, Corynebacerium spp., Klebsiella spp., Stomatococcus, Streptoccoci, and Veillonella spp. It is interesting to note that the majority of these bacteria come from oropharyngeal sources, with exception to Bacteroides, E. Coli and Klebsiella. Of interest Bifidobacterium, often used in commercial probiotics, was reduced in the fecal flora of cirrhotics[64].
The reason for bacterial overgrowth in liver disease is not known. One hypothesis suggests that impaired bile flow results in bacterial proliferation and mucosal injury in the small intestines. Conjugated bile acids induce expression of antimicrobial proteins angiogenin 1 and RNAse family member 4 in the enterocytes, which then prevents bacterial overgrowth and promotes epithelial cell integrity in experimental mouse models[65]. As liver dysfunction progresses, less conjugated bile acids are produced and available in the small intestines, which may then contribute to small intestinal bacterial overgrowth.
Dysmotility has also been proposed as a mechanism for stasis and bacterial overgrowth in patients with cirrhosis. Alcohol has been shown to reduce gastrointestinal motility, which may result in a higher number of luminal bacteria[66]. A delayed transit time, characterized by alteration of the migrating motor complex in the small intestine, has been observed in cirrhotics[67-69]. A recent cross-sectional study also demonstrated a higher prevalence of small intestinal bacterial overgrowth with prolonged orocecal transit time in cirrhotic patients with hepatic encephalopathy[70]. Further evidence linking overgrowth and intestinal transit time includes the use of the pro-motility agent cisapride, which reversed small intestinal dysmotiliy and bacterial overgrowth in patients with cirrhosis[71]. These patients also exhibited further clinical improvement in Child-Pugh scores and hepatic encephalopathy.
Moreover, a higher gastric pH may possibly contribute to bacterial overgrowth and translocation. Hypochlorhydria has been associated with an increase in bacteria in the jejunum in patients with cirrhosis[61,72]. A retrospective case-control study also noted that cirrhotic patients with spontaneous bacterial peritonitis had a significantly higher pre-hospital use of proton pump inhibitors compared with controls[73]. Less gastric acid secretion with higher transit times from intestinal dysmotility enables bacterial colonization of the upper gastrointestinal tract. With a reduction in protective conjugated bile acids, cirrhotic patients are predisposed to bacterial overgrowth and translocation.
Intestinal microbiome changes in alcoholic liver disease
The enteric microbial communities are complex and harbor 10 different bacterial phyla with more than 15 000 species-level bacterial phylotypes[74]. Firmicutes and Bacteroidetes make up the vast majority of these phylotypes in mice[75] and humans[76,77]. In healthy individuals, the microflora maintains a symbiotic relationship with the human intestine. This balance of number, distribution, and composition is regulated by the innate and adaptive immune system including host antimicrobial proteins secreted from paneth cells and intestinal epithelial cells. Dysbiosis is known as the disruption and enteric disequilibrium between the microbiota and colonized host, and has been associated with disease including inflammatory bowel disease[78].
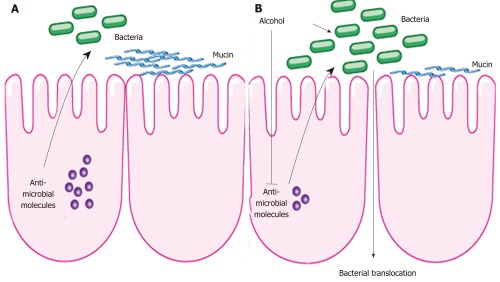
One of the first animal studies in alcoholism and enteric dysbiosis utilized length heterogeneity polymerase chain reaction fingerprinting, and demonstrated differing enteric microbiota composition in rats after 10 wk of intragastric feeding of alcohol[79]. A subgroup of animals given probiotics or prebiotics prevented dysbiosis in animals treated with alcohol. A recent animal study examined changes in the intestinal microbial community with the use of deep DNA pyrosequencing of the bacterial 16S rRNA during the early stages of ALD[75]. Following continuous intragastric feeding of alcohol for 3 wk, there was a relative predominance of the Bacteroidetes and Verrucomicrobia phylotypes in mice fed alcohol compared with an abundance of Firmicutes bacteria in the control group. Interestingly, Lactobacillus was significantly reduced in alcohol fed mice, which now explains the beneficial effect of probiotic Lactobacillus in alcoholic steatohepatitis shown in several reports[79,80,81]. In addition, Akkermansia muciniphila, a gram-negative anaerobic bacteria belonging to the bacterial phylum Verrucomicrobia[82], was strongly induced in animals fed alcohol for 3 wk. Akkermansia muciniphila is a common bacterial component of the human intestinal tract and has been found to degrade mucin in pure culture[83,84]. By degrading the intestinal mucus as part of the innate immune system, bacterial translocation might be facilitated. However, a causal relationship between an increase in intestinal Akkermansia muciniphila and bacterial translocation needs to be investigated in ALD in future studies. Antimicrobial effector molecules secreted by enterocytes and paneth cells not only protect against pathogenic organisms, but also play a role in maintaining a symbiotic relationship between the host and the commensal enteric microflora. A suppression of bactericidal protein expression regenerating islet derived (Reg)-3b and Reg3g were observed in the small intestines following alcohol administration in mice, suggesting a possible mechanism for qualitative and quantitative changes within the microbiome (Figure 1). Furthermore, the treatment with prebiotics partially restored Reg3g protein levels, while mitigating bacterial overgrowth and attenuating the severity of alcoholic steatohepatitis[76].
Figure 1.
Intestinal changes following alcohol administration. A: In health, antimicrobial molecules as part of the innate immune response are secreted by intestinal epithelial cells and kill enteric bacteria. B: Alcohol suppresses the expression of these molecules resulting in intestinal bacterial overgrowth and dysbiosis. This might contribute to bacterial translocation observed after alcohol. Alcohol might also exert a direct effect on the intestinal microflora.
Although changes in the enteric microbial composition has not been studied in patients with alcohol abuse, intestinal dysbiosis as a consequence of chronic alcohol consumption is speculated to be a possible precursor to bacterial translocation and a contributing factor to the initiation and progression of liver disease. It is likely that changes to the microbiota modulate mucosal barrier function and antimicrobial regulators within the host intestinal epithelium.
ARE ANTIBIOTICS, PROBIOTICS, PREBIOTICS OR SYNBIOTICS USEFUL IN ALCOHOLIC LIVER DISEASE?
The beneficial effect of antibiotics on alcohol-induced liver disease in animal models has been discussed above. In patients, antibiotics have mostly been used to decontaminate the intestine for the treatment of hepatic encephalopathy in end-stage liver disease. Antibiotic treatment for up to 6 mo has also been associated with improvement in liver function and Child-Pugh classification of patients with alcoholic cirrhosis[71]. Antibiotic-induced changes in the composition of the gastrointestinal microflora can influence the susceptibility of the host to specific enteric pathogens, including the induction of antibiotic resistance to other microorganisms. Antibiotics disturb the normal mechanisms of microbial community regulation, compromising the mucosal innate immune defense mechanism, which can result in pathogen colonization and antibiotic resistance[85].
Probiotics are dietary supplements of live microbes consumed by the host that benefit the health, and includes the microorganisms Lactobacillus and Bifidobacterium[86]. These microbes are thought to enhance production of anti-inflammatory cytokines, stimulate the secretion of antibacterial proteins, and alter the intestinal microflora, ultimately reducing production and translocation of endotoxin[87]. Pretreatment of animals with Lactobacillus decreases plasma LPS and reduces the severity of liver injury. As discussed above, Lactobacillus was depleted after 3 wk of intragastric alcohol administration in mice[75]. Feeding a gram-positive probiotic lactobacillus strain (species GG) with concomitant displacement of gram-negative bacteria also protected rats from ethanol-induced liver injury with a decrease in systemic endotoxin levels[80,81]. Thus, a possible mechanism for preventing ALD is reversing the enteric dysbiosis associated with alcohol abuse.
There have been multiple studies examining the benefits of probiotics in patients with ALD. Treatment of 20 patients with alcoholic liver cirrhosis with a probiotic mixture containing Streptococcus thermophilus, Bifidobacterium breve, Bifidobacterium longum, Bifidobacterium infantis, Lactobacillus acidophilus, Lactobacillus plantarum, Lactobacillus casei and Lactobacillus bulgaricus (VSL#3) for up to 4 mo, results in decreased plasma markers for oxidative stress and markedly reduced liver enzymes[88]. A randomized prospective trial demonstrated that probiotic treatment (Bifidobacterium bifidum and Lactobacillus plantarum) for five days results in restoration of colonic bowel flora and improvement of serum liver tests in patients with alcoholic liver injury[89]. As a defective innate immune response to enteric pathogens likely contributes to increased infections in patients with cirrhosis, another mechanism that explains the beneficial effects of probiotics could be a restoration of neutrophil function. And in fact, administration of Lactobacillus casei for 4 wk restores neutrophil phagocytic activity in patients with alcoholic cirrhosis[90]. However, care must be applied when probiotics are administered to patients with a preexisting gut barrier leakage due to a higher risk of infection. In patients with predicted severe acute pancreatitis, probiotic prophylaxis with a combination of six different strains of probiotic strains (Lactobacillus acidophilus, Lactobacillus casei, Lactobacillus salivarius, Lactococcus lactis, Bifidobacterium bifidum, and Bifidobacterium infantis) did not reduce the risk of infectious complications and was associated with an increased risk of mortality[91].
Prebiotics are complex carbohydrates that cannot be digested by pancreatic or intestinal enzymes and are ultimately metabolized by the gut microflora. Common prebiotics are fructo-oligosaccharides (FOS), galacto-oligosaccharides, or lactulose[86]. The later is of special interest, as it is commonly used in patients with decompensated cirrhosis for the treatment and prevention of hepatic encephalopathy. As mentioned earlier, oats supplementation as a prebiotic not only prevents alcohol-induced dysbiosis following 10 wk of alcohol treatment, but reduces gut leakiness with subsequent reduction in endotoxin levels and attenuation of liver damage in rats[79,92]. Similarly, FOS reduces intestinal bacterial overgrowth and alcoholic steatohepatitis following intragastric feeding of alcohol for 3 wk in mice[75]. The beneficial effect of prebiotics is suggested to prevent quantitative and qualitative changes in the microbiome associated with alcohol use. At this point, it is not entirely clear whether prebiotics might prevent intestinal barrier leakage independent from changes to the microbiome. Prebiotics are very promising for future use in patients with ALD and further clinical studies are needed.
Synbiotics are combinations of prebiotics and probiotics. Pretreatment with synbiotics (Lactobacillus acidophilus, Lactobacillus helveticus and Bifidobacterium in an enriched medium) effectively protects against endotoxemia and bacterial translocation, as well as liver damage in the course of acute pancreatitis and concomitant heavy alcohol consumption in rats[93].
CONCLUSION
Alcoholic liver disease remains a leading cause of morbidity and mortality worldwide. The progression of alcoholic liver disease to fibrosis and cirrhosis is partly mediated through an inflammatory response to the translocation of bacteria and endotoxin. Bacterial translocation is also a contributing factor to the complications arising from alcoholic liver disease including spontaneous bacterial peritonitis, hepatic encephalopathy, portal hypertension, and sepsis. There are many possible mechanisms for which this occurs. Bacterial overgrowth of pathogenic organisms and intestinal dysmotility both occur in alcoholic liver disease, and may predispose the intestines for bacterial translocation. The oxidative stress resulting from exposure of the intestines to alcohol and its metabolites disrupts the integrity of the intestinal wall, increasing permeability to gut-derived endotoxin. There have also been recent advances in diagnostic technologies for research in molecular genetics. The use of 454 pyrosequencing has enabled the qualitative and quantitative examination of the enteric microbiome. In an experimental model of alcoholic liver disease, shifts in the composition of the intestinal flora have been observed along with downregulation of intestinal antimicrobial proteins. These changes can modulate mucosal barrier function by disrupting the innate immune system and alter antimicrobial regulators in the host intestinal epithelium. This process can facilitate bacterial translocation and more animal studies will be needed to firmly establish this mechanism. There are few therapeutic options for patients suffering from alcoholic liver disease, aside from abstinence and liver transplantation for end-stage liver disease. There is some limited evidence on the use of antibiotics, probiotics, and prebiotics to attenuate disease activity in patients with alcoholic liver disease by altering the intestinal microflora. However, large scale clinical studies examining the potential benefits of probiotics and prebiotics are still needed before routine use of these supplements can be recommended.
Footnotes
Supported by NIH grants No. K08 DK081830 and No. R01 AA020703; the Pilot Project Program of the Southern California Research Center for ALPD and Cirrhosis No. P50AA11999 funded by the National Institute on Alcohol Abuse and Alcoholism; and ABMRF/The Foundation for Alcohol Research to Schnabl B.
Peer reviewers: Dr. Ali Sazci, Professor, Department of Medical Biology and Genetics, University of Kocaeli, Faculty of Medicine, Umuttepe, Kocaeli 41380, Turkey; Felix Dias Dias Carvalho, Professor, Department of Toxicology, Faculty of Pharmacy, University of Porto, Rua Anibal Cunha, 164, Porto 4099-030, Portugal; JC Perazzo, Professor, Department of Biological Science, School of Pharmacy and Biochemistry, University of Buenos Aires, Buenos Aires 1113, Argentina
S- Editor Wu X L- Editor A E- Editor Wu X
References
- 1.Savolainen VT, Liesto K, Männikkö A, Penttilä A, Karhunen PJ. Alcohol consumption and alcoholic liver disease: evidence of a threshold level of effects of ethanol. Alcohol Clin Exp Res. 1993;17:1112–1117. doi: 10.1111/j.1530-0277.1993.tb05673.x. [DOI] [PubMed] [Google Scholar]
- 2.Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115:209–218. doi: 10.1172/JCI24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ginès P, Cárdenas A, Arroyo V, Rodés J. Management of cirrhosis and ascites. N Engl J Med. 2004;350:1646–1654. doi: 10.1056/NEJMra035021. [DOI] [PubMed] [Google Scholar]
- 4.Paula H, Asrani SK, Boetticher NC, Pedersen R, Shah VH, Kim WR. Alcoholic liver disease-related mortality in the United States: 1980-2003. Am J Gastroenterol. 2010;105:1782–1787. doi: 10.1038/ajg.2010.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brann OS. Infectious complications of cirrhosis. Curr Gastroenterol Rep. 2001;3:285–292. doi: 10.1007/s11894-001-0051-2. [DOI] [PubMed] [Google Scholar]
- 6.Christou L, Pappas G, Falagas ME. Bacterial infection-related morbidity and mortality in cirrhosis. Am J Gastroenterol. 2007;102:1510–1517. doi: 10.1111/j.1572-0241.2007.01286.x. [DOI] [PubMed] [Google Scholar]
- 7.Wong F, Bernardi M, Balk R, Christman B, Moreau R, Garcia-Tsao G, Patch D, Soriano G, Hoefs J, Navasa M. Sepsis in cirrhosis: report on the 7th meeting of the International Ascites Club. Gut. 2005;54:718–725. doi: 10.1136/gut.2004.038679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Francés R, Zapater P, González-Navajas JM, Muñoz C, Caño R, Moreu R, Pascual S, Bellot P, Pérez-Mateo M, Such J. Bacterial DNA in patients with cirrhosis and noninfected ascites mimics the soluble immune response established in patients with spontaneous bacterial peritonitis. Hepatology. 2008;47:978–985. doi: 10.1002/hep.22083. [DOI] [PubMed] [Google Scholar]
- 9.Steffen EK, Berg RD, Deitch EA. Comparison of translocation rates of various indigenous bacteria from the gastrointestinal tract to the mesenteric lymph node. J Infect Dis. 1988;157:1032–1038. doi: 10.1093/infdis/157.5.1032. [DOI] [PubMed] [Google Scholar]
- 10.Fernández J, Navasa M, Gómez J, Colmenero J, Vila J, Arroyo V, Rodés J. Bacterial infections in cirrhosis: epidemiological changes with invasive procedures and norfloxacin prophylaxis. Hepatology. 2002;35:140–148. doi: 10.1053/jhep.2002.30082. [DOI] [PubMed] [Google Scholar]
- 11.Fukui H, Brauner B, Bode JC, Bode C. Plasma endotoxin concentrations in patients with alcoholic and non-alcoholic liver disease: reevaluation with an improved chromogenic assay. J Hepatol. 1991;12:162–169. doi: 10.1016/0168-8278(91)90933-3. [DOI] [PubMed] [Google Scholar]
- 12.Bode C, Kugler V, Bode JC. Endotoxemia in patients with alcoholic and non-alcoholic cirrhosis and in subjects with no evidence of chronic liver disease following acute alcohol excess. J Hepatol. 1987;4:8–14. doi: 10.1016/s0168-8278(87)80003-x. [DOI] [PubMed] [Google Scholar]
- 13.Schäfer C, Parlesak A, Schütt C, Bode JC, Bode C. Concentrations of lipopolysaccharide-binding protein, bactericidal/permeability-increasing protein, soluble CD14 and plasma lipids in relation to endotoxaemia in patients with alcoholic liver disease. Alcohol Alcohol. 2002;37:81–86. doi: 10.1093/alcalc/37.1.81. [DOI] [PubMed] [Google Scholar]
- 14.Guarner C, González-Navajas JM, Sánchez E, Soriando G, Francés R, Chiva M, Zapater P, Benlloch S, Muñoz C, Pascual S, et al. The detection of bacterial DNA in blood of rats with CCl4-induced cirrhosis with ascites represents episodes of bacterial translocation. Hepatology. 2006;44:633–639. doi: 10.1002/hep.21286. [DOI] [PubMed] [Google Scholar]
- 15.Wiest R, Garcia-Tsao G. Bacterial translocation (BT) in cirrhosis. Hepatology. 2005;41:422–433. doi: 10.1002/hep.20632. [DOI] [PubMed] [Google Scholar]
- 16.Uesugi T, Froh M, Arteel GE, Bradford BU, Thurman RG. Toll-like receptor 4 is involved in the mechanism of early alcohol-induced liver injury in mice. Hepatology. 2001;34:101–108. doi: 10.1053/jhep.2001.25350. [DOI] [PubMed] [Google Scholar]
- 17.Yin M, Bradford BU, Wheeler MD, Uesugi T, Froh M, Goyert SM, Thurman RG. Reduced early alcohol-induced liver injury in CD14-deficient mice. J Immunol. 2001;166:4737–4742. doi: 10.4049/jimmunol.166.7.4737. [DOI] [PubMed] [Google Scholar]
- 18.Iimuro Y, Gallucci RM, Luster MI, Kono H, Thurman RG. Antibodies to tumor necrosis factor alfa attenuate hepatic necrosis and inflammation caused by chronic exposure to ethanol in the rat. Hepatology. 1997;26:1530–1537. doi: 10.1002/hep.510260621. [DOI] [PubMed] [Google Scholar]
- 19.Mason CM, Dobard E, Kolls J, Nelson S. Effect of alcohol on bacterial translocation in rats. Alcohol Clin Exp Res. 1998;22:1640–1645. [PubMed] [Google Scholar]
- 20.Fleming S, Toratani S, Shea-Donohue T, Kashiwabara Y, Vogel SN, Metcalf ES. Pro- and anti-inflammatory gene expression in the murine small intestine and liver after chronic exposure to alcohol. Alcohol Clin Exp Res. 2001;25:579–589. [PubMed] [Google Scholar]
- 21.Napolitano LM, Koruda MJ, Zimmerman K, McCowan K, Chang J, Meyer AA. Chronic ethanol intake and burn injury: evidence for synergistic alteration in gut and immune integrity. J Trauma. 1995;38:198–207. doi: 10.1097/00005373-199502000-00008. [DOI] [PubMed] [Google Scholar]
- 22.Adachi Y, Moore LE, Bradford BU, Gao W, Thurman RG. Antibiotics prevent liver injury in rats following long-term exposure to ethanol. Gastroenterology. 1995;108:218–224. doi: 10.1016/0016-5085(95)90027-6. [DOI] [PubMed] [Google Scholar]
- 23.Nanji AA, Khettry U, Sadrzadeh SM, Yamanaka T. Severity of liver injury in experimental alcoholic liver disease. Correlation with plasma endotoxin, prostaglandin E2, leukotriene B4, and thromboxane B2. Am J Pathol. 1993;142:367–373. [PMC free article] [PubMed] [Google Scholar]
- 24.Tamai H, Kato S, Horie Y, Ohki E, Yokoyama H, Ishii H. Effect of acute ethanol administration on the intestinal absorption of endotoxin in rats. Alcohol Clin Exp Res. 2000;24:390–394. [PubMed] [Google Scholar]
- 25.Tabata T, Tani T, Endo Y, Hanasawa K. Bacterial translocation and peptidoglycan translocation by acute ethanol administration. J Gastroenterol. 2002;37:726–731. doi: 10.1007/s005350200118. [DOI] [PubMed] [Google Scholar]
- 26.Lin RS, Lee FY, Lee SD, Tsai YT, Lin HC, Lu RH, Hsu WC, Huang CC, Wang SS, Lo KJ. Endotoxemia in patients with chronic liver diseases: relationship to severity of liver diseases, presence of esophageal varices, and hyperdynamic circulation. J Hepatol. 1995;22:165–172. doi: 10.1016/0168-8278(95)80424-2. [DOI] [PubMed] [Google Scholar]
- 27.Bode C, Bode JC. Activation of the innate immune system and alcoholic liver disease: effects of ethanol per se or enhanced intestinal translocation of bacterial toxins induced by ethanol? Alcohol Clin Exp Res. 2005;29:166S–171S. doi: 10.1097/01.alc.0000189280.19073.28. [DOI] [PubMed] [Google Scholar]
- 28.Parlesak A, Schäfer C, Schütz T, Bode JC, Bode C. Increased intestinal permeability to macromolecules and endotoxemia in patients with chronic alcohol abuse in different stages of alcohol-induced liver disease. J Hepatol. 2000;32:742–747. doi: 10.1016/s0168-8278(00)80242-1. [DOI] [PubMed] [Google Scholar]
- 29.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 30.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 31.Enomoto N, Yamashina S, Kono H, Schemmer P, Rivera CA, Enomoto A, Nishiura T, Nishimura T, Brenner DA, Thurman RG. Development of a new, simple rat model of early alcohol-induced liver injury based on sensitization of Kupffer cells. Hepatology. 1999;29:1680–1689. doi: 10.1002/hep.510290633. [DOI] [PubMed] [Google Scholar]
- 32.Enomoto N, Ikejima K, Yamashina S, Hirose M, Shimizu H, Kitamura T, Takei Y, Sato And N, Thurman RG. Kupffer cell sensitization by alcohol involves increased permeability to gut-derived endotoxin. Alcohol Clin Exp Res. 2001;25:51S–54S. doi: 10.1097/00000374-200106001-00012. [DOI] [PubMed] [Google Scholar]
- 33.Isayama F, Hines IN, Kremer M, Milton RJ, Byrd CL, Perry AW, McKim SE, Parsons C, Rippe RA, Wheeler MD. LPS signaling enhances hepatic fibrogenesis caused by experimental cholestasis in mice. Am J Physiol Gastrointest Liver Physiol. 2006;290:G1318–G1328. doi: 10.1152/ajpgi.00405.2005. [DOI] [PubMed] [Google Scholar]
- 34.Hritz I, Mandrekar P, Velayudham A, Catalano D, Dolganiuc A, Kodys K, Kurt-Jones E, Szabo G. The critical role of toll-like receptor (TLR) 4 in alcoholic liver disease is independent of the common TLR adapter MyD88. Hepatology. 2008;48:1224–1231. doi: 10.1002/hep.22470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gäbele E, Mühlbauer M, Dorn C, Weiss TS, Froh M, Schnabl B, Wiest R, Schölmerich J, Obermeier F, Hellerbrand C. Role of TLR9 in hepatic stellate cells and experimental liver fibrosis. Biochem Biophys Res Commun. 2008;376:271–276. doi: 10.1016/j.bbrc.2008.08.096. [DOI] [PubMed] [Google Scholar]
- 36.Huang H, Shiffman ML, Friedman S, Venkatesh R, Bzowej N, Abar OT, Rowland CM, Catanese JJ, Leong DU, Sninsky JJ, et al. A 7 gene signature identifies the risk of developing cirrhosis in patients with chronic hepatitis C. Hepatology. 2007;46:297–306. doi: 10.1002/hep.21695. [DOI] [PubMed] [Google Scholar]
- 37.Guo J, Loke J, Zheng F, Hong F, Yea S, Fukata M, Tarocchi M, Abar OT, Huang H, Sninsky JJ, et al. Functional linkage of cirrhosis-predictive single nucleotide polymorphisms of Toll-like receptor 4 to hepatic stellate cell responses. Hepatology. 2009;49:960–968. doi: 10.1002/hep.22697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Keshavarzian A, Choudhary S, Holmes EW, Yong S, Banan A, Jakate S, Fields JZ. Preventing gut leakiness by oats supplementation ameliorates alcohol-induced liver damage in rats. J Pharmacol Exp Ther. 2001;299:442–448. [PubMed] [Google Scholar]
- 39.Rao RK, Seth A, Sheth P. Recent Advances in Alcoholic Liver Disease I. Role of intestinal permeability and endotoxemia in alcoholic liver disease. Am J Physiol Gastrointest Liver Physiol. 2004;286:G881–G884. doi: 10.1152/ajpgi.00006.2004. [DOI] [PubMed] [Google Scholar]
- 40.Purohit V, Bode JC, Bode C, Brenner DA, Choudhry MA, Hamilton F, Kang YJ, Keshavarzian A, Rao R, Sartor RB, et al. Alcohol, intestinal bacterial growth, intestinal permeability to endotoxin, and medical consequences: summary of a symposium. Alcohol. 2008;42:349–361. doi: 10.1016/j.alcohol.2008.03.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mathurin P, Deng QG, Keshavarzian A, Choudhary S, Holmes EW, Tsukamoto H. Exacerbation of alcoholic liver injury by enteral endotoxin in rats. Hepatology. 2000;32:1008–1017. doi: 10.1053/jhep.2000.19621. [DOI] [PubMed] [Google Scholar]
- 42.Ferrier L, Bérard F, Debrauwer L, Chabo C, Langella P, Buéno L, Fioramonti J. Impairment of the intestinal barrier by ethanol involves enteric microflora and mast cell activation in rodents. Am J Pathol. 2006;168:1148–1154. doi: 10.2353/ajpath.2006.050617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Keshavarzian A, Farhadi A, Forsyth CB, Rangan J, Jakate S, Shaikh M, Banan A, Fields JZ. Evidence that chronic alcohol exposure promotes intestinal oxidative stress, intestinal hyperpermeability and endotoxemia prior to development of alcoholic steatohepatitis in rats. J Hepatol. 2009;50:538–547. doi: 10.1016/j.jhep.2008.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.French SW. Intragastric ethanol infusion model for cellular and molecular studies of alcoholic liver disease. J Biomed Sci. 2001;8:20–27. doi: 10.1007/BF02255967. [DOI] [PubMed] [Google Scholar]
- 45.Turner JR. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol. 2009;9:799–809. doi: 10.1038/nri2653. [DOI] [PubMed] [Google Scholar]
- 46.Such J, Guardiola JV, de Juan J, Casellas JA, Pascual S, Aparicio JR, Solá-Vera J, Pérez-Mateo M. Ultrastructural characteristics of distal duodenum mucosa in patients with cirrhosis. Eur J Gastroenterol Hepatol. 2002;14:371–376. doi: 10.1097/00042737-200204000-00006. [DOI] [PubMed] [Google Scholar]
- 47.Bjarnason I, Peters TJ, Wise RJ. The leaky gut of alcoholism: possible route of entry for toxic compounds. Lancet. 1984;1:179–182. doi: 10.1016/s0140-6736(84)92109-3. [DOI] [PubMed] [Google Scholar]
- 48.Rao RK. Acetaldehyde-induced increase in paracellular permeability in Caco-2 cell monolayer. Alcohol Clin Exp Res. 1998;22:1724–1730. [PubMed] [Google Scholar]
- 49.Basuroy S, Sheth P, Mansbach CM, Rao RK. Acetaldehyde disrupts tight junctions and adherens junctions in human colonic mucosa: protection by EGF and L-glutamine. Am J Physiol Gastrointest Liver Physiol. 2005;289:G367–G375. doi: 10.1152/ajpgi.00464.2004. [DOI] [PubMed] [Google Scholar]
- 50.Lichtman SN, Sartor RB, Keku J, Schwab JH. Hepatic inflammation in rats with experimental small intestinal bacterial overgrowth. Gastroenterology. 1990;98:414–423. doi: 10.1016/0016-5085(90)90833-m. [DOI] [PubMed] [Google Scholar]
- 51.Riordan SM, McIver CJ, Thomas DH, Duncombe VM, Bolin TD, Thomas MC. Luminal bacteria and small-intestinal permeability. Scand J Gastroenterol. 1997;32:556–563. doi: 10.3109/00365529709025099. [DOI] [PubMed] [Google Scholar]
- 52.Parks RW, Clements WD, Pope C, Halliday MI, Rowlands BJ, Diamond T. Bacterial translocation and gut microflora in obstructive jaundice. J Anat. 1996;189(Pt 3):561–565. [PMC free article] [PubMed] [Google Scholar]
- 53.Guarner C, Runyon BA, Young S, Heck M, Sheikh MY. Intestinal bacterial overgrowth and bacterial translocation in cirrhotic rats with ascites. J Hepatol. 1997;26:1372–1378. doi: 10.1016/s0168-8278(97)80474-6. [DOI] [PubMed] [Google Scholar]
- 54.Sánchez E, Casafont F, Guerra A, de Benito I, Pons-Romero F. Role of intestinal bacterial overgrowth and intestinal motility in bacterial translocation in experimental cirrhosis. Rev Esp Enferm Dig. 2005;97:805–814. doi: 10.4321/s1130-01082005001100005. [DOI] [PubMed] [Google Scholar]
- 55.Garcia-Tsao G, Lee FY, Barden GE, Cartun R, West AB. Bacterial translocation to mesenteric lymph nodes is increased in cirrhotic rats with ascites. Gastroenterology. 1995;108:1835–1841. doi: 10.1016/0016-5085(95)90147-7. [DOI] [PubMed] [Google Scholar]
- 56.Runyon BA, Squier S, Borzio M. Translocation of gut bacteria in rats with cirrhosis to mesenteric lymph nodes partially explains the pathogenesis of spontaneous bacterial peritonitis. J Hepatol. 1994;21:792–796. doi: 10.1016/s0168-8278(94)80241-6. [DOI] [PubMed] [Google Scholar]
- 57.Casafont Morencos F, de las Heras Castaño G, Martín Ramos L, López Arias MJ, Ledesma F, Pons Romero F. Small bowel bacterial overgrowth in patients with alcoholic cirrhosis. Dig Dis Sci. 1996;41:552–556. doi: 10.1007/BF02282340. [DOI] [PubMed] [Google Scholar]
- 58.Bode JC, Bode C, Heidelbach R, Dürr HK, Martini GA. Jejunal microflora in patients with chronic alcohol abuse. Hepatogastroenterology. 1984;31:30–34. [PubMed] [Google Scholar]
- 59.Pande C, Kumar A, Sarin SK. Small-intestinal bacterial overgrowth in cirrhosis is related to the severity of liver disease. Aliment Pharmacol Ther. 2009;29:1273–1281. doi: 10.1111/j.1365-2036.2009.03994.x. [DOI] [PubMed] [Google Scholar]
- 60.Morencos FC, de las Heras Castaño G, Martín Ramos L, López Arias MJ, Ledesma F, Pons Romero F. Small bowel bacterial overgrowth in patients with alcoholic cirrhosis. Dig Dis Sci. 1995;40:1252–1256. doi: 10.1007/BF02065533. [DOI] [PubMed] [Google Scholar]
- 61.Bauer TM, Steinbrückner B, Brinkmann FE, Ditzen AK, Schwacha H, Aponte JJ, Pelz K, Kist M, Blum HE. Small intestinal bacterial overgrowth in patients with cirrhosis: prevalence and relation with spontaneous bacterial peritonitis. Am J Gastroenterol. 2001;96:2962–2967. doi: 10.1111/j.1572-0241.2001.04668.x. [DOI] [PubMed] [Google Scholar]
- 62.Jun DW, Kim KT, Lee OY, Chae JD, Son BK, Kim SH, Jo YJ, Park YS. Association between small intestinal bacterial overgrowth and peripheral bacterial DNA in cirrhotic patients. Dig Dis Sci. 2010;55:1465–1471. doi: 10.1007/s10620-009-0870-9. [DOI] [PubMed] [Google Scholar]
- 63.Papp M, Norman GL, Vitalis Z, Tornai I, Altorjay I, Foldi I, Udvardy M, Shums Z, Dinya T, Orosz P, et al. Presence of anti-microbial antibodies in liver cirrhosis--a tell-tale sign of compromised immunity? PLoS One. 2010;5:e12957. doi: 10.1371/journal.pone.0012957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhao HY, Wang HJ, Lu Z, Xu SZ. Intestinal microflora in patients with liver cirrhosis. Chin J Dig Dis. 2004;5:64–67. doi: 10.1111/j.1443-9573.2004.00157.x. [DOI] [PubMed] [Google Scholar]
- 65.Inagaki T, Moschetta A, Lee YK, Peng L, Zhao G, Downes M, Yu RT, Shelton JM, Richardson JA, Repa JJ, et al. Regulation of antibacterial defense in the small intestine by the nuclear bile acid receptor. Proc Natl Acad Sci U S A. 2006;103:3920–3925. doi: 10.1073/pnas.0509592103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bode C, Bode JC. Effect of alcohol consumption on the gut. Best Pract Res Clin Gastroenterol. 2003;17:575–592. doi: 10.1016/s1521-6918(03)00034-9. [DOI] [PubMed] [Google Scholar]
- 67.Chang CS, Chen GH, Lien HC, Yeh HZ. Small intestine dysmotility and bacterial overgrowth in cirrhotic patients with spontaneous bacterial peritonitis. Hepatology. 1998;28:1187–1190. doi: 10.1002/hep.510280504. [DOI] [PubMed] [Google Scholar]
- 68.Madrid AM, Cumsille F, Defilippi C. Altered small bowel motility in patients with liver cirrhosis depends on severity of liver disease. Dig Dis Sci. 1997;42:738–742. doi: 10.1023/a:1018899611006. [DOI] [PubMed] [Google Scholar]
- 69.Gunnarsdottir SA, Sadik R, Shev S, Simrén M, Sjövall H, Stotzer PO, Abrahamsson H, Olsson R, Björnsson ES. Small intestinal motility disturbances and bacterial overgrowth in patients with liver cirrhosis and portal hypertension. Am J Gastroenterol. 2003;98:1362–1370. doi: 10.1111/j.1572-0241.2003.07475.x. [DOI] [PubMed] [Google Scholar]
- 70.Gupta A, Dhiman RK, Kumari S, Rana S, Agarwal R, Duseja A, Chawla Y. Role of small intestinal bacterial overgrowth and delayed gastrointestinal transit time in cirrhotic patients with minimal hepatic encephalopathy. J Hepatol. 2010;53:849–855. doi: 10.1016/j.jhep.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 71.Madrid AM, Hurtado C, Venegas M, Cumsille F, Defilippi C. Long-Term treatment with cisapride and antibiotics in liver cirrhosis: effect on small intestinal motility, bacterial overgrowth, and liver function. Am J Gastroenterol. 2001;96:1251–1255. doi: 10.1111/j.1572-0241.2001.03636.x. [DOI] [PubMed] [Google Scholar]
- 72.Shindo K, Machida M, Miyakawa K, Fukumura M. A syndrome of cirrhosis, achlorhydria, small intestinal bacterial overgrowth, and fat malabsorption. Am J Gastroenterol. 1993;88:2084–2091. [PubMed] [Google Scholar]
- 73.Bajaj JS, Zadvornova Y, Heuman DM, Hafeezullah M, Hoffmann RG, Sanyal AJ, Saeian K. Association of proton pump inhibitor therapy with spontaneous bacterial peritonitis in cirrhotic patients with ascites. Am J Gastroenterol. 2009;104:1130–1134. doi: 10.1038/ajg.2009.80. [DOI] [PubMed] [Google Scholar]
- 74.Camp JG, Kanther M, Semova I, Rawls JF. Patterns and scales in gastrointestinal microbial ecology. Gastroenterology. 2009;136:1989–2002. doi: 10.1053/j.gastro.2009.02.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yan AW, Fouts DE, Brandl J, Stärkel P, Torralba M, Schott E, Tsukamoto H, Nelson KE, Brenner DA, Schnabl B. Enteric dysbiosis associated with a mouse model of alcoholic liver disease. Hepatology. 2011;53:96–105. doi: 10.1002/hep.24018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Eckburg PB, Bik EM, Bernstein CN, Purdom E, Dethlefsen L, Sargent M, Gill SR, Nelson KE, Relman DA. Diversity of the human intestinal microbial flora. Science. 2005;308:1635–1638. doi: 10.1126/science.1110591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444:1022–1023. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- 78.Frank DN, St Amand AL, Feldman RA, Boedeker EC, Harpaz N, Pace NR. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci USA. 2007;104:13780–13785. doi: 10.1073/pnas.0706625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mutlu E, Keshavarzian A, Engen P, Forsyth CB, Sikaroodi M, Gillevet P. Intestinal dysbiosis: a possible mechanism of alcohol-induced endotoxemia and alcoholic steatohepatitis in rats. Alcohol Clin Exp Res. 2009;33:1836–1846. doi: 10.1111/j.1530-0277.2009.01022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nanji AA, Khettry U, Sadrzadeh SM. Lactobacillus feeding reduces endotoxemia and severity of experimental alcoholic liver (disease) Proc Soc Exp Biol Med. 1994;205:243–247. doi: 10.3181/00379727-205-43703. [DOI] [PubMed] [Google Scholar]
- 81.Forsyth CB, Farhadi A, Jakate SM, Tang Y, Shaikh M, Keshavarzian A. Lactobacillus GG treatment ameliorates alcohol-induced intestinal oxidative stress, gut leakiness, and liver injury in a rat model of alcoholic steatohepatitis. Alcohol. 2009;43:163–172. doi: 10.1016/j.alcohol.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Derrien M, Vaughan EE, Plugge CM, de Vos WM. Akkermansia muciniphila gen. nov., sp. nov., a human intestinal mucin-degrading bacterium. Int J Syst Evol Microbiol. 2004;54:1469–1476. doi: 10.1099/ijs.0.02873-0. [DOI] [PubMed] [Google Scholar]
- 83.Derrien M, Collado MC, Ben-Amor K, Salminen S, de Vos WM. The Mucin degrader Akkermansia muciniphila is an abundant resident of the human intestinal tract. Appl Environ Microbiol. 2008;74:1646–1648. doi: 10.1128/AEM.01226-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Collado MC, Derrien M, Isolauri E, de Vos WM, Salminen S. Intestinal integrity and Akkermansia muciniphila, a mucin-degrading member of the intestinal microbiota present in infants, adults, and the elderly. Appl Environ Microbiol. 2007;73:7767–7770. doi: 10.1128/AEM.01477-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Brandl K, Plitas G, Mihu CN, Ubeda C, Jia T, Fleisher M, Schnabl B, DeMatteo RP, Pamer EG. Vancomycin-resistant enterococci exploit antibiotic-induced innate immune deficits. Nature. 2008;455:804–807. doi: 10.1038/nature07250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gibson GR. Prebiotics as gut microflora management tools. J Clin Gastroenterol. 2008;42 Suppl 2:S75–S79. doi: 10.1097/MCG.0b013e31815ed097. [DOI] [PubMed] [Google Scholar]
- 87.Gratz SW, Mykkanen H, El-Nezami HS. Probiotics and gut health: a special focus on liver diseases. World J Gastroenterol. 2010;16:403–410. doi: 10.3748/wjg.v16.i4.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Loguercio C, Federico A, Tuccillo C, Terracciano F, D'Auria MV, De Simone C, Del Vecchio Blanco C. Beneficial effects of a probiotic VSL#3 on parameters of liver dysfunction in chronic liver diseases. J Clin Gastroenterol. 2005;39:540–543. doi: 10.1097/01.mcg.0000165671.25272.0f. [DOI] [PubMed] [Google Scholar]
- 89.Kirpich IA, Solovieva NV, Leikhter SN, Shidakova NA, Lebedeva OV, Sidorov PI, Bazhukova TA, Soloviev AG, Barve SS, McClain CJ, et al. Probiotics restore bowel flora and improve liver enzymes in human alcohol-induced liver injury: a pilot study. Alcohol. 2008;42:675–682. doi: 10.1016/j.alcohol.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Stadlbauer V, Mookerjee RP, Hodges S, Wright GA, Davies NA, Jalan R. Effect of probiotic treatment on deranged neutrophil function and cytokine responses in patients with compensated alcoholic cirrhosis. J Hepatol. 2008;48:945–951. doi: 10.1016/j.jhep.2008.02.015. [DOI] [PubMed] [Google Scholar]
- 91.Besselink MG, van Santvoort HC, Buskens E, Boermeester MA, van Goor H, Timmerman HM, Nieuwenhuijs VB, Bollen TL, van Ramshorst B, Witteman BJ, et al. Probiotic prophylaxis in predicted severe acute pancreatitis: a randomised, double-blind, placebo-controlled trial. Lancet. 2008;371:651–659. doi: 10.1016/S0140-6736(08)60207-X. [DOI] [PubMed] [Google Scholar]
- 92.Tang Y, Forsyth CB, Banan A, Fields JZ, Keshavarzian A. Oats supplementation prevents alcohol-induced gut leakiness in rats by preventing alcohol-induced oxidative tissue damage. J Pharmacol Exp Ther. 2009;329:952–958. doi: 10.1124/jpet.108.148643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Marotta F, Barreto R, Wu CC, Naito Y, Gelosa F, Lorenzetti A, Yoshioka M, Fesce E. Experimental acute alcohol pancreatitis-related liver damage and endotoxemia: synbiotics but not metronidazole have a protective effect. Chin J Dig Dis. 2005;6:193–197. doi: 10.1111/j.1443-9573.2005.00230.x. [DOI] [PubMed] [Google Scholar]