Abstract
Nitric oxide (NO), the smallest signalling molecule known, is produced by three isoforms of NO synthase (NOS; EC 1.14.13.39). They all utilize l-arginine and molecular oxygen as substrates and require the cofactors reduced nicotinamide-adenine-dinucleotide phosphate (NADPH), flavin adenine dinucleotide (FAD), flavin mononucleotide (FMN), and (6R-)5,6,7,8-tetrahydrobiopterin (BH4). All NOS bind calmodulin and contain haem. Neuronal NOS (nNOS, NOS I) is constitutively expressed in central and peripheral neurons and some other cell types. Its functions include synaptic plasticity in the central nervous system (CNS), central regulation of blood pressure, smooth muscle relaxation, and vasodilatation via peripheral nitrergic nerves. Nitrergic nerves are of particular importance in the relaxation of corpus cavernosum and penile erection. Phosphodiesterase 5 inhibitors (sildenafil, vardenafil, and tadalafil) require at least a residual nNOS activity for their action. Inducible NOS (NOS II) can be expressed in many cell types in response to lipopolysaccharide, cytokines, or other agents. Inducible NOS generates large amounts of NO that have cytostatic effects on parasitic target cells. Inducible NOS contributes to the pathophysiology of inflammatory diseases and septic shock. Endothelial NOS (eNOS, NOS III) is mostly expressed in endothelial cells. It keeps blood vessels dilated, controls blood pressure, and has numerous other vasoprotective and anti-atherosclerotic effects. Many cardiovascular risk factors lead to oxidative stress, eNOS uncoupling, and endothelial dysfunction in the vasculature. Pharmacologically, vascular oxidative stress can be reduced and eNOS functionality restored with renin- and angiotensin-converting enzyme-inhibitors, with angiotensin receptor blockers, and with statins.
Keywords: (6R-)5,6,7,8-Tetrahydrobiopterin; l-Arginine; Asymmetric dimethyl-l-arginine; NADPH oxidase; Peroxynitrite
Introduction
Nitric oxide (NO) is an unorthodox messenger molecule, which has numerous molecular targets. NO controls servoregulatory functions such as neurotransmission1,2 or vascular tone3,4 (by stimulating NO-sensitive guanylyl cyclase), regulates gene transcription5,6 and mRNA translation (e.g. by binding to iron-responsive elements),7,8 and produces post-translational modifications of proteins (e.g. by ADP ribosylation).9,10 An important mode of inactivation of NO is its reaction with superoxide anion (O2−•) to form the potent oxidant peroxynitrite (ONOO−). This compound can cause oxidative damage, nitration, and S-nitrosylation of biomolecules including proteins, lipids, and DNA.11,12 Nitrosative stress by ONOO− has been implicated in DNA single-strand breakage, followed by poly-ADP-ribose polymerase (PARP) activation.13
In mammals, NO can be generated by three different isoforms of the enzyme NO synthase (NOS; l-arginine, NADPH:oxygen oxidoreductases, NO forming; EC 1.14.13.39). The isozymes are referred to as neuronal ‘n’NOS (or NOS I), inducible ‘i’NOS (or NOS II), and endothelial ‘e’NOS (or NOS III) (Figure 1). This review will cover all three isoforms. However, a focus of the article will be on eNOS and functions of NO in the cardiovascular system.
Figure 1.
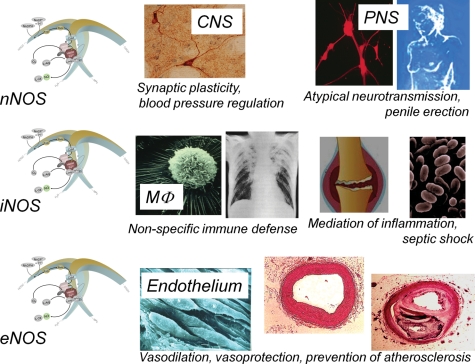
Important functions of the different NOS isoforms. (Top panel) Neuronal NOS is expressed in specific neurons of the central nervous system (CNS). It has been implicated in synaptic plasticity (i.e. phenomena such a long-term potentiation and long-term inhibition). These phenomena are involved in learning and memory formation. Neuronal NOS-derived NO also participates in central control of blood pressure. In the peripheral nervous system (PNS), neuronal NOS-derived NO acts as an atypical neurotransmitter, which mediates relaxing components of gut peristalsis, vasodilation, and penile erection. At least a minimal stimulation of soluble guanylyl cyclase in corpus cavernosum by nNOS-derived NO, and the subsequent formation of small amounts of cyclic GMP, is a prerequisite for the pro-erectile effects of the phosphodiesterase 5 inhibitors sildenafil (Viagra®), vardenafil (Levitra®), and tadalafil (Cialis®). (Middle panel) Inducible NOS expression can be induced by cytokines and other agents in almost any cell type. This had initially been shown for macrophages (MΦ). The induction of inducible NOS in MΦ is essential for the control of intracellular bacteria such as Mycobacterium tuberculosis156,157 or the parasite Leishmania.158,159 However, inducible NOS is also up-regulated in various types of inflammatory disease, and the NO generated by the enzyme mediates various symptoms of inflammation.160,161 Finally, inducible NOS-derived NO is the predominant mediator of vasodilation and fall in blood pressure seen in septic shock.161 In fact, mice with a disrupted inducible NOS gene are protected from many symptoms of septic shock.159 (Bottom panel) Endothelial NOS-derived NO is a physiological vasodilator, but can also convey vasoprotection in several ways. NO released towards the vascular lumen is a potent inhibitor of platelet aggregation and adhesion to the vascular wall. Besides protection from thrombosis, this also prevents the release of platelet-derived growth factors that stimulate smooth muscle proliferation and its production of matrix molecules. Endothelial NO also controls the expression of genes involved in atherogenesis. NO decreases the expression of chemoattractant protein MCP-1 and of a number of surface adhesion molecules, thereby preventing leucocyte adhesion to vascular endothelium and leucocyte migration into the vascular wall. This offers protection against early phases of atherogenesis. Also the decreased endothelial permeability, the reduced influx of lipoproteins into the vascular wall and the inhibition of low-density lipoprotein oxidation may contribute to the anti-atherogenic properties of endothelial NOS-derived NO. Finally, NO has been shown to inhibit DNA synthesis, mitogenesis, and proliferation of vascular smooth muscle cells as well as smooth muscle cell migration, thereby protecting against a later phase of atherogenesis. Based on the combination of those effects, NO produced in endothelial cells can be considered an anti-atherosclerotic principle (for review, see Li and Förstermann162).
Mechanisms of nitric oxide synthesis
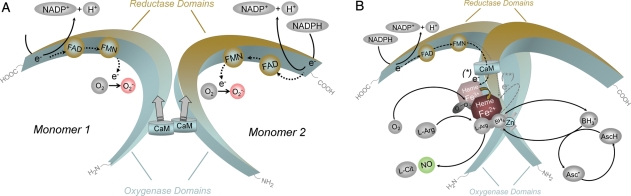
All isoforms of NOS utilize l-arginine as the substrate, and molecular oxygen and reduced nicotinamide-adenine-dinucleotide phosphate (NADPH) as co-substrates. Flavin adenine dinucleotide (FAD), flavin mononucleotide (FMN), and (6R-)5,6,7,8-tetrahydro-l-biopterin (BH4) are cofactors of all isozymes. All NOS proteins are homodimers (Figure 2). A functional NOS transfers electrons from NADPH, via the flavins FAD and FMN in the carboxy-terminal reductase domain, to the haem in the amino-terminal oxygenase domain. The oxygenase domain also binds the essential cofactor BH4, molecular oxygen, and the substrate l-arginine14,15 (Figure 2). At the haem site, the electrons are used to reduce and activate O2 and to oxidize l-arginine to l-citrulline and NO (Figure 2). Sequences located near the cysteine ligand of the haem are also apparently involved in l-arginine and BH4 binding.16 In order to synthesize NO, the NOS enzyme goes through two steps. In a first step, NOS hydroxylates l-arginine to Nω-hydroxy-l-arginine (which remains largely bound to the enzyme). In a second step, NOS oxidizes Nω-hydroxy-l-arginine to l-citrulline and NO.17,18 All isoforms of NOS bind calmodulin (Figure 2). In nNOS and eNOS, calmodulin binding is brought about by an increase in intracellular Ca2+ (half-maximal activity between 200 and 400 nM). When calmodulin affinity to NOS increases, it facilitates the flow of electrons from NADPH in the reductase domain to the haem in the oxygenase domain. In inducible NOS (iNOS), calmodulin already binds at extremely low intracellular Ca2+ concentrations (below 40 nM) due to a different amino acid structure of the calmodulin-binding site.19,20 All NOS proteins contain a zinc–thiolate cluster formed by a zinc ion that is tetrahedrally coordinated to two CysXXXXCys motifs (one contributed by each monomer) at the NOS dimer interface.21–23 Zinc in NOS has a structural rather than a catalytic function.21
Figure 2.
Structure and catalytic mechanisms of functional NOS. (A) NOS monomers are capable of transferring electrons from reduced nicotinamide-adenine-dinucleotide phosphate (NADPH), to flavin-adenine-dinucleotide (FAD) and flavin-mononucleotide (FMN) and have a limited capacity to reduce molecular oxygen to superoxide (O2−•).18,163,164 Monomers and isolated reductase domains can bind calmodulin (CaM), which enhances electron transfer within the reductase domain.165 NOS monomers are unable to bind the cofactor (6R-)5,6,7,8-tetrahydrobiopterin (BH4) or the substrate l-arginine and cannot catalyze NO production.163,166 (B) In the presence of haem, NOS can form a functional dimer.163,166 Haem is essential for the interdomain electron transfer from the flavins to the haem of the opposite monomer.165,167 Due to differences in the calmodulin-binding domain, elevated Ca2+ is required for calmodulin binding (and thus catalytic activity) in nNOS and eNOS, whereas calmodulin binds to inducible NOS with high affinity even in the absence of Ca2+. When sufficient substrate l-arginine (l-Arg) and cofactor BH4 are present, intact NOS dimers couple their haem and O2 reduction to the synthesis of NO (fully functional NOS). l-Citrulline (l-Cit) is formed as the byproduct. For clarity, the flow of electrons is only shown from the reductase domain of one monomer to the oxygenase domain of the other monomer. NOS enzymes perform two separate oxidation steps, one to form Nω-hydroxy-l-arginine and a second to convert this intermediate to NO.18 All NOS isoforms contain a zinc ion (Zn) coordinated in a tetrahedral conformation with pairs of CXXXXC motifs at the dimer interface. This site is of major importance for the binding of BH4 and l-arginine. Electron transfer from the reductase domain (*) enables NOS ferric (Fe3+) haem to bind O2 and form a ferrous (Fe2+)-dioxy species. This species may receive a second electron (**) preferentially from BH4 or from the reductase domain. The nature of the resulting oxidized BH4 has been identified as the trihydrobiopterin radical (BH3•) or the trihydropterin radical cation protonated at N5 (BH3•H+). The BH3• radical (or radical cation) can be recycled to BH4 by the NOS itself (using an electron supplied by the flavins). Alternatively, there is evidence that reducing agents such as ascorbic acid (AscH, which is present in cells in millimolar concentrations) can reduce the BH3• radical back to BH4103 (Asc·, ascorbate radical).
The NO formed by NOS can act on a number of target enzymes and proteins. The most important physiological signalling pathway stimulated by NO is the activation of soluble guanylyl cyclase and the generation of cyclic GMP.3,4,24–26
Neuronal nitric oxide synthase
Neuronal NOS is constitutively expressed in specific neurons of the brain (Figure 1). Enzyme activity is regulated by Ca2+ and calmodulin. Brain nNOS is found in particulate and soluble forms in cells and the differential subcellular localization of nNOS may contribute to its diverse functions. Neuronal NOS contains a PDZ domain and can interact directly with the PDZ domains of other proteins. These interactions determine the subcellular distribution and the activity of the enzyme.27 In addition to brain tissue, nNOS has been identified by immunohistochemistry in the spinal cord, in the sympathetic ganglia and adrenal glands, in peripheral nitrergic nerves, in epithelial cells of various organs, in kidney macula densa cells, in pancreatic islet cells, and in the vascular smooth muscle.28 In mammalians, the largest source of nNOS in terms of tissue mass is the skeletal muscle.28,29
Physiological functions of neuronal nitric oxide synthase
In the past years, an increasing number of reports have confirmed the significance of nNOS in a variety of synaptic signalling events. Neuronal NOS has been implicated in modulating physiological functions such as learning, memory, and neurogenesis.27 In the central nervous system (CNS), nNOS mediates long-term regulation of synaptic transmission (long-term potentiation, long-term inhibition),1,2,30,31 whereas there is no evidence for an involvement of nNOS-derived NO in acute neurotransmission (Figure 1). Retrograde communication across synaptic junctions is presumed to be involved in memory formation, and there is evidence that inhibitors of NOS impair learning and produce amnesia in animal models.32,33 There is also evidence that NO formed in the CNS by nNOS is involved in the central regulation of blood pressure34–36 (Figure 1). Blockade of nNOS activity in the medulla and hypothalamus causes systemic hypertension.37
In the periphery, many smooth muscle tissues are innervated by nitrergic nerves, i.e. nerves that contain nNOS and generate and release NO (Figure 1). Nitric oxide produced by nNOS in nitrergic nerves can be viewed as an unusual neurotransmitter that stimulates NO-sensitive guanylyl cyclase in its effector cells, thereby decreasing the tone of various types of smooth muscle including blood vessels.28,38 The conventional notion that eNOS is mostly responsible for the regulation of vascular tone in the periphery (see later in this article) has been challenged by a human study with S-methyl-l-thiocitrulline (SMTC), a selective inhibitor of nNOS. S-Methyl-l-thiocitrulline reduces basal blood flow in the human forearm and in the coronary circulation. This effect can be reversed by l-arginine. Interestingly, SMTC does not affect classical eNOS-mediated vasodilatation in response to acetylcholine, substance P, or fluid shear stress. These data are coherent with the notion that nNOS plays an important role in the regulation of vascular tone, independent of effects of nNOS in the CNS. Thus, eNOS and nNOS may have distinct roles in the physiological regulation of human microvascular tone in vivo.39 Interestingly, vascular smooth muscle cells also express low levels of nNOS, which have been shown to maintain some degree of vasodilation, when the predominant eNOS becomes dysfunctional.40
By mediating the relaxation of the corpus cavernosum smooth muscle, nNOS-containing nitrergic nerves are responsible for penile erection41,42 (Figure 1). Also in the corpus cavernosum, NO-induced smooth muscle relaxation is mediated by cyclic GMP.42 Cyclic GMP is degraded by phosphodiesterases. The predominant isoform in the corpus cavernosum is isoform 5.43 Thus, a residual nNOS activity is essential for the proerectile effect of selective phosphodiesterase 5 inhibitors such as sildenafil (Viagra®), vardenafil (Levitra®), and tadalafil (Cialis®).43,44 Interestingly, because phosphodiesterase 5 is also significantly expressed in pulmonary arteries, sildenafil (under the trade name Revatio®) and tadalafil (under the trade name Adcirca®) have also been approved for the treatment of pulmonary arterial hypertension.
Role of neuronal nitric oxide synthase in pathophysiology
Abnormal NO signalling is likely to contribute to a variety of neurodegenerative pathologies such as excitotoxicity following stroke, multiple sclerosis, Alzheimer's, and Parkinson's diseases.45 Hyperactive nNOS, stimulated by massive Ca2+ influx into neuronal cells, has been implicated in N-methyl-d-aspartate receptor-mediated neuronal death in cerebrovascular stroke.46 Under those conditions, NO can contribute to excitotoxicity, probably via peroxynitrite activation of PARP and/or mitochondrial permeability transition. High levels of NO can also produce energy depletion, due to inhibition of mitochondrial respiration and inhibition of glycolysis.47
Some disturbances of smooth muscle tone within the gastrointestinal tract (e.g. gastro-oesophageal reflux disease) may also be related to an overproduction of NO by nNOS in peripheral nitrergic nerves.48,49
Inducible nitric oxide synthase
Inducible NOS is not usually expressed in cells, but its expression can be induced by bacterial lipopolysaccharide, cytokines, and other agents. Although primarily identified in macrophages (Figure 1), expression of the enzyme can be stimulated in virtually any cell or tissue, provided that the appropriate inducing agents have been identified.28,38 Once expressed, iNOS is constantly active and not regulated by intracellular Ca2+ concentrations.
Physiological functions of inducible nitric oxide synthase
Inducible NOS, when induced in macrophages, produces large amounts of NO, which represent a major cytotoxic principle of those cells.50 Due to its affinity to protein-bound iron, NO can inhibit key enzymes that contain iron in their catalytic centres. These include iron–sulfur cluster-dependent enzymes (complexes I and II) involved in mitochondrial electron transport, ribonucleotide reductase (the rate-limiting enzyme in DNA replication), and cis-aconitase (a key enzyme in the citric acid cycle).50 In addition, higher concentrations of NO, as produced by induced macrophages, can directly interfere with the DNA of target cells and cause strand breaks and fragmentation.51,52 A combination of these effects is likely to form the basis of the cytostatic and cytotoxic effects of NO on parasitic microorganisms and certain tumour cells (Figure 1). Interestingly, non-immune cells can also be induced with cytokines to release amounts of NO large enough to affect the neighbouring cells. Cytokine-activated endothelial cells, for example, have been shown to lyse tumour cells,53 and induced hepatocytes can use NO to kill malaria sporozoites.54 Inducible NOS activity is likely to be responsible for all of these effects.
Role of inducible nitric oxide synthase in pathophysiology
The high levels of NO produced by activated macrophages (and probably neutrophils and other cells) may not only be toxic to undesired microbes, parasites, or tumour cells, but—when released at the wrong site—may also harm healthy cells. In vivo, cell and tissue damage can be related to the NO radical itself or an interaction of NO with O2−• leading to the formation of peroxynitrite (ONOO−). The large majority of inflammatory and autoimmune lesions are characterized by an abundance of activated macrophages and neutrophils. Significant amounts of NO can be secreted by those cells, leading to damage of the surrounding tissue52,55 (Figure 1). Inducible NOS-derived NO is also likely to be involved in non-specific allograft rejection.56
Inflammatory neurodegeneration contributes to a number of brain pathologies. Mechanisms by which activated microglia and astrocytes kill neurons have been identified in cell culture. These mechanisms include the activation of the phagocyte NADPH oxidase in microglia and expression of iNOS in glia. This combination produces apoptosis via ONOO− production. Inducible NOS-derived NO also synergizes with hypoxia to induce neuronal death because NO inhibits cytochrome oxidase. This can result in glutamate release and excitotoxicity57,58 (see the Role of neuronal nitric oxide synthase in pathophysiology section on nNOS and excitotoxicity).
Lastly, excessive NO production by iNOS plays a crucial role in septic shock (Figure 1). This condition is characterized by massive arteriolar vasodilatation, hypotension, and microvascular damage. Bacterial endotoxins usually initiate the symptoms. A number of mediators such as platelet-activating factor, thromboxane A2, prostanoids, and cytokines such as interleukin-1, tumour necrosis factor-α, and interferon-γ are elevated in septic shock and have been implicated in its pathophysiology. However, the fall in blood pressure is predominantly due to excess NO production by iNOS induced in the vascular wall.59,60
Endothelial nitric oxide synthase
Endothelial NOS is mostly expressed in endothelial cells (Figure 1). However, the isozyme has also been detected in cardiac myocytes, platelets, certain neurons of the brain, in syncytio-trophoblasts of the human placenta and in LLC-PK1 kidney tubular epithelial cells.28,38
Similar to nNOS, Ca2+-activated calmodulin is important for the regulation of eNOS activity. Endothelial NOS synthesizes NO in a pulsatile manner with eNOS activity markedly increasing when intracellular Ca2+ rises. Ca2+ induces the binding of calmodulin to the enzyme.20 However, several other proteins also interact with eNOS and regulate its activity. For example, heat shock protein 90 (hsp90) has been found associated with eNOS and serves as an allosteric modulator activating the enzyme61 and promoting eNOS (re)coupling62,63 (see later in the text). The fraction of eNOS that is localized in caveolae64 can interact with the caveolae coat protein, caveolin-1. Caveolin-1 is a tonic inhibitor of eNOS activity. This concept has been proven genetically because blood vessels from caveolin-1-deficient mice show enhanced endothelium-dependent relaxations.65 Mechanistically, the recruitment of calmodulin and hsp90 to eNOS can displace caveolin-1 from the enzyme thereby leading to enzyme activation.66
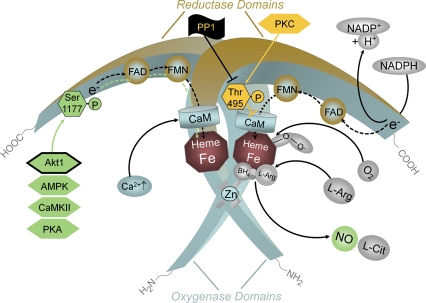
However, eNOS can also be activated by stimuli that do not produce sustained increases in intracellular Ca2+, but still induce a long-lasting release of NO. The best established such stimulus is fluid shear stress. This activation is mediated by phosphorylation of the enzyme.67,68 The eNOS protein can be phosphorylated on several serine (Ser), threonine (Thr), and tyrosine (Tyr) residues. Phosphorylation of Ser1177 stimulates the flux of electrons within the reductase domain, increases the Ca2+ sensitivity of the enzyme, and represents an additional and independent mechanism of eNOS activation.68,69 Oestrogen and vascular endothelial growth factor (VEGF) phosphorylate eNOS mainly via the Ser/Thr kinase Akt, insulin probably activates both Akt and the AMP-activated protein kinase (AMPK), the bradykinin-induced phosphorylation of Ser1177 is mediated by Ca2+/calmodulin-dependent protein kinase II (CaMKII), and shear stress elicits phosphorylation mainly by activating protein kinase A (PKA) (Figure 3). Recent evidence using Akt1-deficient mice carrying knock-in mutations of the critical Akt1 phosphorylation site on eNOS has proven that kinase Akt1 is a critical regulator of eNOS function also in vivo.70 Ser1176 is the Akt1 phosphorylation site in the mouse that corresponds to Ser1177 in the human species. The phosphomimetic mutation Ser1176Asp rendered the enzyme constitutively active, whereas the mutation Ser1176Ala reduced enzyme activity.70 Thus, although all the kinases mentioned can regulate eNOS Ser1177 in vitro, Akt1 is the only kinase proven to regulate eNOS function in vivo.
Figure 3.
Regulation of endothelial NOS activity by intracellular Ca2+ and phosphorylation. An increase in intracellular Ca2+ leads to an enhanced binding of calmodulin (CaM) to the enzyme, which in turn displaces an auto-inhibitory loop and facilitates the flow of electrons from NADPH in the reductase domain to the haem in the oxygenase domain. Established functionally important phosphorylation sites in human endothelial NOS are Ser1177 and Thr495. In resting endothelial cells, Ser1177 is usually not phosphorylated. Phosphorylation is induced when the cells are exposed to oestrogens, vascular endothelial growth factor (VEGF), insulin, bradykinin or fluid shear stress. The kinases responsible for phosphorylation (green hexagons) depend on the primary stimulus. Oestrogen and vascular endothelial growth factor elicit phosphorylation of Ser1177 by activating serine/threonine kinase Akt. So far, Akt1 is the only kinase proven to regulate endothelial NOS function in vivo (framed green hexagon). Insulin probably activates both Akt and the AMP-activated protein kinase (AMPK), the bradykinin-induced phosphorylation of Ser1177 is mediated by Ca2+/calmodulin-dependent protein kinase II (CaMKII), and shear stress leads to phosphorylation of endothelial NOS mainly via protein kinase A (PKA). Phosphorylation of the Ser1177 residue increases the flux of electrons through the reductase domain and thus enzyme activity. The Thr495 residue of human endothelial NOS tends to be constitutively phosphorylated in endothelial cells. Thr495 is a negative regulatory site, and its phosphorylation is associated with a decreased electron flux and enzyme activity. The constitutively active kinase that phosphorylates endothelial NOS Thr495 is most probably protein kinase C (PKC, yellow hexagon). Phosphorylation of Thr495 reduces endothelial NOS activity (yellow block arrow). The phosphatase that dephosphorylates Thr495 appears to be protein phosphatase1 (PP1, black flag with black block arrow).
Thr495 tends to be phosphorylated under non-stimulated conditions (most probably by protein kinase C). Phosphorylation of Thr495 is likely to interfere with the binding of calmodulin to the calmodulin-binding domain. In fact, dephosphorylation of Thr495 is associated with stimuli that elevate intracellular Ca2+ concentrations and increase eNOS activity. Substantially more calmodulin binds to eNOS when Thr495 is dephosphorylated.68 However, dephosphorylation of Thr495 has also been shown to favour eNOS uncoupling (see below).71
Other phosphorylation sites of human eNOS include Ser114, Ser633, Tyr81, and Tyr657 residues. Phosphorylation of these residues is an intensively studied area and may have important consequences for enzyme activity as recently reviewed.72
Physiological functions of endothelial nitric oxide synthase
Vasodilation and inhibition of platelet aggregation and adhesion
Endothelial NOS appears to be a homeostatic regulator of numerous essential cardiovascular functions. Endothelial NOS-derived NO dilates all types of blood vessels by stimulating soluble guanylyl cyclase and increasing cyclic GMP in smooth muscle cells.3,4,73 Deletion of the eNOS gene leads to elevated blood pressure.74,75 Nitric oxide released towards the vascular lumen is a potent inhibitor of platelet aggregation and adhesion to the vascular wall.76–78 Besides protection from thrombosis, this also prevents the release of platelet-derived growth factors that stimulate smooth muscle proliferation and its production of matrix molecules. Endothelial NOS is also critical for adaptive vascular remodelling to chronic changes in flow.79
Inhibition of leucocyte adhesion and vascular inflammation
Endothelial NO controls the expression of genes involved in atherogenesis. Nitric oxide decreases the expression of chemoattractant protein MCP-1.80 Nitric oxide can also inhibit leucocyte adhesion to the vessel wall by either interfering with the ability of the leucocyte adhesion molecule CD11/CD18 to bind to the endothelial cell surface or by suppressing CD11/CD18 expression on leucocytes.81,82 Leucocyte adherence is an early event in the development of atherosclerosis, and therefore, NO may protect against the onset of atherogenesis.
A disturbed integrity of the endothelial monolayer barrier can initiate proinflammatory events. Endothelium-derived NO prevents endothelial cell apoptosis induced by proinflammatory cytokines and proatherosclerotic factors including reactive oxygen species (ROS) and angiotensin II (AT). The suppression of apoptosis may also contribute to the antiinflammatory and anti-atherosclerotic effects of endothelium-derived NO.83
Control of vascular smooth muscle proliferation
Furthermore, NO has been shown to inhibit DNA synthesis, mitogenesis, and proliferation of vascular smooth muscle cells.84–87 These antiproliferative effects are likely to be mediated by cyclic GMP.84,85,88 The inhibition of platelet aggregation and adhesion protects smooth muscle from exposure to platelet-derived growth factor(s). Therefore, NO also prevents a later step in atherogenesis, fibrous plaque formation. Based on the combination of those effects, NO produced in endothelial cells can be considered an anti-atherosclerotic principle89 (Figure 1).
Stimulation of angiogenesis by endothellial nitric oxide synthase-derived NO
Endothelial NOS-derived NO plays a critical role in post-natal angiogenesis, mediating signals downstream of angiogenic factors. Recent findings in eNOS-deficient mice point to a novel and previously unrecognized role of NO in foetal lung development and lung morphogenesis. The lung phenotype of eNOS-deficient mice closely resembles alveolar capillary dysplasia in humans, a form of malignant pulmonary hypertension of the newborn that presents with defective lung vascular development and respiratory distress.90 Similarly, eNOS had been found to be critical for collateral formation and angiogenesis post-ischaemia.91 Furthermore, the positive effects of NO on endothelial cell survival are likely to also contribute to the pro-angiogenic effects of NO.83
Activation of endothelial progenitor cells by endothelial nitric oxide synthase-derived nitric oxide
Mice with a deleted eNOS gene show an impaired neovascularization. This was related to a defect in progenitor cell mobilization. Mobilization of endothelial progenitor cells by VEGF is reduced in eNOS-deficient mice. In a model of hind-limb ischaemia in these mice, intravenous infusion of wild-type progenitor cells, but not bone marrow transplantation, can rescue the defective neovascularization. This suggests that mobilization of progenitor cells from the bone marrow is impaired in eNOS-deficient mice. Indeed, matrix metalloproteinase-9, which is required for stem cell mobilization, was reduced in the bone marrow of eNOS-deficient mice. Thus, eNOS expressed by bone marrow stromal cells influences recruitment of stem and progenitor cells. Reduced systemic NO bioactivity seen in ischaemic heart disease may therefore contribute to impaired neovascularization.92
Also in patients with ischaemic cardiomyopathy, bone marrow mononuclear cells show a reduced neovascularization capacity in vivo. As mentioned above, NO plays an important role in neovascularization and NO bioavailability is typically reduced in patients with ischaemic heart disease. Pre-treatment of bone marrow cells from these patients with the enhancer of eNOS expression and activity 4-fluoro-N-indan-2-yl-benzamide (AVE9488)93 significantly increased eNOS expression and activity.94 This is associated with an enhanced migratory capacity of the bone marrow cells in vitro and improved neovascularization capacity of these cells in a mouse ischaemic hind-limb model in vivo. This improved limb perfusion by AVE9488-treated bone marrow cells was NO mediated because it was abrogated by pre-treatment of the cells with the eNOS inhibitor NG-nitro-l-arginine methyl ester. Also, compound AVE9488 showed no effect on the impaired migratory capacity of bone marrow cells from eNOS-deficient mice. Thus, pharmacological enhancement of eNOS expression and activity at least partially reverses the impaired functional activity of bone marrow cells from patients with ischaemic cardiomyopathy.94 Similarly, the eNOS stimulator simvastatin (see below) enhanced the number of functionally active endothelial progenitor cells in patients with myocardial infarction.95
Gene therapy with nitric oxide synthase
Gene therapy refers to the transfer of a specific gene to the host tissue to intervene in a disease process, with resultant alleviation of the symptoms. Nitric oxide synthase gene therapy has been the focus of numerous studies as dysfunction of this enzyme has been implicated in several types of cardiovascular diseases. Research has concentrated on effects of gene delivery of NOS isoforms (eNOS, iNOS, or nNOS) in animal models of vascular tone, ischaemia–reperfusion injury, intimal hyperplasia, and restenosis. In many pre-clinical models of cardiovascular disease, vascular gene delivery proved to be therapeutically beneficial. Endothelial NOS appears particularly promising as it inhibits intimal hyperplasia and enhances reendothelialization in injured blood vessels. The obvious long-term goal is to translate the benefits of NOS gene therapy seen in animal models into clinical practice. However, further work is required along this way to improve delivery systems and to minimize negative side effects.96,97
Role of endothelial nitric oxide synthase in pathophysiology
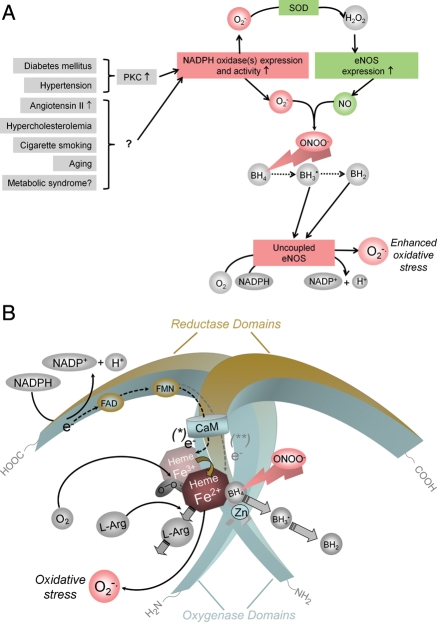
Patients with cardiovascular risk factors (such as hypertension, hypercholesterolaemia, diabetes mellitus, cigarette smoking, etc.) and patients with vascular disease show endothelial dysfunction, i.e. the inability of the endothelium to generate adequate amounts of bioactive NO (and to produce NO-mediated vasodilation). Cardiovascular risk factors and vascular disease are also associated with an increased production of ROS. There are several enzyme systems that can potentially produce ROS in the vessel wall. These include the NADPH oxidases, xanthine oxidase, enzymes of the mitochondrial respiratory chain, and uncoupled eNOS (see below).98 Of these, NADPH oxidases are of primary importance for ROS generation (Figure 4A). Several isoforms of O2−•-producing NADPH oxidase exist in the vascular wall. They are expressed in endothelial and smooth muscle cells, as well as in the adventitia.
Figure 4.
Potential mechanisms by which cardiovascular risk factors lead to oxidative stress and endothelial NOS uncoupling. (A) In many types of vascular disease, NADPH oxidases are up-regulated in the vascular wall and generate superoxide (O2−•). In experimental diabetes mellitus and angiotensin II-induced hypertension, this has been shown to be mediated by protein kinase C (PKC).168,169 Expression of endothelial NOS is also increased in vascular disease. H2O2, the dismutation product of O2−• can increase endothelial NOS expression via transcriptional and post-transcriptional mechanisms (SOD, superoxide dismutase).170 In addition, also protein kinase C activation can enhance endothelial NOS expression,171 and protein kinase C inhibitors reduce endothelial NOS expression levels in vascular disease.169 The products of NADPH oxidases and endothelial NOS, O2−• and NO·, rapidly recombine to form peroxynitrite (ONOO−). This can oxidize the essential cofactor of endothelial NOS (6R-)5,6,7,8-tetrahydrobiopterin (BH4) to trihydrobiopterin radical (BH3•).172,173 BH3• can disproportionate to the quinonoid 6,7-[8H]-H2-biopterin (BH2). As a consequence, oxygen reduction and O2 reduction by endothelial NOS are uncoupled from NO· formation, and a functional NOS is converted into a dysfunctional O2−•-generating enzyme that contributes to vascular oxidative stress. The enhanced endothelial NOS expression (see above) aggravates the situation. (B) Oxidation of BH4 to biologically inactive products such as the BH3• radical or BH2 also reduces the affinity of the substrate l-arginine (l-Arg) to NO, and NOS catalyzes the uncoupled reduction in O2, leading to the production of O2−• (and possibly also H2O2).
Molecular basis of endothelial dysfunction in vascular disease: inactivation of bioactive nitric oxide and endothelial nitric oxide synthase uncoupling
Due to the enhanced oxidative stress seen in vascular disease, an increased degradation of NO by its reaction with O2−• will occur. However, oxidative stress has also been shown to convert eNOS from an NO-producing enzyme to an enzyme that generates O2−•. This process has been referred to as NOS uncoupling (Figure 4B). Mechanisms implicated in eNOS uncoupling include oxidation of the critical NOS cofactor BH4, depletion of l-arginine, and accumulation of endogenous methylarginines. More recently, S-glutathionylation of eNOS has been proposed as yet another mechanism leading to eNOS uncoupling.
The (6R-)5,6,7,8-tetrahydrobiopterin hypothesis
As detailed above, a functional eNOS transfers electrons from NADPH, via the flavins FAD and FMN to the haem, where the substrate l-arginine is oxidized to l-citrulline and NO.99 The reaction product of NO and O2−•, ONOO−, can uncouple oxygen reduction from NO generation in NOS. Oxidation or removal of the essential cofactor BH499–101 or oxidative damage of the zinc–thiolate cluster involved in BH4 and l-arginine binding102 may be the cause of eNOS uncoupling in this situation (Figure 4A and B). ONOO− can oxidize BH4 to the biologically inactive BH3• radical that can disproportionate to the quinonoid 6,7-[8H]-H2-biopterin.103,104 It has been shown that NO production by eNOS correlates closely with the intracellular concentration of BH4,105,106 and BH4 levels have been found decreased in many models of cardiovascular disease107–109,101 and in patients with endothelial dysfunction.110–112
l-Arginine supply of endothelial nitric oxide synthase and endothelial nitric oxide synthase uncoupling
In animal and human pathophysiology (hypercholesterolaemia and hypertension), l-arginine supplementation can improve endothelial dysfunction.113–116 Normal l-arginine plasma concentrations are ∼100 µmol/L. Even in pathophysiology, they hardly fall below 60 µmol/L, and there is an up to 10-fold accumulation of l-arginine within cells.117 On the other hand, the Km of eNOS for l-arginine is only ∼3 µmol/L.118 Also, human endothelial cells are not even dependent on l-arginine uptake from the extracellular space; they can effectively recycle l-citrulline to l-arginine and can also obtain l-arginine from proteolysis.119,120
However, endothelial cells express arginases that can compete with eNOS for substrate and, if highly expressed, ‘starve’ eNOS.121–124 A relative l-arginine deficiency in the vicinity of eNOS caused by excessive arginase activity is conceivable and could explain part of the beneficial effects of l-arginine supplementation. Also non-substrate effects of l-arginine can contribute to these effects. These include potential direct radical-scavenging properties of the guanidino nitrogen group, the cooperativity between l-arginine and BH4-binding sites of NOS,125,126 or the competition of l-arginine with asymmetric dimethyl-l-arginine (ADMA).127
Asymmetrical dimethyl-l-arginine and endothelial nitric oxide synthase uncoupling
Asymmetric dimethyl-l-arginine is considered a risk factor for all-cause cardiovascular mortality.128 Asymmetric dimethyl-l-arginine is an endogenous inhibitor of eNOS, but elevated ADMA has also been associated with eNOS uncoupling.129 The activities (not the expression) of the key enzyme for ADMA production, protein arginine N-methyltransferase (PRMT, type I),130 and of the ADMA-degrading enzyme dimethylarginine dimethylaminohydrolase (DDAH)131 are redox-sensitive. In various models, oxidative stress has been shown to increase the activity of PRMT(s) and decrease that of DDAH, thereby leading to increased ADMA concentrations.127,130,131 Thus, an increased production of ROS could trigger increased ADMA levels.
S-Glutathionylation of endothelial nitric oxide synthase: yet another mechanism leading to endothelial nitric oxide synthase uncoupling
In several disease conditions associated with oxidative stress (see above), BH4 supplementation only partly restores eNOS functionality. Cysteine residues are important for eNOS function. Protein thiols can be subject to S-glutathionylation, a protein modification involved in cell signalling. Conditions of oxidative stress promote S-glutathionylation of proteins. S-Glutathionylation of eNOS reversibly decreases NO production and increases O2−• generation primarily from the reductase domain. Two highly conserved cysteine residues in the reductase domain have been identified as sites of S-glutathionylation.132 Endothelial NOS S-glutathionylation in endothelial cells goes along with an impaired endothelium-dependent vasodilation. In blood vessels from hypertensive animals, eNOS S-glutathionylation is increased and endothelium-mediated vasodilation is reduced. That condition is reversed by thiol-specific reducing agents, which reverse S-glutathionylation. Thus, S-glutathionylation of eNOS is likely to represent an additional mechanism involved in eNOS uncoupling.132,133
Pleiotropic actions of conventional cardiovascular drugs that improve endothelial function
Drugs interfering with the renin–angiotensin–aldosterone system and statins (3-hydroxy-3-methylgultaryl-coenzyme A reductase inhibitors) are able to prevent endothelial dysfunction and eNOS uncoupling.
Drugs interfering with the renin–angiotensin–aldosterone system
Several components of the renin–angiotensin–aldosterone system are up-regulated in atherosclerotic vessels. Angiotensin II and aldosterone both promote endothelial dysfunction.134 Angiotensin II activates NADPH oxidases via AT1 stimulation.135 In addition, the AT1 receptor is up-regulated in vitro by low-density lipoprotein.136
In Watanabe heritable hyperlipidaemic rabbits, the renin inhibitor aliskiren increases eNOS expression, enhances eNOS phosphorylation at Ser1177 (thereby increasing activity), decreases NADPH oxidase expression, augments vascular BH4 levels, and restores eNOS uncoupling.137
Angiotensin-converting enzyme-inhibitors and AT1 receptor blockers have indirect antioxidant effects by preventing the activation of NADPH oxidase.138–141 In addition, they can also increase the activity of extracellular superoxide dismutase (SOD3).142 Angiotensin-converting enzyme-inhibitors significantly reduce cardiovascular events in patients with established coronary artery disease or at high risk for the disease.143 The AT1 receptor blocker losartan restores glomerular NO production by increasing protein expression of GTP cyclohydrolase1 (the rate-limiting enzyme for BH4 synthesis) and elevating BH4 levels in diabetic rats.144
The selective aldosterone antagonist eplerenone and enalapril reduce NADPH oxidase activity, elevate vascular BH4 levels, and enhance eNOS expression and NO bioavailability. Eplerenone also increases eNOS phosphorylation at Ser1177. Both drugs decrease atherosclerotic plaque formation.134
These pleiotropic effects of compounds interfering with the renin–angiotensin–aldosterone system may contribute significantly to the therapeutic benefit of such drugs.
Statins
The cholesterol-lowering statins have additional cholesterol-independent or pleiotropic effects in cardiovascular disease.145 These properties include the improvement of endothelial function, stabilization of atherosclerotic plaques, inhibition of oxidative stress and inflammation, and reduction in thrombogenic responses.146 These effects of statins are, in part, mediated by an effect on eNOS, because they can be inhibited by eNOS inhibitors147 and are absent in eNOS-deficient mice.95
Statins increase the expression of eNOS,148 but also enhance eNOS activity by decreasing caveolin abundance149 and by activation of the phosphatidylinositol 3-kinase/Akt pathway.150
Several statins inhibit endothelial O2−• formation by reducing the expression and/or activity of NADPH oxidase and by preventing the isoprenylation of p21 Rac, which is critical for a functional NADPH oxidase.151 In addition, SOD3 activity is more than doubled by simvastatin.
Statins have also been shown to increase GTP cyclohydrolase1 mRNA expression in endothelial cells and to elevate intracellular BH4 levels.152 Atorvastatin has been shown to normalize endothelial function and reduces oxidative stress by inhibiting vascular NADPH oxidases and preventing eNOS uncoupling by an up-regulation of GTP cyclohydrolase1.153 Together, these effects may contribute to the anti-atherogenic action of statins.154,155
Conclusions
All three NOS isozymes have regulatory functions in the cardiovascular system. Neuronal NOS is involved in central regulation of blood pressure, and nNOS-containing (nitrergic) nerves can dilate certain vascular beds. Nitrergic nerves are of particular importance in the relaxation of corpus cavernosum and penile erection. Phosphodiesterase 5 inhibitors require at least a residual nNOS activity for their action. Inducible NOS is found expressed in atherosclerotic plaque and is an important mediator of the fall in blood pressure in septic shock. The most important isoform is eNOS, which keeps blood vessels dilated, controls blood pressure, and has numerous other vasoprotective and anti-atherosclerotic effects. Although there is no evidence that eNOS is a ‘disease gene’, many cardiovascular risk factors lead to oxidative stress, eNOS uncoupling, and endothelial dysfunction in the vasculature. Drugs interfering with the renin–angiotensin–aldosterone system as well as statins are useful in preventing endothelial dysfunction. Further elucidation of how these therapeutic agents promote eNOS coupling, in face of elevated oxidative stress, may yield insights into other potential avenues leading to the beneficial actions of NO in the cardiovascular system.
Funding
Original work from our laboratories contributing to this review was supported by the Integrated Research and Treatment Center ‘Thrombosis and Hemostasis’ of the German Federal Ministry of Education and Research (BMBF), by grant LI-1042/1-1 from the German Research Foundation (Deutsche Forschungsgemeinschaft), and by grants R01 HL64793, R01 HL61371, R01 HL081190, R01 HL096670, and P01 HL70295 from the National Institutes of Health, USA.
Conflict of interest: none declared.
References
- 1.O'Dell TJ, Hawkins RD, Kandel ER, Arancio O. Tests of the roles of two diffusible substances in long-term potentiation: evidence for nitric oxide as a possible early retrograde messenger. Proc Natl Acad Sci USA. 1991;88:11285–11289. doi: 10.1073/pnas.88.24.11285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schuman EM, Madison DV. A requirement for the intercellular messenger nitric oxide in long-term potentiation. Science. 1991;254:1503–1506. doi: 10.1126/science.1720572. [DOI] [PubMed] [Google Scholar]
- 3.Rapoport RM, Draznin MB, Murad F. Endothelium-dependent relaxation in rat aorta may be mediated through cyclic GMP-dependent protein phosphorylation. Nature. 1983;306:174–176. doi: 10.1038/306174a0. [DOI] [PubMed] [Google Scholar]
- 4.Förstermann U, Mülsch A, Böhme E, Busse R. Stimulation of soluble guanylate cyclase by an acetylcholine-induced endothelium-derived factor from rabbit and canine arteries. Circ Res. 1986;58:531–538. doi: 10.1161/01.res.58.4.531. [DOI] [PubMed] [Google Scholar]
- 5.Khan BV, Harrison DG, Olbrych MT, Alexander RW, Medford RM. Nitric oxide regulates vascular cell adhesion molecule 1 gene expression and redox-sensitive transcriptional events in human vascular endothelial cells. Proc Natl Acad Sci USA. 1996;93:9114–9119. doi: 10.1073/pnas.93.17.9114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gudi T, Hong GK, Vaandrager AB, Lohmann SM, Pilz RB. Nitric oxide and cGMP regulate gene expression in neuronal and glial cells by activating type II cGMP-dependent protein kinase. FASEB J. 1999;13:2143–2152. [PubMed] [Google Scholar]
- 7.Pantopoulos K, Hentze MW. Nitric oxide signaling to iron-regulatory protein: direct control of ferritin mRNA translation and transferrin receptor mRNA stability in transfected fibroblasts. Proc Natl Acad Sci USA. 1995;92:1267–1271. doi: 10.1073/pnas.92.5.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu XB, Hill P, Haile DJ. Role of the ferroportin iron-responsive element in iron and nitric oxide dependent gene regulation. Blood Cells Mol Dis. 2002;29:315–326. doi: 10.1006/bcmd.2002.0572. [DOI] [PubMed] [Google Scholar]
- 9.Pozdnyakov N, Lloyd A, Reddy VN, Sitaramayya A. Nitric oxide-regulated endogenous ADP-ribosylation of rod outer segment proteins. Biochem Biophys Res Commun. 1993;192:610–615. doi: 10.1006/bbrc.1993.1459. [DOI] [PubMed] [Google Scholar]
- 10.Brune B, Dimmeler S, Molina y Vedia L, Lapetina EG. Nitric oxide: a signal for ADP-ribosylation of proteins. Life Sci. 1994;54:61–70. doi: 10.1016/0024-3205(94)00775-6. [DOI] [PubMed] [Google Scholar]
- 11.Mikkelsen RB, Wardman P. Biological chemistry of reactive oxygen and nitrogen and radiation-induced signal transduction mechanisms. Oncogene. 2003;22:5734–5754. doi: 10.1038/sj.onc.1206663. [DOI] [PubMed] [Google Scholar]
- 12.Lee JH, Yang ES, Park JW. Inactivation of NADP+-dependent isocitrate dehydrogenase by peroxynitrite. Implications for cytotoxicity and alcohol-induced liver injury. J Biol Chem. 2003;278:51360–51371. doi: 10.1074/jbc.M302332200. [DOI] [PubMed] [Google Scholar]
- 13.Ridnour LA, Thomas DD, Mancardi D, Espey MG, Miranda KM, Paolocci N, Feelisch M, Fukuto J, Wink DA. The chemistry of nitrosative stress induced by nitric oxide and reactive nitrogen oxide species. Putting perspective on stressful biological situations. Biol Chem. 2004;385:1–10. doi: 10.1515/BC.2004.001. [DOI] [PubMed] [Google Scholar]
- 14.Crane BR, Arvai AS, Ghosh DK, Wu C, Getzoff ED, Stuehr DJ, Tainer JA. Structure of nitric oxide synthase oxygenase dimer with pterin and substrate. Science. 1998;279:2121–2126. doi: 10.1126/science.279.5359.2121. [DOI] [PubMed] [Google Scholar]
- 15.Alderton WK, Cooper CE, Knowles RG. Nitric oxide synthases: structure, function and inhibition. Biochem J. 2001;357:593–615. doi: 10.1042/0264-6021:3570593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nishimura JS, Martasek P, McMillan K, Salerno J, Liu Q, Gross SS, Masters BS. Modular structure of neuronal nitric oxide synthase: localization of the arginine binding site and modulation by pterin. Biochem Biophys Res Commun. 1995;210:288–294. doi: 10.1006/bbrc.1995.1659. [DOI] [PubMed] [Google Scholar]
- 17.Noble MA, Munro AW, Rivers SL, Robledo L, Daff SN, Yellowlees LJ, Shimizu T, Sagami I, Guillemette JG, Chapman SK. Potentiometric analysis of the flavin cofactors of neuronal nitric oxide synthase. Biochemistry. 1999;38:16413–16418. doi: 10.1021/bi992150w. [DOI] [PubMed] [Google Scholar]
- 18.Stuehr D, Pou S, Rosen GM. Oxygen reduction by nitric-oxide synthases. J Biol Chem. 2001;276:14533–14536. doi: 10.1074/jbc.R100011200. [DOI] [PubMed] [Google Scholar]
- 19.Cho HJ, Xie QW, Calaycay J, Mumford RA, Swiderek KM, Lee TD, Nathan C. Calmodulin is a subunit of nitric oxide synthase from macrophages. J Exp Med. 1992;176:599–604. doi: 10.1084/jem.176.2.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hemmens B, Mayer B. Enzymology of nitric oxide synthases. Methods Mol Biol. 1998;100:1–32. doi: 10.1385/1-59259-749-1:1. [DOI] [PubMed] [Google Scholar]
- 21.Hemmens B, Goessler W, Schmidt K, Mayer B. Role of bound zinc in dimer stabilization but not enzyme activity of neuronal nitric-oxide synthase. J Biol Chem. 2000;275:35786–35791. doi: 10.1074/jbc.M005976200. [DOI] [PubMed] [Google Scholar]
- 22.Raman CS, Li H, Martasek P, Kral V, Masters BS, Poulos TL. Crystal structure of constitutive endothelial nitric oxide synthase: a paradigm for pterin function involving a novel metal center. Cell. 1998;95:939–950. doi: 10.1016/s0092-8674(00)81718-3. [DOI] [PubMed] [Google Scholar]
- 23.Li H, Raman CS, Glaser CB, Blasko E, Young TA, Parkinson JF, Whitlow M, Poulos TL. Crystal structures of zinc-free and -bound heme domain of human inducible nitric-oxide synthase. Implications for dimer stability and comparison with endothelial nitric-oxide synthase. J Biol Chem. 1999;274:21276–21284. doi: 10.1074/jbc.274.30.21276. [DOI] [PubMed] [Google Scholar]
- 24.Furchgott RF, Cherry PD, Zawadzki JV, Jothianandan D. Endothelial cells as mediators of vasodilation of arteries. J Cardiovasc Pharmacol. 1984;53:557–573. doi: 10.1097/00005344-198406002-00008. [DOI] [PubMed] [Google Scholar]
- 25.Knowles RG, Palacios M, Palmer RM, Moncada S. Formation of nitric oxide from l-arginine in the central nervous system: a transduction mechanism for stimulation of the soluble guanylate cyclase. Proc Natl Acad Sci USA. 1989;86:5159–5162. doi: 10.1073/pnas.86.13.5159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garthwaite J. Glutamate, nitric oxide and cell–cell signalling in the nervous system. Trends Neurosci. 1991;14:60–67. doi: 10.1016/0166-2236(91)90022-m. [DOI] [PubMed] [Google Scholar]
- 27.Zhou L, Zhu DY. Neuronal nitric oxide synthase: structure, subcellular localization, regulation, and clinical implications. Nitric Oxide. 2009;20:223–230. doi: 10.1016/j.niox.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 28.Förstermann U, Closs EI, Pollock JS, Nakane M, Schwarz P, Gath I, Kleinert H. Nitric oxide synthase isozymes. Characterization, purification, molecular cloning, and functions. Hypertension. 1994;23:1121–1131. doi: 10.1161/01.hyp.23.6.1121. [DOI] [PubMed] [Google Scholar]
- 29.Nakane M, Schmidt HH, Pollock JS, Förstermann U, Murad F. Cloned human brain nitric oxide synthase is highly expressed in skeletal muscle. FEBS Lett. 1993;316:175–180. doi: 10.1016/0014-5793(93)81210-q. [DOI] [PubMed] [Google Scholar]
- 30.Izumi Y, Clifford DB, Zorumski CF. Inhibition of long-term potentiation by NMDA-mediated nitric oxide release. Science. 1992;257:1273–1276. doi: 10.1126/science.1519065. [DOI] [PubMed] [Google Scholar]
- 31.Izumi Y, Zorumski CF. Nitric oxide and long-term synaptic depression in the rat hippocampus. Neuroreport. 1993;4:1131–1134. [PubMed] [Google Scholar]
- 32.Holscher C, Rose SP. An inhibitor of nitric oxide synthesis prevents memory formation in the chick. Neurosci Lett. 1992;145:165–167. doi: 10.1016/0304-3940(92)90012-v. [DOI] [PubMed] [Google Scholar]
- 33.Bohme GA, Bon C, Lemaire M, Reibaud M, Piot O, Stutzmann JM, Doble A, Blanchard JC. Altered synaptic plasticity and memory formation in nitric oxide synthase inhibitor-treated rats. Proc Natl Acad Sci USA. 1993;90:9191–9194. doi: 10.1073/pnas.90.19.9191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Togashi H, Sakuma I, Yoshioka M, Kobayashi T, Yasuda H, Kitabatake A, Saito H, Gross SS, Levi R. A central nervous system action of nitric oxide in blood pressure regulation. J Pharmacol Exp Ther. 1992;262:343–347. [PubMed] [Google Scholar]
- 35.Sakuma I, Togashi H, Yoshioka M, Saito H, Yanagida M, Tamura M, Kobayashi T, Yasuda H, Gross SS, Levi R. NG-methyl-l-arginine, an inhibitor of l-arginine-derived nitric oxide synthesis, stimulates renal sympathetic nerve activity in vivo. A role for nitric oxide in the central regulation of sympathetic tone? Circ Res. 1992;70:607–611. doi: 10.1161/01.res.70.3.607. [DOI] [PubMed] [Google Scholar]
- 36.Elkarib AO, Sheng JJ, Betz AL, Malvin RL. The central effects of a nitric oxide synthase inhibitor (N-omega-nitro-l-arginine) on blood pressure and plasma renin. Clin Exp Hypertension. 1993;15:819–832. doi: 10.3109/10641969309041644. [DOI] [PubMed] [Google Scholar]
- 37.Toda N, Ayajiki K, Okamura T. Control of systemic and pulmonary blood pressure by nitric oxide formed through neuronal nitric oxide synthase. J Hypertens. 2009;27:1929–1940. doi: 10.1097/HJH.0b013e32832e8ddf. [DOI] [PubMed] [Google Scholar]
- 38.Förstermann U. Regulation of nitric oxide synthase expression and activity. In: Mayer B, editor. Handbook of Experimental Pharmacology—Nitric Oxide. Berlin: Springer; 2000. pp. 71–91. [Google Scholar]
- 39.Melikian N, Seddon MD, Casadei B, Chowienczyk PJ, Shah AM. Neuronal nitric oxide synthase and human vascular regulation. Trends Cardiovasc Med. 2009;19:256–262. doi: 10.1016/j.tcm.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schwarz PM, Kleinert H, Förstermann U. Potential functional significance of brain-type and muscle-type nitric oxide synthase I expressed in adventitia and media of rat aorta. Arterioscler Thromb Vasc Biol. 1999;19:2584–2590. doi: 10.1161/01.atv.19.11.2584. [DOI] [PubMed] [Google Scholar]
- 41.Kim N, Azadzoi KM, Goldstein I, Saenz de Tejada I. A nitric oxide-like factor mediates nonadrenergic-noncholinergic neurogenic relaxation of penile corpus cavernosum smooth muscle. J Clin Invest. 1991;88:112–118. doi: 10.1172/JCI115266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rajfer J, Aronson WJ, Bush PA, Dorey FJ, Ignarro LJ. Nitric oxide as a mediator of relaxation of the corpus cavernosum in response to nonadrenergic, noncholinergic neurotransmission. N Engl J Med. 1992;326:90–94. doi: 10.1056/NEJM199201093260203. [DOI] [PubMed] [Google Scholar]
- 43.Turko IV, Ballard SA, Francis SH, Corbin JD. Inhibition of cyclic GMP-binding cyclic GMP-specific phosphodiesterase (Type 5) by sildenafil and related compounds. Mol Pharmacol. 1999;56:124–130. doi: 10.1124/mol.56.1.124. [DOI] [PubMed] [Google Scholar]
- 44.Rosen RC, Kostis JB. Overview of phosphodiesterase 5 inhibition in erectile dysfunction. Am J Cardiol. 2003;92:9M–18M. doi: 10.1016/s0002-9149(03)00824-5. [DOI] [PubMed] [Google Scholar]
- 45.Steinert JR, Chernova T, Forsythe ID. Nitric oxide signaling in brain function, dysfunction, and dementia. Neuroscientist. 2010;16:435–452. doi: 10.1177/1073858410366481. [DOI] [PubMed] [Google Scholar]
- 46.Lipton SA, Choi YB, Pan ZH, Lei SZ, Chen HS, Sucher NJ, Loscalzo J, Singel DJ, Stamler JS. A redox-based mechanism for the neuroprotective and neurodestructive effects of nitric oxide and related nitroso-compounds. Nature. 1993;364:626–632. doi: 10.1038/364626a0. [DOI] [PubMed] [Google Scholar]
- 47.Brown GC. Nitric oxide and neuronal death. Nitric Oxide. 2010;23:153–165. doi: 10.1016/j.niox.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 48.Tøttrup A, Svane D, Forman A. Nitric oxide mediating NANC inhibition in opossum lower esophageal sphincter. Am J Physiol. 1991;260:G385–G389. doi: 10.1152/ajpgi.1991.260.3.G385. [DOI] [PubMed] [Google Scholar]
- 49.Lefebvre RA. Pharmacological characterization of the nitrergic innervation of the stomach. Verh K Acad Geneeskd Belg. 2002;64:151–166. [PubMed] [Google Scholar]
- 50.Nathan CF, Hibbs JB. Role of nitric oxide synthesis in macrophage antimicrobial activity. Curr Opin Immunol. 1991;3:65–70. doi: 10.1016/0952-7915(91)90079-g. [DOI] [PubMed] [Google Scholar]
- 51.Wink DA, Kasprzak KS, Maragos CM, Elespuru RK, Misra M, Dunams TM, Cebula TA, Koch WH, Andrews AW, Allen JS, Keefer JK. DNA deaminating ability and genotoxicity of nitric oxide and its progenitors. Science. 1991;254:1001–1003. doi: 10.1126/science.1948068. [DOI] [PubMed] [Google Scholar]
- 52.Fehsel K, Jalowy A, Qi S, Burkart V, Hartmann B, Kolb H. Islet cell DNA is a target of inflammatory attack by nitric oxide. Diabetes. 1993;42:496–500. doi: 10.2337/diab.42.3.496. [DOI] [PubMed] [Google Scholar]
- 53.Li LM, Kilbourn RG, Adams J, Fidler IJ. Role of nitric oxide in lysis of tumor cells by cytokine-activated endothelial cells. Cancer Res. 1991;51:2531–2535. [PubMed] [Google Scholar]
- 54.Green SJ, Mellouk S, Hoffman SL, Meltzer MS, Nacy CA. Cellular mechanisms of nonspecific immunity to intracellular infection: cytokine-induced synthesis of toxic nitrogen oxides from l-arginine by macrophages and hepatocytes. Immunol Lett. 1990;25:15–19. doi: 10.1016/0165-2478(90)90083-3. [DOI] [PubMed] [Google Scholar]
- 55.Kröncke KD, Kolb-Bachofen V, Berschick B, Burkart V, Kolb H. Activated macrophages kill pancreatic syngeneic islet cells via arginine-dependent nitric oxide generation. Biochem Biophys Res Commun. 1991;175:752–758. doi: 10.1016/0006-291x(91)91630-u. [DOI] [PubMed] [Google Scholar]
- 56.Langrehr JM, Hoffman RA, Billiar TR, Lee KK, Schraut WH, Simmons RL. Nitric oxide synthesis in the in vivo allograft response: a possible regulatory mechanism. Surgery. 1991;110:335–342. [PubMed] [Google Scholar]
- 57.Kanwar JR, Kanwar RK, Burrow H, Baratchi S. Recent advances on the roles of NO in cancer and chronic inflammatory disorders. Curr Med Chem. 2009;16:2373–2394. doi: 10.2174/092986709788682155. [DOI] [PubMed] [Google Scholar]
- 58.Brown GC, Neher JJ. Inflammatory neurodegeneration and mechanisms of microglial killing of neurons. Mol Neurobiol. 2010;41:242–247. doi: 10.1007/s12035-010-8105-9. [DOI] [PubMed] [Google Scholar]
- 59.MacMicking JD, Nathan C, Hom G, Chartrain N, Fletcher DS, Trumbauer M, Stevens K, Xie QW, Sokol K, Hutchinson N, et al. Altered responses to bacterial infection and endotoxic shock in mice lacking inducible nitric oxide synthase. Cell. 1995;81:641–650. doi: 10.1016/0092-8674(95)90085-3. [DOI] [PubMed] [Google Scholar]
- 60.Lange M, Enkhbaatar P, Nakano Y, Traber DL. Role of nitric oxide in shock: the large animal perspective. Front Biosci. 2009;14:1979–1989. doi: 10.2741/3357. [DOI] [PubMed] [Google Scholar]
- 61.Garcia-Cardena G, Fan R, Shah V, Sorrentino R, Cirino G, Papapetropoulos A, Sessa WC. Dynamic activation of endothelial nitric oxide synthase by Hsp90. Nature. 1998;392:821–824. doi: 10.1038/33934. [DOI] [PubMed] [Google Scholar]
- 62.Pritchard KA, Jr, Ackerman AW, Gross ER, Stepp DW, Shi Y, Fontana JT, Baker JE, Sessa WC. Heat shock protein 90 mediates the balance of nitric oxide and superoxide anion from endothelial nitric-oxide synthase. J Biol Chem. 2001;276:17621–17624. doi: 10.1074/jbc.C100084200. [DOI] [PubMed] [Google Scholar]
- 63.Song Y, Cardounel AJ, Zweier JL, Xia Y. Inhibition of superoxide generation from neuronal nitric oxide synthase by heat shock protein 90: implications in NOS regulation. Biochemistry. 2002;41:10616–10622. doi: 10.1021/bi026060u. [DOI] [PubMed] [Google Scholar]
- 64.Sowa G, Pypaert M, Sessa WC. Distinction between signaling mechanisms in lipid rafts vs. caveolae. Proc Natl Acad Sci USA. 2001;98:14072–14077. doi: 10.1073/pnas.241409998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Drab M, Verkade P, Elger M, Kasper M, Lohn M, Lauterbach B, Menne J, Lindschau C, Mende F, Luft FC, Schedl A, Haller H, Kurzchalia TV. Loss of caveolae, vascular dysfunction, and pulmonary defects in caveolin-1 gene-disrupted mice. Science. 2001;293:2449–2452. doi: 10.1126/science.1062688. [DOI] [PubMed] [Google Scholar]
- 66.Gratton JP, Fontana J, O'Connor DS, Garcia-Cardena G, McCabe TJ, Sessa WC. Reconstitution of an endothelial nitric-oxide synthase (eNOS), hsp90, and caveolin-1 complex in vitro. Evidence that hsp90 facilitates calmodulin stimulated displacement of eNOS from caveolin-1. J Biol Chem. 2000;275:22268–22272. doi: 10.1074/jbc.M001644200. [DOI] [PubMed] [Google Scholar]
- 67.Fulton D, Gratton JP, McCabe TJ, Fontana J, Fujio Y, Walsh K, Franke TF, Papapetropoulos A, Sessa WC. Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature. 1999;399:597–601. doi: 10.1038/21218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fleming I, Busse R. Molecular mechanisms involved in the regulation of the endothelial nitric oxide synthase. Am J Physiol Regul Integr Comp Physiol. 2003;284:R1–R12. doi: 10.1152/ajpregu.00323.2002. [DOI] [PubMed] [Google Scholar]
- 69.McCabe TJ, Fulton D, Roman LJ, Sessa WC. Enhanced electron flux and reduced calmodulin dissociation may explain ‘calcium-independent’ eNOS activation by phosphorylation. J Biol Chem. 2000;275:6123–6128. doi: 10.1074/jbc.275.9.6123. [DOI] [PubMed] [Google Scholar]
- 70.Schleicher M, Yu J, Murata T, Derakhshan B, Atochin D, Qian L, Kashiwagi S, Di Lorenzo A, Harrison KD, Huang PL, Sessa WC. The Akt1-eNOS axis illustrates the specificity of kinase-substrate relationships in vivo. Sci Signal. 2009;2:ra41. doi: 10.1126/scisignal.2000343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lin MI, Fulton D, Babbitt R, Fleming I, Busse R, Pritchard KA, Jr, Sessa WC. Phosphorylation of threonine 497 in endothelial nitric-oxide synthase coordinates the coupling of l-arginine metabolism to efficient nitric oxide production. J Biol Chem. 2003;278:44719–44726. doi: 10.1074/jbc.M302836200. [DOI] [PubMed] [Google Scholar]
- 72.Fleming I. Molecular mechanisms underlying the activation of eNOS. Pflugers Arch. 2010;459:793–806. doi: 10.1007/s00424-009-0767-7. [DOI] [PubMed] [Google Scholar]
- 73.Ignarro LJ, Harbison RG, Wood KS, Kadowitz PJ. Activation of purified soluble guanylate cyclase by endothelium-derived relaxing factor from intrapulmonary artery and vein: stimulation by acetylcholine, bradykinin and arachidonic acid. J Pharmacol Exp Ther. 1986;237:893–900. [PubMed] [Google Scholar]
- 74.Shesely EG, Maeda N, Kim HS, Desai KM, Krege JH, Laubach VE, Sherman PA, Sessa WC, Smithies O. Elevated blood pressures in mice lacking endothelial nitric oxide synthase. Proc Natl Acad Sci USA. 1996;93:13176–13181. doi: 10.1073/pnas.93.23.13176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Huang PL, Huang Z, Mashimo H, Bloch KD, Moskowitz MA, Bevan JA, Fishman MC. Hypertension in mice lacking the gene for endothelial nitric oxide synthase. Nature. 1995;377:239–242. doi: 10.1038/377239a0. [DOI] [PubMed] [Google Scholar]
- 76.Alheid U, Frölich JC, Förstermann U. Endothelium-derived relaxing factor from cultured human endothelial cells inhibits aggregation of human platelets. Thromb Res. 1987;47:561–571. doi: 10.1016/0049-3848(87)90361-6. [DOI] [PubMed] [Google Scholar]
- 77.Busse R, Luckhoff A, Bassenge E. Endothelium-derived relaxant factor inhibits platelet activation. Naunyn Schmiedebergs Arch Pharmacol. 1987;336:566–571. doi: 10.1007/BF00169315. [DOI] [PubMed] [Google Scholar]
- 78.Radomski MW, Palmer RM, Moncada S. The anti-aggregating properties of vascular endothelium: interactions between prostacyclin and nitric oxide. Br J Pharmacol. 1987;92:639–646. doi: 10.1111/j.1476-5381.1987.tb11367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rudic RD, Shesely EG, Maeda N, Smithies O, Segal SS, Sessa WC. Direct evidence for the importance of endothelium-derived nitric oxide in vascular remodeling. J Clin Invest. 1998;101:731–736. doi: 10.1172/JCI1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zeiher AM, Fisslthaler B, Schray-Utz B, Busse R. Nitric oxide modulates the expression of monocyte chemoattractant protein 1 in cultured human endothelial cells. Circ Res. 1995;76:980–986. doi: 10.1161/01.res.76.6.980. [DOI] [PubMed] [Google Scholar]
- 81.Arndt H, Smith CW, Granger DN. Leukocyte-endothelial cell adhesion in spontaneously hypertensive and normotensive rats. Hypertension. 1993;21:667–673. doi: 10.1161/01.hyp.21.5.667. [DOI] [PubMed] [Google Scholar]
- 82.Kubes P, Suzuki M, Granger DN. Nitric oxide: an endogenous modulator of leukocyte adhesion. Proc Natl Acad Sci USA. 1991;88:4651–4655. doi: 10.1073/pnas.88.11.4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dimmeler S, Zeiher AM. Nitric oxide—an endothelial cell survival factor. Cell Death Differ. 1999;6:964–968. doi: 10.1038/sj.cdd.4400581. [DOI] [PubMed] [Google Scholar]
- 84.Garg UC, Hassid A. Nitric oxide-generating vasodilators and 8-bromo-cyclic guanosine monophosphate inhibit mitogenesis and proliferation of cultured rat vascular smooth muscle cells. J Clin Invest. 1989;83:1774–1777. doi: 10.1172/JCI114081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nakaki T, Nakayama M, Kato R. Inhibition by nitric oxide and nitric oxide-producing vasodilators of DNA synthesis in vascular smooth muscle cells. Eur J Pharmacol. 1990;189:347–353. doi: 10.1016/0922-4106(90)90031-r. [DOI] [PubMed] [Google Scholar]
- 86.Nunokawa Y, Tanaka S. Interferon-gamma inhibits proliferation of rat vascular smooth muscle cells by nitric oxide generation. Biochem Biophys Res Commun. 1992;188:409–415. doi: 10.1016/0006-291x(92)92400-r. [DOI] [PubMed] [Google Scholar]
- 87.Hogan M, Cerami A, Bucala R. Advanced glycosylation endproducts block the antiproliferative effect of nitric oxide. Role in the vascular and renal complications of diabetes mellitus. J Clin Invest. 1992;90:1110–1115. doi: 10.1172/JCI115928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Southgate K, Newby AC. Serum-induced proliferation of rabbit aortic smooth muscle cells from the contractile state is inhibited by 8-Br-cAMP but not 8-Br-cGMP. Atherosclerosis. 1990;82:113–123. doi: 10.1016/0021-9150(90)90150-h. [DOI] [PubMed] [Google Scholar]
- 89.Förstermann U. Oxidative stress in vascular disease: causes, defense mechanisms and potential therapies. Nat Clin Pract Cardiovasc Med. 2008;5:338–349. doi: 10.1038/ncpcardio1211. [DOI] [PubMed] [Google Scholar]
- 90.Han RN, Stewart DJ. Defective lung vascular development in endothelial nitric oxide synthase-deficient mice. Trends Cardiovasc Med. 2006;16:29–34. doi: 10.1016/j.tcm.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 91.Murohara T, Asahara T, Silver M, Bauters C, Masuda H, Kalka C, Kearney M, Chen D, Symes JF, Fishman MC, Huang PL, Isner JM. Nitric oxide synthase modulates angiogenesis in response to tissue ischemia. J Clin Invest. 1998;101:2567–2578. doi: 10.1172/JCI1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Aicher A, Heeschen C, Mildner-Rihm C, Urbich C, Ihling C, Technau-Ihling K, Zeiher AM, Dimmeler S. Essential role of endothelial nitric oxide synthase for mobilization of stem and progenitor cells. Nat Med. 2003;9:1370–1376. doi: 10.1038/nm948. [DOI] [PubMed] [Google Scholar]
- 93.Wohlfart P, Xu H, Endlich A, Habermeier A, Closs EI, Hubschle T, Mang C, Strobel H, Suzuki T, Kleinert H, Förstermann U, Ruetten H, Li H. Antiatherosclerotic effects of small-molecular-weight compounds enhancing endothelial nitric-oxide synthase (eNOS) expression and preventing eNOS uncoupling. J Pharmacol Exp Ther. 2008;325:370–379. doi: 10.1124/jpet.107.128009. [DOI] [PubMed] [Google Scholar]
- 94.Sasaki K, Heeschen C, Aicher A, Ziebart T, Honold J, Urbich C, Rossig L, Koehl U, Koyanagi M, Mohamed A, Brandes RP, Martin H, Zeiher AM, Dimmeler S. Ex vivo pretreatment of bone marrow mononuclear cells with endothelial NO synthase enhancer AVE9488 enhances their functional activity for cell therapy. Proc Natl Acad Sci USA. 2006;103:14537–14541. doi: 10.1073/pnas.0604144103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Landmesser U, Engberding N, Bahlmann FH, Schaefer A, Wiencke A, Heineke A, Spiekermann S, Hilfiker-Kleiner D, Templin C, Kotlarz D, Mueller M, Fuchs M, Hornig B, Haller H, Drexler H. Statin-induced improvement of endothelial progenitor cell mobilization, myocardial neovascularization, left ventricular function, and survival after experimental myocardial infarction requires endothelial nitric oxide synthase. Circulation. 2004;110:1933–1939. doi: 10.1161/01.CIR.0000143232.67642.7A. [DOI] [PubMed] [Google Scholar]
- 96.Chen AF, Ren J, Miao CY. Nitric oxide synthase gene therapy for cardiovascular disease. Jpn J Pharmacol. 2002;89:327–336. doi: 10.1254/jjp.89.327. [DOI] [PubMed] [Google Scholar]
- 97.O'Connor DM, O'Brien T. Nitric oxide synthase gene therapy: progress and prospects. Expert Opin Biol Ther. 2009;9:867–878. doi: 10.1517/14712590903002047. [DOI] [PubMed] [Google Scholar]
- 98.Mueller CF, Laude K, McNally JS, Harrison DG. Redox mechanisms in blood vessels. Arterioscler Thromb Vasc Biol. 2005;25:274–278. doi: 10.1161/01.ATV.0000149143.04821.eb. [DOI] [PubMed] [Google Scholar]
- 99.Vasquez-Vivar J, Kalyanaraman B, Martasek P, Hogg N, Masters BS, Karoui H, Tordo P, Pritchard KA., Jr Superoxide generation by endothelial nitric oxide synthase: the influence of cofactors. Proc Natl Acad Sci USA. 1998;95:9220–9225. doi: 10.1073/pnas.95.16.9220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Milstien S, Katusic Z. Oxidation of tetrahydrobiopterin by peroxynitrite: implications for vascular endothelial function. Biochem Biophys Res Commun. 1999;263:681–684. doi: 10.1006/bbrc.1999.1422. [DOI] [PubMed] [Google Scholar]
- 101.Landmesser U, Dikalov S, Price SR, McCann L, Fukai T, Holland SM, Mitch WE, Harrison DG. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J Clin Invest. 2003;111:1201–1209. doi: 10.1172/JCI14172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zou MH, Shi C, Cohen RA. Oxidation of the zinc–thiolate complex and uncoupling of endothelial nitric oxide synthase by peroxynitrite. J Clin Invest. 2002;109:817–826. doi: 10.1172/JCI14442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Werner ER, Gorren AC, Heller R, Werner-Felmayer G, Mayer B. Tetrahydrobiopterin and nitric oxide: mechanistic and pharmacological aspects. Exp Biol Med. 2003;228:1291–1302. doi: 10.1177/153537020322801108. [DOI] [PubMed] [Google Scholar]
- 104.Heller R, Werner-Felmayer G, Werner ER. Antioxidants and endothelial nitric oxide synthesis. Eur J Clin Pharmacol. 2006;62(Suppl. 13)):21–28. [Google Scholar]
- 105.Werner-Felmayer G, Werner ER, Fuchs D, Hausen A, Reibnegger G, Schmidt K, Weiss G, Wachter H. Pteridine biosynthesis in human endothelial cells. Impact on nitric oxide-mediated formation of cyclic GMP. J Biol Chem. 1993;268:1842–1846. [PubMed] [Google Scholar]
- 106.Rosenkranz-Weiss P, Sessa WC, Milstien S, Kaufman S, Watson CA, Pober JS. Regulation of nitric oxide synthesis by proinflammatory cytokines in human umbilical vein endothelial cells. Elevations in tetrahydrobiopterin levels enhance endothelial nitric oxide synthase specific activity. J Clin Invest. 1994;93:2236–2243. doi: 10.1172/JCI117221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Shinozaki K, Kashiwagi A, Nishio Y, Okamura T, Yoshida Y, Masada M, Toda N, Kikkawa R. Abnormal biopterin metabolism is a major cause of impaired endothelium-dependent relaxation through nitric oxide/O2− imbalance in insulin-resistant rat aorta. Diabetes. 1999;48:2437–2445. doi: 10.2337/diabetes.48.12.2437. [DOI] [PubMed] [Google Scholar]
- 108.Hong H-J, Hsiao G, Cheng T-H, Yen M-H. Supplemention with tetrahydrobiopterin suppresses the development of hypertension in spontaneously hypertensive rats. Hypertension. 2001;38:1044–1048. doi: 10.1161/hy1101.095331. [DOI] [PubMed] [Google Scholar]
- 109.Laursen JB, Somers M, Kurz S, McCann L, Warnholtz A, Freeman BA, Tarpey M, Fukai T, Harrison DG. Endothelial regulation of vasomotion in apoE-deficient mice: implications for interactions between peroxynitrite and tetrahydrobiopterin. Circulation. 2001;103:1282–1288. doi: 10.1161/01.cir.103.9.1282. [DOI] [PubMed] [Google Scholar]
- 110.Stroes E, Kastelein J, Cosentino F, Erkelens W, Wever R, Koomans H, Luscher T, Rabelink T. Tetrahydrobiopterin restores endothelial function in hypercholesterolemia. J Clin Invest. 1997;99:41–46. doi: 10.1172/JCI119131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Heitzer T, Krohn K, Albers S, Meinertz T. Tetrahydrobiopterin improves endothelium-dependent vasodilation by increasing nitric oxide activity in patients with Type II diabetes mellitus. Diabetologia. 2000;43:1435–1438. doi: 10.1007/s001250051551. [DOI] [PubMed] [Google Scholar]
- 112.Higashi Y, Sasaki S, Nakagawa K, Fukuda Y, Matsuura H, Oshima T, Chayama K. Tetrahydrobiopterin enhances forearm vascular response to acetylcholine in both normotensive and hypertensive individuals. Am J Hypertens. 2002;15:326–332. doi: 10.1016/s0895-7061(01)02317-2. [DOI] [PubMed] [Google Scholar]
- 113.Hishikawa K, Nakaki T, Suzuki H, Kato R, Saruta T. Role of l-arginine nitric oxide pathway in hypertension. J Hypertens. 1993;11:639–645. doi: 10.1097/00004872-199306000-00008. [DOI] [PubMed] [Google Scholar]
- 114.Imaizumi T, Hirooka Y, Masaki H, Harada S, Momohara M, Tagawa T, Takeshita A. Effects of l-arginine on forearm vessels and responses to acetylcholine. Hypertension. 1992;20:511–517. doi: 10.1161/01.hyp.20.4.511. [DOI] [PubMed] [Google Scholar]
- 115.Drexler H, Zeiher AM, Meinzer K, Just H. Correction of endothelial dysfunction in coronary microcirculation of hypercholesterolaemic patients by l-arginine. Lancet. 1991;338:1546–1550. doi: 10.1016/0140-6736(91)92372-9. [DOI] [PubMed] [Google Scholar]
- 116.Rossitch E, Alexander E, Black PM, Cooke JP. l-Arginine normalizes endothelial function in cerebral vessels from hypercholesterolemic rabbits. J Clin Invest. 1991;87:1295–1299. doi: 10.1172/JCI115132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Closs EI, Scheld JS, Sharafi M, Förstermann U. Substrate supply for nitric-oxide synthase in macrophages and endothelial cells: role of cationic amino acid transporters. Mol Pharmacol. 2000;57:68–74. [PubMed] [Google Scholar]
- 118.Pollock JS, Förstermann U, Mitchell JA, Warner TD, Schmidt HH, Nakane M, Murad F. Purification and characterization of particulate endothelium-derived relaxing factor synthase from cultured and native bovine aortic endothelial cells. Proc Natl Acad Sci USA. 1991;88:10480–10484. doi: 10.1073/pnas.88.23.10480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Simon A, Plies L, Habermeier A, Martine U, Reining M, Closs EI. Role of neutral amino acid transport and protein breakdown for substrate supply of nitric oxide synthase in human endothelial cells. Circ Res. 2003;93:813–820. doi: 10.1161/01.RES.0000097761.19223.0D. [DOI] [PubMed] [Google Scholar]
- 120.Hecker M, Sessa WC, Harris HJ, Anggard EE, Vane JR. The metabolism of l-arginine and its significance for the biosynthesis of endothelium-derived relaxing factor: cultured endothelial cells recycle l-citrulline to l-arginine. Proc Natl Acad Sci USA. 1990;87:8612–8616. doi: 10.1073/pnas.87.21.8612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bivalacqua TJ, Hellstrom WJ, Kadowitz PJ, Champion HC. Increased expression of arginase II in human diabetic corpus cavernosum: in diabetic-associated erectile dysfunction. Biochem Biophys Res Commun. 2001;283:923–927. doi: 10.1006/bbrc.2001.4874. [DOI] [PubMed] [Google Scholar]
- 122.Xu W, Kaneko FT, Zheng S, Comhair SA, Janocha AJ, Goggans T, Thunnissen FB, Farver C, Hazen SL, Jennings C, Dweik RA, Arroliga AC, Erzurum SC. Increased arginase II and decreased NO synthesis in endothelial cells of patients with pulmonary arterial hypertension. FASEB J. 2004;18:1746–1748. doi: 10.1096/fj.04-2317fje. [DOI] [PubMed] [Google Scholar]
- 123.Berkowitz DE, White R, Li D, Minhas KM, Cernetich A, Kim S, Burke S, Shoukas AA, Nyhan D, Champion HC, Hare JM. Arginase reciprocally regulates nitric oxide synthase activity and contributes to endothelial dysfunction in aging blood vessels. Circulation. 2003;108:2000–2006. doi: 10.1161/01.CIR.0000092948.04444.C7. [DOI] [PubMed] [Google Scholar]
- 124.Ming XF, Barandier C, Viswambharan H, Kwak BR, Mach F, Mazzolai L, Hayoz D, Ruffieux J, Rusconi S, Montani JP, Yang Z. Thrombin stimulates human endothelial arginase enzymatic activity via RhoA/ROCK pathway: implications for atherosclerotic endothelial dysfunction. Circulation. 2004;110:3708–3714. doi: 10.1161/01.CIR.0000142867.26182.32. [DOI] [PubMed] [Google Scholar]
- 125.Gorren AC, List BM, Schrammel A, Pitters E, Hemmens B, Werner ER, Schmidt K, Mayer B. Tetrahydrobiopterin-free neuronal nitric oxide synthase: evidence for two identical highly anticooperative pteridine binding sites. Biochemistry. 1996;35:16735–16745. doi: 10.1021/bi961931j. [DOI] [PubMed] [Google Scholar]
- 126.Martasek P, Miller RT, Liu Q, Roman LJ, Salerno JC, Migita CT, Raman CS, Gross SS, Ikeda-Saito M, Masters BS. The C331A mutant of neuronal nitric-oxide synthase is defective in arginine binding. J Biol Chem. 1998;273:34799–34805. doi: 10.1074/jbc.273.52.34799. [DOI] [PubMed] [Google Scholar]
- 127.Sydow K, Munzel T. ADMA and oxidative stress. Atheroscler Suppl. 2003;4:41–51. doi: 10.1016/s1567-5688(03)00033-3. [DOI] [PubMed] [Google Scholar]
- 128.Boger RH, Sullivan LM, Schwedhelm E, Wang TJ, Maas R, Benjamin EJ, Schulze F, Xanthakis V, Benndorf RA, Vasan RS. Plasma asymmetric dimethylarginine and incidence of cardiovascular disease and death in the community. Circulation. 2009;119:1592–1600. doi: 10.1161/CIRCULATIONAHA.108.838268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Antoniades C, Shirodaria C, Leeson P, Antonopoulos A, Warrick N, Van-Assche T, Cunnington C, Tousoulis D, Pillai R, Ratnatunga C, Stefanadis C, Channon KM. Association of plasma asymmetrical dimethylarginine (ADMA) with elevated vascular superoxide production and endothelial nitric oxide synthase uncoupling: implications for endothelial function in human atherosclerosis. Eur Heart J. 2009;30:1142–1150. doi: 10.1093/eurheartj/ehp061. [DOI] [PubMed] [Google Scholar]
- 130.Lin KY, Ito A, Asagami T, Tsao PS, Adimoolam S, Kimoto M, Tsuji H, Reaven GM, Cooke JP. Impaired nitric oxide synthase pathway in diabetes mellitus: role of asymmetric dimethylarginine and dimethylarginine dimethylaminohydrolase. Circulation. 2002;106:987–992. doi: 10.1161/01.cir.0000027109.14149.67. [DOI] [PubMed] [Google Scholar]
- 131.Böger RH, Sydow K, Borlak J, Thum T, Lenzen H, Schubert B, Tsikas D, Bode-Böger SM. LDL cholesterol upregulates synthesis of asymmetrical dimethylarginine in human endothelial cells: involvement of S-adenosylmethionine-dependent methyltransferases. Circ Res. 2000;87:99–105. doi: 10.1161/01.res.87.2.99. [DOI] [PubMed] [Google Scholar]
- 132.Chen CA, Wang TY, Varadharaj S, Reyes LA, Hemann C, Talukder MA, Chen YR, Druhan LJ, Zweier JL. S-glutathionylation uncouples eNOS and regulates its cellular and vascular function. Nature. 2010;468:1115–1118. doi: 10.1038/nature09599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zweier J, Chen CA, Druhan LJ. S-glutathionylation reshapes our understanding of eNOS Uncoupling and NO/ROS-mediated signaling. Antioxid Redox Signal. 2011;14:1769–1775. doi: 10.1089/ars.2011.3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Imanishi T, Ikejima H, Tsujioka H, Kuroi A, Kobayashi K, Muragaki Y, Mochizuki S, Goto M, Yoshida K, Akasaka T. Addition of eplerenone to an angiotensin-converting enzyme inhibitor effectively improves nitric oxide bioavailability. Hypertension. 2008;51:734–741. doi: 10.1161/HYPERTENSIONAHA.107.104299. [DOI] [PubMed] [Google Scholar]
- 135.Griendling KK, Sorescu D, Ushio-Fukai M. NAD(P)H oxidase: role in cardiovascular biology and disease. Circ Res. 2000;86:494–501. doi: 10.1161/01.res.86.5.494. [DOI] [PubMed] [Google Scholar]
- 136.Nickenig G, Baumer AT, Temur Y, Kebben D, Jockenhovel F, Bohm M. Statin-sensitive dysregulated AT1 receptor function and density in hypercholesterolemic men. Circulation. 1999;100:2131–2134. doi: 10.1161/01.cir.100.21.2131. [DOI] [PubMed] [Google Scholar]
- 137.Imanishi T, Tsujioka H, Ikejima H, Kuroi A, Takarada S, Kitabata H, Tanimoto T, Muragaki Y, Mochizuki S, Goto M, Yoshida K, Akasaka T. Renin inhibitor aliskiren improves impaired nitric oxide bioavailability and protects against atherosclerotic changes. Hypertension. 2008;52:563–572. doi: 10.1161/HYPERTENSIONAHA.108.111120. [DOI] [PubMed] [Google Scholar]
- 138.Klingbeil AU, John S, Schneider MP, Jacobi J, Handrock R, Schmieder RE. Effect of AT1 receptor blockade on endothelial function in essential hypertension. Am J Hypertens. 2003;16:123–128. doi: 10.1016/s0895-7061(02)03154-0. [DOI] [PubMed] [Google Scholar]
- 139.Wassmann S, Hilgers S, Laufs U, Bohm M, Nickenig G. Angiotensin II type 1 receptor antagonism improves hypercholesterolemia-associated endothelial dysfunction. Arterioscler Thromb Vasc Biol. 2002;22:1208–1212. doi: 10.1161/01.atv.0000022847.38083.b6. [DOI] [PubMed] [Google Scholar]
- 140.Warnholtz A, Nickenig G, Schulz E, Macharzina R, Brasen JH, Skatchkov M, Heitzer T, Stasch JP, Griendling KK, Harrison DG, Bohm M, Meinertz T, Munzel T. Increased NADH-oxidase-mediated superoxide production in the early stages of atherosclerosis: evidence for involvement of the renin–angiotensin system. Circulation. 1999;99:2027–2033. doi: 10.1161/01.cir.99.15.2027. [DOI] [PubMed] [Google Scholar]
- 141.Mancini GB, Henry GC, Macaya C, O'Neill BJ, Pucillo AL, Carere RG, Wargovich TJ, Mudra H, Luscher TF, Klibaner MI, Haber HE, Uprichard AC, Pepine CJ, Pitt B. Angiotensin-converting enzyme inhibition with quinapril improves endothelial vasomotor dysfunction in patients with coronary artery disease. The TREND (Trial on Reversing ENdothelial Dysfunction) Study. Circulation. 1996;94:258–265. doi: 10.1161/01.cir.94.3.258. [DOI] [PubMed] [Google Scholar]
- 142.Hornig B, Landmesser U, Kohler C, Ahlersmann D, Spiekermann S, Christoph A, Tatge H, Drexler H. Comparative effect of ace inhibition and angiotensin II type 1 receptor antagonism on bioavailability of nitric oxide in patients with coronary artery disease: role of superoxide dismutase. Circulation. 2001;103:799–805. doi: 10.1161/01.cir.103.6.799. [DOI] [PubMed] [Google Scholar]
- 143.Bauersachs J, Fraccarollo D. More NO—no more ROS: combined selective mineralocorticoid receptor blockade and angiotensin-converting enzyme inhibition for vascular protection. Hypertension. 2008;51:624–625. doi: 10.1161/HYPERTENSIONAHA.107.106625. [DOI] [PubMed] [Google Scholar]
- 144.Satoh M, Fujimoto S, Arakawa S, Yada T, Namikoshi T, Haruna Y, Horike H, Sasaki T, Kashihara N. Angiotensin II type 1 receptor blocker ameliorates uncoupled endothelial nitric oxide synthase in rats with experimental diabetic nephropathy. Nephrol Dial Transplant. 2008;23:3806–3813. doi: 10.1093/ndt/gfn357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Liao JK. Beyond lipid lowering: the role of statins in vascular protection. Int J Cardiol. 2002;86:5–18. doi: 10.1016/s0167-5273(02)00195-x. [DOI] [PubMed] [Google Scholar]
- 146.Liao JK, Laufs U. Pleiotropic effects of statins. Annu Rev Pharmacol Toxicol. 2005;45:89–118. doi: 10.1146/annurev.pharmtox.45.120403.095748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.John S, Schlaich M, Langenfeld M, Weihprecht H, Schmitz G, Weidinger G, Schmieder RE. Increased bioavailability of nitric oxide after lipid-lowering therapy in hypercholesterolemic patients: a randomized, placebo-controlled, double-blind study. Circulation. 1998;98:211–216. doi: 10.1161/01.cir.98.3.211. [DOI] [PubMed] [Google Scholar]
- 148.Laufs U, Liao JK. Post-transcriptional regulation of endothelial nitric oxide synthase mRNA stability by Rho GTPase. J Biol Chem. 1998;273:24266–24271. doi: 10.1074/jbc.273.37.24266. [DOI] [PubMed] [Google Scholar]
- 149.Feron O, Dessy C, Desager JP, Balligand JL. Hydroxy-methylglutaryl-coenzyme A reductase inhibition promotes endothelial nitric oxide synthase activation through a decrease in caveolin abundance. Circulation. 2001;103:113–118. doi: 10.1161/01.cir.103.1.113. [DOI] [PubMed] [Google Scholar]
- 150.Kureishi Y, Luo Z, Shiojima I, Bialik A, Fulton D, Lefer DJ, Sessa WC, Walsh K. The HMG-CoA reductase inhibitor simvastatin activates the protein kinase Akt and promotes angiogenesis in normocholesterolemic animals. Nat Med. 2000;6:1004–1010. doi: 10.1038/79510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Wagner AH, Kohler T, Ruckschloss U, Just I, Hecker M. Improvement of nitric oxide-dependent vasodilatation by HMG-CoA reductase inhibitors through attenuation of endothelial superoxide anion formation. Arterioscler Thromb Vasc Biol. 2000;20:61–69. doi: 10.1161/01.atv.20.1.61. [DOI] [PubMed] [Google Scholar]
- 152.Hattori Y, Nakanishi N, Akimoto K, Yoshida M, Kasai K. HMG-CoA reductase inhibitor increases GTP cyclohydrolase I mRNA and tetrahydrobiopterin in vascular endothelial cells. Arterioscler Thromb Vasc Biol. 2003;23:176–182. doi: 10.1161/01.atv.0000054659.72231.a1. [DOI] [PubMed] [Google Scholar]
- 153.Wenzel P, Daiber A, Oelze M, Brandt M, Closs E, Xu J, Thum T, Bauersachs J, Ertl G, Zou MH, Förstermann U, Münzel T. Mechanisms underlying recoupling of eNOS by HMG-CoA reductase inhibition in a rat model of streptozotocin-induced diabetes mellitus. Atherosclerosis. 2008;198:65–76. doi: 10.1016/j.atherosclerosis.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Nissen SE, Nicholls SJ, Sipahi I, Libby P, Raichlen JS, Ballantyne CM, Davignon J, Erbel R, Fruchart JC, Tardif JC, Schoenhagen P, Crowe T, Cain V, Wolski K, Goormastic M, Tuzcu EM. Effect of very high-intensity statin therapy on regression of coronary atherosclerosis: the ASTEROID trial. JAMA. 2006;295:1556–1565. doi: 10.1001/jama.295.13.jpc60002. [DOI] [PubMed] [Google Scholar]
- 155.Patel TN, Shishehbor MH, Bhatt DL. A review of high-dose statin therapy: targeting cholesterol and inflammation in atherosclerosis. Eur Heart J. 2007;28:664–672. doi: 10.1093/eurheartj/ehl445. [DOI] [PubMed] [Google Scholar]
- 156.Nicholson S, Bonecini-Almeida Mda G, Lapa e Silva JR, Nathan C, Xie QW, Mumford R, Weidner JR, Calaycay J, Geng J, Boechat N, Linhares C, Rom W, Ho JL. Inducible nitric oxide synthase in pulmonary alveolar macrophages from patients with tuberculosis. J Exp Med. 1996;183:2293–2302. doi: 10.1084/jem.183.5.2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.MacMicking J, Xie QW, Nathan C. Nitric oxide and macrophage function. Annu Rev Immunol. 1997;15:323–350. doi: 10.1146/annurev.immunol.15.1.323. [DOI] [PubMed] [Google Scholar]
- 158.Stenger S, Thuring H, Rollinghoff M, Bogdan C. Tissue expression of inducible nitric oxide synthase is closely associated with resistance to Leishmania major. J Exp Med. 1994;180:783–793. doi: 10.1084/jem.180.3.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Wei XQ, Charles IG, Smith A, Ure J, Feng GJ, Huang FP, Xu D, Muller W, Moncada S, Liew FY. Altered immune responses in mice lacking inducible nitric oxide synthase. Nature. 1995;375:408–411. doi: 10.1038/375408a0. [DOI] [PubMed] [Google Scholar]
- 160.Rafiee P, Ogawa H, Heidemann J, Li MS, Aslam M, Lamirand TH, Fisher PJ, Graewin SJ, Dwinell MB, Johnson CP, Shaker R, Binion DG. Isolation and characterization of human esophageal microvascular endothelial cells: mechanisms of inflammatory activation. Am J Physiol Gastrointest Liver Physiol. 2003;285:G1277–G1292. doi: 10.1152/ajpgi.00484.2002. [DOI] [PubMed] [Google Scholar]
- 161.Wong JM, Billiar TR. Regulation and function of inducible nitric oxide synthase during sepsis and acute inflammation. Adv Pharmacol. 1995;34:155–170. doi: 10.1016/s1054-3589(08)61084-4. [DOI] [PubMed] [Google Scholar]
- 162.Li H, Förstermann U. Nitric oxide in the pathogenesis of vascular disease. J Pathol. 2000;190:244–254. doi: 10.1002/(SICI)1096-9896(200002)190:3<244::AID-PATH575>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 163.Klatt P, Pfeiffer S, List BM, Lehner D, Glatter O, Bachinger HP, Werner ER, Schmidt K, Mayer B. Characterization of heme-deficient neuronal nitric-oxide synthase reveals a role for heme in subunit dimerization and binding of the amino acid substrate and tetrahydrobiopterin. J Biol Chem. 1996;271:7336–7342. doi: 10.1074/jbc.271.13.7336. [DOI] [PubMed] [Google Scholar]
- 164.Kotsonis P, Frohlich LG, Shutenko ZV, Horejsi R, Pfleiderer W, Schmidt HH. Allosteric regulation of neuronal nitric oxide synthase by tetrahydrobiopterin and suppression of auto-damaging superoxide. Biochem J. 2000;346(Pt 3):767–776. [PMC free article] [PubMed] [Google Scholar]
- 165.Abu-Soud HM, Stuehr DJ. Nitric oxide synthases reveal a role for calmodulin in controlling electron transfer. Proc Natl Acad Sci USA. 1993;90:10769–10772. doi: 10.1073/pnas.90.22.10769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.List BM, Klosch B, Volker C, Gorren AC, Sessa WC, Werner ER, Kukovetz WR, Schmidt K, Mayer B. Characterization of bovine endothelial nitric oxide synthase as a homodimer with down-regulated uncoupled NADPH oxidase activity: tetrahydrobiopterin binding kinetics and role of haem in dimerization. Biochem J. 1997;323(Pt 1):159–165. doi: 10.1042/bj3230159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Brunner K, Tortschanoff A, Hemmens B, Andrew PJ, Mayer B, Kungl AJ. Sensitivity of flavin fluorescence dynamics in neuronal nitric oxide synthase to cofactor-induced conformational changes and dimerization. Biochemistry. 1998;37:17545–17553. doi: 10.1021/bi981138l. [DOI] [PubMed] [Google Scholar]
- 168.Mollnau H, Wendt M, Szocs K, Lassegue B, Schulz E, Oelze M, Li H, Bodenschatz M, August M, Kleschyov AL, Tsilimingas N, Walter U, Förstermann U, Meinertz T, Griendling K, Munzel T. Effects of angiotensin II infusion on the expression and function of NAD(P)H oxidase and components of nitric oxide/cGMP signaling. Circ Res. 2002;90:E58–E65. doi: 10.1161/01.res.0000012569.55432.02. [DOI] [PubMed] [Google Scholar]
- 169.Hink U, Li H, Mollnau H, Oelze M, Matheis E, Hartmann M, Skatchkov M, Thaiss F, Stahl RA, Warnholtz A, Meinertz T, Griendling K, Harrison DG, Förstermann U, Münzel T. Mechanisms underlying endothelial dysfunction in diabetes mellitus. Circ Res. 2001;88:E14–E22. doi: 10.1161/01.res.88.2.e14. [DOI] [PubMed] [Google Scholar]
- 170.Drummond GR, Cai H, Davis ME, Ramasamy S, Harrison DG. Transcriptional and posttranscriptional regulation of endothelial nitric oxide synthase expression by hydrogen peroxide. Circ Res. 2000;86:347–354. doi: 10.1161/01.res.86.3.347. [DOI] [PubMed] [Google Scholar]
- 171.Li H, Oehrlein SA, Wallerath T, Ihrig-Biedert I, Wohlfart P, Ulshöfer T, Jessen T, Herget T, Förstermann U, Kleinert H. Activation of protein kinase C alpha and/or epsilon enhances transcription of the human endothelial nitric oxide synthase gene. Mol Pharmacol. 1998;53:630–637. doi: 10.1124/mol.53.4.630. [DOI] [PubMed] [Google Scholar]
- 172.Gorren AC, Kungl AJ, Schmidt K, Werner ER, Mayer B. Electrochemistry of pterin cofactors and inhibitors of nitric oxide synthase. Nitric Oxide. 2001;5:176–186. doi: 10.1006/niox.2001.0332. [DOI] [PubMed] [Google Scholar]
- 173.Bec N, Gorren AFC, Mayer B, Schmidt PP, Andersson KK, Lange R. The role of tetrahydrobiopterin in the activation of oxygen by nitric-oxide synthase. J Inorg Biochem. 2000;81:207–211. doi: 10.1016/s0162-0134(00)00104-5. [DOI] [PubMed] [Google Scholar]