Abstract
Modern cardiology was born at the turn of the nineteenth to twentieth centuries with three great discoveries: the X ray, the sphygmomanometer, and the electrocardiograph. This was followed by cardiac catheterization, which led to coronary angiography and to percutaneous coronary intervention. The coronary care units and early reperfusion reduced the early mortality owing to acute myocardial infarction, and the discovery of coronary risk factors led to the development of Preventive Cardiology. Other major advances include several cardiac imaging techniques, the birth and development of cardiac surgery, and the control of cardiac arrhythmias. The treatment of heart failure, although greatly improved, remains a challenge. Current cardiology practice is evidence-based and global in scope. Research and practice are increasingly conducted in cardiovascular centres and institutes. It is likely that in the future, a greater emphasis will be placed on prevention, which will be enhanced by genetic information.
Keywords: Heart failure, Myocardial infarction, Arrhythmias
The beginning
The conception
Huang Ti Na-Ching Su Wen, China's Yellow Emperor's Classic on Medicine, published circa 2600 BCE,1 was aware of the heart and circulation, writing: ‘The blood current flows continuously in a circle and never stops’. The importance of the heart to the sustenance of life was appreciated by the ancient Egyptians as early as 2000 BCE. In London, William Harvey conducted the first hypothesis-driven experiments in biology. In 1628, decades before Isaac Newton, Harvey2 published his monumental book De motu cordis, in which he described the circulation and the function of the heart.
Interest in various manifestations of heart disease grew in the eighteenth and early nineteenth centuries. Following Heberden's3 classic description of angina pectoris in 1772, the first paper in the first issue of the New England Journal of Medicine was a paper by Warren4 on the same subject. In 1785, Withering, a British physician, described foxglove (digitalis), its use in ‘dropsy’ (oedema), and in strengthening and slowing the heart. Otherwise, no useful cardiovascular therapy appeared for another century, when Brunton described the use of amyl nitrite in the treatment of angina. Physical examination of the heart was greatly facilitated by the invention, in 1819, of the stethoscope by the renowned French Professor René Laennec, a device which refined the diagnosis of valvular heart disease.
The birth
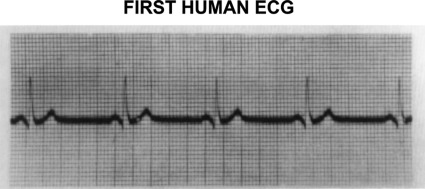
A technical revolution swept over the medical world at the turn of the twentieth century with three discoveries, each of which contributed importantly to the birth of cardiology. The first of these, in 1895, was diagnostic X-ray by Wilhelm Roentgen,5 a German physicist who received the first Nobel prize in physics for this accomplishment. Many early radiographs showed the cardiac silhouette, the size and shape of which were of intense interest to physicians; fluoroscopy then allowed assessment of cardiac motion. The second was a practical, non-invasive measurement of blood pressure made possible by the twin developments of the blood pressure cuff by Riva Rocci in Turin, Italy in 1896, and its use in the auscultatory measurement of blood pressure by Korotkoff in St. Petersburg, Russia in 1905. The third was the string galvanometer for recording cardiac potentials on the surface of the body, the electrocardiograph (Figure 1), which was described by Einthoven6 in 1902; he, too, was rewarded with a Nobel Prize. The earliest application of electrocardiography was in the recognition of a variety of arrhythmias. Physicians who used all three of these new tools in addition to clinical findings to study their patients became identified as the first ‘heart specialists’, i.e. cardiologists.
Figure 1.
First human ECG. From Einthoven W. The galvanometric registration of the human electrocardiogram. Pfluger's Arch f.d. ges Physiol 1903;99:472–480.
Animal experiments in the nineteenth century showed that ligation of a major coronary artery in the dog was rapidly fatal and the same was thought to be the case in humans who suffered acute thrombotic coronary occlusion. However, in 1901, Krehl,7 a Viennese physician, appears to have been the first (or among the first) to report that coronary occlusion in patients is associated with chest pain, that it causes acute myocardial infarction (AMI), and that it is not uniformly fatal. In 1910, Obratzov and Strazhenko8 in Kiev, described five patients with AMI. They reported that unusually heavy exertion and intense emotion could be precipitants. Herrick,9,10 a Chicago physician, who alerted the English speaking world to AMI was also the first to describe electrocardiographic changes in this condition.
Biochemistry and experimental pathology were the next sciences to exert important impacts on cardiology. In 1910, Adolf Windhaus, the Nobel Prize-winning German organic chemist, described the presence of cholesterol in human aortic plaques and in 1913, two young Russians, Anitschkov and Chalatow,11 carried out what would become recognized as one of the most important experiments in the early history of cardiology. They fed large quantities of cholesterol to rabbits, raising the concentration of plasma cholesterol to ∼1000 mg/dL. At sacrifice, these rabbits had early aortic plaques that contained cholesterol. These dual observations gave rise to the lipid theory of atherogenesis.
Thus, modern cardiology was born in Europe and it then spread rapidly to the rest of the world.
The early years
Cardiology quickly developed its own journals and societies, both of which are features of a separate medical specialty. In 1908, both the Archives des Maladies du Coeur in France and the Zentralblatt fur Herzkrankheiten in Germany commenced publication. In 1909, Heart, the first English language journal, was started. In Italy, Malatti du cuore began publication in 1916 and the American Heart Journal in 1925. Reflecting continental and global interests, the European Heart Journal was begun in 1980 and the International Journal of Cardiology in 1981. At the present time, 114 print cardiology journals are listed in the Index Medicus, and the number of online-only journals is growing rapidly.
The British Cardiac Club was founded in 1922 and became the British Cardiac Society in 1937. The American Heart Association was begun in 1924, while the Deutsche Gesellschaft fur Kreislaufforschung was started in 1927. The Belgian, Italian, French and Swiss Cardiac Societies were formed in 1934, 1935, 1937 and 1948, respectively. In the US, a second important organization, the American College of Cardiology was established in 1949. The need for resources to support cardiovascular research became widely recognized and many cardiac societies developed foundations to help provide the necessary funds. National governments also began to support cardiac research. In the United States, the National Heart Institute (now the National Heart, Lung and Blood Institute) was established in 1948, with robust intramural and extramural programmes. The pharmaceutical industry perceived great scientific and financial opportunities in developing new treatments for cardiovascular disease and began to devote substantial resources to this effort as well.
Cardiac catheterization
In 1929 Werner Forssmann,12 a resident in urologic surgery in Eberswalde, Germany, carried out the first cardiac catheterization, and on himself! His goal was to develop a method of injecting drugs into the heart. Learning of what he considered to be a stunt, his chief forbade him from repeating this procedure (on anyone). Cardiac catheterization, first as a research, and later as a diagnostic tool, was begun in 1941 at Columbia University/Bellevue Hospital in New York by Andre Cournand (formerly a French pulmonary physiologist) and Dickinson Richards, who systematically investigated the haemodynamics in every important cardiac condition.13 They and Forssmann shared a Nobel Prize. By the 1960s, cardiac catheterization had spread to most large hospitals in the industrialized world and had become an indispensible cardiac diagnostic procedure.
Cardiac catheterization spawned several important diagnostic and therapeutic techniques, including clinical electrophysiology. Catheter-based treatments of arrhythmias, including pacemakers and internal cardioverter defibrillators followed, as did cardiac resynchronization therapy by means of biventricular pacing for the treatment of systolic heart failure, and catheter-based replacement of the aortic valve (Figure 2).
Figure 2.
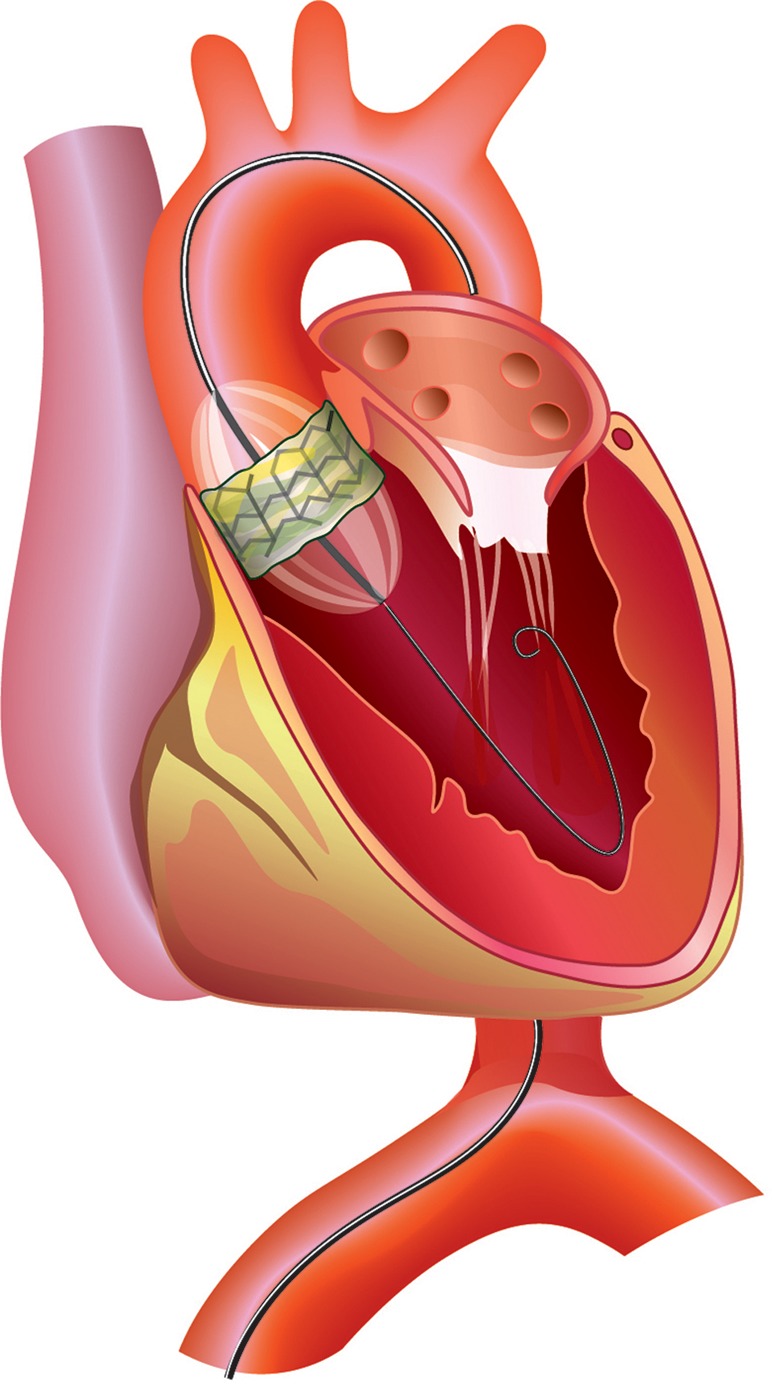
Transfemoral implantation of the Edwards Sapien prosthesis. From Buellesfeld L, Windecker S. Transcatheter aortic valve implantation: the evidence is catching up with reality. Eur Heart J 2011;32:133–137.
Coronary arteriography, first carried out accidentally and then developed by Sones in Cleveland in 1958, arose directly from retrograde left ventricular catheterization. Coronary arteriography allowed the diagnosis and aided in the management of patients with ischaemic heart disease. Coronary arteriography was critical in the selection of patients for coronary bypass surgery and for the development, by Gruntzig and coworkers14 in Zurich, of transluminal coronary angioplasty; this was followed by the development of stents and other refinements of percutaneous coronary intervention.
The science base
From its birth, cardiology has been a science-based clinical specialty. The work of the great cardiac physiologists of the late nineteenth and first half of the twentieth century, such as Frank in Munich, Waller and Starling in London, and Wiggers in Cleveland, provided the basis for understanding the heart—its electrical activity and its pumping function. The effects of inotropic stimuli and of changes in the preload, afterload and frequency of contraction tied the performance of the intact heart to the contractile behaviour of isolated cardiac muscle. By the middle of the twentieth century, cardiovascular disorders were seen increasingly as disturbances of normal physiology.
More recently, cardiovascular science has focused on studies of: (i) the structure, function and dysfunction of the endothelium and of other components of the vascular wall; (ii) the structure and function of ion channels and of receptors on myocytes, vascular smooth muscle cells and platelets; and (iii) the ultrastructure of myocytes, the chemistry of contractile proteins and of intracellular signalling. Increasingly, cardiovascular disorders are understood at a molecular level and the genetic bases of these molecular abnormalities are being uncovered. Thus far, these advances have had their greatest impact on the cardiomyopathies and inherited myocyte ion channel disorders.
Milestones in the evolution of cardiology
Treatment of acute myocardial infarction
In his classic 1912 paper on AMI, Herrick stated ‘the importance of absolute rest in bed … is clear’9 and for five decades bed rest was the cornerstone of therapy. Shortly thereafter, it was recognized that AMI could cause arrhythmias and pulmonary oedema. Digitalis was given and fluid intake was restricted. By mid-century, it had become clear that AMI was not a rarity but was, in fact, the most common cause of death in the industrialized world, that cardiac rupture was a relatively uncommon complication, and that strict limitation of physical activity did not reduce the incidence of this dreaded event; indeed, it appeared that long-term bed rest might itself be hazardous and associated with venous thrombosis and fatal pulmonary embolism. Practice gradually changed; ambulation was accelerated and convalescence shortened.
The development of the coronary care unit (CCU) was one of a pair of signal advances in the treatment of AMI. The idea of the CCU was proposed in 1961 by Julian,15 then a medical registrar at the Royal Infirmary, Edinburgh. Four separate concepts made the CCU possible: (i) aggregating patients into a single area of the hospital where trained personnel, equipment and drugs are all available; (ii) continuous monitoring of the ECG; (iii) development of closed-chest cardiac resuscitation for ventricular fibrillation; and (iv) delegation of the immediate treatment of ventricular fibrillation and other life-threatening arrhythmias to trained nurses, in the absence of a physician.16
The concept of the treatment of AMI in CCUs spread like wildfire and rapidly became the standard of care worldwide. As control of deaths from arrhythmias in the CCU cut the early mortality by half (from about 30% to 15%), pump failure secondary to extensive myocardial damage emerged as the principal cause of death. Animal experiments demonstrated that the extent of ischaemic damage of the myocardium following coronary artery occlusion could be modified by altering myocardial oxygen needs as well as by reperfusion.17
In the 1970s, the Soviet cardiologist Chazov and coworkers18 ushered in the modern era of management of AMI with the second signal advance in the care of patients with AMI, i.e. reperfusion of the ischaemic myocardium by infusion of streptokinase directly into the thrombotically occluded coronary artery in an effort to limit infarct size. Chazov's paper was not translated and had little impact outside the Soviet Union. In 1979, Rentrop et al.19 introduced this treatment to the rest of the world. The next major advance came a decade later, when the GISSI group20 and the ISIS-2 group21 showed that early intravenous administration of streptokinase reduced mortality in patients with AMI. Thus, limiting infarct size and thereby reducing mortality had moved from the experimental laboratory to the hospital emergency room.
Balloon angioplasty,22 followed by stent insertion23 further improved outcome. Reduction of infarct size, and hence in mortality, was critically dependent on early reperfusion; therefore, attention has been focused in minimizing the time between the onset of symptoms and presentation to the hospital and the subsequent ‘door to balloon’ time. Early reperfusion reduced mortality further so that in many countries, the hospital mortality has declined to about 5%. Remodelling of the left ventricle following AMI, a cause of late heart failure and death could be reduced by angiotensin-converting enzyme inhibition, reducing late mortality.24
Acute coronary syndromes
In the first half of the twentieth century, ischaemia was clearly divided into two separate categories, transient ischaemia, which caused chronic angina—usually owing to exertion-induced increases in myocardial oxygen demands in the presence of subtotal coronary obstruction—and prolonged ischaemia at rest, likely owing to totally occlusive coronary thrombosis, which was responsible for AMI. In the late-1930s, clinicians began to identify a third syndrome, having some characteristics of both of these conditions. This condition, ultimately named unstable angina, was considered to be severe angina occurring at rest.25 With ever increasing sensitivity and specificity of biomarkers of myocardial necrosis, it has become apparent that the majority of cases of unstable angina are, in fact, small non-ST segment elevation AMIs (NSTEMI). In these patients, coronary arteriography often shows multi-vessel obstructive coronary artery disease without recent total occlusion. These patients are not helped by fibrinolytic therapy and those that are deemed to be at high risk and have the appropriate findings on coronary arteriography require prompt, but usually not emergent coronary revascularization.26,27 Registries have shown that NSTEMI is, in fact, more frequent than STEMI, and is now one of the most common reasons for hospitalization of adults. The long-term prognosis is not better and in some series actually worse than in patients with STEMI.
Imaging
Since the days of William Harvey, basic scientists and clinicians alike have marvelled at both the simplicity of the heart's pumping function and the complexity of its structure. Until the end of the nineteenth century, the disordered structure and function of the heart could not be ascertained in living humans. The first approaches to cardiac imaging, radiography and fluoroscopy, followed by peripheral angiocardiography and then selective angiocardiography, have overcome this barrier. During the past 40 years, clinical cardiovascular investigators, specialists in imaging techniques, nuclear physicists, and engineers have cooperated in the development of a broad array of non-invasive imaging techniques. As a consequence, we now have various modes of echocardiography available, a technique first described in 1954 by Edler and Hertz28 in Sweden, computed X-ray tomography, magnetic resonance imaging and spectroscopy, radionuclide imaging, and positron emission tomography. The development of these techniques constitutes one of the major pillars of modern cardiology. They aid immensely in diagnosis, and as they are non-invasive, they can be repeated at intervals to assess disease progression or the response to therapy.
Cardiac surgery
The modern era of cardiac surgery began in the early 1950s with the development of open heart surgery using cardiopulmonary bypass, which was necessary for the successful repair of most congenital and many acquired cardiac disorders.29 This ushered in a series of spectacular collaborations between surgeons and engineers. The development of prosthetic heart valves led to astounding benefits in the lives of patients with severe valvular heart disease.
Coronary artery bypass grafting, begun by DeBakey et al.30 in Houston in 1964, and greatly advanced by Favolaro31 in Cleveland provides relief from angina pectoris that is refractory to medical management, and has improved the survival in subgroups of patients, such as those with left main coronary artery disease and three-vessel disease with impaired left ventricular function.
Arrhythmias
Serious bradyarrhythmias—particularly advanced atrioventricular block—was the first arrhythmia to be controlled, initially by an external pacemaker by Zoll32 in Boston in 1952 and then an implanted cardiac pacemaker, developed by Elmqvist and Senning33 in Stockholm in 1958. This was followed by the development of external cardioversion of ventricular fibrillation, ventricular tachycardia, and atrial fibrillation. For several decades, pharmacological agents were used chronically, but unsuccessfully (see below) in an attempt to prevent ventricular fibrillation and sudden cardiac death in subjects at high risk of these disorders. The development of implantable cardioverter-defibrillators by Mirowski et al.34 has proved to be life-saving in such patients. Progressive improvements in electrophysiological testing and endocardial electrical mapping have led to the abolition of a number of arrhythmias, including paroxysmal supraventricular and ventricular tachycardias. Electrical isolation of the origins of the pulmonary veins in patients with atrial fibrillation can abolish or reduce the frequency of this important arrhythmia.35
Valvular heart disease
Despite the recent marked decline in the prevalence of rheumatic heart disease in Europe, North America, and Australia, the total number of patients with valvular heart disease in these regions appears to have levelled-off because of a reciprocal increase in degenerative valvular diseases, especially aortic stenosis, which accompanies the ageing of the population. The development of transcatheter aortic valve replacement36 is a promising technique (Figure 2); catheter-based reduction of mitral regurgitation is also possible.
The numbers of patients with valvular heart disease in developing countries is now rising because the incidence of new cases of rheumatic heart disease has not (yet) fallen to the low levels observed in the industrialized nations, while the number of elderly individuals at risk of degenerative valvular disease is increasing. The care of patients with valvular heart disease requires the assessment of the course of the disease in each patient to select the appropriate time for intervention. Randomized trials of surgical vs. catheter-based therapy must continue.
Heart failure
Although heart failure was recognized as a clinical entity in the eighteenth century, the causes were obscure; no effective therapies other than digitalis were available, and the prognosis was dismal. Mercurial diuretics were introduced in the 1920s, but these drugs were only of modest efficacy and required painful, deep intramuscular injections.
No real advances occurred until the development of effective oral diuretics; the benzothiadiazines in 1957,37 the aldosterone inhibitors in 1959,38 and the powerful ‘loop diuretics’ in 1962.39 The next important step was vasodilator therapy, which was first administered intravenously in acute heart failure. Cohn et al.40 then led a multicentre trial, the VHEFT trial, which showed improved survival in patients with chronic heart failure who received the combination of hydralazine and a long-acting nitrate. Next came the application of neurohormonal blockers—angiotensin-converting enzyme inhibitors,24,41 beta-adrenergic receptor blockers,42 and aldosterone inhibitors—each of which was shown to improve prognosis. These drugs are now begun earlier in the course of heart failure and increasingly in patients at risk of developing heart failure identified by modern imaging and biomarkers. Cardiac transplantation, introduced in 1967,43 is effective in prolonging life by about 10 years in end-stage heart failure, but because of a donor shortage, it is available only to a small fraction of patients who could benefit. Approximately one-half of the deaths of patients with heart failure are sudden and are caused by ventricular fibrillation and the other half by pump failure. Both of these causes have been addressed by the development of three classes of devices—the implanted cardioverter-defibrillators to prevent sudden cardiac death;34 special pacemakers to provide cardiac resynchronization to enhance cardiac contractility;44 and left ventricular assist devices as a bridge to cardiac transplantation, and increasingly as destination therapy.45 All three device classes have been shown to improve survival.
Despite the dazzling progress in the diagnosis and therapy of all forms of cardiac disease, the prevalence, incidence, and deaths from heart failure are all climbing steadily, albeit in patients who are progressively older. As patients with a variety of cardiac diseases including hypertension, iscahemic heart disease, and various cardiomyopathies are increasingly managed successfully, although rarely cured, the damage to their cardiac muscle persists and sometimes progresses as mechanisms which are initially adaptive become maladaptive. With the progressive prolongation of the life span and the growing pandemics of diabetes, obesity, and atrial fibrillation, the stage is now set for further large increases in heart failure, which may be considered to be the last great battleground of advanced cardiac disease in industrialized nations.
Prevention
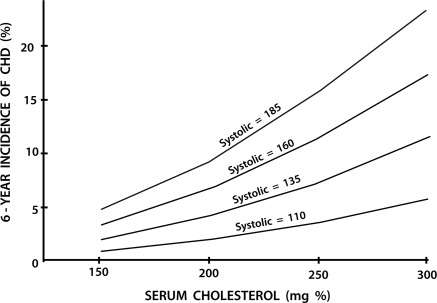
Up to the middle of the twentieth century, the practice of cardiology focused on the treatment of patients with established, often advanced, cardiovascular disease and most treatment were symptomatic. Prevention of cardiovascular disease was not deemed possible, and rarely mentioned. At mid-century, the occurrence of an AMI was usually totally unexpected. Most commonly it struck down apparently healthy persons in their most productive years. However, by 1961, investigators in the Framingham Heart Study had shown that overtly healthy subjects with hypertension and hypercholesterolaemia were at higher risk of developing AMI46 (Figure 3); they coined the term ‘coronary risk factors’. An array of additional coronary risk factors is now recognized. They include age, gender, family history, cigarette smoking, low HDL-cholesterol, elevated lipoprotein(a), diabetes, inflammation, and chronic kidney disease. The identification of these risk factors has provided the basis for prevention of atherosclerotic vascular disease. Healthy lifestyles, weight control, cessation of smoking, reduction of low-density lipoprotein cholesterol, and control of blood pressure have substantially reduced the incidence and recurrence of AMI, acute coronary syndromes, stroke, and coronary deaths (see below).
Figure 3.
Synergistic effects of two risk factors. Six-year incidence of coronary heart disease according to cholesterol levels at specified systolic blood pressures (men 45–62 years). From Kannel WB, Dawber TR, Kagan A, Revotskie N, Stokes J III. Factors of risk in the development of coronary heart disease—six-year follow-up experience. The Framingham Study. Ann Int Med 1961;55:33–50.
The burgeoning evidence supporting the clinical benefit of statins,47–49 first described by Endo and coworkers50 in 1976, has evolved so that practice guidelines now recommend these drugs for both primary and secondary prevention of atherosclerotic vascular disease. Perhaps, no other class of drugs has been responsible for greater reduction of cardiovascular death and morbidity.
The present
Evidence-based cardiological practice
For the first three-quarters of the twentieth century, cardiovascular therapy was based primarily on the relief of symptoms, and intermediate, surrogate ‘softer’ endpoints such as improvement in left ventricular ejection fraction, or reduction in the frequency of ventricular extrasystoles. Perhaps, the most common basis of therapeutics was encapsulated in the letters IMCE (In My Clinical Experience). Such time-honored approaches began to be subjected to widespread critical testing in the mid-1970s, using the randomized clinical trial, an approach pioneered by British investigators in 1948.51 When these ‘softer’ endpoints were compared with ‘hard’ clinical outcomes, such as mortality, (re)hospitalization for heart failure, the development (or recurrence) of AMI, and stroke, they were found to be wanting.
The cardiology community (including this author) was startled by the results of two clinical trials. The first was CAST,52 designed to document the benefit of three anti-arrhythmic agents in post-AMI patients in whom frequent ventricular extrasystoles could be suppressed by these drugs. However, when compared with placebo, these agents were found not to reduce sudden cardiac death, but actually to increase it! The second trial, the PROMISE trial, examined the effect of the oral phosphodiesterase-3-inhibitor milrinone on the survival of patients with heart failure. In phase 1 and 2 trials, milrinone had been shown to reverse the abnormal haemodynamics and reduced symptoms of heart failure. Despite these favourable effects, mortality actually increased in patients randomized to milrinone in the pivotal phase 3 trial.53
It is now clear that to establish the clinical efficacy of therapeutic interventions (devices as well as drugs), improvement in hard clinical endpoints is necessary. Regulatory agencies, clinical practice guidelines writers, and those responsible for reimbursement of therapeutic interventions are now focused predominantly on these outcomes, while the softer endpoints remain useful in elucidating some of the mechanisms of action of interventions.
While the prospective, double-blind, multi-centre, adequately sized clinical trial remains the gold standard for obtaining bias-free evidence, this approach is not without drawbacks. Eligibility criteria are often drawn so narrowly that the results of the trial apply to only a small proportion of the patients with the condition under study. Prospective registries that broaden the patient population can be used to complement clinical trials. A relatively new field, comparative effectiveness research, is designed to determine which interventions are effective in the ‘real world’.54
The rise of global cardiology
Shortly following World War II, cardiology began to transcend national boundaries, with the formation of regional, continental, and international societies of cardiology, all of which hold regular meetings, thereby facilitating communication among scientists, clinicians, and trainees. The first International Congress of Cardiology was held in Paris in 1950; subsequent congresses have been held on a regular basis on all continents. The European Society of Cardiology has become the largest and most active continental society.
International collaborations have become increasingly important, especially in the case of large, multi-centre clinical trials that require the recruitment of tens of thousands of patients in upwards of 50 countries, on all inhabited continents. Registries, meta-analyses, data banks and analyses of biomarkers often involve thousands of patients, and are enhancing the understanding of the natural history of cardiac disorders and their management. Genomic technologies that identify patients at risk for various cardiovascular disorders are not limited by political or geographical boundaries. The development of new devices requires the collaboration of experts that span nations and continents. Cardiology has become a global enterprise,55 facilitated by the personal computer, the internet, and the adoption of English as the lingua franca of medicine and medical science. Most importantly, these collaborative efforts have created global communities of basic scientists, clinical investigators, and clinicians.
Cardiology has also been an important agent for world peace. Paul D. White, the great American cardiologist, arranged the first non-governmental meeting between Soviets and Americans—between cardiologists of both nations in 1961, at the height of the cold war! The Soviet cardiologist Eugene Chazov and the American cardiologist Bernard Lown accepted the Nobel Peace Prize on behalf of the International Physicians for the Prevention of Nuclear War in 1984; this organization helped to pull the superpowers back from the nuclear precipice.
The growth of cardiovascular centres
With the rapid growth of knowledge and of technologies, cardiologists have sub-specialized in various branches (and twigs) of the specialty, becoming interventionalists, electrophysiologists, echocardiographers, nuclear cardiologists, specialists in other imaging modalities, heart failure, acute coronary care, lipid disorders, hypertension, peripheral vascular disease, prevention, geriatric cardiology, pulmonary hypertension, etc. Although sub-specialization has certainly improved care, it is a two-edged sword, because sometimes it fragments cardiac care.
The need for close collaboration among cardiac specialists in different departments has led to the creation of multi-disciplinary cardiac centres which are usually a part of, or are closely affiliated with a medical school.56 These centres are also the site of clinical and fundamental research and training. In order for such large cardiovascular centres or institutes to be financially viable, they must be able to attract a critical mass of patients. These institutes are becoming multimillion euro/dollar enterprises, often housed in separate buildings, with surgical and cardiovascular imaging suites, catheterization and electrophysiological laboratories, inpatient beds, outpatient clinics, rehabilitation units, and research divisions, staffed by a wide range of professionals including clinicians, nurses, nutritionists, technologists, clinical and basic scientists, epidemiologists, bioengineers as well as trainees at many levels, administrators, and (alas) accountants.
The future
The near term future, i.e. approximately the next decade, is likely to see an increasing prevalence of atherosclerotic disease worldwide, with the ageing of the population and the rise in obesity and diabetes. The total costs of caring for these patients, direct and indirect, will be staggering.57 Efforts will be intensified to start preventive therapy, such as changes in diet and the use of statins, progressively earlier in life. The greatest challenge will be to apply the enormous advances that have occurred in the diagnosis, management, and prevention of cardiovascular disease to the large and growing majority of the world's population that has not yet enjoyed their benefits. Perhaps, inexpensive ‘polypills’ containing aspirin, an angiotensin-converting enzyme inhibitor, a thiazide diuretic and a statin, or some variation thereof, will prove to be of value for primary and secondary prevention in selected persons in developing nations.58
Successful use of stem-cell therapy in the prevention and treatment of heart failure following AMI and in some forms of chronic heart failure is likely.59 Pluripotent stem cells derived from the patient's own cell types, including fibroblasts,60 are particularly interesting.
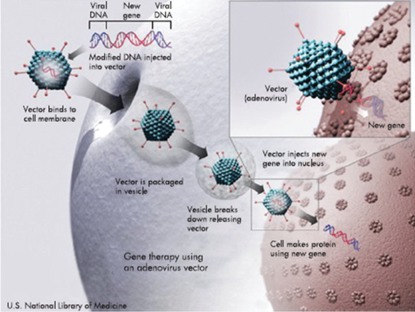
There will be enormous advances and rapidly growing use of smaller, more reliable, safer, and less-expensive implanted ventricular assist pumps as ‘destination therapy’. These devices are likely to be used for weeks, perhaps in combination with cell therapy as ‘bridges to recovery’ from heart failure. When attempts to wean the patient from the device are unsuccessful, they will be left in place as destination therapy. When all else fails, gene therapy may be employed61 (Figure 4). It will be necessary to set up new, specialized clinics to care for patients who receive cell-based, device-based, and combination therapy for heart failure and AMI.
Figure 4.
Gene transfer. Viral vectors bind to cell surface receptors, initiating endocytosis. Once internalized, the viral particles avoid degradation and travel to ‘dock’ on the nuclear envelope membrane pores, and the genome is delivered into the cell nucleus. (Created by the US National Library of Medicine.) From Lyon AR, Sato M, Hajjar RJ, Samulski RJ, Harding SE. Gene therapy: targeting the myocardium. Heart 2008;94:89–99.
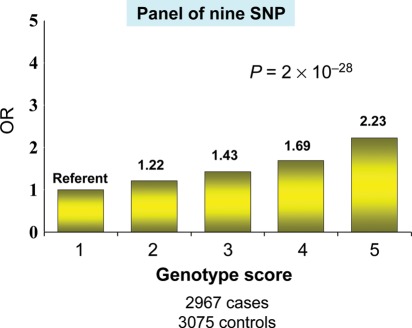
The long-term future, i.e. beyond the next decade, is more difficult to discern. However, it appears likely that the fruits of the biological revolution will be applied to cardiology with ever greater vigour. Pharmacogenomics will expand. Genomics will enhance the sub-classification of disease, allowing truly ‘personalized’ care62 (Figure 5). RNA, long a stepchild, is poised to take centre stage. MicroRNAs (miRNAs) are small RNAs that are key regulators of gene expression. Changes in the expression of MiRNAs have been found to be important in cardiac development, myocardial hypertrophy, and heart failure.63 RNA interference (RNAi) is an efficient and versatile mechanism capable of silencing specific genes. RNAi has the potential to provide novel drugs in many areas of medicine, including the treatment of heart failure.64 Primordial prevention, i.e. prevention of the future development of risk factors,65 will become the dominant form of prevention and will focus on children and adolescents.
Figure 5.
Genetics predict risk of first myocardial infarction. Quintiles of allelic dosage score comprises nine validated single nucleotide polymorphisms and risk of early onset myocardial infarction. Data from Kathiresan S, Voight BF, Purcell S, Musunuru K, Ardissino D, Mannucci PM et al. for the Myocardial Infarction Genetics Consortium. Genome-wide association of early-onset myocardial infarction with single nucleotide polymorphisms and copy number variants. Nat Genet 2009;41:334–341.
It is likely that the role of cardiologists will change. Although most cardiologists today still deal predominantly with patients who have established disease, the primary goal of cardiologists of the future may become the interpretation of their patients' characteristics determined by genomic and other ‘omic’ techniques in a move from personalized care to personalized primordial prevention. The greatest possible victory that cardiology could aspire to would be to greatly reduce or even eliminate coronary artery disease, the most common form of cardiovascular disease, and to delay substantially the development of degenerative heart disease. The world cardiology community should set these important goals for 2052, the sesquicentennial of Einthoven's description of the string galvanometer in 1902, and the birth of modern cardiology.
Acknowledgements
The author thanks Drs Elliott M. Antman, W. Bruce Fye, and Peter Libby for their helpful comments.
Conflict of interest: none declared.
References
- 1.Veith I. Huang Ti Nei Ching Su Wen—The Yellow Emperor's Classic of Internal Medicine. Berkeley, Los Angeles: University of California Press; 1986. p. 260. [Google Scholar]
- 2.Harvey W. An anatomical disputation concerning the movement of the heart and blood in living creatures. Oxford: Blackwell Scientific Publications; 1976. pp. 1–135. Translation by Whitteridge G. [Google Scholar]
- 3.Heberden W. Some account of a disorder of the breast. Med Trans. 1772;2:59–67. [Google Scholar]
- 4.Warren J. Remarks on angina pectoris. N Engl J Med. 1812;1:1–11. doi: 10.1056/NEJM196201042660101. [DOI] [PubMed] [Google Scholar]
- 5.Roentgen WC. On a new kind of rays. Gesellschaft: Sitzungsberichte der Wurzburgery Physik-medic; 1895. [Google Scholar]
- 6.Einthoven W. Herinneringsbundel Professor S. S. Rosenstein. Leiden: Edouard Ijdo; 1902. Galvonometrische registratie van het menschelijk electrocardiogram; pp. 101–107. [Google Scholar]
- 7.Krehl L. Die Ekrankungen des Herzmuskels und die Nervosen Herzkrankheiten. Vienna: Alfred Holder; 1901. [Google Scholar]
- 8.Obrastzov WP, Straschesko ND. Zur kenntnis der thrombose der koronararterien des herzens. Z Klin Med. 1910;71:116–132. [Google Scholar]
- 9.Herrick JB. Certain clinical features of sudden obstruction of the coronary arteries. JAMA. 1912;59:2015–2020. [PubMed] [Google Scholar]
- 10.Herrick JB. Thrombosis of the coronary arteries. JAMA. 1919;72:387–390. [Google Scholar]
- 11.Anitschkow N, Chalatow S. On experimental cholesterin steatosis and its significance in the origin of some pathological processes. ATVB. 1983;3:178–182. Translated by Maria Z. Pelias. [PubMed] [Google Scholar]
- 12.Forssman W. Catheterization of the right heart. Klin Wochenshr. 1929;8:2085–2087. doi:10.1007/BF01875120. [Google Scholar]
- 13.Cournand AF, Ranges HS. Catheterization of the right auricle in man. Proc Soc Exp Biol Med. 1941;46:462–466. [Google Scholar]
- 14.Gruntzig AR, Senning A, Siegenthaler WE. Nonoperative dilation of coronary-artery stenosis: percutaneous transluminal coronary angioplasty. N Engl J Med. 1979;301:61–68. doi: 10.1056/NEJM197907123010201. doi:10.1056/NEJM197907123010201. [DOI] [PubMed] [Google Scholar]
- 15.Julian DG. Treatment of cardiac arrest in acute myocardial ischemia and infarction. Lancet. 1961;ii:840–844. doi: 10.1016/s0140-6736(61)90738-3. doi:10.1016/S0140-6736(61)90738-3. [DOI] [PubMed] [Google Scholar]
- 16.Fye WB. Resuscitating a Circulation abstract to celebrate the 50th anniversary of the coronary care unit concept. Circulation. 2011;124:1886–1893. doi: 10.1161/CIRCULATIONAHA.111.033597. doi:10.1161/CIRCULATIONAHA.111.033597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maroko PR, Kjekshus JK, Sobel BE, Watanabe T, Covell JW, Ross J, Jr, Braunwald E. Factors influencing infarct size following experimental coronary artery occlusion. Circulation. 1971;43:67–82. doi: 10.1161/01.cir.43.1.67. [DOI] [PubMed] [Google Scholar]
- 18.Chazov EI, Mateeva LS, Mazaev AV. Intracoronary administration of fibrinolysin in acute myocardial infarction. Ter Arkh. 1976;48:8–19. [PubMed] [Google Scholar]
- 19.Rentrop KP, Blanke H, Karsch KR, Wiegand V, Köstering H, Oster H, Leitz K. Acute myocardial infarction: Intracoronary application of nitroglycerin and streptokinase. Clin Card. 1979;2:354–63. doi: 10.1002/clc.4960020507. [DOI] [PubMed] [Google Scholar]
- 20.Gruppo Italiano per lo Studio della Streptochinasi nell'Infarto Miocardico (GISSI) Effectiveness of intravenous thrombolytic treatment in acute myocardial infarction. Lancet. 1986;1:397–402. [PubMed] [Google Scholar]
- 21.ISIS-2 Collaborative Group. Randomized trial of intravenous streptokinase, oral aspirin, both, or neither among 17 187 cases of suspected acute myocardial infarction: ISIS-2. Lancet. 1988;1:349–360. [PubMed] [Google Scholar]
- 22.Zijlstra F, de Boer MJ, Hoorntje JCA, Reiffers S, Reiber JHC, Suryapranata H. A comparison of immediate coronary angioplasty with intravenous streptokinase in acute myocardial infarction. N Engl J Med. 1993;328:680–684. doi: 10.1056/NEJM199303113281002. doi:10.1056/NEJM199303113281002. [DOI] [PubMed] [Google Scholar]
- 23.Zhu MM, Feit A, Chadow H, Alam M, Kwan T, Clark LT. Primary stent implantation compared with primary balloon angioplasty for acute myocardial infarction. A meta analysis of randomized clinical trials. Am J Cardiol. 2001;88:297–301. doi: 10.1016/s0002-9149(01)01645-9. doi:10.1016/S0002-9149(01)01645-9. [DOI] [PubMed] [Google Scholar]
- 24.Pfeffer MA, Braunwald E, Moyé LA, Basta L, Brown EJ, Jr, Cuddy TE, Davis BR, Geltman EM, Goldman S, Flaker GC, Klein M, Lamas GA, Packer M, Rouleau J, Rutherford J, Wertheimer JH, Hawkins CM on behalf of the SAVE investigators. Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction. Results of the survival and ventricular enlargement trial. The SAVE Investigators. SAVE TRIAL. N Engl J Med. 1992;327:669–677. doi: 10.1056/NEJM199209033271001. doi:10.1056/NEJM199209033271001. [DOI] [PubMed] [Google Scholar]
- 25.Braunwald E. Unstable angina–A classification. Circulation. 1989;80:410–414. doi: 10.1161/01.cir.80.2.410. doi:10.1161/01.CIR.80.2.410. [DOI] [PubMed] [Google Scholar]
- 26.Wallentin L, Lagerqvist B, Husted S, Kontny F, Stahle E, Swahn E for the FRISC II Investigators. Outcome at 1 year after an invasive compared with a non-invasive strategy in unstable coronary-artery disease: the FRISC II invasive randomized trial. Lancet. 2000;356:9–16. doi: 10.1016/s0140-6736(00)02427-2. doi:10.1016/S0140-6736(00)02427-2. [DOI] [PubMed] [Google Scholar]
- 27.Cannon CP, Weintraub WS, Demopoulos LA, Vicari R, Frey MJ, Lakkis N, Neumann FJ, Robertson DH, DeLucca PT, DiBattiste PM, Gibson CM, Braunwald E. Comparison of early invasive and conservative strategies in patients with unstable coronary syndromes treated with the glycoprotein IIb/IIIa inhibitor tirofiban. N Engl J Med. 2001;344:1879–1887. doi: 10.1056/NEJM200106213442501. doi:10.1056/NEJM200106213442501. [DOI] [PubMed] [Google Scholar]
- 28.Edler I, Hertz CH. Use of ultrasonic reflectoscope for the continuous recording of movements of heart walls. Kungl Fysiografiska Sallskapets i Lund Forhandlingar. 1954;24:40–58. doi: 10.1111/j.1475-097X.2004.00539.x. [DOI] [PubMed] [Google Scholar]
- 29.Gibbon JH., Jr Application of a mechanical heart and lung apparatus to cardiac surgery. Minn Med. 1954;37:171–175. [PubMed] [Google Scholar]
- 30.DeBakey M, Garrett HE, Dennis EW. Aorto-coronary bypass with saphenous vein graft. Seven-year follow-up. JAMA. 1973;223:792. doi:10.1001/jama.223.7.792. [PubMed] [Google Scholar]
- 31.Favaloro RG. Saphenous vein graft in the surgical treatment of coronary artery disease. Operative technique. J Thorac Cardiovasc Surg. 1969;58:178–85. [PubMed] [Google Scholar]
- 32.Zoll PM. Resuscitation of the heart in ventricular standstill by external electrical stimulation. N Engl J Med. 1952;247:768–771. doi: 10.1056/NEJM195211132472005. doi:10.1056/NEJM195211132472005. [DOI] [PubMed] [Google Scholar]
- 33.Elmqvist R. Review of early pacemaker development. PACE. 1978;1:535–536. doi: 10.1111/j.1540-8159.1978.tb03518.x. [DOI] [PubMed] [Google Scholar]
- 34.Mirowski M, Reid PR, Mower MM, Watkins L, Gott VL, Schauble JF, Langer A, Heilman MS, Kolenik SA, Fischell RE, Weisfeldt ML. Termination of malignant ventricular arrhythmias with an implanted automatic defibrillator in human beings. N Engl J Med. 1980;303:322–324. doi: 10.1056/NEJM198008073030607. doi:10.1056/NEJM198008073030607. [DOI] [PubMed] [Google Scholar]
- 35.Haissaguerre M, Jais P, Shah DC, Garrigue S, Takahasi A, Lavergne T, Hocini M, Peng JT, Roudaut R, Clementy J. Electrophysiological end point for catheter ablation of atrial fibrillation initiated from multiple pulmonary venous foci. Circulation. 2000;101:1409–1417. doi: 10.1161/01.cir.101.12.1409. [DOI] [PubMed] [Google Scholar]
- 36.Smith CR, Leon MB, Mack MJ, Miller DC, Moses JW, Svensson LG, Tuzcu EM, Webb JG, Fontana GP, Makkar RR, Williams M, Dewey T, Kapadia S, Babaliaros V, Thourani VH, Corso P, Pichard AD, Bavaria JE, Herrmann HC, Akin JJ, Anderson WN, Wang D, Pocock SJ. Transcatheter versus surgical aortic valve replacement in high-risk patients. N Engl J Med. 2011;364:2187–2198. doi: 10.1056/NEJMoa1103510. doi:10.1056/NEJMoa1103510. [DOI] [PubMed] [Google Scholar]
- 37.Novello SC, Sprague JM. Benzothiadiazine dioxides as novel diuretics. J Am Chem Soc. 1957;79:2028. doi:10.1021/ja01565a079. [Google Scholar]
- 38.Kagawa CM, Sturtevant FM, van Arman CG. Pharmacology of a new steroid that blocks salt activity of aldosterone and desoxycorticosterone. J Pharm Exp Ther. 1959;126:123–130. [PubMed] [Google Scholar]
- 39.Schultz EM, Chragoe EJ, Bicking JB, Bolhofer WA, Sprague JM. α,β-Unsaturated ketone derivatives of arloxyacetic acids, a new class of diuretics. J Med Pharm Chem. 1962;5:642–646. doi: 10.1021/jm01238a030. doi:10.1021/jm01238a025. [DOI] [PubMed] [Google Scholar]
- 40.Cohn JN, Archibald DG, Ziesche S, Franciosa JA, Harston WE, Tristani FE, Dunkman WB, Jacobs W, Francis GS, Flohr KH, Goldman S, Cobb FR, Shah PM, Saunders R, Fletcher RD, Loeb HS, Hughes VC, Baker B. Effect of vasodilator therapy on mortality in chronic congestive heart failure. Results of a Veterans Administration Cooperative Study. N Engl J Med. 1986;314:1547–1552. doi: 10.1056/NEJM198606123142404. doi:10.1056/NEJM198606123142404. [DOI] [PubMed] [Google Scholar]
- 41.The CONSENSUS trial study group. Effects of enalapril on mortality in severe congestive heart failure. N Engl J Med. 1987;316:1429–1435. doi: 10.1056/NEJM198706043162301. doi:10.1056/NEJM198706043162301. [DOI] [PubMed] [Google Scholar]
- 42.Waagstein F, Hjalmarson A, Swedeberg K, Wallentin I. Beta-blockers in dilated cardiomyopathies: they work. Eur Heart J. 1983;4:173–178. doi: 10.1093/eurheartj/4.suppl_a.173. [DOI] [PubMed] [Google Scholar]
- 43.Barnard CN. The operation. S Afr Med J. 1967;41:1271. [PubMed] [Google Scholar]
- 44.Cazeau S, Ritter P, Lazarus A, Gras D, Backdach H, Mundler O, Mugica J. Multisite pacing for end-stage heart failure: early experience. Pacing Clin Electrophysiol. 1996;19:1748–1757. doi: 10.1111/j.1540-8159.1996.tb03218.x. doi:10.1111/j.1540-8159.1996.tb03218.x. [DOI] [PubMed] [Google Scholar]
- 45.Kirklin JK, Naftel DC, Kormos RL, Stevenson LW, Pagani FD, Miller MA, Ulisney KL, Baldwin JT, Young JB. Third INTERMACS annual report: the evolution of destination therapy in the United States. J Heart Lung Transplant. 2011;30:115–123. doi: 10.1016/j.healun.2010.12.001. doi:10.1016/j.healun.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 46.Kannel WB, Dawber TR, Kagan A, Revotskie N, Stokes J., 3rd Factors of risk in the development of coronary heart disease—six-year follow-up experience. The Framingham Study. Ann Int Med. 1961;55:33–50. doi: 10.7326/0003-4819-55-1-33. [DOI] [PubMed] [Google Scholar]
- 47.Scandinavian Simvastatin Survival Study Group. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simbastatin Survival Study. Lancet. 1994;344:1383–1389. [PubMed] [Google Scholar]
- 48.Cholesterol Treatment Trialists’ (CTT) Collaboration. Efficacy and safety of more intensive lowering of LDL cholesterol: a metaanalysis of data from 170 000 participants in 26 randomised trials. Lancet. 2010;376:1670–1681. doi: 10.1016/S0140-6736(10)61350-5. doi:10.1016/S0140-6736(10)61350-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM, Jr, Kastelein JJ, Koenig W, Libby P, Lorenzatti AJ, MacFadyen JG, Nordestgaard BG, Shepherd J, Willerson JT, Glynn RJ JUPITER Study Group. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359:2195–2207. doi: 10.1056/NEJMoa0807646. doi:10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 50.Endo A, Kuroda M, Tsujita Y. ML-236A, ML-236B, and ML-236C, new inhibitors of cholesterogenesis produced by Penicillium citrinum. J Antibiot (Jpn) 1976;29:1346–1348. doi: 10.7164/antibiotics.29.1346. [DOI] [PubMed] [Google Scholar]
- 51.Medical Research Council Streptomycin in Tuberculosis Trials Committee. Streptomycin treatment for pulmonary tuberculosis. BMJ. 1948;ii:769–782. [Google Scholar]
- 52.The Cardiac Arrhythmia Suppression Trial (CAST) Investigators. Preliminary report: effect on encainide and flecainide on mortality in a randomized trial of arrhythmia suppression after myocardial infarction. N Engl J Med. 1989;321:406–412. doi: 10.1056/NEJM198908103210629. doi:10.1056/NEJM198908103210629. [DOI] [PubMed] [Google Scholar]
- 53.Packer M, Carver JR, Rodeheffer RJ, Ivanhoe RJ, DiBianco R, Zeldis SM, Hendrix GH, Bommer WJ, Elkayam U, Kukin ML, Mallis GI, Sollano JA, Shannon J, Tandon PK, DeMets DL The PROMISE Study Research Group. Effect of oral milrinone on mortality in severe chronic heart failure. N Engl J Med. 1991;325:1468–1475. doi: 10.1056/NEJM199111213252103. doi:10.1056/NEJM199111213252103. [DOI] [PubMed] [Google Scholar]
- 54.Antman EM. Evidence and education. Circulation. 2011;123:681–685. doi: 10.1161/CIRCULATIONAHA.110.015255. doi:10.1161/CIRCULATIONAHA.110.015255. [DOI] [PubMed] [Google Scholar]
- 55.Holmes DR., Jr The Global College of Cardiology: it's a small world after all. J Am Coll Cardiol. 2011;58:194–195. doi: 10.1016/j.jacc.2011.06.002. doi:10.1016/j.jacc.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 56.Braunwald E. Departments, divisions and centers in the evolution of medical schools. Am J Med. 2006;119:457–462. doi: 10.1016/j.amjmed.2005.11.025. doi:10.1016/j.amjmed.2005.11.025. [DOI] [PubMed] [Google Scholar]
- 57.Heidenreich PA, Trogdon JG, Khavjou OA, Butler J, Dracup K, Ezekowitz MD, Finkelstein EA, Hong Y, Johnston SC, Khera A, Lloyd-Jones DM, Nelson SA, Nichol G, Orenstein D, Wilson PW, Woo YJ. Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation. 2011;123:933–944. doi: 10.1161/CIR.0b013e31820a55f5. doi:10.1161/CIR.0b013e31820a55f5. [DOI] [PubMed] [Google Scholar]
- 58.Lonn E, Bosch J, Teo KK, Pais P, Xavier D, Yusuf S. The polypill in the prevention of cardiovascular diseases: key concepts, current status, challenges, and future directions. Circulation. 2010;122:2078–2088. doi: 10.1161/CIRCULATIONAHA.109.873232. doi:10.1161/CIRCULATIONAHA.109.873232. [DOI] [PubMed] [Google Scholar]
- 59.Segers VFM, Lee RT. Stem-cell therapy for cardiac disease. Nature. 2008;451:937–942. doi: 10.1038/nature06800. doi:10.1038/nature06800. [DOI] [PubMed] [Google Scholar]
- 60.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. doi:10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 61.Jessup M, Greenberg B, Mancini D, Cappola T, Pauly DF, Jaski B, Yaroshinsky A, Zsebo KM, Dittrich H, Hajjar RJ. Calcium upregulation by percutaneous administration of gene therapy in cardiac disease (CUPID). A phase 2 trial of intracoronary gene therapy of sarcoplasmic reticulum Ca2+-ATPase in patients with advanced heart failure. Circulation. 2011;124:304–313. doi: 10.1161/CIRCULATIONAHA.111.022889. doi:10.1161/CIRCULATIONAHA.111.022889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Myocardial Infarction Genetics Consortium. Genome-wide association of early-onset myocardial infarction with single nucleotide polymorphisms and copy number variants. Nat Genet. 2009;41:334–341. doi: 10.1038/ng.327. doi:10.1038/ng.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sucharov C, Bristow MR, Port JD. miRNA expression in the failing human heart: functional correlates. J Mol Cell Biol. 2008;45:185–192. doi: 10.1016/j.yjmcc.2008.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Suckau L, Fechner H, Chemaly E, Krohn S, Hadri L, Kockskämper J, Westermann D, Bisping E, Ly H, Wang X, Kawase Y, Chen J, Liang L, Sipo I, Vetter R, Weger S, Kurreck J, Erdmann V, Tschope C, Pieske B, Lebeche D, Schultheiss HP, Hajjar RJ, Poller WC. Long-term cardiac-targeted RNA interference for the treatment of heart failure restores cardiac function and reduces pathological hypertrophy. Circulation. 2009;119:1241–1252. doi: 10.1161/CIRCULATIONAHA.108.783852. doi:10.1161/CIRCULATIONAHA.108.783852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Weintraub WS, Daniels SR, Burke LE, Franklin BA, Goff DC, Jr, Hayman LL, Lloyd-Jones D, Pandey DK, Sanchez EJ, Schram AP, Whitsel LP on behalf of the American Heart Association Advocacy Coordinating Committee. Value of primordial and primary prevention for cardiovascular disease: a policy statement from the American Heart Association. Circulation. 2011;124:967–990. doi: 10.1161/CIR.0b013e3182285a81. [DOI] [PubMed] [Google Scholar]