Abstract
Aims
To evaluate the effects of cardiac resynchronization therapy (CRT) on long-term survival of patients without baseline left ventricular (LV) mechanical dyssynchrony.
Methods and results
A total of 290 heart failure patients (age 67 ± 10 years, 77% males) without significant baseline LV dyssynchrony (<60 ms as assessed with tissue Doppler imaging) were treated with CRT. Patients were divided according to the median LV dyssynchrony measured after 48 h of CRT into two groups. All-cause mortality was compared between the subgroups. In addition, the all-cause mortality rates of these subgroups were compared with the all-cause mortality of 290 heart failure patients treated with CRT who showed significant LV dyssynchrony (≥60 ms) at baseline. In the group of patients without significant LV dyssynchrony, median LV dyssynchrony increased from 22 ms (inter-quartile range 16–34 ms) at baseline to 40 ms (24–56 ms) 48 h after CRT. The cumulative mortality rates at 1-, 2-, and 3-year follow-up of patients with LV dyssynchrony ≥40 ms 48 h after CRT implantation were significantly higher when compared with patients with LV dyssynchrony <40 ms (10, 17, and 23 vs. 3, 8, and 10%, respectively; log-rank P< 0.001). Finally, the cumulative mortality rates at 1-, 2-, and 3-year follow-up of patients with baseline LV dyssynchrony were 3, 8, and 11%, respectively (log-rank P= 0.375 vs. patients with LV dyssynchrony <40 ms). Induction of LV dyssynchrony after CRT was an independent predictor of mortality (hazard ratio: 1.247; P= 0.009).
Conclusion
In patients without significant LV dyssynchrony, the induction of LV dyssynchrony after CRT may be related to a less favourable long-term outcome.
Keywords: Heart failure, Echocardiography, Cardiac resynchronization therapy, Prognosis
See page 816 for the editorial comment on this article (doi:10.1093/eurheartj/ehr478)
Introduction
Cardiac resynchronization therapy (CRT) is currently indicated for patients with drug-refractory heart failure, left ventricular ejection fraction (LVEF) ≤35%, and wide QRS complex (≥120 ms).1,2 Observational studies have shown that the presence of significant left ventricular (LV) mechanical dyssynchrony (as assessed with different imaging techniques) is related to a favourable response to CRT and improved outcome.3–7 Indeed, restoration of LV synchronicity by biventricular pacing has been related to LV reverse remodelling, decrease in mitral regurgitation, and improvement in clinical outcome.8 In contrast, lack of significant LV mechanical dyssynchrony has been related to a high rate of non-response to CRT.9,10 As much as 30% of heart failure patients presenting with a wide QRS complex do not show LV mechanical dyssynchrony.11 It has been suggested that CRT could induce LV mechanical dyssynchrony in this subgroup of patients, leading to impaired LV performance and, subsequently, poor clinical outcome.12 However, to date, no study has evaluated the potential induction of LV mechanical dyssynchrony after CRT implantation in this subgroup of patients and, more important, the long-term clinical consequences of this acutely induced LV dyssynchrony remain unknown. Accordingly, the aims of the present study were:
to evaluate the acute effects of CRT on LV synchronicity in heart failure patients fulfilling the current inclusion criteria for CRT but without baseline LV mechanical dyssynchrony;
to study the impact of acutely induced LV dyssynchrony on long-term survival of this specific subgroup of patients. The long-term survival of heart failure patients with and without induced LV dyssynchrony after CRT implantation was compared with the long-term survival of a group of patients with baseline LV dyssynchrony treated with CRT.
Methods
Patient population and data collection
Patients with New York Heart Association (NYHA) functional class III or IV heart failure symptoms despite optimal medical therapy, LVEF ≤ 35%, and QRS duration ≥120 ms were selected for CRT.2 Before CRT implantation, clinical status was evaluated and two-dimensional echocardiography was performed to measure LV volumes and LVEF. In addition, LV mechanical dyssynchrony was evaluated with tissue Doppler imaging (TDI).13 A total of 290 consecutive patients without significant LV mechanical dyssynchrony before CRT (LV dyssynchrony value at baseline <60 ms) were selected from an ongoing registry.14 Patients with recent myocardial infarction (<3 months) and decompensated heart failure requiring continuous intravenous therapy were excluded from the present analysis. Patient data were prospectively recorded in the departmental Cardiology Information System (EPD vision®, Leiden University Medical Centre) and retrospectively analysed.
According to the clinical protocol, within 48 h after CRT implantation, the patients underwent repeat echocardiography to evaluate whether a significant change in LV mechanical dyssynchrony occurred. Subsequently, patients were divided into two groups according to the median value of LV dyssynchrony assessed at 48 h after CRT implantation. Patients with LV dyssynchrony equal or superior to the median value formed the induced LV dyssynchrony subgroup.
At 6-month follow-up, the clinical evaluation was repeated and LV volumes, LVEF, and LV dyssynchrony were re-assessed. Response to CRT was defined by ≥15% reduction in LV end-systolic volume (LVESV) at 6-month follow-up.
Baseline characteristics, CRT response rate at 6-month follow-up, and long-term outcome of patients with induced LV dyssynchrony after CRT implantation were compared with patients without induced LV dyssynchrony.
In addition, a group of 290 patients with overt LV mechanical dyssynchrony before CRT implantation (LV dyssynchrony value at baseline ≥60 ms) were selected from the ongoing registry. These patients formed the control group and were matched with the group of patients without baseline LV mechanical dyssynchrony according to age, gender, baseline LVEF, and NYHA functional class. The patients received CRT during the same time period as the group of patients without baseline LV dyssynchrony. The long-term survival of patients with and without significant induction of LV dyssynchrony at 48 h after CRT implantation was compared with the outcome of patients with overt LV dyssynchrony at baseline.
Clinical evaluation
Baseline clinical evaluation included the assessment of NYHA functional class, quality-of-life score (using the Minnesota living with Heart Failure Questionnaire), and evaluation of exercise capacity using the 6 min walk distance test.13,15,16
Echocardiography
Patients were imaged in the left lateral decubitus position using a commercially available system (Vingmed system Seven, General Electric-Vingmed, Milwaukee, WI, USA). Images were obtained using a 3.5 MHz transducer, at a depth of 16 cm in the parasternal and apical views (standard long-axis and two- and four-chamber images). Standard two-dimensional and colour Doppler data, triggered to the QRS complex, were saved in a cine-loop format.
The end-systolic and end-diastolic LV volumes and LVEF were measured from the conventional apical two- and four-chamber images, using the biplane Simpson's technique.17 All echocardiographic data acquisitions and analyses were performed blinded to the patients' baseline characteristics and clinical outcome. The intra-observer reproducibility for left ventricular end-diastolic volume (LVEDV), LVESV, and LVEF was 7.4 ± 11.2, 7.0 ± 10.1 mL, and 1.9 ± 4.4%, respectively.18 The inter-observer reproducibility for LVEDV, LVESV, and LVEF was 12.9 ± 14.7, 11.3 ± 13.9 mL, and 2.5 ± 4.9%, respectively.18
Left ventricular mechanical dyssynchrony assessment
In addition to the conventional echocardiographic examination, colour-coded TDI was performed to assess LV dyssynchrony. For TDI data acquisition, colour Doppler frame rates were set between 80 and 220 frames/s; pulse repetition frequencies were between 500 Hz and 1 KHz, resulting in aliasing velocities between 16 and 32 cm/s. TDI parameters were measured off-line from colour-coded images of three consecutive heartbeats. Data were analysed using commercial software (EchoPac 108.1.5, General Electric/Vingmed Ultrasound).
To determine LV dyssynchrony, the sample volume (6 mm × 12 mm) was placed in the LV basal parts of the septal and lateral walls (four-chamber apical view) and the time interval between the onset of the QRS complex and the peak systolic velocity was derived for each region. Left ventricular dyssynchrony was defined as the maximum delay between peak systolic velocities of the septal and the lateral walls.13 The analysis of peak systolic velocities was limited to the LV ejection period and post-systolic peaks were not included. Previously reported inter- and intra-observer agreement for assessment of LV dyssynchrony with colour-coded TDI was 90 and 96%, respectively.11
Pacemaker implantation
The LV pacing lead was inserted transvenously via the subclavian route. A coronary sinus venogram was obtained using a balloon catheter. Next, the LV pacing lead (Easytrak, Guidant Corporation, St Paul, MN, USA; Attain, Medtronic Inc., Minneapolis, MN, USA; or Corox, Biotronik, Berlin, Germany) was inserted through the coronary sinus with the help of an 8 Fr-guiding catheter and positioned in the venous system, preferably in a posterolateral vein. The right atrial and right ventricular leads were positioned conventionally. The CRT device and lead implantation were successful in all patients without major complications (Contak Renewal, Guidant Corporation; Insync III or Insync Sentry, Medtronic Inc.; or Lumax, Biotronik).
Within 24 h after CRT device implantation, the atrioventricular delay was adjusted to optimize LV diastolic filling as assessed with pulsed-wave Doppler echocardiography. The interventricular delay was set at 0 ms and was not systematically adjusted during the first 6 months of follow-up.
Outcome at long-term follow-up
Long-term follow-up was performed by chart review and telephone contact. All-cause mortality was the primary endpoint. Data on mortality were collected by reviewing medical records and retrieval of survival status through the municipal civil registries. All clinical variables were collected by independent observers blinded to the echocardiographic results. The long-term survival was compared between the following three groups of patients:
patients without significant LV dyssynchrony at baseline and after CRT implantation,
patients without significant LV dyssynchrony at baseline but induced LV dyssynchrony at 48 h after CRT implantation, and
patients with significant LV dyssynchrony at baseline.
Statistical analysis
Continuous data are expressed as mean ± SD or median and inter-quartile range, as appropriate, and were compared with the two-tailed Student's t-test for paired and unpaired data or a non-parametric test (Mann–Whitney U-test) when appropriate. Categorical variables are expressed as number and frequencies and were compared using the χ2 test. Changes in LV dyssynchrony along the three different time points of follow-up were compared with the Friedman test. Survival curves were determined according to the Kaplan–Meier method and comparisons of cumulative event rates were performed by the log-rank test. Cox's proportional hazard analysis was used to determine the value of acute LV dyssynchrony induction to predict long-term survival. First, univariate analysis of baseline clinical and echocardiographic characteristics was performed using all-cause mortality as an endpoint. For each variable, the hazard ratio (HR) and the 95% confidence intervals (CI) were calculated. In the multivariate analysis, the predictive values of acute LV dyssynchrony induction was corrected by those variables with a P-value of <0.20 in the univariate analysis. All statistical analyses were performed with SPSS software (version 17.0, SPSS Inc., Chicago, IL, USA). For all tests, a P-value of <0.05 was considered statistically significant.
Results
Patient population
A total of 290 consecutive patients without baseline LV mechanical dyssynchrony (<60 ms) and 290 patients with overt LV mechanical dyssynchrony (≥60 ms) at baseline were selected from an ongoing registry.14 Baseline characteristics of all patients are summarized in Table 1. The population comprised mostly men (77%), with a mean age of 67 ± 10 years. Heart failure was of ischaemic aetiology in 173 (59%) patients. The mean QRS duration was 152 ± 31 ms.
Table 1.
Baseline characteristics of patients with and without significant left ventricular dyssynchrony (≥60 and <60 ms, respectively)
| Variables | Baseline LV dyssynchrony |
P-value | |
|---|---|---|---|
| ≥60 ms (n= 290) | <60 ms (n= 290) | ||
| Age (years) | 65 ± 11 | 67 ± 10 | 0.196 |
| Gender (male/female) | 216 /74 | 225 /65 | 0.218 |
| Ischaemic aetiology, n (%) | 161 (56%) | 173 (59%) | 0.413 |
| NYHA | 3.04 ± 0.21 | 3.08 ± 0.32 | 0.236 |
| 6MWT (m) | 310 ± 113 | 310 ± 118 | 0.983 |
| QoL score | 35 ± 18 | 34 ± 19 | 0.695 |
| QRS duration (ms) | 157 ± 34 | 152 ± 31 | 0.016 |
| QRS < 150 ms, n (%) | 109 (38%) | 131 (45%) | 0.064 |
| QRS morphology | |||
| LBBB | 209 (72%) | 201 (69%) | 0.262 |
| RBBB | 13 (5%) | 26 (9%) | 0.023 |
| IVCD | 68 (23%) | 63 (22%) | 0.383 |
| LVEDV | 224 ± 78 | 206 ± 74 | 0.005 |
| LVESV | 169 ± 68 | 156 ± 67 | 0.016 |
| LVEF (%) | 25 ± 8 | 25 ± 8 | 0.745 |
| LV dyssynchrony (ms) | 100 (80–120) | 22 (16–34) | <0.001 |
Continuous variables are expressed as mean ± SD. Left ventricular dyssynchrony is expressed as median and inter-quartile range. Categorical variables are expressed as n (%).
6MWT, 6 min walk distance test; IVCD, unspecified intraventricular conduction delay; LBBB, left bundle branch block; LVEDV, left ventricle end-diastolic volume; LVEF, left ventricle ejection fraction; LVESV, left ventricle end-systolic volume; NYHA, New York Heart functional class; QoL, quality-of-life questionnaire (Minnesota living with Heart Failure); RBBB, right bundle branch block.
Changes in left ventricular mechanical dyssynchrony after cardiac resynchronization therapy
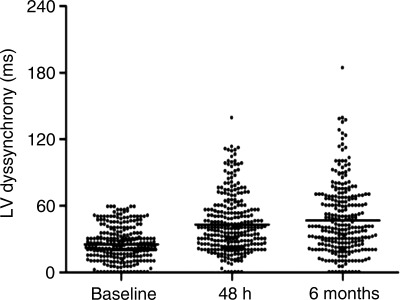
In patients without significant LV dyssynchrony at baseline, the median baseline LV dyssynchrony was 22 ms (inter-quartile range: 16–34 ms). After 48 h of continuous CRT, LV dyssynchrony increased to 40 ms (inter-quartile range: 24–56 ms) and remained unchanged at 6-month follow-up [40 ms (inter-quartile range: 24–67 ms)] (P< 0.001; Figure 1). Conversely, in the group of patients with significant LV dyssynchrony at baseline, the median LV dyssynchrony was 100 ms (inter-quartile range: 80–120 ms) and decreased to 45 ms (inter-quartile range: 20–70 ms) at 48 h and remained unchanged at 6-month follow-up [39 ms (inter-quartile range: 19–63 ms)] (P< 0.001).
Figure 1.
Assessment of LV dyssynchrony at baseline, 48 h after implantation, and at 6-month follow-up in patients without baseline LV dyssynchrony. At baseline, all patients showed LV dyssynchrony <60 ms [22 ms (inter-quartile range: 16–34 ms)]. At 48 h after cardiac resynchronization therapy implantation, LV dyssynchrony increased to 40 ms (inter-quartile range: 24–56 ms) and at 6-month follow-up remained unchanged [40 ms (inter-quartile range: 24–67 ms)] (P< 0.001).
Cardiac resynchronization therapy response in patients without baseline left ventricular mechanical dyssynchrony
According to the median value of LV dyssynchrony measured 48 h after CRT implantation, patients without significant baseline LV dyssynchrony were divided into two groups: patients with LV dyssynchrony ≥40 ms and patients showing LV dyssynchrony <40 ms.
Baseline characteristics of these two groups are presented in Table 2. Patients with LV dyssynchrony ≥40 ms were older (68 ± 9 vs. 65 ± 11 years, P= 0.004) and had more frequently ischaemic aetiology of heart failure (73 vs. 46%, P< 0.001) compared with patients with LV dyssynchrony <40 ms.
Table 2.
Baseline characteristics of the group of patients with and without induced left ventricular dyssynchrony after cardiac resynchronization therapy implantation
| Variables at baseline | Induced LV dyssynchrony ≥40 ms (n= 145) | Non-induced LV dyssynchrony <40 ms (n= 145) | P-value |
|---|---|---|---|
| Age (years) | 68 ± 11 | 65 ± 9 | 0.004 |
| Gender (male/female) | 113 /32 | 112 /33 | 0.432 |
| Ischaemic aetiology, n (%) | 106 (73%) | 67 (46%) | <0.001 |
| NYHA | 3.02 ± 0.14 | 3.02 ± 0.16 | 0.121 |
| 6MWT (m) | 277 ± 117 | 344 ± 108 | <0.001 |
| QoL | 33 ± 21 | 35 ± 19 | 0.310 |
| QRS duration (ms) | 150 ± 30 | 153 ± 31 | 0.443 |
| QRS < 150 ms, n (%) | 66 (46%) | 65 (45%) | 0.906 |
| QRS morphology | |||
| LBBB | 101 (69%) | 100 (69%) | 0.378 |
| RBBB | 16 (11%) | 10 (7%) | |
| IVCD | 28 (20%) | 35 (24%) | |
| LVEDV (mL) | 203 ± 71 | 210 ± 76 | 0.417 |
| LVESV (mL) | 154 ± 62 | 159 ± 69 | 0.512 |
| LVEF (%) | 25 ± 9 | 26 ± 9 | 0.540 |
| Baseline LV dyssynchrony (ms) | 23 (17–38) | 21 (15–30) | 0.457 |
Continuous variables are expressed as mean ± SD. Left ventricular dyssynchrony is expressed as median and inter-quartile range. Categorical variables are expressed as n (%).
6MWT, 6 min walk distance test; IVCD, unspecified intra-ventricular conduction delay; LBBB, left bundle branch block; LVEDV, left ventricle end-diastolic volume; LVEF, left ventricular ejection fraction; LVESV, left ventricle end-systolic volume; NYHA, New York Heart functional class; QoL, quality-of-life questionnaire (Minnesota living with Heart Failure); RBBB, right bundle branch block.
At 6-month follow-up, there were 81 patients (28%) who responded to CRT (defined by a decrease of ≥15% in LVESV) and 209 patients (72%) who did not respond to CRT. A total of 40 patients showed an increase in LVESV ≥ 15% at 6-month follow-up. The percentage of non-responders was significantly higher in the group of patients with LV dyssynchrony ≥40 ms after 48 h of CRT compared with the group of patients with LV dyssynchrony <40 ms (93 vs. 51%, P< 0.001).
Long-term outcome of patients with vs. without induced left ventricular dyssynchrony
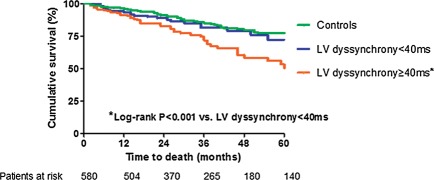
During a median follow-up of 34 months, a total of 73 (25.1%) patients without baseline LV dyssynchrony died. When the patient population was divided according to the median LV dyssynchrony assessed 48 h after CRT implantation (<40 vs. ≥40 ms), a cumulative 10, 17, and 23% of patients with LV dyssynchrony ≥40 ms died by 1-, 2-, and 3-year follow-up, respectively. In contrast, the group of patients with LV dyssynchrony <40 ms at 48 h after CRT implantation had superior outcome and, respectively, 3, 8, and 10% of patients died during the same time period (log-rank P< 0.001; Figure 2). Finally, in the group of patients with significant LV dyssynchrony at baseline, 57 (19.7%) patients died during follow-up. The cumulative mortality rate in this group of patients at 1-, 2-, and 3-year follow-up was 3, 8, and 11%, respectively, and compared favourably with the group of patients with LV dyssynchrony <40 ms at 48 h after CRT implantation (log-rank P= 0.375).
Figure 2.
The Kaplan–Meier estimates of time to all-cause mortality in patients without induced left ventricular dyssynchrony (n= 145) and induced left ventricular dyssynchrony (n= 145) and control group (baseline left ventricular dyssynchrony ≥60 ms, n= 290). LV, left ventricular. *Log-rank P < 0.001 vs. left ventricular dyssynchrony <40 ms.
Among the patients without baseline LV dyssynchrony and who exhibited an increase in LVESV ≥ 15% at 6-month follow-up, a total of 13 patients died after a median follow-up of 34 months. In this group of patients, cumulative mortality rates at 1-, 2-, and 3-year follow-up were 5, 26, and 36%, respectively.
Baseline clinical and echocardiographic parameters were evaluated to predict all-cause mortality for patients without baseline LV dyssynchrony. In the univariate analysis, age, ischaemic aetiology of heart failure, NYHA functional class, LVEF, and acute LV dyssynchrony were significant predictors of all-cause mortality (Table 3). In the multivariate Cox regression analysis, induced LV dyssynchrony was an independent predictor of all-cause mortality with an HR of 1.247 for each 20 ms increase (95% CI: 1.056–1.474, P= 0.009). Age, ischaemic aetiology of heart failure, baseline NYHA functional class, and baseline LVEF were also significantly related to all-cause mortality (Table 3).
Table 3.
Univariate and multivariate Cox's regression analysis to identify predictors of long-term mortality
| Variables | Univariate |
Multivariate |
||
|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Age (years) | 1.42 (1.016–1.062) | 0.010 | 1.037 (1.009–1.067) | 0.010 |
| Gender (male) | 0.749 (0.445–1.262) | 0.278 | — | — |
| Ischaemic aetiology | 1.766 (1.066–2.927) | 0.027 | 1.926 (1.088–3.407) | 0.024 |
| NYHA | 2.999 (1.895–4.798) | <0.001 | 2.412 (1.515–3.842) | <0.001 |
| LVESV (mL) | 1.000 (0.997–1.003) | 0.935 | — | — |
| LVEF (%) | 0.962 (0.935–0.990) | 0.007 | 0.951 (0.923–0.981) | 0.001 |
| QRS width (ms) | 1.004 (0.996–1.011) | 0.358 | — | — |
| Induced LV dyssynchrony (per 20 ms increase) | 1.013 (1.005–1.021) | 0.001 | 1.247 (1.056–1.474) | 0.009 |
6MWT, 6 min walk distance test; LV, left ventricle; LVEF, left ventricle ejection fraction; LVEDV, left ventricle end-diastolic volume; LVESV, left ventricle end-systolic volume; NYHA, New York Heart functional class; QoL, quality-of-life questionnaire (Minnesota living with Heart Failure).
Discussion
The present observational study demonstrated that in heart failure patients without significant LV mechanical dyssynchrony at baseline, CRT may induce significant LV dyssynchrony inasmuch as 50% of the patients. In addition, the patients who experienced more extensive LV dyssynchrony after CRT implantation showed less LV reverse remodelling at 6-month follow-up and worse long-term outcome than patients without induced LV dyssynchrony.
Importance of assessing left ventricular dyssynchrony before cardiac resynchronization therapy implantation
QRS complex width is presently used to select patients for CRT as a measure of LV dyssynchrony.1,2 However, electrical dyssynchrony is not equivalent to LV mechanical dyssynchrony and a poor correlation between QRS duration and LV dyssynchrony has been reported.11 Furthermore, heart failure patients with narrow QRS complex may show echocardiographic mechanical LV dyssynchrony amenable to be corrected with CRT.19
The additional value of echocardiographic LV dyssynchrony assessment before CRT implantation has been demonstrated in many single-centre trials.3,5,20 The presence of baseline LV dyssynchrony predicts favourable response to CRT and improved long-term outcome.21 In addition, recent subanalysis of the PROSPECT trial, including 286 patients treated with CRT, demonstrated that larger baseline LV dyssynchrony as assessed with TDI was strongly associated with larger reduction in LVESV at 6-month follow-up (P= 0.0022).22 Furthermore, it has been suggested that the persistence of LV dyssynchrony after CRT may be a reason for suboptimal response at follow-up.23 Based on this evidence, CRT appears to exert beneficial effects in those patients with baseline LV dyssynchrony and induces a favourable LV reverse remodelling with improved outcome at mid- and long-term follow-up.24,25 However, the effects of CRT on LV dyssynchrony in patients without significant baseline LV mechanical dyssynchrony have not been extensively studied. In addition, it was unclear whether an eventually induced LV mechanical dyssynchrony after CRT may impact on long-term prognosis of heart failure patients. The present study demonstrated for the first time that CRT may induce LV mechanical dyssynchrony which was associated with less favourable LV reverse remodelling and worse long-term prognosis.
Effects of cardiac resynchronization therapy on left ventricular dyssynchrony
In heart failure patients fulfilling the current inclusion criteria for CRT, the prevalence of LV mechanical dyssynchrony as assessed with TDI is around 69–75%.14,26 In this subgroup of patients, after CRT implantation, the majority of patients show a significant reduction in LV mechanical dyssynchrony which has been associated with favourable LV reverse remodelling.8 However, in 5% of patients with overt LV mechanical dyssynchrony at baseline, CRT may induce worsening of LV dyssynchrony and prevent LV reverse remodelling.8 In contrast, 25–31% of the patients who may eventually receive a CRT device do not show LV mechanical dyssynchrony at baseline.14,26 In this subgroup of patients, CRT may induce LV mechanical dyssynchrony, as demonstrated in the present study.
As previously mentioned, QRS duration is not the optimal criterion to identify the patients who will respond to CRT. In contrast, QRS morphology may provide further assessment of the pattern of activation within the LV. The results of a recent subanalysis of the MADIT-CRT trial have shown that the patients with left bundle branch block (LBBB) exhibit a greater clinical benefit from CRT compared with patients with other QRS complex morphologies (i.e. right bundle branch block or unspecific interventricular conduction delay).27 However, LBBB is a heterogeneous conduction disorder yielding different LV activation time delays as assessed by surface ECG.28 Indeed, despite a wide QRS complex with LBBB morphology, Sweeney et al.28 showed that patients with shorter LV activation time delays (≤80 ms) had a 51% response rate compared with 73% response in patients with larger LV activation time delays (≥125 ms). These differences in LV activation time delays may result in different LV mechanical activation patterns that determine a different response to CRT. Therefore, the presence of LV mechanical dyssynchrony amenable to be corrected with CRT rather than only the width or morphology of the QRS complex may be more important to predict a favourable response to CRT.21 In the present study, despite showing a QRS width of ≥120 ms, a substantial number of patients did not show LV mechanical dyssynchrony on TDI echocardiography. After CRT, a significant percentage of patients showed increased LV dyssynchrony which remained at 6-month follow-up. The group of patients with induced LV dyssynchrony after CRT showed a lower response rate and worse long-term outcome when compared with patients without induced LV dyssynchrony. In contrast, the control group formed by heart failure patients with overt LV mechanical dyssynchrony at baseline showed a significant decrease in LV mechanical dyssynchrony and improved long-term outcome. Several trials have demonstrated the relationship between restoration of LV synchrony and CRT response and improved long-term outcome.8,29 In contrast, the results of the present evaluation provide novel insights by demonstrating that the lack of response to CRT may be explained by induction of LV dyssynchrony in patients who show LV synchronous contraction despite wide QRS complex.
Clinical implications
Previous studies have shown the long-term benefits of CRT implantation in advanced heart failure patients.30,31 CRT induces LV reverse remodelling and improves survival by restoring LV synchrony.8 However, in a significant percentage of patients without LV dyssynchrony at baseline, CRT induced LV dyssynchrony acutely. This group of patients had worse outcome than patients without induced LV dyssynchrony. Therefore, in a patient selection process for CRT, accurate assessment of LV mechanical dyssynchrony seems to be clinically relevant, since the induction of LV dyssynchrony after CRT may prevent LV reverse remodelling and portend worse long-term outcome. In addition, close monitoring of LV mechanical dyssynchrony after CRT implantation and further adjustments of the device settings, such as interventricular delay, to correct LV dyssynchrony may help to increase the favourable response rate to CRT and to improve long-term survival.
Several limitations have to be acknowledged. First, the present evaluation is a retrospective analysis and, accordingly, we cannot conclude whether withdrawal of CRT should be indicated in patients who show worsening of LV dyssynchrony after CRT. A post hoc analysis from the MADIT-CRT trial has recently demonstrated that LV mechanical dyssynchrony worsening was associated with an increased risk for the occurrence of primary endpoint (death or heart failure event).29 Despite the study was not primarily designed to demonstrate the relationship between changes in LV synchrony and outcomes, the data come from one of the largest series of patients included in a randomized controlled trial and are in line with the results of the current evaluation. However, these data should be interpreted with caution and additional randomized controlled trials are needed to confirm this hypothesis. Secondly, the assessment of LV dyssynchrony was performed only with TDI. Reproducibility was one of the main limitations of this technique in the PROSPECT trial.32 However, in high experienced centres, the reproducibility of TDI-derived LV dyssynchrony is good.11 The results of the PROSPECT trial promoted the research of novel LV dyssynchrony measurements that are currently tested in randomized control trials including heart failure patients who fulfil (TARGET trial) and do not fulfil the current inclusion criteria for CRT (i.e. EchoCRT trial).33–35 The use of radial strain imaging with speckle tracking, for example, may be more appropriate since it overcomes some of the limitations of TDI (angle insonation dependency, tethering and traction from other myocardial regions, and cardiac translational artefacts) (see supplementary material). Finally, the baseline characteristics of patients with and without induced LV dyssynchrony were not completely comparable. The group of patients with induced LV dyssynchrony after CRT had more frequently ischaemic heart failure and poorer exercise capacity compared with patients without induced LV dyssynchrony.
Conclusion
In heart failure patients without LV mechanical dyssynchrony at baseline, induction of LV dyssynchrony after CRT may be associated with a less favourable response rate and less favourable long-term outcome. Therefore, LV mechanical dyssynchrony assessment at baseline in heart failure patients undergoing CRT implantation could be crucial in order to anticipate the results of the therapy. The absence of LV mechanical dyssynchrony at baseline may anticipate the need of further adjustments of the device settings in order to minimize the induction of LV dyssynchrony after CRT implantation.
Supplementary material
Supplementary material is available at European Heart Journal online.
Funding
D.A. is financially supported by the ‘Programme de bourse de perfectionnement et de fellowship du Centre Hospitalier de l'Université de Montréal (CHUM) et de la Fondation du CHUM’. S.H.E. is financially supported by the Ministry of Health Training Scholarship, Singapore. T.G.W. is financially supported by the Research Fellowship of the European Society of Cardiology.
Conflict of interest: The department of cardiology received research grants from Biotronik, Medtronic, Boston Scientific, St Jude Medical, GE Healthcare, Lantheus Medical Imaging, and Edwards Lifesciences. V.D. received consulting fees from St Jude medical.
Supplementary Material
References
- 1.Epstein AE, DiMarco JP, Ellenbogen KA, Estes NA, 3rd, Freedman RA, Gettes LS, Gillinov AM, Gregoratos G, Hammill SC, Hayes DL, Hlatky MA, Newby LK, Page RL, Schoenfeld MH, Silka MJ, Stevenson LW, Sweeney MO, Smith SC, Jr, Jacobs AK, Adams CD, Anderson JL, Buller CE, Creager MA, Ettinger SM, Faxon DP, Halperin JL, Hiratzka LF, Hunt SA, Krumholz HM, Kushner FG, Lytle BW, Nishimura RA, Ornato JP, Riegel B, Tarkington LG, Yancy CW. ACC/AHA/HRS 2008 Guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices): developed in collaboration with the American Association for Thoracic Surgery and Society of Thoracic Surgeons. Circulation. 2008;117:e350–e408. doi: 10.1161/CIRCUALTIONAHA.108.189742. [DOI] [PubMed] [Google Scholar]
- 2.Dickstein K, Vardas PE, Auricchio A, Daubert JC, Linde C, McMurray J, Ponikowski P, Priori SG, Sutton R, van Veldhuisen DJ, Vahanian A, Bax J, Ceconi C, Dean V, Filippatos G, Funck-Brentano C, Hobbs R, Kearney P, McDonagh T, Popescu BA, Reiner Z, Sechtem U, Sirnes PA, Tendera M, Vardas P, Widimsky P, Anker SD, Blanc JJ, Gasparini M, Hoes AW, Israel CW, Kalarus Z, Merkely B, Swedberg K, Camm AJ. 2010 Focused Update of ESC Guidelines on device therapy in heart failure: an update of the 2008 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure and the 2007 ESC guidelines for cardiac and resynchronization therapy. Developed with the special contribution of the Heart Failure Association and the European Heart Rhythm Association. Eur Heart J. 2010;31:2677–2687. doi: 10.1093/eurheartj/ehq337. [DOI] [PubMed] [Google Scholar]
- 3.Bilchick KC, Dimaano V, Wu KC, Helm RH, Weiss RG, Lima JA, Berger RD, Tomaselli GF, Bluemke DA, Halperin HR, Abraham T, Kass DA, Lardo AC. Cardiac magnetic resonance assessment of dyssynchrony and myocardial scar predicts function class improvement following cardiac resynchronization therapy. JACC Cardiovasc Imaging. 2008;1:561–568. doi: 10.1016/j.jcmg.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Delgado V, Ypenburg C, van Bommel RJ, Tops LF, Mollema SA, Marsan NA, Bleeker GB, Schalij MJ, Bax JJ. Assessment of left ventricular dyssynchrony by speckle tracking strain imaging comparison between longitudinal, circumferential, and radial strain in cardiac resynchronization therapy. J Am Coll Cardiol. 2008;51:1944–1952. doi: 10.1016/j.jacc.2008.02.040. [DOI] [PubMed] [Google Scholar]
- 5.Henneman MM, Chen J, Dibbets-Schneider P, Stokkel MP, Bleeker GB, Ypenburg C, van der Wall EE, Schalij MJ, Garcia EV, Bax JJ. Can LV dyssynchrony as assessed with phase analysis on gated myocardial perfusion SPECT predict response to CRT? J Nucl Med. 2007;48:1104–1111. doi: 10.2967/jnumed.107.039925. [DOI] [PubMed] [Google Scholar]
- 6.Marsan NA, Bleeker GB, Ypenburg C, Van Bommel RJ, Ghio S, Van de Veire NR, Delgado V, Holman ER, van der Wall EE, Schalij MJ, Bax JJ. Real-time three-dimensional echocardiography as a novel approach to assess left ventricular and left atrium reverse remodeling and to predict response to cardiac resynchronization therapy. Heart Rhythm. 2008;5:1257–1264. doi: 10.1016/j.hrthm.2008.05.021. [DOI] [PubMed] [Google Scholar]
- 7.Yu CM, Gorcsan J, 3rd, Bleeker GB, Zhang Q, Schalij MJ, Suffoletto MS, Fung JW, Schwartzman D, Chan YS, Tanabe M, Bax JJ. Usefulness of tissue Doppler velocity and strain dyssynchrony for predicting left ventricular reverse remodeling response after cardiac resynchronization therapy. Am J Cardiol. 2007;100:1263–1270. doi: 10.1016/j.amjcard.2007.05.060. [DOI] [PubMed] [Google Scholar]
- 8.Bleeker GB, Mollema SA, Holman ER, Van de Veire N, Ypenburg C, Boersma E, van der Wall EE, Schalij MJ, Bax JJ. Left ventricular resynchronization is mandatory for response to cardiac resynchronization therapy: analysis in patients with echocardiographic evidence of left ventricular dyssynchrony at baseline. Circulation. 2007;116:1440–1448. doi: 10.1161/CIRCULATIONAHA.106.677005. [DOI] [PubMed] [Google Scholar]
- 9.Kaszala K, Ellenbogen KA. When right may not be right: right bundle-branch block and response to cardiac resynchronization therapy. Circulation. 2010;122:1999–2001. doi: 10.1161/CIRCULATIONAHA.110.986943. [DOI] [PubMed] [Google Scholar]
- 10.Gorcsan J, 3rd, Oyenuga O, Habib PJ, Tanaka H, Adelstein EC, Hara H, McNamara DM, Saba S. Relationship of echocardiographic dyssynchrony to long-term survival after cardiac resynchronization therapy. Circulation. 2010;122:1910–1918. doi: 10.1161/CIRCULATIONAHA.110.954768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bleeker GB, Schalij MJ, Molhoek SG, Verwey HF, Holman ER, Boersma E, Steendijk P, Van Der Wall EE, Bax JJ. Relationship between QRS duration and left ventricular dyssynchrony in patients with end-stage heart failure. J Cardiovasc Electrophysiol. 2004;15:544–549. doi: 10.1046/j.1540-8167.2004.03604.x. [DOI] [PubMed] [Google Scholar]
- 12.Tanabe M, Dohi K, Onishi K, Nakata T, Sato Y, Nakajima H, Takamura T, Miyahara M, Nakamura M, Takeda K, Ito M. Biventricular pacing worsened dyssynchrony in heart failure patient with right-bundle branch block. Int J Cardiol. 2010;138:e47–50. doi: 10.1016/j.ijcard.2008.06.063. [DOI] [PubMed] [Google Scholar]
- 13.Bax JJ, Marwick TH, Molhoek SG, Bleeker GB, van Erven L, Boersma E, Steendijk P, van der Wall EE, Schalij MJ. Left ventricular dyssynchrony predicts benefit of cardiac resynchronization therapy in patients with end-stage heart failure before pacemaker implantation. Am J Cardiol. 2003;92:1238–1240. doi: 10.1016/j.amjcard.2003.06.016. [DOI] [PubMed] [Google Scholar]
- 14.van Bommel RJ, Borleffs CJ, Ypenburg C, Marsan NA, Delgado V, Bertini M, van der Wall EE, Schalij MJ, Bax JJ. Morbidity and mortality in heart failure patients treated with cardiac resynchronization therapy: influence of pre-implantation characteristics on long-term outcome. Eur Heart J. 2010;31:2783–2790. doi: 10.1093/eurheartj/ehq252. [DOI] [PubMed] [Google Scholar]
- 15.Lipkin G, Knecht ME, Rosenberg M. A potent inhibitor of normal and transformed cell growth derived from contact-inhibited cells. Cancer Res. 1978;38:635–643. [PubMed] [Google Scholar]
- 16.Rector TS, Kubo SH, Cohn JN. Validity of the Minnesota Living with Heart Failure questionnaire as a measure of therapeutic response to enalapril or placebo. Am J Cardiol. 1993;71:1106–1107. doi: 10.1016/0002-9149(93)90582-w. [DOI] [PubMed] [Google Scholar]
- 17.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise J, Solomon S, Spencer KT, St John Sutton M, Stewart W. Recommendations for chamber quantification. Eur J Echocardiogr. 2006;7:79–108. doi: 10.1016/j.euje.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 18.Bertini M, Marsan NA, Delgado V, van Bommel RJ, Nucifora G, Borleffs CJ, Boriani G, Biffi M, Holman ER, van der Wall EE, Schalij MJ, Bax JJ. Effects of cardiac resynchronization therapy on left ventricular twist. J Am Coll Cardiol. 2009;54:1317–1325. doi: 10.1016/j.jacc.2009.05.063. [DOI] [PubMed] [Google Scholar]
- 19.van Bommel RJ, Tanaka H, Delgado V, Bertini M, Borleffs CJ, Ajmone Marsan N, Holzmeister J, Ruschitzka F, Schalij MJ, Bax JJ, Gorcsan J., 3rd Association of intraventricular mechanical dyssynchrony with response to cardiac resynchronization therapy in heart failure patients with a narrow QRS complex. Eur Heart J. 2010;31:3054–3062. doi: 10.1093/eurheartj/ehq334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu CM, Fung JW, Zhang Q, Chan CK, Chan YS, Lin H, Kum LC, Kong SL, Zhang Y, Sanderson JE. Tissue Doppler imaging is superior to strain rate imaging and postsystolic shortening on the prediction of reverse remodeling in both ischemic and nonischemic heart failure after cardiac resynchronization therapy. Circulation. 2004;110:66–73. doi: 10.1161/01.CIR.0000133276.45198.A5. [DOI] [PubMed] [Google Scholar]
- 21.Bax JJ, Gorcsan J., 3rd Echocardiography and noninvasive imaging in cardiac resynchronization therapy: results of the PROSPECT (Predictors of Response to Cardiac Resynchronization Therapy) study in perspective. J Am Coll Cardiol. 2009;53:1933–1943. doi: 10.1016/j.jacc.2008.11.061. [DOI] [PubMed] [Google Scholar]
- 22.van Bommel RJ, Bax JJ, Abraham WT, Chung ES, Pires LA, Tavazzi L, Zimetbaum PJ, Gerritse B, Kristiansen N, Ghio S. Characteristics of heart failure patients associated with good and poor response to cardiac resynchronization therapy: a PROSPECT (Predictors of Response to CRT) sub-analysis. Eur Heart J. 2009;30:2470–2477. doi: 10.1093/eurheartj/ehp368. [DOI] [PubMed] [Google Scholar]
- 23.Mullens W, Grimm RA, Verga T, Dresing T, Starling RC, Wilkoff BL, Tang WH. Insights from a cardiac resynchronization optimization clinic as part of a heart failure disease management program. J Am Coll Cardiol. 2009;53:765–773. doi: 10.1016/j.jacc.2008.11.024. [DOI] [PubMed] [Google Scholar]
- 24.Ypenburg C, van Bommel RJ, Borleffs CJ, Bleeker GB, Boersma E, Schalij MJ, Bax JJ. Long-term prognosis after cardiac resynchronization therapy is related to the extent of left ventricular reverse remodeling at midterm follow-up. J Am Coll Cardiol. 2009;53:483–490. doi: 10.1016/j.jacc.2008.10.032. [DOI] [PubMed] [Google Scholar]
- 25.Van Bommel RJ, Ypenburg C, Borleffs CJ, Delgado V, Marsan NA, Bertini M, Holman ER, Schalij MJ, Bax JJ. Value of tissue Doppler echocardiography in predicting response to cardiac resynchronization therapy in patients with heart failure. Am J Cardiol. 2010;105:1153–1158. doi: 10.1016/j.amjcard.2009.12.018. [DOI] [PubMed] [Google Scholar]
- 26.Chan CP, Zhang Q, Yip GW, Fung JW, Lam YY, Lee PW, Wu EB, Shang Q, Liang Y, Yu CM. Relation of left ventricular systolic dyssynchrony in patients with heart failure to left ventricular ejection fraction and to QRS duration. Am J Cardiol. 2008;102:602–605. doi: 10.1016/j.amjcard.2008.04.032. [DOI] [PubMed] [Google Scholar]
- 27.Zareba W, Klein H, Cygankiewicz I, Hall WJ, McNitt S, Brown M, Cannom D, Daubert JP, Eldar M, Gold MR, Goldberger JJ, Goldenberg I, Lichstein E, Pitschner H, Rashtian M, Solomon S, Viskin S, Wang P, Moss AJ. Effectiveness of cardiac resynchronization therapy by QRS morphology in the Multicenter Automatic Defibrillator Implantation Trial-Cardiac Resynchronization Therapy (MADIT-CRT) Circulation. 2011;123:1061–1072. doi: 10.1161/CIRCULATIONAHA.110.960898. [DOI] [PubMed] [Google Scholar]
- 28.Sweeney MO, van Bommel RJ, Schalij MJ, Borleffs CJ, Hellkamp AS, Bax JJ. Analysis of ventricular activation using surface electrocardiography to predict left ventricular reverse volumetric remodeling during cardiac resynchronization therapy. Circulation. 2010;121:626–634. doi: 10.1161/CIRCULATIONAHA.109.894774. [DOI] [PubMed] [Google Scholar]
- 29.Pouleur AC, Knappe D, Shah AM, Uno H, Bourgoun M, Foster E, McNitt S, Hall WJ, Zareba W, Goldenberg I, Moss AJ, Pfeffer MA, Solomon SD. Relationship between improvement in left ventricular dyssynchrony and contractile function and clinical outcome with cardiac resynchronization therapy: the MADIT-CRT trial. Eur Heart J. 2011;32:1720–1729. doi: 10.1093/eurheartj/ehr185. [DOI] [PubMed] [Google Scholar]
- 30.Cleland JG, Daubert JC, Erdmann E, Freemantle N, Gras D, Kappenberger L, Tavazzi L. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;352:1539–1549. doi: 10.1056/NEJMoa050496. [DOI] [PubMed] [Google Scholar]
- 31.Abraham WT, Fisher WG, Smith AL, Delurgio DB, Leon AR, Loh E, Kocovic DZ, Packer M, Clavell AL, Hayes DL, Ellestad M, Trupp RJ, Underwood J, Pickering F, Truex C, McAtee P, Messenger J. Cardiac resynchronization in chronic heart failure. N Engl J Med. 2002;346:1845–1853. doi: 10.1056/NEJMoa013168. [DOI] [PubMed] [Google Scholar]
- 32.Chung ES, Leon AR, Tavazzi L, Sun JP, Nihoyannopoulos P, Merlino J, Abraham WT, Ghio S, Leclercq C, Bax JJ, Yu CM, Gorcsan J, 3rd, St John Sutton M, De Sutter J, Murillo J. Results of the Predictors of Response to CRT (PROSPECT) trial. Circulation. 2008;117:2608–2616. doi: 10.1161/CIRCULATIONAHA.107.743120. [DOI] [PubMed] [Google Scholar]
- 33.Echocardiography Guided Cardiac Resynchronization Therapy (EchoCRT) 30 August 2007. http://clinicaltrials.gov/ct2/show/NCT00683696?term=EchoCRT&rank=1. (31 May 2011) [Google Scholar]
- 34.Khan F, Virdee MS, Begley D, Pugh PJ, Read PA, Fynn SP, Dutka DP. Targeted left ventricular lead placement using speckle tracking echocardiography improves the acute hemodynamic response to cardiac resynchronization therapy: a randomized controlled trial. J Am Coll Cardiol. 2011;57:E2033. doi: 10.1016/j.jacc.2011.12.030. [DOI] [PubMed] [Google Scholar]
- 35.TARgeted left ventricular lead placement to Guide cardiac rEsynchronisation Therapy in patients with heart failure: a randomised prospective study (TARGET Study) September 2010. http://www.controlled-trials.com/ISRCTN19717943. (February 2011) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.