Abstract
Aims
Although acute venous thrombo-embolism (VTE) often afflicts patients with advanced age, the predictors of in-hospital mortality for elderly VTE patients are unknown.
Methods and results
Among 1247 consecutive patients with acute VTE from the prospective SWIss Venous ThromboEmbolism Registry (SWIVTER), 644 (52%) were elderly (≥65 years of age). In comparison to younger patients, the elderly more often had pulmonary embolism (PE) (60 vs. 42%; P< 0.001), cancer (30 vs. 20%; P< 0.001), chronic lung disease (14 vs. 8%; P= 0.001), and congestive heart failure (12 vs. 2%; P< 0.001). Elderly VTE patients were more often hospitalized (75 vs. 52%; P< 0.001), and there was no difference in the use of thrombolysis, catheter intervention, or surgical embolectomy between the elderly and younger PE patients (5 vs. 6%; P= 0.54), despite a trend towards a higher rate of massive PE in the elderly (8 vs. 4%; P= 0.07). The overall in-hospital mortality rate was 6.6% in the elderly vs. 3.2% in the younger VTE patients (P= 0.033). Cancer was associated with in-hospital death both in the elderly [hazard ratio (HR) 4.91, 95% confidence interval (CI) 2.32–10.38; P< 0.001] and in the younger patients (HR 4.90, 95% CI 1.37–17.59; P= 0.015); massive PE was a predictor of in-hospital death in the elderly only (HR 3.77, 95% CI 1.63–8.74; P= 0.002).
Conclusion
Elderly patients had more serious VTE than younger patients, and massive PE was particularly life-threatening in the elderly.
Keywords: Age, Deep vein thrombosis, Mortality, Pulmonary embolism, Venous thrombo-embolism
Introduction
The incidence of venous thrombo-embolism (VTE), comprising deep vein thrombosis (DVT) and pulmonary embolism (PE), increases exponentially with advancing age.1 The elderly, for whom the cut-off point of 65 years was adopted by the International Conference on Harmonisation of Technical Requirements of Pharmaceuticals for Human Use (ICH) E7 guideline on geriatric patients, represent the fastest growing demographic segment of the patient population in the industrial world.2 High incidences of concomitant diseases and co-medications, impaired hepatic or renal function, and changes in pharmacokinetic and pharmacodynamic profiles make elderly patients especially vulnerable. The current consensus guidelines of the American College of Chest Physicians (ACCP) do not issue specific recommendations devoted to the acute management of elderly VTE patients.3
Intermediate and long-term survival of elderly VTE patients is poor, and the elderly are especially prone to bleeding complications from anticoagulation or reperfusion treatment.4–8 It is unknown whether differences in VTE severity exist between the elderly and younger patients, and whether VTE severity impacts on short-term clinical outcomes. We also aimed to explore the clinical predictors of early mortality in elderly hospitalized patients with acute objectively confirmed VTE.
Methods
Patients
The prospective SWIss Venous ThromboEmbolism Registry (SWIVTER) enrolled 1247 consecutive patients with acute DVT or PE from 4 academic and 14 non-academic acute care hospitals in Switzerland from January 2009 to May 2010. Inclusion criteria were age ≥18 years and objectively confirmed acute VTE event, by compression ultrasound or phlebography in the case of DVT, and by contrast-enhanced chest computed tomography, ventilation perfusion scan, or conventional pulmonary angiography in the case of PE. There were no exclusion criteria. Eligible patients were enrolled during clinical inpatient or outpatient visits at participating hospitals. SWIVTER issued no recommendations on VTE diagnosis or management. The study was approved by the local Ethics Committees of the participating hospitals.
Data, definitions, and statistical analysis
Anonymous data on patient demographics, co-morbidities, localization of VTE, risk factors for VTE and bleeding, management, and clinical outcomes such as in-hospital mortality, recurrent VTE, and bleeding requiring treatment were collected in a standardized case report form (CRF). Data were directly entered into the electronic CRF by the physician in charge of the patient's care or by a study physician or a study nurse and then securely transmitted to a central database monitored by an independent data coordinating centre (La Volta Statistics, Zurich, Switzerland).
Elderly age was defined as an age of 65 or more years. Massive PE was predefined as PE with systolic arterial hypotension (blood pressure levels of <90 mmHg) or as PE requiring cardiopulmonary resuscitation or the administration of catecholamines.9 Provoked VTE was defined as thrombosis associated with surgery, hospitalization, immobilization for more than 3 days, oestrogen therapy, pregnancy, or prolonged travel of more than 5 h, all within 30 days prior to VTE diagnosis.3 Reperfusion therapy for PE included systemic thrombolysis, catheter intervention with or without thrombolysis, or surgical embolectomy. Reperfusion therapy for DVT included catheter-directed thrombolysis, catheter intervention with or without thrombolysis, or surgical thrombectomy. Recurrent VTE was defined as symptomatic non-fatal PE and/or symptomatic DVT after the initial VTE diagnosis.
Group comparisons for continuous variables with a normal distribution were performed using the t-test and the data were described as means with standard deviations (SD), group comparisons for continuous variables with a skewed distribution were performed using the Wilcoxon rank-sum test and the data were presented as median values with inter-quartile ranges (IQRs), and group comparisons for discrete variables were performed using the χ2 or Fisher exact test and the data were presented as frequencies and percentages.
For identifying clinical factors associated with in-hospital mortality, univariate Cox's regression analysis reporting hazard ratios (HRs) with 95% confidence intervals (CIs) was performed. All reported P-values are two-tailed. The data were analysed using STATA 10 software (STATACorp LP, College Station, TX, USA).
Results
Patient characteristics
Overall, 1247 patients were enrolled; 644 (52%) were elderly and 603 (48%) younger than 65 years of age. In comparison to younger patients, the elderly were more often hospitalized at the time of VTE diagnosis or immobilized for more than 3 days, more frequently had an acute infection, acute respiratory or heart failure, diabetes, chronic respiratory or heart disease, renal failure, and a history of stroke, but less often were obese and less frequently had recent surgery or trauma (Table 1).
Table 1.
Patient characteristics, co-morbidities, and venous thrombo-embolism therapy
| Total (n= 1247) | Age ≥65 (n= 644) | Age <65 (n= 603) | P-value | |
|---|---|---|---|---|
| Demographics | ||||
| Age (years), mean ± SD | 61 ± 18 | 76 ± 7 | 46 ± 13 | <0.001 |
| Women, n (%) | 605 (48.5) | 321 (49.8) | 284 (47.1) | 0.33 |
| Inpatient at the time of diagnosis, n (%) | 478 (38.3) | 304 (47.2) | 174 (28.9) | <0.001 |
| Prior hospitalization, n (%) | 332 (26.6) | 179 (27.8) | 153 (25.4) | 0.33 |
| Co-morbidities | ||||
| Cancer, n (%) | 315 (25.3) | 192 (29.8) | 123 (20.4) | <0.001 |
| Metastatic disease, n (%) | 179 (65.1) | 97 (59.9) | 82 (72.6) | 0.030 |
| Recent cancer surgery, n (%) | 83 (27.8) | 37 (20.9) | 46 (37.7) | 0.001 |
| Recent chemotherapy, n (%) | 154 (52.2) | 77 (44.0) | 77 (64.2) | 0.001 |
| Prior VTE, n (%) | 288 (23.1) | 147 (22.8) | 141 (23.4) | 0.82 |
| Bed rest for >3 days, n (%) | 231 (18.5) | 146 (22.7) | 85 (14.1) | <0.001 |
| Obesity, n (%) | 174 (14.0) | 76 (11.8) | 98 (16.3) | 0.023 |
| Surgery, n (%) | 170 (13.6) | 74 (11.5) | 96 (15.9) | 0.023 |
| Varicosis, n (%) | 164 (13.2) | 102 (15.8) | 62 (10.3) | 0.004 |
| Acute infection/sepsis n (%) | 148 (11.9) | 92 (14.3) | 56 (9.3) | 0.006 |
| Chronic lung disease, n (%) | 140 (11.2) | 91 (14.1) | 49 (8.1) | 0.001 |
| Diabetes, n (%) | 114 (9.1) | 90 (14.0) | 24 (4.0) | <0.001 |
| Ongoing chemotherapy, n (%) | 114 (9.1) | 59 (9.2) | 55 (9.1) | 0.98 |
| Trauma/fracture n (%) | 91 (7.3) | 34 (5.3) | 57 (9.5) | 0.005 |
| Renal failure, n (%) | 89 (7.1) | 61 (9.5) | 28 (4.6) | 0.001 |
| Inflammatory/rheumatic disease, n (%) | 89 (7.1) | 50 (7.8) | 39 (6.5) | 0.37 |
| Acute respiratory failure, n (%) | 83 (6.7) | 59 (9.2) | 24 (4.0) | <0.001 |
| Congestive heart failure, n (%) | 81 (6.5) | 71 (11.0) | 10 (1.7) | <0.001 |
| ICU admission, n (%) | 69 (5.5) | 41 (6.4) | 28 (4.6) | 0.18 |
| History of stroke/TIA, n (%) | 67 (5.4) | 61 (9.5) | 6 (1.0) | <0.001 |
| Bleeding requiring treatment within 30 days prior to VTE diagnosis, n (%) | 60 (4.8) | 34 (5.3) | 26 (4.3) | 0.43 |
| Acute heart failure, n (%) | 37 (3.0) | 31 (4.8) | 6 (1.0) | <0.001 |
| VTE therapy | ||||
| Inpatient therapy, n (%) | 800 (64.2) | 484 (75.2) | 316 (52.4) | <0.001 |
| Compression therapy, n (%) | 1'031 (82.7) | 548 (85.1) | 483 (80.1) | 0.020 |
| IVC filter, n (%) | 20 (1.6) | 12 (1.9) | 8 (1.3) | 0.45 |
| UFH or LMWH, n (%) | 1'200 (96.2) | 619 (96.1) | 581 (96.4) | 0.83 |
| Vitamin K antagonist, n (%) | 970 (77.8) | 509 (79.0) | 461 (76.5) | 0.27 |
| Planned duration of anticoagulation | ||||
| ≤3 months, n (%) | 198 (15.9) | 85 (13.2) | 113 (18.7) | 0.007 |
| >3–6 months, n (%) | 483 (38.7) | 237 (36.8) | 246 (40.8) | 0.15 |
| >6–12 months, n (%) | 243 (19.5) | 128 (19.9) | 115 (19.1) | 0.72 |
| >12 months or indefinitely, n (%) | 323 (25.9) | 194 (30.1) | 129 (21.4) | <0.001 |
VTE, venous thrombo-embolism; ICU, intensive care unit; TIA, transient ischemic attack; IVC, inferior vena cava; UFH, unfractionated heparin; LMWH, low-molecular-weight heparin.
The elderly more often had acute PE when compared with the younger patients (60 vs. 42%; P< 0.001), whereas they less often had first isolated distal DVT (11 vs. 18%; P= 0.001) and upper-extremity DVT (5 vs. 8%; P= 0.011). In comparison to younger patients, the elderly more frequently had cancer-associated VTE (30 vs. 20%; P< 0.001) and less often had provoked VTE (38 vs. 45%; P= 0.009). Among patients who were diagnosed during an outpatient visit, the elderly more often had acute PE than younger patients (54 vs. 37%; P< 0.001). Among patients who were diagnosed during an inpatient visit, the elderly more often had acute PE when compared with the younger patients (67 vs. 56%; P= 0.013). Among patients who were treated on an outpatient basis, acute PE was similarly often present in the elderly and younger patients (21 vs. 18%; P= 0.46). Among patients who were hospitalized after VTE diagnosis, the elderly more often had acute PE when compared with the younger patients (74 vs. 65%; P= 0.007).
Among patients with acute PE, there was a trend towards a higher rate of massive PE in the elderly vs. younger patients, and the elderly more frequently had positive cardiac biomarkers, troponin T or I or (N-terminal pro) B-type natriuretic peptide (BNP), oxygen saturation in room air below 90%, dyspnoea, and a high simplified pulmonary embolism severity index (sPESI) when compared with the younger patients (Table 2). Among PE patients ≤80 years of age, patients between 65 and 80 years of age more often had a high sPESI than patients below 65 years of age (69 vs. 49%; P< 0.001). Among patients with DVT alone, DVT of the inferior vena cava (IVC) or the iliac veins was similarly often present in both age groups (18 vs. 17%; P= 0.80). Among patients with cancer-associated VTE, younger patients more frequently had metastatic disease, recent chemotherapy, or surgery (Table 1).
Table 2.
Patient characteristics and reperfusion therapy in patients with pulmonary embolism
| Total (n =644) | Age ≥65 (n =389) | Age <65 (n= 255) | P-value | |
|---|---|---|---|---|
| Dyspnoea, n (%) | 527 (82.5) | 331 (85.5) | 196 (77.8) | 0.012 |
| Positive (N-terminal pro) BNP,an (%) | 150/249 (60.2) | 121/180 (67.2) | 29/69 (42.0) | <0.001 |
| Right heart strain by ECG,bn (%) | 233/499 (46.7) | 149/305 (48.9) | 84/194 (43.3) | 0.23 |
| Concomitant DVT, n (%) | 227 (35.3) | 126 (32.4) | 101 (39.6) | 0.06 |
| Oxygen saturation in room air <90%, n (%) | 174/577 (30.2) | 124/340 (36.5) | 50/237 (21.1) | <0.001 |
| Positive troponin T or I,an (%) | 99/387 (25.6) | 79/251 (31.5) | 20/136 (14.7) | <0.001 |
| Heart rate ≥110 b.p.m., n (%) | 158/619 (25.5) | 100/369 (27.1) | 58/250 (23.2) | 0.28 |
| Syncope, n (%) | 64 (10.0) | 44 (11.3) | 20 (7.9) | 0.16 |
| Massive PE,cn (%) | 39 (6.1) | 29 (7.5) | 10 (3.9) | 0.07 |
| High sPESI,dn (%) | 430 (66.8) | 304 (78.2) | 126 (49.4) | <0.001 |
| Reperfusion therapy, n (%) | 36 (5.6) | 20 (5.1) | 16 (6.3) | 0.54 |
| Systemic thrombolysis, n (%) | 21 (3.3) | 13 (3.3) | 8 (3.1) | 0.89 |
| Catheter intervention, n (%) | 10 (1.6) | 5 (1.3) | 5 (2.0) | 0.50 |
| Surgical thrombectomy, n (%) | 9 (1.4) | 3 (0.8) | 6 (2.4) | 0.09 |
aA positive biomarker test result was defined as a quantitative biomarker level above the manufacturer's assay threshold.
bRight heart strain by electrocardiography (ECG) was defined as the presence of at least one of the following signs: sinus tachycardia, incomplete or complete right bundle branch block, SI-QIII type, or negative T-waves in V2, V3, or V4 within 24 h of VTE diagnosis.
cMassive PE was defined as PE with systolic arterial hypotension (systolic blood pressure of <90 mmHg) or as PE requiring cardiopulmonary resuscitation or the administration of catecholamines.
dA high simplified pulmonary embolism severity index (sPESI) was defined as the presence of at least one of the following criteria: age >80 years, systolic systemic pressure <100 mmHg, heart rate >110 b.p.m., oxygen saturation <90%, cancer, heart failure, and chronic lung disease.9
Initial and long-term treatment of VTE
Overall, 75% of the elderly and 52% of younger VTE patients were hospitalized (Table 1). In total, the median duration of hospital stay was 11 (IQR 7–20) days; it was 12 (7–22) days in the elderly and 10 (5–19) days in the younger patients (P= 0.022). Among the 644 patients with acute PE, elderly patients were more often hospitalized than younger patients (92 vs. 80%; P< 0.001). Among the 603 patients with acute DVT alone, elderly patients were more often treated on an inpatient basis than younger patients (50 vs. 32%; P< 0.001). Among VTE patients who were diagnosed during an outpatient visit, the elderly were more often hospitalized when compared with the younger patients (55 vs. 34%; P< 0.001). Among PE patients who were diagnosed during an outpatient visit, the elderly were more often hospitalized when compared with the younger patients (83 vs. 68%; P= 0.001).
There was no difference in the use of reperfusion therapy, including systemic thrombolysis, catheter intervention, and surgical thrombectomy between the elderly and younger PE patients (Table 2). There was also no difference in the use of reperfusion therapy (31 vs. 20%; P= 0.50), including systemic thrombolysis (21 vs. 20%; P= 0.96), catheter intervention (7 vs. 0%; P= 0.39), and surgical thrombectomy (7 vs. 0%; P= 0.39) between the elderly and younger patients with massive PE. Reperfusion therapy (0.8 vs. 4.6%; P= 0.007), including catheter-directed thrombolysis (0.4 vs. 3.5%; P= 0.011) and surgical thrombectomy (0.8 vs. 2.5%; P= 0.10), was less often administered in the elderly when compared with the younger patients with DVT alone.
Overall, the elderly more frequently received antithrombotic therapy with unfractionated heparin (31 vs. 18%; P< 0.001) and less often with low-molecular-weight heparin (80 vs. 88%; P< 0.001); twice-daily regimens were prescribed more frequently in the elderly (27 vs. 18%; P< 0.001). Mechanical therapy with vascular compression stockings or bandages was more often prescribed to elderly than to younger patients; there was no difference in the use of IVC filters between both age groups (Table 1).
In-hospital clinical outcomes
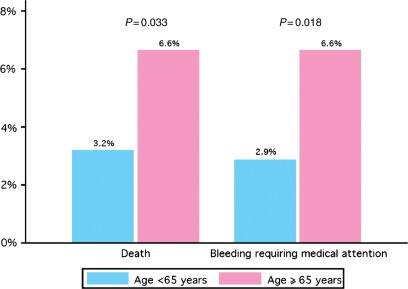
The overall rate of in-hospital mortality was 5.3%; this rate was greater in the elderly when compared with the younger patients (6.6 vs. 3.2%; P= 0.033; Figure 1). In total, the proportion of patients with in-hospital recurrent non-fatal VTE was 2.3%; it was 1.5% in the elderly and 3.5% in the younger patients (P= 0.06). The overall rate of in-hospital bleeding requiring treatment was 5.1%; this rate was greater in the elderly when compared with the younger patients (6.6 vs. 2.9%; P= 0.018).
Figure 1.
In-hospital mortality and bleeding requiring treatment (n= 800).
In hospitalized patients, cancer (HR 5.15, 95% CI 2.71–9.82; P< 0.001), massive PE (HR 4.25, 95% CI 1.96–9.20; P< 0.001), hypoxia (HR 4.17, 95% CI 2.10–8.25; P< 0.001), chronic lung disease (HR 3.16, 95% CI 1.68–5.96; P< 0.001), tachycardia (HR 2.52, 95% CI 1.36–4.67; P= 0.003), and an increasing age (HR 1.04, 95% CI 1.02–1.07; P=0.001) were univariate predictors of in-hospital death. In elderly hospitalized VTE patients, cancer, massive PE, hypoxia, and chronic lung disease were univariately associated with in-hospital death (Table 3). In younger hospitalized VTE patients, hypoxia, tachycardia, and cancer were univariate predictors of in-hospital death.
Table 3.
Clinical factors univariately associated with in-hospital mortality
| Factor | HR | 95% CI | P-value |
|---|---|---|---|
| Patients ≥65 years of age | |||
| Cancer | 4.91 | 2.32–10.38 | <0.001 |
| Massive PE | 3.77 | 1.63–8.74 | 0.002 |
| Oxygen saturation in room air <90% | 3.58 | 1.65–7.77 | 0.001 |
| Chronic lung disease | 2.89 | 1.42–5.85 | 0.003 |
| Heart rate ≥110 b.p.m. | 1.98 | 0.97–4.05 | 0.06 |
| Congestive heart failure | 1.32 | 0.54–3.22 | 0.54 |
| Recurrent VTE | 0.23 | 0.05–0.96 | 0.044 |
| Patients <65 years of age | |||
| Oxygen saturation in room air <90% | 6.90 | 1.34–35.69 | 0.021 |
| Heart rate ≥110 b.p.m. | 5.82 | 1.53–22.14 | 0.010 |
| Cancer | 4.90 | 1.37–17.59 | 0.015 |
| Congestive heart failure | 4.42 | 0.55–35.41 | 0.16 |
| Massive PE | 4.02 | 0.49–32.68 | 0.19 |
| Chronic lung disease | 3.31 | 0.67–16.38 | 0.14 |
| Recurrent VTE | 1.79 | 0.49–6.60 | 0.38 |
HR, hazard ratio; CI, confidence interval; PE, pulmonary embolism; VTE, venous thrombo-embolism.
Discussion
In the SWIVTER, elderly patients over 65 years of age had more serious VTE than younger patients. First, PE occurred more frequently in the elderly when compared with the younger VTE patients. In addition, PE severity was pronounced in the elderly as evidenced by higher rates of an increased sPESI,10 of increased cardiac biomarkers, and a trend towards a higher rate of massive PE. Secondly, massive PE was particularly life-threatening in the elderly. Finally, potentially life-saving reperfusion treatment was used in less than one-third of patients with massive PE from both age groups. Overall, our data suggest but do not prove that there might be an underuse of reperfusion therapy among patients with massive PE. High rates of co-morbidities and an increased risk of bleeding may partly explain the omission of reperfusion therapy, particularly in elderly patients with massive PE. However, an increased risk of bleeding may prohibit the administration of systemic thrombolysis but not necessarily a catheter intervention, with or without the use of catheter-directed thrombolysis, or surgical embolectomy.
The clinical manifestation of VTE between the elderly and younger patients differed substantially in SWIVTER; the elderly more often had cancer and less frequently had provoked VTE and first isolated distal or upper-extremity DVT when compared with the younger patients. The higher incidence of cancer-associated VTE and the lower incidence of upper-extremity DVT in the elderly in our study are consistent with the results of the Worcester Venous Thromboembolism Study11 and previous registries.12,13 The finding of a lower proportion of provoked VTE in the elderly vs. younger patients contrasts with the Worcester Venous Thromboembolism Study.11
When compared with a recent review of studies of elderly patients with acute PE, the most common clinical PE symptoms in our study were dyspnoea (86 vs. 59–92%) and tachycardia (27 vs. 29–76%).14 The overall proportion of massive PE in SWIVTER and in the International Cooperative Pulmonary Embolism Registry (ICOPER) was similar (6 vs. 5%), and both registries reported that more than two-thirds of patients with massive PE did not receive reperfusion therapy.9
Our study offers important insights regarding the relationship between age and management of unselected patients with acute VTE. Two-thirds of the elderly and half of the younger patients were treated in a hospital setting, an observation that likely corresponds to higher rates of PE and co-morbidities in the elderly. The elderly more frequently received intravenous unfractionated heparin as an initial antithrombotic agent, probably because of a higher rate of massive PE, renal failure, or other bleeding risk factors. When compared with younger patients, the rate of reperfusion therapy for acute DVT was lower in the elderly patients (1 vs. 5%), reflecting an increased risk of bleeding complications and a shorter life expectancy in the elderly.
Elderly VTE patients had a higher rate of early death when compared with younger VTE patients, a finding that confirms the results of ICOPER with an age of >70 years as an independent predictor of 3-month mortality.15 The rate of bleeding complications exceeded the rate of recurrent VTE among elderly patients in our study (in-hospital bleeding requiring medical attention: 6.5% vs. in-hospital VTE recurrence: 1.4%) and in the Registro Informatizado de la Enfermedad TromboEmbólica (RIETE) (major bleeding at 3 months: 3.4% vs. VTE recurrence at 3 months: 2.1%).16 Similar results were also observed in the Worcester Venous Thromboembolism Study.11
The main strength of our study is the multicentric prospective inclusion of unselected VTE patients in a real-world setting that provides a representative picture of the age distribution in patients with acute VTE. A weakness of the study is that no systematic follow-up beyond hospital discharge was obtained. The high proportion of PE patients in our study population of hospital-managed VTE patients reflects that many DVT patients were likely not enrolled in SWIVTER because in Switzerland, DVT patients are increasingly being managed on an outpatient basis from private practices.17 Finally, our findings may not necessarily be valid in other countries; particularly, the novel finding of an increased VTE severity in the elderly requires confirmation from other national or international surveys.
In conclusion, elderly patients had more serious VTE than younger patients, and massive PE was particularly life-threatening in the elderly. Future PE management studies should consider the inclusion of elderly patients where clinical benefit from reperfusion therapy, including catheter-based interventions with or without thrombolysis, may potentially be present.
Funding
This study was funded by Sanofi-Aventis (Suisse) SA, Meyrin, Switzerland. Data collection, data management, database entry, and data analysis were independent from the sponsor.
Conflict of interest: D.S. is an employee of sanofi-aventis (Suisse) SA, Meyrin, Switzerland.
Acknowledgements
We thank the following site investigators for participating in the study: Marietta Puck (University Hospital Zurich), Jörg Ugi (University Hospital Bern), D.H. (Cantonal Hospital Fribourg), Peter Rupp (Salem Hospital Bern), T.B. (University Hospital Basel), Alexander Imhof (Regional Hospital Langenthal), Anita Lebeda (Cantonal Hospital Frauenfeld), Michaela Heidemann-Zabel (Cantonal Hospital Lucerne), Stephanie Witzig (University Hospital Lausanne), Robert Escher (Regional Hospital Burgdorf), Barbara Federspiel (Hospital Zimmerberg Wädenswil), Thierry Fumeaux (Regional Hospital Nyon), Ulrich Frank (Cantonal Hospital Chur), Thomas Kaeslin (Cantonal Hospital Sarnen), Andreas Walser (Regional Hospital Flawil), Heinz Josef Schaad (Regional Hospital Interlaken), Urs Marbet (Cantonal Hospital Altdorf), and Herve Duplain (Regional Hospital Porrentruy).
References
- 1.Anderson FA, Wheeler HB, Goldberg RJ, Hosmer DW, Patwardhan NA, Jovanovic B, Forcier A, Dalen JE. A population-based perspective of the hospital incidence and case-fatality rates of deep vein thrombosis and pulmonary embolism: the Worchester DVT Study. Arch Intern Med. 1991;151:933–938. [PubMed] [Google Scholar]
- 2.International Conference on Harmonisation of Technical Requirements of Pharmaceuticals for Human Use (ICH) Topic E7 Note for Guidance on Studies to Support of Special Populations: Geriatrics. London: European Medicine Agency; 1994. CPMP/ICH/379/95. [Google Scholar]
- 3.Kearon C, Kahn SR, Agnelli G, Goldhaber S, Raskob GE, Comerota AJ. Antithrombotic therapy for venous thromboembolic disease. American College of Chest Physicians evidence-based clinical practice guidelines (8th Edition) Chest. 2008;133:454S–545S. doi: 10.1378/chest.08-0658. [DOI] [PubMed] [Google Scholar]
- 4.Spencer FA, Gore JM, Reed G, Lessard D, Pacifico L, Emery C, Crowther MA, Goldberg RJ. Venous thromboembolism and bleeding in a community setting: The Worcester Venous Thromboembolism Study. Thromb Haemost. 2009;101:878–885. [PMC free article] [PubMed] [Google Scholar]
- 5.Heit JA, Mohr DN, Silverstein MD, Petterson TM, O'Fallon WM, Melton LJ., III Predictors of recurrence after deep vein thrombosis and pulmonary embolism. A population-based cohort study. Arch Intern Med. 2000;160:761–768. doi: 10.1001/archinte.160.6.761. [DOI] [PubMed] [Google Scholar]
- 6.White RH, Zhou H, Romano PS. Length of hospital stay for treatment of deep venous thrombosis and the incidence of recurrent thromboembolism. Arch Intern Med. 1998;158:1005–1010. doi: 10.1001/archinte.158.9.1005. [DOI] [PubMed] [Google Scholar]
- 7.Ahmed S, Antman EM, Murphy SA, Giugliano RP, Cannon CP, White H, Morrow DA, Braunwald E. Poor outcomes after fibrinolytic therapy for ST-segment elevation myocardial infarction: impact of age (a meta-analysis of a decade of trials) J Thromb Thrombolysis. 2006;21:119–129. doi: 10.1007/s11239-006-5485-9. [DOI] [PubMed] [Google Scholar]
- 8.Sinnaeve PR, Huang Y, Bogaerts K, Vahanian A, Adgey J, Armstrong PW, Wallentin L, Van de Werf FJ, Granger CB. Age, outcomes, and treatment effects of fibrinolytic and antithrombotic combinations: findings from Assessment of the Safety and Efficacy of a New Thrombolytic (ASSENT)-3 and ASSENT-3 PLUS. Am Heart J. 2006;152:684–689. doi: 10.1016/j.ahj.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 9.Kucher N, Rossi E, De Rosa M, Goldhaber SZ. Massive pulmonary embolism. Circulation. 2006;113:577–582. doi: 10.1161/CIRCULATIONAHA.105.592592. [DOI] [PubMed] [Google Scholar]
- 10.Jimenez D, Aujesky D, Moores L, Gomez V, Lobo JL, Uresandi F, Otero R, Monreal M, Muriel A, Yusen RD. Simplification of the pulmonary embolism severity index for prognostication in patients with acute symptomatic pulmonary embolism. Arch Intern Med. 2010;170:1383–1389. doi: 10.1001/archinternmed.2010.199. [DOI] [PubMed] [Google Scholar]
- 11.Spencer FA, Gore JM, Lessard D, Emery C, Pacifico L, Reed G, Gurwitz JH, Goldberg RJ. Venous thromboembolism in the elderly: a community-based perspective. Thromb Haemost. 2008;100:780–788. [PMC free article] [PubMed] [Google Scholar]
- 12.Joffe HV, Kucher N, Tapson VF, Goldhaber SZ. Upper-extremity deep vein thrombosis: a prospective registry of 592 patients. Circulation. 2004;110:1605–1611. doi: 10.1161/01.CIR.0000142289.94369.D7. [DOI] [PubMed] [Google Scholar]
- 13.Muñoz FJ, Mismetti P, Poggio R, Valle R, Barron M, Guil M, Monreal M. Clinical outcome of patients with upper extremity deep vein thrombosis: results from the RIETE Registry. Chest. 2008;133:143–148. doi: 10.1378/chest.07-1432. [DOI] [PubMed] [Google Scholar]
- 14.Masotti L, Ray P, Righini M, le Gal G, Antonelli F, Landini G, Cappelli R, Prisco D, Rottoli P. Pulmonary embolism in the elderly: a review on clinical, instrumental and laboratory presentation. Vasc Health Risk Manag. 2008;4:629–636. doi: 10.2147/vhrm.s2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldhaber SZ, Visani L, De Rosa M. Acute pulmonary embolism: clinical outcomes in the International Cooperative Pulmonary Embolism Registry (ICOPER) Lancet. 1999;353:1375–1376. doi: 10.1016/s0140-6736(98)07534-5. [DOI] [PubMed] [Google Scholar]
- 16.Lopez-Jimenez L, Montero M, Gonzalez-Fajardo JA, Arcelus JI, Suarez C, Lobo JL, Monreal M. Venous thromboembolism in very elderly patients: findings from a prospective registry (RIETE) Haematologica. 2006;91:1046–1051. [PubMed] [Google Scholar]
- 17.Spirk D, Banyai M, Jacomella V, Frank U, Baldi T, Baumgartner I, Amann-Vesti B, Kucher N, Husmann M. Outpatient management of acute deep vein thrombosis: results from the OTIS-DVT registry. Thromb Res. 2011;127:406–410. doi: 10.1016/j.thromres.2011.01.006. [DOI] [PubMed] [Google Scholar]