Abstract
Aims
The activation of cannabinoid receptor type 2 (CB2)-mediated pathways might represent a promising anti-atherosclerotic treatment. Here, we investigated the expression of the endocannabinoid system in human carotid plaques and the impact of CB2 pharmacological activation on markers of plaque vulnerability in vivo and in vitro.
Methods and results
The study was conducted using all available residual human carotid tissues (upstream and downstream the blood flow) from our cohort of patients symptomatic (n = 13) or asymptomatic (n = 27) for ischaemic stroke. Intraplaque levels of 2-arachidonoylglycerol, anandamide N-arachidonoylethanolamine, N-palmitoylethanolamine, N-oleoylethanolamine, and their degrading enzymes (fatty acid amide hydrolase and monoacylglycerol lipase) were not different in human plaque portions. In the majority of human samples, CB1 (both mRNA and protein levels) was undetectable. In downstream symptomatic plaques, CB2 protein expression was reduced when compared with asymptomatic patients. In these portions, CB2 levels were inversely correlated (r = −0.4008, P = 0.0170) with matrix metalloprotease (MMP)-9 content and positively (r = 0.3997, P = 0.0174) with collagen. In mouse plaques, CB2 co-localized with neutrophils and MMP-9. Treatment with the selective CB2 agonist JWH-133 was associated with the reduction in MMP-9 content in aortic root and carotid plaques. In vitro, pre-incubation with JWH-133 reduced tumour necrosis factor (TNF)-α-mediated release of MMP-9. This effect was associated with the reduction in TNF-α-induced ERK1/2 phosphorylation in human neutrophils.
Conclusion
Cannabinoid receptor type 2 receptor is down-regulated in unstable human carotid plaques. Since CB2 activation prevents neutrophil release of MMP-9 in vivo and in vitro, this treatment strategy might selectively reduce carotid vulnerability in humans.
Keywords: Carotid arteries, Metalloproteinase-9, Neutrophils
Introduction
The endocannabinoid signalling system comprises two G-protein-coupled receptors, the cannabinoid receptor type 1 (CB1) and 2 (CB2), the endogenous agonists at these receptors, also known as endocannabinoids [the two most studied of which are the lipids anandamide N-arachidonoylethanolamine (AEA) and 2-arachidonoylglycerol (2-AG)], and enzymes for endocannabinoid biosynthesis and degradation.1 The endocannabinoid system participates in a plethora of physiological and pathological conditions. Its overall role, at the onset of nearly any type of cell perturbation, is to be activated ‘on demand’ to help the cell, and hence the organism, to re-establish the homeostasis to a level as similar as possible to the one existing before that perturbation, through the local regulation of the levels and activity of other chemical signals, such as neurotransmitters, hormones, or cytokines.1 A typical example of such role is represented by endocannabinoid function in re-establishing energy homeostasis following a brief period of food deprivation, mostly exerted via CB1 receptors.2 Likewise, endocannabinoid signalling at CB2 receptors is often triggered as a response to inflammation to either facilitate or suppress immune cell function depending on whether or not the inflammatory status is still beneficial.3 However, especially during chronic pathological states, the endocannabinoid system may become deranged and start contributing to the disorders accompanying hyperphagia, obesity, and inflammatory diseases, such as atherosclerosis.4
Despite some controversies, early data pointed to CB2 and CB1 receptors as a protective and contributing factor, respectively, in atherogenic inflammation.5,6 Here, we investigated the potential role of the ‘intraplaque’ endocannabinoid system on local mediators of vulnerability, with emphasis on the CB2 putative control of plaque levels of inflammatory cells, matrix metalloprotease (MMP)-9, and collagen, in patients with severe carotid stenosis (symptomatic or asymptomatic for ischaemic stroke), in a mouse model of shear stress-induced atherogenesis and plaque vulnerability in carotid artery and in vitro in neutrophils (a cell type particularly infiltrating vulnerable plaques).7
Methods
For additional details, see Supplementary material online.
Patients and study design
All patients with available residual carotid tissues and previously enrolled in the unmatched case–control study (performed at San Martino Hospital, Genoa, Italy) were included in the present substudy.7 Among the total cohort [81 patients with extracranial high-grade internal carotid stenosis (>70% luminal narrowing)8 and symptomatic (n = 18) or asymptomatic (n = 63) for ischaemic stroke], biopsy material was available for 13 symptomatic and 27 asymptomatic patients. Patients were classified as symptomatic for ischaemic stroke after the first episode of ipsilateral ischaemic stroke (focal neurological deficit of acute onset lasting more than 24 h occurring between 30 and 10 days prior to endarterectomy). Patients were defined as asymptomatic when they had no history of ischaemic symptoms and in the absence of signs of cerebral necrosis at magnetic resonance imaging with diffusion sequences. Both asymptomatic and symptomatic patients underwent carotid endarterectomy according to the recommendations published by the Asymptomatic Carotid Surgery Trial (ACST), the European Carotid Surgery Trial (ECST), and the North American Symptomatic Carotid Endarterectomy Trial (NASCET).8–10 The day prior to endarterectomy, serum samples were obtained to measure circulating markers of cardiovascular vulnerability. Medications reported in Supplementary material online, Table S1, were not modified in the 2 months prior to enrolment. Exclusion criteria were: spontaneous cerebral embolism during 30 min preoperatively and during the dissection phase of the operation, malignant hypertension, acute coronary artery disease, any cardiac arrhythmias, congestive heart failure (II, III, and IV NYHA classes), liver or renal disorder or function abnormalities, acute and chronic infectious diseases, autoimmune and rheumatic diseases, cancer, endocrine diseases, inflammatory bowel diseases and anti-inflammatory (other than aspirin) medications, oral anticoagulant treatments, and hormone, cytokine, or growth factor therapies.
The Medical Ethics Committee of San Martino Hospital approved the study and participants provided written informed consent. The study was conducted in compliance with the Declaration of Helsinki.
Human carotid plaque specimen processing
Shortly after surgical excision, the internal carotid plaque specimens were cut perpendicularly to the long axis through the point of maximum stenosis to obtain two portions (upstream and downstream of the blood flow; see Supplementary material online, Figure S1A).7 Each portion was further divided perpendicularly to the long axis in the middle into two subsegments. One half was snap-frozen in liquid nitrogen and stored at −80°C (for mRNA isolation and endocannabinoid measurements), and the other half was frozen in cryoembedding medium for histological analysis (see Supplementary material online, Figure S2A).
Mouse model of shear stress-induced atherogenesis and plaque vulnerability
Apolipoprotein E-deficient mice (apoE−/−) in a C57BL/6J background were obtained from The Charles River Laboratories. Animals at 15–20 weeks of age were randomly assigned to receive either vehicle (n = 6) or the selective CB2 agonist JWH-133 (n = 7) treatment. During the experimental period, all animals were fed a Western-type diet consisting of 15% (wt/wt) cocoa butter and 0.25% (wt/wt) cholesterol (Diet W; abDiets). After a 2-week period of Western diet, shear stress in the right common carotid artery was altered by cast surgical placement.11 This model was reported in detail in the Supplementary material online. Nine weeks after surgery, the animals were euthanized to collect tissue and serum samples. During the last 3 weeks before euthanizing (from Weeks 6 to 9 of cast implantation), mice were intraperitoneally injected with the selective CB2 agonist JWH-133 (5 mg/kg/day for 5 consecutive days per week, Tocris Bioscience, Bristol, UK) or respective vehicle control (Tocrisolve™ 100, Tocris Bioscience). In selective experiments, to assess the CB2 levels in mouse carotid and aortic root plaques, animals (n = 4) were intraperitoneally and daily injected with phosphate-buffered saline (PBS) (for 5 consecutive days per week from Weeks 6 to 9 with cast). This animal study was approved by local Ethics Committee and Swiss authorities and conformed to the ‘position of the American Heart Association on Research Animal Use’.
Immunohistochemistry in human carotid plaques, mouse carotid plaques, and mouse aortic sinus
Frozen upstream and downstream human carotid specimens, mouse carotid arteries, and aortic sinus were serially cut and stained as described in the Supplementary material online.
Oil Red O staining for lipid content
Oil Red O staining was performed as described in the Supplementary material online.
Sirius red staining for collagen content
Sirius red staining was performed as described in detail in the Supplementary material online.
Real-time RT–PCR
Real-time PCR was performed as described in detail in the Supplementary material online. Specific primers and probes were used to determine the mRNA expression of CNR1 (CB1 receptor), fatty acid amide hydrolase, monoacylglycerol lipase, matrix metalloproteinase-8 (MMP-8), and GAPDH (housekeeping gene) and are reported in the Supplementary material online, Table S3.
Quantification of endocannabinoids
After lipid extraction and pre-purification, endocannabinoid fractions were assessed as described in detail in the Supplementary material online.
Human primary neutrophil isolation and culture
Human neutrophils, obtained from healthy volunteers after informed consent, were isolated and cultured as described in detail in the Supplementary material online.
Apoptosis assay
Apoptosis rates of neutrophils after 2 h of treatment with JWH-133 (1 µM) and AM630 (1 µM) were determined via the analysis of phosphatidylserine externalization using an annexin V-FITC apoptosis detection kit (Medical and Biological Laboratories Co., Ltd, Woburn, MA, USA).
Flow cytometry
Flow cytometry on human neutrophils was assessed as described in detain in the Supplementary material online.
Detection of inflammatory mediators in human serum and cell supernatants
Inflammatory markers were assessed in human serum and cell supernatants as described in detail in the Supplementary material online.
Pro-matrix metalloprotease-9 zymographic assay
Pro-matrix metalloprotease-9 zymographic activity was assessed in human serum and cell supernatants as described in detail in the Supplementary material online.
Western blot analysis
Western blot on human neutrophils were performed as described in detail in the Supplementary material online.
Statistical analysis
Patient characteristics were described 1 day before endarterectomy. Patients asymptomatic for ischaemic stroke were compared with symptomatic patients using Pearson's χ2 test or Fisher's exact test (when appropriate) for the comparison of qualitative variables. The Mann–Whitney non-parametric test (the normality assumption of the variables' distribution in both groups was violated) was used for comparisons of continuous variables. Comparisons between parameters of mouse plaque vulnerability in JWH-133- and vehicle-treated mice were performed using the Mann–Whitney test. For continuous variables, results were expressed as medians [inter-quartile range (IQR)]. Spearman's rank correlation coefficients were used to assess correlations between CB2 levels and, respectively, intraplaque contents of lipids, collagen, vascular and inflammatory cells, MMP-9, or MMP-8 mRNA expression (ΔCT) in both upstream and downstream regions of carotid atherosclerotic plaques. In vitro results were expressed as means (±SD) (ERK phosphorylation in human neutrophils) and as medians (IQR) (neutrophil release of MMP-9 and zymography). One-way ANOVA was used for multiple group comparison, and the unpaired Student t-test was used for two-group comparison. Values of P < 0.05 (two-tailed) were considered significant. All analyses were done with Statistica™ software (StatSoft, Tulsa, OK, USA).
Results
Patient characteristics and systemic levels of cardiovascular risk biomarkers
Based on the criterion of the residual availability of carotid plaque samples for measuring the mediators of the endocannabinoid system, we included in the present substudy 27 patients asymptomatic and 13 symptomatic for ischaemic stroke. Their clinical and biological characteristics, as well as medications, are reported in Supplementary material online, Table S1. No significant difference between asymptomatic and symptomatic in terms of age, sex, co-morbidities, laboratory parameters, and medications was found. Then, we focused on serum inflammatory cardiovascular risk biomarkers, recently suggested to be potentially useful in the assessment of the global cardiovascular vulnerability.12 Patients symptomatic for ischaemic stroke had increased serum levels of MMP-8 when compared with the asymptomatic group (see Supplementary material online, Table S4). No significant differences in the serum levels of MMP-9 and their tissue inhibitors were observed between the two groups (see Supplementary material online, Table S4).
Intraplaque levels of cannabinoid receptor type 2 receptor are reduced in downstream portions of symptomatic plaques and inversely associated with parameters of vulnerability
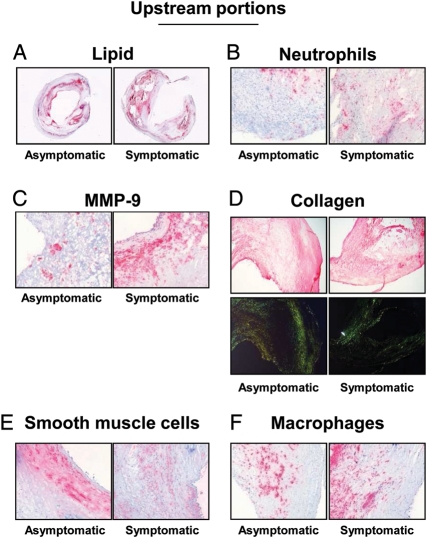
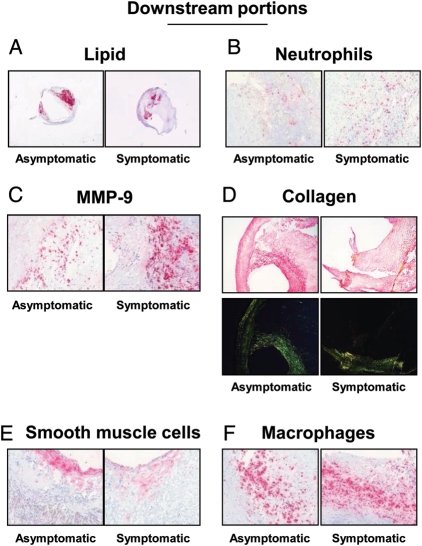
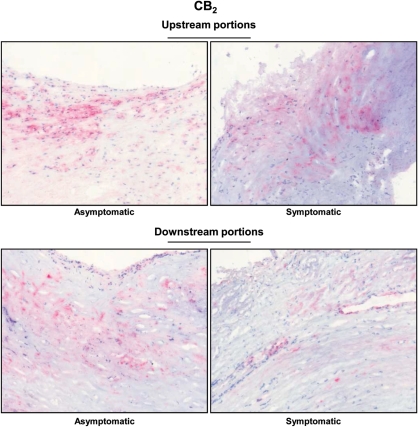
The levels of mediators of the endocannabinoid system and plaque vulnerability were investigated in the upstream and downstream portions of human carotid plaques. In agreement with our previously published data from the entire cohort,7 upstream portions of symptomatic plaques had increased lipid, neutrophil, and MMP-9 contents when compared with corresponding regions in asymptomatic patients (Table 1 andFigure 1A–C). On the other hand, collagen and smooth muscle cell contents were decreased in the symptomatic upstream portion when compared with asymptomatic (Table 1 and Figure 1D and E). No significant differences between the symptomatic and asymptomatic groups in macrophage infiltration, MMP-8 mRNA expression, as well as in all endocannabinoid system components were found in upstream plaque regions (Figure 1F and Table 1). In downstream portions of carotid plaques, intraplaque neutrophil and MMP-9 contents were significantly increased in symptomatic patients when compared with asymptomatic (Table 1 andFigure 2B and C). Accordingly, collagen content was decreased in symptomatic patients when compared with asymptomatic (Table 1 and Figure 2D). Interestingly, intraplaque levels of CB2 were strongly reduced in downstream portions of symptomatic patients when compared with asymptomatic (Table 1 and Figure 3). No significant differences were observed in the expression of other intraplaque parameters, MMP-8 mRNA expression, endocannabinoids (AEA or 2-AG), or endocannabinoid-like molecules [N-oleoylethanolamine (OEA) and N-palmitoylethanolamine (PEA)] (Table 1). In human carotid plaque samples, CB1 content was almost undetectable at both mRNA {detectable in 13 asymptomatic and 5 symptomatic upstream plaques [median (IQR) of fold increase in upstream plaque, respectively: 0.94 (0.52–0.34) vs. 0.24 (0.23–1.19), P = 0.37]; detectable in 15 asymptomatic and 3 symptomatic downstream plaques [median (IQR) of fold increase in asymptomatic upstream, respectively: 0.49 (0.28–0.92) vs. 0.29 (0.21–0.41), P = 0.17]} and protein levels (detectable in 3 asymptomatic and 1 symptomatic patients, data not shown). In downstream portions, CB2 content was positively correlated with protective intraplaque collagen (r = 0.3997, P = 0.0174) and inversely with MMP-9 content (r = −0.4008, P = 0.0170) (see Supplementary material online, Table S5). No significant correlations were observed between intraplaque CB2 levels and other parameters of plaque vulnerability (see Supplementary material online, Table S5). By confirming that human carotid plaques are highly heterogeneous tissues,7 these results indicate that intraplaque levels of CB2 receptor are reduced in the most vulnerable regions where MMP-9 is increased and collagen is reduced.
Table 1.
Parameters of intraplaque vulnerability
| Carotid intraplaque parameters | Asymptomatic (n = 27) | Symptomatic (n = 13) | P-value vs. asymptomatic |
|---|---|---|---|
| Upstream portion | |||
| % lipid | 7.0 (4.0–9.0) | 9.4 (7.9–14.8) | 0.005 |
| % total collagen | 25.2 (18.4–33.3) | 14.2 (9.3–17.4) | 0.0008 |
| % collagen I | 13.3 (10.2–16.4) | 5.5(3.3–8.1) | 0.0003 |
| % collagen III | 12.9 (9.3–18.3) | 7.0 (5.4–8.2) | 0.0002 |
| % of smooth muscle cell-rich area | 7.9 (4.5–10.3) | 3.3 (5.6–2.4) | 0.004 |
| % of macrophage-rich area | 5.7 (2.8–10.3) | 7.4 (6.3–9.3) | 0.15 |
| Neutrophils/mm2 | 2.4 (1.0–2.7) | 5.4 (4.3–7.5) | 0.0006 |
| MMP-8 mRNA, fold increase | 0.46 (0.22–1.20) | 0.49 (0.12–1.05) | 0.60 |
| % MMP-9 | 4.0 (2.0–5.3) | 11.1 (7.4–13.2) | 0.005 |
| % CB2 receptor-rich area | 1.7 (0.3–3.0) | 1.5 (0.1–2.4) | 0.48 |
| MAGL mRNA, fold increase | 0.84 (0.64–1.08) | 0.80 (0.73–0.87) | 0.54 |
| FAAH mRNA, fold increase | 0.69 (0.46–1.12) | 0.52 (0.30–0.95) | 0.20 |
| AEA, pmol/g | 38.5 (26.8–52.9) | 39.7 (29.9–50.9) | 0.87 |
| 2-AG, pmol/mg | 0.70 (0.46–1.03) | 0.89 (0.61–1.31) | 0.36 |
| PEA, pmol/mg | 0.21 (0.16–0.39) | 0.24 (0.18–0.46) | 0.76 |
| OEA, pmol/mg | 0.12 (0.09–0.21) | 0.18 (0.16–0.26) | 0.17 |
| Downstream portion | |||
| % lipid | 2.7 (1.5–4.4) | 3.3 (2.2–5.9) | 0.47 |
| % total collagen | 18.8 (10.3–24.4) | 11.3 (10.3–13.3) | 0.05 |
| % collagen I | 10.8 (5.5–12.9) | 5.5 (5.3–7.5) | 0.02 |
| % collagen III | 9.1 (4.6–11.7) | 5.8 (3.4–6.4) | 0.04 |
| % of smooth muscle cell-rich area | 3.3 (2.0–5.5) | 2.3 (1.8–3.2) | 0.06 |
| % of macrophage-rich area | 7.1 (2.3–17.5) | 15.3 (6.3–17.5) | 0.32 |
| Neutrophils/mm2 | 6.1 (1.8–11.9) | 11.6 (10.2–15.3) | 0.005 |
| MMP-8 mRNA, fold increase | 0.62 (0.20–1.50) | 0.60 (0.07–1.55) | 0.49 |
| % MMP-9 | 5.3 (2.8–8.0) | 10.3 (7.4–19.9) | 0.01 |
| % CB2 receptor-rich area | 1.3 (0.6–5.0) | 0.2 (0.01–1.1) | 0.01 |
| MAGL mRNA, fold increase | 0.76 (0.58–0.97) | 0.64 (0.56–0.81) | 0.36 |
| FAAH mRNA, fold increase | 0.40 (0.25–0.80) | 0.30 (0.23–0.67) | 0.48 |
| AEA, pmol/g | 32.3 (23.8–54.8) | 37.3 (27.2–48.2) | 0.78 |
| 2-AG, pmol/mg | 0.73 (0.52–10.2) | 0.95 (0.74–1.23) | 0.18 |
| PEA, pmol/mg | 0.32 (0.22–0.44) | 0.32 (0.27–0.38) | 0.87 |
| OEA, pmol/mg | 0.21 (0.13–0.30) | 0.25 (0.18–0.27) | 0.35 |
Data are expressed as median (inter-quartile range). CB, cannabinoid; MAGL, monoacylglycerol lipase; FAAH, fatty acid amide hydrolase; AEA, anandamide; 2-AG, 2-arachidonoylglycerol; PEA, N-palmitoylethanolamine; OEA, N-oleoylethanolamine.
Figure 1.
Parameters of plaque vulnerability in upstream portions of carotid plaques. Representative sections from human asymptomatic and symptomatic carotid plaques showing staining for lipids (A), neutrophils (B), matrix metalloprotease-9 (C), collagen (D), smooth muscle cells (E), and macrophages (F). Collagen specificity is shown under bright illumination without polarization (upper part of panel D) and under polarized light illumination (lower part of panel D).
Figure 2.
Parameters of plaque vulnerability in downstream portions of carotid plaques. Representative sections from human asymptomatic and symptomatic carotid plaques showing staining for lipids (A), neutrophils (B), matrix metalloprotease-9 (C), collagen (D), smooth muscle cells (E), and macrophages (F). Collagen specificity is shown under bright illumination without polarization (upper part of panel D) and under polarized light illumination (lower part of panel D).
Figure 3.
Cannabinoid receptor type 2 expression is reduced in downstream portions of symptomatic plaques. Representative sections from human carotid plaques showing staining for cannabinoid receptor type 2.
Treatment with selective cannabinoid receptor type 2 agonist JWH-133 reduces matrix metalloprotease-9 content in atherosclerotic plaques in ApoE−/− mice
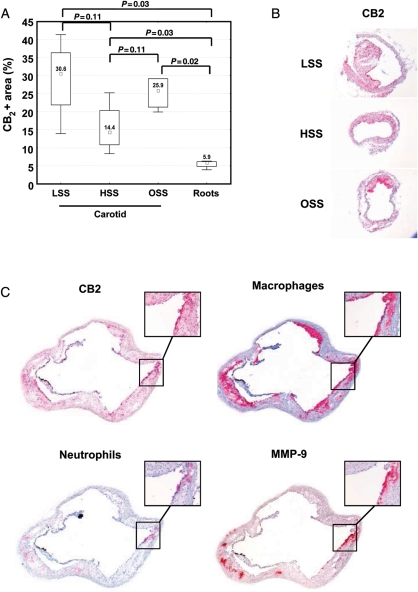
We first investigated the expression of CB2 in atherosclerotic vessels in a mouse model of shear stress-induced atherogenesis and plaque vulnerability.11 This experiment was done in mice treated with PBS (control) to be next to disease pathophysiology and avoid any artifact potentially induced by the vehicle (Tocrisolve). Cannabinoid receptor type 2 protein expression was higher in carotid arteries when compared with aortic roots (Figure 4A–C). In plaques from mouse aortic roots, CB2 particularly co-localized with both neutrophils and MMP-9-rich areas (Figure 4C). Only partial co-localization was observed between CB2 and macrophages (Figure 4C). Then, the potential protective effect of selective CB2 pharmacological activation was assessed in mice treated with JWH-133 or the corresponding vehicle (Tocrisolve) for the last 3 weeks (6 weeks after the cast placement) before analysing. Intraplaque vulnerability was assessed in the aortic roots and in the different shear stress regions of carotid artery [low shear stress (LSS) and oscillatory shear stress (OSS)]. At sacrifice, mouse body weight, serum total cholesterol, and triglycerides were similar in the two experimental groups (see Supplementary material online, Table S6). In LSS carotid plaques, JWH-133 treatment did not induce any change in relative plaque content of lipids, macrophages, neutrophils, collagen, and MMP-9 (Table 2; see Supplementary material online, Figures S3A–C and S4A and B). Similarly, no difference was observed in lipid content and macrophage infiltration in OSS carotid and aortic root plaques (Table 2; see Supplementary material online, Figure S3A and B). However, neutrophil infiltration was reduced in aortic root plaques of JWH-133-treated mice when compared with controls (Table 2; see Supplementary material online, Figure S3C). No significant difference in intraplaque collagen content was shown between JWH-133 and control-treated mice (Table 2; see Supplementary material online, Figure S4A). Conversely, JWH-133 treatment was associated with lower MMP-9 plaque content in both OSS carotid and aortic root plaques (Table 2; see Supplementary material online, Figure S4B). These results indicate that in vivo CB2 activation with the selective agonist JWH-133 was associated with a weak reduction in MMP-9 content in mouse plaques considered more stable.11
Figure 4.
Cannabinoid receptor type 2 is expressed in mouse carotid and aortic root plaques. (A) Cannabinoid receptor type 2 protein expression in low shear stress, high shear stress (HSS), oscillatory shear stress carotid and aortic root plaques of phosphate-buffered saline-treated mice (n = 4). Data are expressed as median (inter-quartile range). (B) Representative microphotographs of CB2 staining in mouse carotid plaques in low shear stress, HSS, and oscillatory shear stress carotid portions. (C) Representative microphotographs of consecutive cryosections from root plaques of phosphate-buffered saline-treated mice for, respectively, CB2, macrophages (CD68+ cells), neutrophils (Ly-6B.2+ cells), and matrix metalloprotease-9.
Table 2.
Parameters of mouse plaque vulnerability
| Parameters | Vehicle-treated mice (n = 6) | JWH-133-treated mice (n = 7) | P-value |
|---|---|---|---|
| Carotid LSS | |||
| Oil-red-O, ×103 μm2 | 179.5 (24.7–198.0) | 22.1 (15.4–49.2) | 0.18 |
| Macrophage+ area, % | 5.1 (0.1–8.7) | 0.6 (0.1–2.4) | 0.42 |
| Neutrophils/mm2 | 1.7 (0.02–4.5) | 0.06 (0.04–0.19) | 0.52 |
| Total collagen, % | 18.1 (17.7–26.7) | 12.9 (7.0–15.2) | 0.05 |
| MMP-9, % | 1.6 (0.4–2.8) | 0.09 (0.05–1.11) | 0.17 |
| Carotid OSS | |||
| Oil-red-O, ×103 μm2 | 155.3 (101.0–163.5) | 179.8 (148.9–212.2) | 0.37 |
| Macrophage+ area, % | 2.6 (0.6–7.4) | 3.6 (1.3–5.5) | 0.68 |
| Neutrophils/mm2 | 1.3 (0.5–3.2) | 0.65 (0.1–1.4) | 0.22 |
| Total collagen, % | 10.4 (9.6–12.9) | 15.2 (10.9–20.5) | 0.17 |
| MMP-9, % | 3.9 (0.5–6.8) | 0.4 (0.2–1.5) | 0.04 |
| Aortic roots | |||
| Oil-red-O, ×103 μm2 | 172.2 (140.0–203.2) | 223.6 (161.2–239.3) | 0.10 |
| Macrophage+ area, % | 17.3 (15.0–20.2) | 18.9 (13.4–19.3) | 0.94 |
| Neutrophils/mm2 | 7.2 (4.7–18.2) | 2.4 (2.0–19.3) | 0.01 |
| Total collagen, % | 33.0 (18.4–40.0) | 39.3 (33.7–55.6) | 0.07 |
| MMP-9, % | 6.0 (4.3–11.9) | 1.9 (1.6–2.3) | 0.001 |
Data are expressed as median (inter-quartile range). MMP, matrix metalloproteinase.
Cannabinoid receptor type 2 activation reduces tumour necrosis factor-α-mediated release of matrix metalloprotease-9 in human primary neutrophils
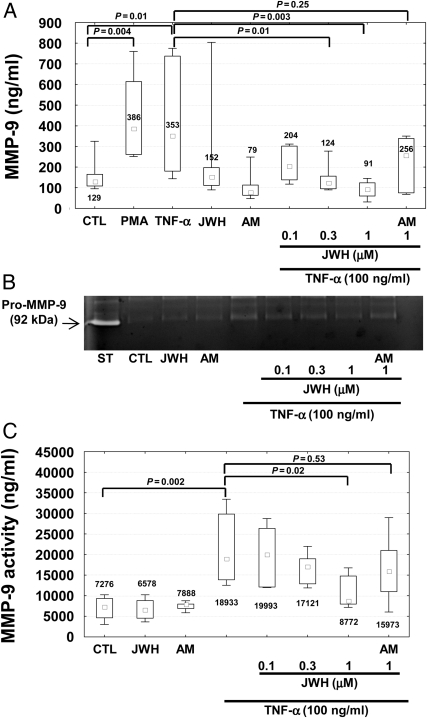
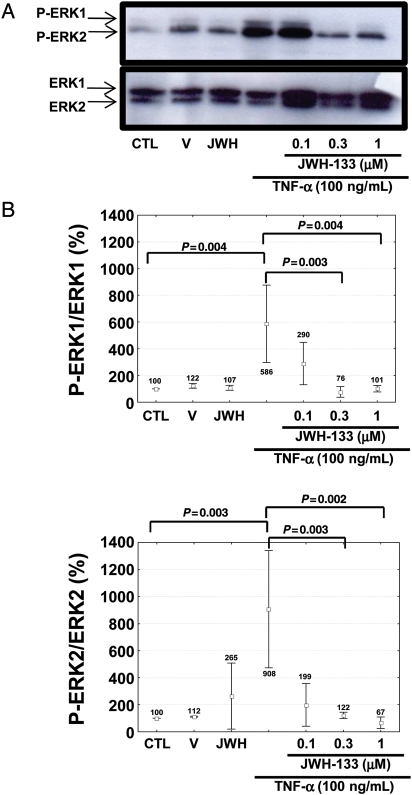
To investigate whether the activation of CB2 cannabinoid receptors might interfere with the release of MMP-9 by cells infiltrating carotid plaques,7 we performed in vitro cultures of human neutrophils. We tested the potential benefits of CB2 activation on cells adherent to polystyrene dishes and stimulated with tumour necrosis factor (TNF)-α (a pro-atherosclerotic cytokine previously shown to induce MMP-9 release by human neutrophils).13 Stimulation with 100 ng/mL TNF-α induced the release of MMP-9 by human neutrophils in a comparable manner to the positive control phorbol-12-myristate-13-acetate (PMA) (Figure 5A). Pre-incubation with the selective CB2 receptor agonist JWH-133 (at 0.3 and 1 μM) significantly reduced TNF-α-mediated release of MMP-9 by human neutrophils (Figure 5A). JWH-133-induced benefits were partially reversed by co-incubation with the selective CB2 antagonist AM630 (Figure 5A). No effects were induced by the incubation with the CB2 agonist or antagonist in the absence of TNF-α. Similar results were observed by assessing pro-MMP-9 gelatinolytic activity in neutrophil supernatants (Figure 5B and C). Cell viability in the presence of JWH-133 or AM630 was also assessed in the cell cultures by flow cytometric analysis of annexin V-FITC and propidium iodide staining. Pre-treatment with the cannabinoid agonist/antagonist did not induce any effects on early apoptosis, nor late apoptosis/necrosis (data not shown). We further investigated the potential molecular mechanism(s) underlying the beneficial effects of CB2 activation on TNF-α-mediated release of MMP-9, focusing on the membrane surface expression of TNF receptors and their downstream intracellular kinase pathways. Treatment for 2 h with different concentrations of JWH-133 did not induce any effect on TNFRI and TNFRII expression when compared with control medium alone (see Supplementary material online, Figure S5A and B). Given the potential role for ERK1/2 in TNF-α-mediated degranulation of MMP-9,13 we investigated whether CB2 pre-activation could modulate this pathway. Pre-incubation with PD98059 (selective inhibitor of MEK, a kinase directly activating ERK1/2) at 25 μM abrogated the increase in MMP-9 release and activity mediated by TNF-α (see Supplementary material online, Figure S6A–C). To confirm these findings, we directly assessed the effects of CB2 activation on ERK1/2 phosphorylation. Pre-treatment with 0.3–1 μM JWH-133 was associated with a strong reduction in ERK1/2 phosphorylation in response to TNF-α (Figure 6A and B), indicating that this intracellular pathway is crucial for CB2-mediated beneficial activity on the neutrophilic response to TNF-α.
Figure 5.
Pre-treatment with JWH-133 reduces tumour necrosis factor-α-mediated exocytosis of matrix metalloprotease-9 by human neutrophils. Matrix metalloprotease-9 release and activity in supernatants of cells incubated in polystyrene dishes in the presence or absence (30 min at 37°C) of control medium (CTL), 10 ng/mL phorbol-12-myristate-13-acetate (PMA, positive control), 100 ng/mL tumour necrosis factor-α, 1 μM JWH-133 (JWH), and 1 μM AM630 (AM). In selective experiments (right part of the histograms and gel), tumour necrosis factor-α stimulation was preceded by 2 h incubation with different doses of JWH-133 (JWH) and 1 μM AM630 (AM). Data are expressed as median (inter-quartile range). (A) Matrix metalloprotease-9 release (n = 7). (B) Representative gel of matrix metalloprotease-9 zymography. White band (arrow) on the gel represents the pro-matrix metalloprotease-9 gelatinolytic activity of standard recombinant pro-matrix metalloprotease-9 (ST), and supernatants of cells. (C) Results of densitometric quantifications of seven different zymography experiments.
Figure 6.
Pre-treatment with the CB2 receptor agonist JWH-133 reduces tumour necrosis factor-α-mediated phosphorylation of ERK1/2 in human neutrophils. Neutrophils were pre-incubated for 2 h at 37°C in the presence or absence of different concentrations of JWH-133 (JWH). Then, without washing, cells were further stimulated for 7 min at 37°C in the presence or absence of control medium (CTL), cannabinoid vehicle (V), 1 μM JWH-133 (JWH), or 100 ng/mL tumour necrosis factor-α. (A) Representative western blot analysis of ERK1/2 phosphorylation. (B) Densitometric analysis of phospho-ERK1/2 (P-ERK1/2). Values were, respectively, normalized to amounts of total ERK1/2 and expressed as percentage of control medium (CTL), defined as 100%. Data are expressed as mean ± SD (n = 3).
Discussion
Human study
Our study shows that the expression of the CB2 receptor protein is markedly reduced in carotid plaque portions downstream the blood flow in patients symptomatic for ischaemic stroke when compared with asymptomatic ones. In the same plaque regions, CB2 protein expression inversely correlated with MMP-9 expression and directly with collagen content (recognized parameters of plaque vulnerability).7,12 These data suggest that CB2 might be down-regulated in the most vulnerable portions of symptomatic plaques. Given these differences between intraplaque parameters in regions upstream or downstream the blood flow, we also confirmed that the high heterogeneity of carotid plaque tissues might be crucially influenced by the mechanical shear stress.7,11,14
Differently from Sugamura et al.15 who showed that CB1 is highly expressed in human coronary plaques, very little and hardly detectable amounts of protein or mRNA of CB1 receptor were retrieved in our human carotid plaque samples. This unexpected result was obtained in both symptomatic and asymptomatic patients. Furthermore, no changes in the local levels of the two major endocannabinoids (anandamide and 2-AG) and two endogenous peroxisome proliferator-activated receptor-α ligands (OEA and PEA)16 were observed between symptomatic and asymptomatic patients, despite them being well above detection limits, and even reaching concentrations potentially effective at cannabinoid receptors (particularly for 2-AG, the concentration of which ranged between 0.73 and 0.95 μM). Our findings were not explained by a lack of power due the largest sample sizes used in our study compared with those of Sugamura et al.15 On the other hand, these results might suggest that unlike coronary atheromas, the endocannabinoid tone in carotid plaques might uniquely reduce plaque vulnerability via CB2 receptor activation and does not also participate in plaque formation via CB1 receptors. Therefore, when CB2 receptor expression is decreased, as we have observed here in carotid plaques from symptomatic patients, this might contribute to the increase in vulnerability via uncontrolled release of MMP-9 and subsequent collagen degradation.
Animal study
We have provided evidence suggesting that intraplaque cannabinoid CB2 receptor activation, for which previous data indicate a protective role against atherosclerotic plaque formation,5,6,17 may also decrease the vulnerability of carotid plaques by reducing MMP-9 release. Since this protease has been indicated as a parameter associated with the ‘cannibal’ intraplaque collagen degradation,18 the potential involvement of the CB2 pathway in MMP-9 regulatory mechanisms might have a pathophysiological relevance. In order to assess the potential benefits of a pharmacological approach activating CB2 to reduce plaque vulnerability, we used a mouse model of shear stress-induced atherogenesis and plaque vulnerability. We observed a weak but significant reduction in MMP-9 intraplaque levels (aortic roots and OSS carotid portions) in animals treated with the selective CB2 agonist JWH-133 when compared with the control vehicle. Extrapolated to humans, these mouse results may suggest a causal relationship between CB2 activity and reduced MMP-9 release.
In vitro study
In order to identify the intraplaque source of MMP-9, we studied the localization of this metalloproteinase in consecutive sections of mouse plaques and observed its co-occurrence with CB2 in infiltrating neutrophils. Since neutrophils have been detected particularly in human unstable plaques7 and indicated as a potent producer of MMP-9 in response to pro-atherosclerotic cytokines (such as TNF-α),13 we envisaged that these cells might be the potential cellular target of the CB2-mediated beneficial stimulation. Results showed that pre-incubation with JWH-133 dose-dependently reduced the neutrophilic release of MMP-9 in response to TNF-α. Intriguingly, selective CB2 activation did not interfere with the surface expression of TNF receptors, but abrogated the phosphorylation of one of the most important inflammatory intracellular pathways (i.e. ERK1/2), activated by TNF-α.19 Via the activation of this kinase, TNF-α has been shown to induce several pro-atherosclerotic functions in inflammatory cells.20 However, ERK1/2 phosphorylation has not been shown before to be responsible for the MMP-9 release by human neutrophils.13 Taking into account our results using different doses of the selective ERK1/2 pathway inhibitor (PD98059) and showing that TNF-α-mediated ERK1/2 phosphorylation preceded MMP-9 release (7 vs. 30 min), we conclude that this intracellular pathway might be also involved in the deleterious neutrophilic release of MMP-9. These findings provide a cellular and molecular mechanism to the previously described enhancement of MMP-9 up-regulation in double low-density lipoprotein receptor (ldlr) and CB2 knockout mice, in the absence of any net effect on atherosclerotic plaque formation.5 Together with this previous report, our data suggest that systemic administration of selective CB2 receptor agonists might perhaps be used as an atherosclerotic plaque pacifier agent in patients with significant but asymptomatic carotid stenosis.
Limitations and conclusions
The present ‘translational’ study has some limitations. The study in humans is mainly an observational study comparing samples collected after the ischaemic event. Thus, we cannot establish whether CB2 reduction in symptomatic downstream plaque is the cause or the effect consequence of a potential plaque rupture. In addition, since the study was not designed as prospective, we cannot also estimate the potential association between CB2 expression and the risk of stroke recurrence. Finally, as the number of subjects in human and mouse studies are low, our conclusions require the confirmation on larger studies. Nonetheless, the study sample size allowed us to show a large difference of 1.6, considered as clinically relevant, on the intraplaque cannabinoid receptor expression between symptomatic and asymptomatic patients.15
In conclusion, our data suggest that CB2 activation rather than CB1 inhibition should be considered to abrogate the intraplaque neutrophilic release of MMP-9. Although the role of CB2 receptors as atherogenic modulators in mice remains marginal and therefore controversial and the benefits associated with the treatment with the selective CB2 agonist JWH-133 were weak in atherosclerotic mice,5,21 our translational results indicate that a treatment strategy targeting CB2 activation might be effective in human beings. Therefore, the selective activation of the CB2 pathway (possibly without interfering with endocannabinoid signalling in the brain) might be appealing as a therapeutic approach for clinical studies to combat atherosclerotic plaque vulnerability through the reduction in MMP-9 neutrophilic degranulation.
Supplementary material
Supplementary material is available at European Heart Journal online.
Funding
This research was funded by the Brazilian Swiss Joint Research Program (BSJRP) to F.Ma., N.S., and R.A.S.S. This research was funded by EU FP7, grant number 201668, AtheroRemo to F.Ma. This work was also supported by the Swiss National Science Foundation Grants to F.Ma. (#310030-118245), F.Mo. (#32002B-134963/1), and S.S. (#310030-130732/1). This work was also funded by a grant from the Swiss Heart Foundation to F.Ma. This work was funded by the ‘Sir Jules Thorn Trust Reg’ fund and Gustave and Simone Prévot fund to F.Mo. This work was funded by a grant by Fondazione Carige to F.D. Finally, this work was partly funded by NIH grant number DA009789 to V.M.
Conflict of interest: none declared.
Supplementary Material
References
- 1.Di Marzo V. Endocannabinoids: synthesis and degradation. Rev Physiol Biochem Pharmacol. 2008;160:1–24. doi: 10.1007/112_0505. doi:10.1007/112_0505. [DOI] [PubMed] [Google Scholar]
- 2.Quarta C, Mazza R, Obici S, Pasquali R, Pagotto U. Energy balance regulation by endocannabinoids at central and peripheral levels. Trends Mol Med. 2011;17:518–526. doi: 10.1016/j.molmed.2011.05.002. doi:10.1016/j.molmed.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 3.Pacher P, Mechoulam R. Is lipid signaling through cannabinoid 2 receptors part of a protective system? Prog Lipid Res. 2011;50:193–211. doi: 10.1016/j.plipres.2011.01.001. doi:10.1016/j.plipres.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Di Marzo V. Targeting the endocannabinoid system: to enhance or reduce? Nat Rev Drug Discov. 2008;7:438–455. doi: 10.1038/nrd2553. doi:10.1038/nrd2553. [DOI] [PubMed] [Google Scholar]
- 5.Netherland CD, Pickle TG, Bales A, Thewke DP. Cannabinoid receptor type 2 (CB2) deficiency alters atherosclerotic lesion formation in hyperlipidemic Ldlr-null mice. Atherosclerosis. 2010;213:102–108. doi: 10.1016/j.atherosclerosis.2010.07.060. doi:10.1016/j.atherosclerosis.2010.07.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dol-Gleizes F, Paumelle R, Visentin V, Marés AM, Desitter P, Hennuyer N, Gilde A, Staels B, Schaeffer P, Bono F. Rimonabant, a selective cannabinoid CB1 receptor antagonist, inhibits atherosclerosis in LDL receptor-deficient mice. Arterioscler Thromb Vasc Biol. 2009;29:12–18. doi: 10.1161/ATVBAHA.108.168757. doi:10.1161/ATVBAHA.108.168757. [DOI] [PubMed] [Google Scholar]
- 7.Montecucco F, Lenglet S, Gayet-Ageron A, Bertolotto M, Pelli G, Palombo D, Pane B, Spinella G, Steffens S, Raffaghello L, Pistoia V, Ottonello L, Pende A, Dallegri F, Mach F. Systemic and intraplaque mediators of inflammation are increased in patients symptomatic for ischemic stroke. Stroke. 2010;41:1394–1404. doi: 10.1161/STROKEAHA.110.578369. doi:10.1161/STROKEAHA.110.578369. [DOI] [PubMed] [Google Scholar]
- 8.North American Symptomatic Carotid Endarterectomy Trial Collaborators. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med. 1991;325:445–453. doi: 10.1056/NEJM199108153250701. doi:10.1056/NEJM199108153250701. [DOI] [PubMed] [Google Scholar]
- 9.Randomised trial of endarterectomy for recently symptomatic carotid stenosis: final results of the MRC European Carotid Surgery Trial (ECST) Lancet. 1998;351:1379–1387. doi:10.1016/S0140-6736(97)09292-1. [PubMed] [Google Scholar]
- 10.Barnett HJ, Taylor DW, Eliasziw M, Fox AJ, Ferguson GG, Haynes RB, Rankin RN, Clagett GP, Hachinski VC, Sackett DL, Thorpe KE, Meldrum HE, Spence JD. Benefit of carotid endarterectomy in patients with symptomatic moderate or severe stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med. 1998;339:1415–1425. doi: 10.1056/NEJM199811123392002. doi:10.1056/NEJM199811123392002. [DOI] [PubMed] [Google Scholar]
- 11.Cheng C, Tempel D, van Haperen R, van der Baan A, Grosveld F, Daemen MJ, Krams R, de Crom R. Atherosclerotic lesion size and vulnerability are determined by patterns of fluid shear stress. Circulation. 2006;113:2744–2753. doi: 10.1161/CIRCULATIONAHA.105.590018. doi:10.1161/CIRCULATIONAHA.105.590018. [DOI] [PubMed] [Google Scholar]
- 12.Montecucco F, Vuilleumier N, Pagano S, Lenglet S, Bertolotto M, Braunersreuther V, Pelli G, Kovari E, Pane B, Spinella G, Pende A, Palombo D, Dallegri F, Mach F, Roux-Lombard P. Anti-apolipoprotein A-1 auto-antibodies are active mediators of atherosclerotic plaque vulnerability. Eur Heart J. 2011;32:412–421. doi: 10.1093/eurheartj/ehq521. doi:10.1093/eurheartj/ehq521. [DOI] [PubMed] [Google Scholar]
- 13.Chakrabarti S, Zee JM, Patel KD. Regulation of matrix metalloproteinase-9 (MMP-9) in TNF-stimulated neutrophils: novel pathways for tertiary granule release. J Leukoc Biol. 2006;79:214–222. doi: 10.1189/jlb.0605353. doi:10.1189/jlb.0605353. [DOI] [PubMed] [Google Scholar]
- 14.Helderman F, Segers D, de Crom R, Hierck BP, Poelmann RE, Evans PC, Krams R. Effect of shear stress on vascular inflammation and plaque development. Curr Opin Lipidol. 2007;18:527–533. doi: 10.1097/MOL.0b013e3282ef7716. doi:10.1097/MOL.0b013e3282ef7716. [DOI] [PubMed] [Google Scholar]
- 15.Sugamura K, Sugiyama S, Nozaki T, Matsuzawa Y, Izumiya Y, Miyata K, Nakayama M, Kaikita K, Obata T, Takeya M, Ogawa H. Activated endocannabinoid system in coronary artery disease and antiinflammatory effects of cannabinoid 1 receptor blockade on macrophages. Circulation. 2009;119:28–36. doi: 10.1161/CIRCULATIONAHA.108.811992. doi:10.1161/CIRCULATIONAHA.108.811992. [DOI] [PubMed] [Google Scholar]
- 16.Lo Verme J, Gaetani S, Fu J, Oveisi F, Burton K, Piomelli D. Regulation of food intake by oleoylethanolamide. Cell Mol Life Sci. 2005;62:708–716. doi: 10.1007/s00018-004-4494-0. doi:10.1007/s00018-004-4494-0. [DOI] [PubMed] [Google Scholar]
- 17.Steffens S, Veillard NR, Arnaud C, Pelli G, Burger F, Staub C, Karsak M, Zimmer A, Frossard JL, Mach F. Low dose oral cannabinoid therapy reduces progression of atherosclerosis in mice. Nature. 2005;434:782–786. doi: 10.1038/nature03389. doi:10.1038/nature03389. [DOI] [PubMed] [Google Scholar]
- 18.Krams R, Verheye S, van Damme LC, Tempel D, Mousavi Gourabi B, Boersma E, Kockx MM, Knaapen MW, Strijder C, van Langenhove G, Pasterkamp G, van der Steen AF, Serruys PW. In vivo temperature heterogeneity is associated with plaque regions of increased MMP-9 activity. Eur Heart J. 2005;26:2200–2205. doi: 10.1093/eurheartj/ehi461. doi:10.1093/eurheartj/ehi461. [DOI] [PubMed] [Google Scholar]
- 19.Drost EM, MacNee W. Potential role of IL-8, platelet-activating factor and TNF-alpha in the sequestration of neutrophils in the lung: effects on neutrophil deformability, adhesion receptor expression, and chemotaxis. Eur J Immunol. 2002;32:393–403. doi: 10.1002/1521-4141(200202)32:2<393::AID-IMMU393>3.0.CO;2-5. doi:10.1002/1521-4141(200202)32:2<393::AID-IMMU393>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 20.Montecucco F, Steffens S, Burger F, Da Costa A, Bianchi G, Bertolotto M, Mach F, Dallegri F, Ottonello L. Tumor necrosis factor-alpha (TNF-alpha) induces integrin CD11b/CD18 (Mac-1) up-regulation and migration to the CC chemokine CCL3 (MIP-1alpha) on human neutrophils through defined signalling pathways. Cell Signal. 2008;20:557–568. doi: 10.1016/j.cellsig.2007.11.008. doi:10.1016/j.cellsig.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 21.Willecke F, Zeschky K, Ortiz Rodriguez A, Colberg C, Auwärter V, Kneisel S, Hutter M, Lozhkin A, Hoppe N, Wolf D, von zur Mühlen C, Moser M, Hilgendorf I, Bode C, Zirlik A. Cannabinoid receptor 2 signaling does not modulate atherogenesis in mice. PLoS One. 2011;6:e19405. doi: 10.1371/journal.pone.0019405. doi:10.1371/journal.pone.0019405. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.