Abstract
Aims
Periodic breathing with central sleep apnoea (CSA) is common in heart failure patients and is associated with poor quality of life and increased risk of morbidity and mortality. We conducted a prospective, non-randomized, acute study to determine the feasibility of using unilateral transvenous phrenic nerve stimulation for the treatment of CSA in heart failure patients.
Methods and results
Thirty-one patients from six centres underwent attempted transvenous lead placement. Of these, 16 qualified to undergo two successive nights of polysomnography—one night with and one night without phrenic nerve stimulation. Comparisons were made between the two nights using the following indices: apnoea–hypopnoea index (AHI), central apnoea index (CAI), obstructive apnoea index (OAI), hypopnoea index, arousal index, and 4% oxygen desaturation index (ODI4%). Patients underwent phrenic nerve stimulation from either the right brachiocephalic vein (n = 8) or the left brachiocephalic or pericardiophrenic vein (n = 8). Therapy period was (mean ± SD) 251 ± 71 min. Stimulation resulted in significant improvement in the AHI [median (inter-quartile range); 45 (39–59) vs. 23 (12–27) events/h, P = 0.002], CAI [27 (11–38) vs. 1 (0–5) events/h, P≤ 0.001], arousal index [32 (20–42) vs. 12 (9–27) events/h, P = 0.001], and ODI4% [31 (22–36) vs. 14 (7–20) events/h, P = 0.002]. No significant changes occurred in the OAI or hypopnoea index. Two adverse events occurred (lead thrombus and episode of ventricular tachycardia), though neither was directly related to phrenic nerve stimulation therapy.
Conclusion
Unilateral transvenous phrenic nerve stimulation significantly reduces episodes of CSA and restores a more natural breathing pattern in patients with heart failure. This approach may represent a novel therapy for CSA and warrants further study.
ClinicalTrials.gov identifier: NCT00909259.
Keywords: Heart failure, Central sleep apnoea, Phrenic nerve stimulation
See page 810 for the editorial comment on this article (doi:10.1093/eurheartj/ehr357)
Introduction
Congestive heart failure is a major public health problem that is associated with substantial morbidity, mortality, and economic cost. A frequent co-morbidity associated with poor outcome in heart failure is sleep-related breathing disorders.1 Among these disorders, central sleep apnoea (CSA) with periodic breathing, known as Hunter–Cheyne–Stokes breathing, occurs in a high proportion of heart failure patients.2–4
Central sleep apnoea is characterized by the temporary withdrawal of brainstem-driven respiratory drive that results in cessation of breathing, hypoxia, and arousals from sleep. Periodic breathing with CSA has a number of adverse effects on cardiac function that contribute to the progression of heart failure, including stimulation of the sympathetic nervous system and arrhythmias.1,5–8 Central sleep apnoea has also been shown to be an independent prognostic indicator of poor survival.9–11
Several devices, including continuous positive airway pressure (CPAP) and adaptive pressure support servoventilation devices, have been used to treat CSA in patients with systolic heart failure.12–16 In contrast to treatment of obstructive sleep apnoea, where application of nasal CPAP invariably results in the virtual elimination of obstructive disordered breathing events, treatment of CSA in systolic heart failure is difficult, and response to therapy is not uniform. In two studies, CPAP failed to eliminate CSA in 43–57% of the patients, although survival improved considerably in those whose CSA was suppressed by CPAP.12,14 In addition to the lack of uniform response, a major limitation with the long-term use of positive airway pressure devices, both in obstructive sleep apnoea and in CSA, is adherence to therapy.14,17
Phrenic nerve stimulation offers an alternative means to regulate breathing by utilizing a physiological approach to initiate respiration. This therapy is currently used to provide respiratory support in select patients with respiratory paralysis from high cervical spinal cord injury and in patients with central alveolar hypoventilation syndrome (Ondine's curse).18–21 Given this experience, phrenic nerve stimulation to regulate breathing may also prove useful in the treatment of CSA in heart failure. However, present means of chronically stimulating the phrenic nerve require transthoracic surgical placement of cuff electrodes on the nerve itself. This surgical procedure may not be well tolerated in patients with advanced heart failure and may result in damage to the nerve either by direct surgical manipulation or by contact with the electrode.22 Transvenous stimulation of the phrenic nerves may offer an alternative approach to avoid these complications. In addition, as a potential implantable device-based therapy, adherence to therapy would be assured. Here, we report the results of the first prospective study to determine the feasibility of a novel transvenous approach to unilateral phrenic nerve stimulation for the treatment of CSA in heart failure patients.
Methods
Patients
Patients were eligible for this acute study if they had a history of sleep apnoea and/or previous polysomnographic (PSG) testing with evidence of periodic breathing with CSA within 6 months prior to enrolment in the study. All potential study participants then underwent two additional full nights of attended PSG study. Patients were included in the study if they had an apnoea–hypopnoea index (AHI) of ≥15 and a central apnoea index (CAI) of ≥5. Patients were excluded from the study if they had a baseline oxygen saturation of <90%; were on supplemental oxygen; had evidence of phrenic nerve palsy; had severe chronic obstructive pulmonary disease; had unstable angina or myocardial infarction within 3 months of the investigative procedure; were pacemaker-dependent; or had inadequate capture of the phrenic nerve during neurostimulation. The study was performed in compliance with the Declaration of Helsinki. The institutional review board of each participating centre approved the study protocol, and all patients gave written informed consent.
Study design
Patients who met the entry criteria and who had successful placement of a temporary transvenous lead for phrenic nerve stimulation underwent two successive nights of PSG study: (i) a control night without phrenic nerve stimulation and (ii) a therapy night with phrenic nerve stimulation. To determine the stages of sleep, we recorded a four-channel electroencephalogram (ML135 Dual Bio Amp, ADInstruments, Bella Vista, New South Wales, Australia) and a two-channel electro-oculogram (ML135 Dual Bio Amp, ADInstruments) during the study. Thoracoabdominal excursions were measured qualitatively by respiratory inductive plethysmography (XactTrace, Embla, Broomfield, CO, USA). Airflow was monitored qualitatively with an oronasal thermocouple (Model TCT1R, Grass Technologies, West Warwick, RI, USA). Arterial blood oxyhaemoglobin saturation was recorded using a finger probe (ML320/MLT321, ADInstruments). These variables were recorded on a multichannel polygraph (PowerLab16/30, ADInstruments) during both the control and therapy nights. Prescribed medications were given as usual. For the purpose of this trial, patients received a hypnotic (such as zolpidem or alprazolam, with the same hypnotic being used both nights) at bedtime per the discretion of the attending physician.
The neurostimulation lead was removed the morning following the study. Twenty-four hours after testing and 5–10 days later, an in-clinic or phone follow-up was conducted to assess for adverse events. Patients then exited from the study.
Procedure description
Venous access was obtained via the axillary or subclavian vein. Based on the patient's anatomy and the implanting physician's preference, stimulation leads (Cardima catheter, Cardima, Inc., Fremont, CA, USA, or proprietary stimulation leads, Respicardia, Inc., Minnetonka, MN, USA) were placed in either the right brachiocephalic vein, the left brachiocephalic vein, or the left pericardiophrenic vein to stimulate the adjacent phrenic nerve. An external pulse generator system (Respicardia, Inc.) was used that provided low-energy nerve stimulation. During the lead implantation procedure, capture was determined by external palpation of diaphragmatic contraction on the side of stimulation. The stimulation lead was then secured to the skin for the duration of the study. Additionally, two patients underwent optional temporary azygous lead implantation for sensing respiration.
During the therapy night, the amount of phrenic nerve stimulation was titrated as needed with the goal of eliminating centrally mediated apnoeic events without arousing the patient from sleep. The sequence of the two study nights was alternated between the patients so that some patients had therapy the first night and some the second night.
Scoring of polysomnographic studies
Polysomnograms from the two nights of the study were scored by two certified sleep technicians using the 2007 American Academy of Sleep Medicine (AASM) Manual for the Scoring of Sleep and Associated Events.23 The technicians were blinded to the subject identifiers, the ordering of the study nights, and the application of stimulation.
An episode of apnoea was defined as the cessation of inspiratory airflow for 10 s or more. An episode of obstructive apnoea was defined as the absence of airflow in the presence of rib cage and abdominal excursions. An episode of central apnoea was defined as the absence of rib cage and abdominal excursions and the absence of airflow. Hypopnoea was defined as a reduction in airflow lasting 10 s or more and associated with at least a 4% decrease in arterial oxyhaemoglobin saturation. An electroencephalographic arousal was defined as the appearance of α- or θ-waves or increase in frequency >16 Hz on the electroencephalogram for at least 3s after at least 10 s of sleep.23 The AHI was defined as the number of episodes of apnoea and hypopnoea per hour of sleep. The study evaluated the effects of phrenic nerve stimulation on the following indices characterizing the severity of CSA as defined by AASM criteria:23 AHI, CAI, obstructive apnoea index (OAI), hypopnoea index, oxygen desaturation index of 4% (ODI4%), and arousal index.
Statistical analysis
A total of 31 patients from six centres in Poland, Germany, and the USA met the study's inclusion criteria and underwent lead placement. Of these patients, 11 were excluded due to technically inadequate capture of the phrenic nerve during neurostimulation. Four patients were also excluded for the following reasons: CAI <5 h−1 on the control night (n = 3), and a single episode of ventricular tachycardia that occurred in a patient independent of the procedure. The remaining 16 patients were included in the statistical analysis.
Related data from the two nights were analysed using the Wilcoxon signed-rank test. All probability values were calculated from two-sided tests, and a P-value of <0.05 was considered statistically significant. All statistical calculations were performed using PASW Statistics Release 18 (SPSS, Inc., Chicago, IL, USA). Discrete variables are expressed as frequencies and percentages, and continuous variables are expressed as mean ± standard deviation or median (inter-quartile range).
Results
Patients
The characteristics of the 16 patients analysed are summarized in Table 1. The group was composed of men, aged 59 ± 12 years, with a body mass index of 27.5 ± 3.3 kg/m2. They were on standard therapy for systolic heart failure and had a mean left ventricular ejection fraction of 30.2 ± 12.0%.
Table 1.
Patient characteristics
| Characteristica | n = 16 |
|---|---|
| Age (years) | 58.6 ± 11.7 |
| Male | 100% (16) |
| Body mass index (kg/m2) | 27.5 ± 3.3 |
| Respiratory rate (breaths/min) | 15.2 ± 4.9 |
| Heart rate (b.p.m.) | 73.3 ± 17.1 |
| Atrial fibrillation | 19.0% (3) |
| Chronic obstructive pulmonary disease | 13.3% (2) |
| Bronchial asthma | 6.7% (1) |
| NYHA classification | |
| I | 18.8% (3) |
| II | 50.0% (8) |
| III | 31.3% (5) |
| Left ventricular ejection fraction (%) | 30.2 ± 12.0 |
| Cardiomyopathy | |
| Ischaemic | 43.8% (7) |
| Non-ischaemic | 53.8% (9) |
| ICD/CRT-D present | 25.0% (4) |
| Medications | |
| ACE-inhibitor/ARB | 87.5% (14) |
| β-Blocker | 93.8% (15) |
| Diuretic | 100% (16) |
| Aldosterone antagonist | 56.3% (9) |
| Anticoagulant | 43.8% (7) |
| Statin | 68.8% (11) |
| Antiarrhythmic | 12.5% (2) |
| Diabetes medication | 25.0% (4) |
NYHA, New York Heart Association; ICD, implantable cardioverter-defibrillator; CRT-D, cardiac resynchronization therapy-defibrillation device; ACE, angiotensin-converting enzyme; ARB, angiotensin II receptor blocker.
aAll values expressed as % (n) or mean ± SD.
Lead implantation and stimulation characteristics
In 8 of 16 (50%) patients, the stimulation lead location was in the right brachiocephalic vein, and in 8 of 16 (50%) patients, it was in either the left pericardiophrenic (n = 7) or left brachiocephalic (n = 1) vein. Lead implantation stimulation thresholds were consistently low on average regardless of whether the right or left phrenic nerve was stimulated (4.7 ± 0.81 vs. 2.8 ± 2.30 mA, respectively). Four patients had previously implanted cardiac devices. Two had an implantable cardiac defibrillator (ICD), and two had a combined cardiac resynchronization therapy-ICD (CRT-ICD) device. Devices were evaluated for possible over- or under-sensing in the presence of phrenic nerve stimulation. The implanted device was programmed to the highest sensitivity setting to assess for crosstalk. At 10 mA (20 Hz, 150 μs pulse width), there was no detection of the phrenic nerve stimulation by the ICD or ICD component of the CRT-ICD.
Therapy results
Six of the 16 patients underwent the therapy study the first night. The therapy period, defined as the time when the patient was asleep and phrenic nerve stimulation was attempted, was 251 ± 71 min. Stimulation levels delivered during the course of the therapy night averaged 5.3 ± 2.1 mA for the right phrenic nerve and 3.5 ± 1.6 mA for the left phrenic nerve.
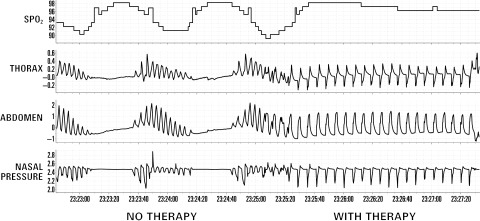
Figure 1 demonstrates the typical effects of unilateral phrenic nerve stimulation in one patient, with neurostimulation resulting in the elimination of CSA.
Figure 1.
Elimination of respiratory instability and improvement in oxygenation during unilateral phrenic nerve stimulation in a heart failure patient with central sleep apnoea.
The stimulation resulted in significant improvement of all evaluated indices characterizing the severity of CSA, with decreases seen in the AHI [45 (39–59) vs. 23 (12–27) events/h, P = 0.002], CAI [27 (11–38) vs. 1 (0–5) events/h, P≤ 0.001], arousal index [32 (20–42) vs. 12 (9–27) events/h, P = 0.001], and ODI4% [31 (22–36) vs. 14 (7–20) events/h, P = 0.002] (Table 2). There were also categorical improvements in the severity of the sleep apnoea seen during unilateral phrenic nerve stimulation, using standard definitions of disease severity based on the AHI (Table 3).
Table 2.
Sleep characteristics: control night vs. therapy night
| Sleep parameter or index | Control nighta (n = 16) | Therapy nighta (n = 16) | P-value |
|---|---|---|---|
| Sleep total (min) | 236 (207–279) | 245 (219–297) | 0.535 |
| Sleep efficiency (%) | 78 (62–83) | 71 (57–84) | 0.796 |
| Time in each sleep stage (%) | |||
| N1 | 18 (11–37) | 17 (9–30) | 0.352 |
| N2 | 46 (41–66) | 53 (40–72) | 0.326 |
| N3 | 3 (0–12) | 0 (0–13) | 0.646 |
| REM | 13 (10–17) | 18 (10–22) | 0.196 |
| Baseline oxygen saturation (%) | 95 (94–96) | 96 (94–97) | 0.056 |
| Average heart rate (b.p.m.) | 71 (59–86) | 70 (57–82) | 0.044 |
| Average respiratory rate (breaths/min) | 15 (12–18) | 16 (15–17) | 0.438 |
| Arousal index (events/h) | 32 (20–42) | 12 (9–27) | 0.001 |
| AHI | 45 (39–59) | 23 (12–27) | 0.002 |
| CAI | 27 (11–38) | 1 (0–5) | ≤0.001 |
| OAI | 1 (0–7) | 4 (1–14) | 0.056 |
| Hypopnoea index | 10 (3–18) | 10 (7–14) | 0.756 |
| ODI4% | 31 (22–36) | 14 (7–20) | 0.002 |
REM, rapid eye movement; AHI, apnoea–hypopnoea index; CAI, central apnoea index; OAI, obstructive apnoea index; ODI4%, oxygen desaturation index 4%.
aAll values expressed as median (inter-quartile range).
Table 3.
Categorical change in the severity of sleep apnoea based on the apnoea-hypopnoea index
| Severity/AHI (events/h) | Control night* (n = 16) | Therapy night* (n = 16) |
|---|---|---|
| Mild (<15) | 0 | 5 (31.3%) |
| Moderate (15–30) | 1 (6.3%) | 8 (50.0%) |
| Severe (>30) | 15 (93.8%) | 3 (18.8%) |
AHI, apnoea–hypopnoea index.
*Wilcoxon's matched pairs signed-rank test, P = 0.001.
Therapy tolerance and adverse events
The procedure was well tolerated. All patients completed their required follow-up visits. Two adverse events occurred. At the time of intended lead removal, one patient, who underwent placement of a sensing lead in the azygous vein to aid sensing of respiration, was noted to have a thrombus on the lead. The patient was started on anticoagulation therapy, and the lead was later removed without sequelae. A second patient had an episode of ventricular tachycardia the day after lead placement, but prior to the stimulation study. The tachycardia was treated appropriately with defibrillation from the patient's implanted ICD. The patient recovered without sequelae, but the patient's study was aborted prior to therapy initiation.
Discussion
The results of the present study demonstrate the feasibility of unilateral transvenous phrenic nerve stimulation for the treatment of CSA in heart failure. Therapy improved the nocturnal breathing pattern in heart failure patients, producing a 48% reduction in AHI due to a 90% reduction in the number of CSA episodes. Consistent with the improvement in the AHI, the ODI4% and arousal index also improved significantly during the therapy night. Since the cardiovascular effects of CSA are thought to be largely mediated by intermittent hypoxia and arousal, the improvement in desaturations and arousals supports the potential beneficial clinical effects of unilateral transvenous phrenic nerve stimulation therapy. It is also worth noting that in both obstructive sleep apnoea and CSA, mortality is highest in those with the most severe sleep apnoea, defined as an AHI of >30 events/h.9,24 In the present study, the number of patients with severe sleep apnoea decreased from 94 to 19% with therapy.
In the long-term Canadian CPAP trial, survival improved in patients achieving an AHI of <15 events/h.14 In the present study, five patients (31%) achieved an AHI of <15 events/h during the therapy night. This result compares favourably with those from an initial study of overnight CPAP therapy, which showed 43% of patients reaching an AHI of <15 events/h.12 Similarly, in the long-term Canadian CPAP trial, response increased to 57% of patients who were able to complete 3 months of CPAP therapy reaching an AHI <15 events/h.14 It is therefore conceivable that with long-term phrenic nerve stimulation, the AHI would decrease further, as full adherence to therapy is expected, and therapy should continue throughout the night.
As an early-phase feasibility trial, this study had several limitations. Most notably, it was a non-randomized, open-label study; nonetheless, patient studies were scored blindly. In addition, the results of only a single night of therapy involving a small number of patients, all of whom were males, are reported. The lack of women in this small study is not surprising though, given the significantly higher frequency of CSA in male heart failure patients.2,3 The study's design also did not allow us to adequately assess potential complications with this therapy, such as its potential for interfering with pre-existing implanted cardiac devices. Additionally, there were a number of patients excluded for lead placement issues; however, this was the first experience with placement of leads in these positions, and adequate placement improved throughout the course of the study. We also prescribed benzodiazepine receptor agonists for patients' convenience during the study. The same agonist was used both nights. While these agonists do not affect AHI in heart failure patients, they do decrease the number of arousals.25 It is also important to note that many heart failure patients with CSA also have some obstructive apnoeas. It is therefore expected that unilateral transvenous phrenic nerve stimulation therapy should only target patients with predominantly CSA. Finally, as an acute, early feasibility study, we did not collect data on the clinical profile of those heart failure patients who are most likely to benefit from this therapy, nor did we obtain potentially important data that may be useful in characterizing the physiological mechanisms underlying the benefits of phrenic nerve stimulation. Future randomized, controlled trials will necessarily need to include these data to identify these patients and to better understand these mechanisms.
In conclusion, the findings from this acute feasibility study provide a strong proof of concept supporting the use of unilateral transvenous phrenic nerve stimulation as a treatment for CSA in heart failure patients by demonstrating significant improvement in major indices of CSA severity, including the AHI, CAI, ODI4%, and arousal index. Therapy was well tolerated. With the development of a permanent, fully implantable system for unilateral transvenous phrenic nerve stimulation, a novel approach to the chronic treatment of CSA in heart failure becomes possible. Large-scale, long-term, randomized controlled trials using an implanted system are now needed to further evaluate the clinical impact of this approach.
Funding
This study was supported by Respicardia, Inc., Minnetonka, MN, USA.
Conflict of interest: W.T.A., R.A., S.J., R.N.K., O.O., and P.P. are paid consultants to Respicardia, Inc. R.G. is employed by Respicardia, Inc.
Acknowledgments
We wish to thank the patients for their participation in this study. We also wish to thank Jerome Dempsey, MD, for his review of the manuscript, and Janice Hoettels, BS, PA-C, for her editorial assistance in the preparation of the manuscript.
References
- 1.Somers VK, White DP, Amin R, Abraham WT, Costa F, Culebras A, Daniels S, Floras JS, Hunt CE, Olson LJ, Pickering TG, Russell R, Woo M, Young T American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology; American Heart Association Stroke Council; American Heart Association Council on Cardiovascular Nursing; American College of Cardiology Foundation. Sleep apnea and cardiovascular disease: an American Heart Association/American College of Cardiology Foundation scientific statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council on Cardiovascular Nursing. Circulation. 2008;118:1080–1111. doi: 10.1161/CIRCULATIONAHA.107.189375. doi:10.1161/CIRCULATIONAHA.107.189420. [DOI] [PubMed] [Google Scholar]
- 2.Javaheri S, Parker TJ, Liming JD, Corbett WS, Nishiyama H, Wexler L, Roselle GA. Sleep apnea in 81 ambulatory male patients with stable heart failure. Types and their prevalences, consequences, and presentations. Circulation. 1998;97:2154–2159. doi: 10.1161/01.cir.97.21.2154. [DOI] [PubMed] [Google Scholar]
- 3.Sin DD, Fitzgerald F, Parker JD, Newton G, Floras JS, Bradley TD. Risk factors for central and obstructive sleep apnea in 450 men and women with congestive heart failure. Am J Respir Crit Care Med. 1999;160:1101–1106. doi: 10.1164/ajrccm.160.4.9903020. [DOI] [PubMed] [Google Scholar]
- 4.Oldenburg O, Lamp B, Faber L, Teschler H, Horstkotte D, Töpfer V. Sleep disordered breathing in patients with symptomatic heart failure: a contemporary study of prevalence in and characteristics of 700 patients. Eur J Heart Fail. 2007;9:251–257. doi: 10.1016/j.ejheart.2006.08.003. doi:10.1016/j.ejheart.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 5.Naughton MT, Benard DC, Liu PP, Rutherford R, Rankin F, Bradley TD. Effects of nasal CPAP on sympathetic activity in patients with heart failure and central sleep apnea. Am J Respir Crit Care Med. 1995;152:473–479. doi: 10.1164/ajrccm.152.2.7633695. [DOI] [PubMed] [Google Scholar]
- 6.Spaak J, Egri ZJ, Kubo T, Yu E, Ando S, Kaneko Y, Usui K, Bradley TD, Floras JS. Muscle sympathetic activity during wakefulness in heart failure patients with and without sleep apnea. Hypertension. 2005;46:1327–1332. doi: 10.1161/01.HYP.0000193497.45200.66. doi:10.1161/01.HYP.0000193497.45200.66. [DOI] [PubMed] [Google Scholar]
- 7.Lanfranchi PA, Somers VK, Braghiroli A, Corra U, Eleuteri E, Giannuzzi P. Central sleep apnea in left ventricular dysfunction: prevalence and implications for arrhythmic risk. Circulation. 2003;107:727–732. doi: 10.1161/01.cir.0000049641.11675.ee. doi:10.1161/01.CIR.0000049641.11675.EE. [DOI] [PubMed] [Google Scholar]
- 8.Bitter T, Westerheide N, Prinz C, Hossain MS, Vogt J, Langer C, Horstkotte D, Oldenburg O. Cheyne–Stokes respiration and obstructive sleep apnea are independent risk factors for malignant ventricular arrhythmias requiring appropriate cardioverter-defibrillator therapies in patients with congestive heart failure. Eur Heart J. 2011;32:61–74. doi: 10.1093/eurheartj/ehq327. doi:10.1093/eurheartj/ehq327. [DOI] [PubMed] [Google Scholar]
- 9.Lanfranchi PA, Braghiroli A, Bosimini E, Mazzuero G, Colombo R, Donner CF, Giannuzzi P. Prognostic value of nocturnal Cheyne–Stokes respiration in chronic heart failure. Circulation. 1999;99:1435–1440. doi: 10.1161/01.cir.99.11.1435. [DOI] [PubMed] [Google Scholar]
- 10.Hanly PJ, Zuberi-Khokhar NS. Increased mortality associated with Cheyne–Stokes respiration in patients with congestive heart failure. Am J Respir Crit Care Med. 1996;153:272–276. doi: 10.1164/ajrccm.153.1.8542128. [DOI] [PubMed] [Google Scholar]
- 11.Javaheri S, Shukla R, Zeigler H, Wexler L. Central sleep apnea, right ventricular dysfunction and low diastolic blood pressure are predictors of mortality in systolic heart failure. J Am Coll Cardiol. 2007;49:2028–2034. doi: 10.1016/j.jacc.2007.01.084. doi:10.1016/j.jacc.2007.01.084. [DOI] [PubMed] [Google Scholar]
- 12.Javaheri S. Effects of continuous positive airway pressure on sleep apnea and ventricular irritability in patients with heart failure. Circulation. 2000;101:392–397. doi: 10.1161/01.cir.101.4.392. [DOI] [PubMed] [Google Scholar]
- 13.Bradley TD, Logan AG, Kimoff RJ, Sériès F, Morrison D, Ferguson K, Belenkie I, Pfeifer M, Fleetham J, Hanly P, Smilovitch M, Tomlinson G, Floras JS CANPAP Investigators. Continuous positive airway pressure for central sleep apnea and heart failure. N Engl J Med. 2005;353:2025–2033. doi: 10.1056/NEJMoa051001. doi:10.1056/NEJMoa051001. [DOI] [PubMed] [Google Scholar]
- 14.Arzt M, Floras JS, Logan AG, Kimoff RJ, Series F, Morrison D, Ferguson K, Belenkie I, Pfeifer M, Fleetham J, Hanly P, Smilovitch M, Ryan C, Tomlinson G, Bradley TD CANPAP Investigators. Suppression of central sleep apnea by continuous positive airway pressure and transplant-free survival in heart failure: a post hoc analysis of the Canadian Continuous Positive Airway Pressure for Patients with Central Sleep Apnoea and Heart Failure Trial (CANPAP) Circulation. 2007;115:3173–3180. doi: 10.1161/CIRCULATIONAHA.106.683482. doi:10.1161/CIRCULATIONAHA.106.683482. [DOI] [PubMed] [Google Scholar]
- 15.Teschler H, Döhring J, Wang YM, Berthon-Jones M. Adaptive pressure support servo-ventilation: a novel treatment for Cheyne–Stokes respiration in heart failure. Am J Respir Crit Care Med. 2001;164:614–619. doi: 10.1164/ajrccm.164.4.9908114. [DOI] [PubMed] [Google Scholar]
- 16.Pepperell JC, Maskell NA, Jones DR, Langford-Wiley BA, Crosthwaite N, Stradling JR, Davies RJ. A randomized controlled trial of adaptive ventilation for Cheyne–Stokes breathing in heart failure. Am J Respir Crit Care Med. 2003;168:1109–1114. doi: 10.1164/rccm.200212-1476OC. doi:10.1164/rccm.200212-1476OC. [DOI] [PubMed] [Google Scholar]
- 17.Barbé F, Durán-Cantolla J, Capote F, de la Peña M, Chiner E, Masa JF, Gonzalez M, Marín JM, Garcia-Rio F, de Atauri JD, Terán J, Mayos M, Monasterio C, del Campo F, Gomez S, de la Torre MS, Martinez M, Montserrat JM Spanish Sleep and Breathing Group. Long-term effect of continuous positive airway pressure in hypertensive patients with sleep apnea. Am J Respir Crit Care Med. 2010;181:718–726. doi: 10.1164/rccm.200901-0050OC. doi:10.1164/rccm.200901-0050OC. [DOI] [PubMed] [Google Scholar]
- 18.Glenn WWL, Holcomb WG, Gee JBL, Rath R. Central hypoventilation: long-term ventilator assistance by radiofrequency electrophrenic respiration. Ann Surg. 1970;172:755–773. doi: 10.1097/00000658-197010000-00020. doi:10.1097/00000658-197010000-00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Glenn WWL, Phelps ML. Diaphragm pacing by electrical stimulation of the phrenic nerve. Neurosurgery. 1985;17:974–984. doi: 10.1227/00006123-198512000-00021. doi:10.1227/00006123-198512000-00021. [DOI] [PubMed] [Google Scholar]
- 20.DiMarco AF. Phrenic nerve stimulation in patients with spinal cord injury. Respir Physiol Neurobiol. 2009;169:200–209. doi: 10.1016/j.resp.2009.09.008. doi:10.1016/j.resp.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 21.Chen ML, Tablizo MA, Kun S, Keens TG. Diaphragm pacers as a treatment for congenital central hypoventilation syndrome. Expert Rev Med Devices. 2005;2:577–585. doi: 10.1586/17434440.2.5.577. doi:10.1586/17434440.2.5.577. [DOI] [PubMed] [Google Scholar]
- 22.Glenn WW, Brouillette RT, Dentz B, Fodstad H, Hunt CE, Keens TG, Marsh HM, Pande S, Piepgras DG, Vanderlinden RG. Fundamental considerations in pacing of the diaphragm for chronic ventilatory insufficiency: a multi-center study. Pacing Clin Electrophysiol. 1988;11:2121–2127. doi: 10.1111/j.1540-8159.1988.tb06360.x. doi:10.1111/j.1540-8159.1988.tb06360.x. [DOI] [PubMed] [Google Scholar]
- 23.Iber C, Ancoli-Israel S, Chesson AL, Quan SF. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology, and Technical Specifications. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 24.Young T, Finn L, Peppard PE, Szklo-Coxe M, Austin D, Nieto FJ, Stubbs R, Hla KM. Sleep disordered breathing and mortality. Sleep. 2008;31:1071–1078. [PMC free article] [PubMed] [Google Scholar]
- 25.Biberdorf DJ, Steens R, Millar TW, Kryger MH. Benzodiazepines in congestive heart failure: effects of temazepam on arousability and Cheyne–Stokes respiration. Sleep. 1993;16:529–538. doi: 10.1093/sleep/16.6.529. [DOI] [PubMed] [Google Scholar]