Abstract
Hemopexin is a serum, CSF, and neuronal protein that is protective after experimental stroke. Its efficacy in the latter has been linked to increased expression and activity of heme oxygenase (HO)-1, suggesting that it facilitates heme degradation and subsequent release of cytoprotective biliverdin and carbon monoxide. In this study, the effect of hemopexin on the rate of hemin breakdown by CNS cells was investigated in established in vitro models. Equimolar hemopexin decreased hemin breakdown, as assessed by gas chromatography, by 60–75% in primary cultures of murine neurons and glia. Extracellular hemopexin reduced cell accumulation of 55Fe-hemin by over 90%, while increasing hemin export or extraction from membranes by four-fold. This was associated with significant reduction in HO-1 expression and neuroprotection. In a cell-free system, hemin breakdown by recombinant HO-1 was reduced over 80% by hemopexin; in contrast, albumin and two other heme-binding proteins had no effect. Although hemopexin was detected on immunoblots of cortical lysates from adult mice, hemopexin knockout per se did not alter HO activity in cortical cells treated with hemin. These results demonstrate that hemopexin decreases the accumulation and catabolism of exogenous hemin by neural cells. Its beneficial effect in stroke models is unlikely to be mediated by increased production of cytoprotective heme breakdown products.
Keywords: heme, hemopexin, intracerebral hemorrhage, ischemia, stroke, subarachnoid hemorrhage
1. Introduction
Hemopexin is a ~60kDa glycoprotein that is synthesized primarily by hepatocytes. It is secreted and is abundant in plasma, where it binds free heme or hemin with extraordinary affinity, thereby preventing its participation in free radical reactions (Tolosano et al., 2010). Several laboratories have independently reported that hemopexin is also expressed by both peripheral and central neurons (Chen et al., 1998; He et al., 2010; Li et al., 2009; Swerts et al., 1992; Tolosano et al., 1996). Immunoreactivity is diffuse in neuronal somata, but is absent in axons and dendrites (Li et al., 2009).
Recent experimental evidence suggests that hemopexin is protective after ischemic and hemorrhagic stroke. When tested in a model of transient middle cerebral artery occlusion, hemopexin knockout mice sustained greater infarct volumes and behavioral deficits than their wild-type counterparts (Li et al., 2009). Beneficial effects of hemopexin have also been observed in both the blood injection and collagenase models of intracerebral hemorrhage (Chen et al., 2011). Hemopexin was reported to reduce injury by facilitating the induction of heme oxygenase (HO)-1 (Li et al., 2009), the inducible HO isozyme (EC 1.14.99.3), which catalyzes the rate-limiting step of heme breakdown to iron, carbon monoxide (CO) and biliverdin. Neurons pretreated with hemin-hemopexin overexpressed HO-1, and were subsequently protected from oxidative injury. Protection was not observed in HO-1 knockout neurons, and was reversed in wild-type cells by the HO inhibitor tin protoporphyrin IX, consistent with a requirement for HO catalytic activity (Li et al., 2009).
This observation that the protective effect of hemopexin in CNS cells is mediated by increased heme breakdown is unexpected for two reasons. First, hemopexin decreases or prevents hemin uptake by most cell populations and directs it to the liver, where it is taken up via receptor-mediated endocytosis (Smith and Morgan, 1979). If hemopexin reduces hemin accumulation in CNS cells, it would likely decrease HO activity, particularly in ischemic stroke, since substrate availability is the rate-limiting factor under non-hemolytic conditions (Sassa, 2004; Sheftel et al., 2007). Second, the very high binding affinity of hemopexin for hemin (Kd ~10−13 M, (Morgan et al., 1976) may prevent its transfer to the catalytic site of the HO's, as has been observed for hemoproteins with similar binding affinities (Abraham et al., 1996); cytosolic hemopexin may therefore further limit HO substrate availability. Despite its relevance to ischemic and hemorrhagic stroke, little is known of the effect of hemopexin on hemin trafficking and breakdown in CNS cells. In the present study, we utilized established models to test the hypotheses that hemopexin attenuated hemin accumulation and catabolism in neural cells, HO-1 induction by hemin, and hemin breakdown by recombinant HO-1.
2. Materials and Methods
2.1. Materials
Recombinant rat HO-1, native rat cytochrome P450 reductase (EC 1.6.2.4), and rabbit anti-HO-1 were purchased from Enzo Life Sciences, Farmingdale, NY, USA. Vendors for hemin binding proteins were as follows: human plasma hemopexin, Athens Research and Technology, Athens, GA, USA; recombinant human peroxiredoxin 1 (heme binding protein 23, EC 1.11.1.15), AbFRONTIER, Seoul, Korea; equine liver glutathione S-transferase (EC 2.5.1.18), bovine and human albumin, Sigma-Aldrich, St. Louis, MO, USA. Hemin was purchased from Frontier Scientific, Logan, UT, USA. 55Fe-hemin was synthesized by and purchased from Perkin Elmer, Waltham, MA, USA. Monoclonal anti-hemopexin was kindly provided by Dr. Emanuela Tolosano, University of Turin, Italy.
Oxidized heme (hemin) was used exclusively in all experiments. Use of the word "heme" denotes both its oxidized and reduced forms.
2.2 Cell cultures
Cortical cell cultures containing both neurons and glia (~2% microglia, >90% GFAP+) were prepared from fetal B6129 mice (gestational age 14–16 days), following a method that has been previously described in detail (Rogers et al., 2003). Cultures were used for experiments on days 12–16 in vitro.
2.3 Hemopexin knockout mice
Founding pairs were provided by Dr. Frank Berger, University of South Carolina, U.S.A. and were descended from knockouts originally produced by Dr. Emanuela Tolosano (Tolosano et al., 1999). Heterozygous knockout mice with a B6129 background were used for breeding. Genotype was determined by PCR of genomic DNA extracted from tail clippings, using previously-published primers (Chen et al., 2011).
2.4 Hemin breakdown assay
Hemin breakdown by recombinant HO-1 was quantified using a modification of the method of Vreman and Stevenson (Vreman and Stevenson, 1988), which quantifies CO production via gas chromatography as an index of HO activity. Each amber, septum-sealed reaction vial contained 1.25 μg recombinant HO-1 and 0.25 μg cytochrome P450 reductase in DPBS (total volume 120 μl). Hemin (final concentration 1.56 μM) and NADPH (1.5 mM) alone or with hemin binding proteins were then added. Vials were rapidly purged for 4 seconds with CO-free air at a flow rate of 250 ml/min. Reactions were then run for 15 minutes at 37°C in a water bath under reduced light, and were terminated by quick-freezing vials on dry ice. CO was quantified in the vial head space using the Peak Performer 1 gas analyzer (Peak Laboratories, Mountain View, CA, USA). CO production was expressed as nanomoles per hour per milligram HO protein.
Hemin breakdown by intact cells was quantified in similar fashion. Cultured cells were harvested by gentle scraping, dissociated by trituration, and placed into reaction vials (140 μg cell protein) containing 10 μM hemin alone in DPBS or with equimolar hemopexin. Alternatively, adult wild-type and hemopexin knockout mice were euthanized by cervical dislocation under isoflurane anesthesia. Brains were immediately removed and cortical tissue was excised under a dissecting microscope. After tissue dissociation by trituration, reactions were run as described above, using 140 μg cell protein/vial. Cell protein was quantified with the Pierce BCA protein assay (Thermo Scientific, Rockford, IL, USA).
2.5. Quantification of cell hemin accumulation and export
Cortical cultures were washed free of serum and into MEM containing 10 mM glucose (MEM10). They were then treated with 5 μM 55Fe-hemin alone or with equimolar hemopexin or albumin. In order to prevent iron-mediated neurotoxicity, all cultures were concomitantly treated with 100 μg/ml human apotransferrin (Chen-Roetling et al., 2011). Control experiments demonstrated that apotransferrin had no significant effect on cell hemin uptake. After 2 hours, cultures were washed four times with MEM10 (750 μl), and then were lysed with 0.1% Triton X-100. Lysate radioactivity was quantified by liquid scintillation counting; cell 55Fe accumulation was calculated from the known specific activity of the isotope. In order to quantify cytosol and membrane signals separately, additional cultures were harvested by gentle scraping, collected by low speed centrifugation (400 x g, 5 minutes), washed in MEM10 and then ruptured by sonication. After centrifugation (15,000 x g, 15 min, 4°C), supernatant and pellet fractions were collected and counted separately.
Additional cultures were incubated for 2 hours after 55Fe-hemin washout in MEM10 alone or with 0.1–5 μM hemopexin or albumin. Medium radioactivity was quantified as above.
2.6. Immunoblotting
Cells were lysed in ice-cold lysis buffer (210 mM mannitol, 70 mM sucrose, 5 mM HEPES, 1 mM EDTA, 0.1 % sodium dodecyl sulfate, 0.1 % Triton X-100). After sonication, debris was removed by centrifugation, and the protein concentration of the supernatant was quantified (BCA method, Pierce, Rockford, IL). Protein separation and transfer followed previously described methods (Chen-Roetling et al., 2009). Membranes were then exposed overnight at 4 °C to rabbit anti-HO-1 (1:5000), monoclonal anti-hemopexin (1:2000), or rabbit anti-actin (1:1500, Sigma-Aldrich) as a gel loading control. After washing, they were then exposed to the appropriate HRP-conjugated anti-IgG secondary antibody (1:3500) for 1h at room temperature. Immunoreactive proteins were visualized using Super Signal West Femto Reagent (Pierce) and Kodak Gel Logic 2200.
2.7. Immunostaining
Cultures were washed with MEM10 and were then fixed in ice-cold 4% paraformaldehyde for one hour. After washing with TBS, cultures were treated serially, at room temperature unless otherwise noted, with: 0.25% Tween 20 for 10 min, 10% normal goat serum for 15 min, polyclonal rabbit anti-HO-1 (1:500 dilution) overnight at 4ºC, biotinylated anti-rabbit IgG (1:200, Vector Laboratories, Burlingame, CA) for 30 min, and NeutrAvidin Rhodamine Red-X conjugate (1:200, Invitrogen) for 30 min.
2.8. Neurotoxicity experiments
Cultures were treated with hemin alone or with hemopexin or albumin following a previously described method (Regan et al., 2004). At the end of the exposure interval, all cultures were examined using phase contrast microscopy. Cell death was then quantified by measurement of lactate dehydrogenase (LDH) activity in the culture medium (Regan and Rogers, 2003). The low LDH activity in sister cultures subjected to medium exchange only was subtracted from all values to yield the signal specific for the neurotoxic insult, according to the protocol of Koh and Choi (Koh and Choi, 1988). To facilitate summation of results of experiments conducted on cultures from different platings, which vary somewhat in neuronal density, LDH values were scaled to the mean value in sister cultures exposed to hemin 3 μM alone for the duration of the exposure (39 hours). The latter treatment is sufficient to release all neuronal LDH but does not injure glial cells (Regan et al., 2004).
2.9. Statistical analysis
Data were analyzed by one way analysis of variance (ANOVA), followed by the post-hoc Bonferroni multiple comparisons test to analyze differences between groups.
3. Results
3.1. Hemopexin reduces cellular hemin accumulation
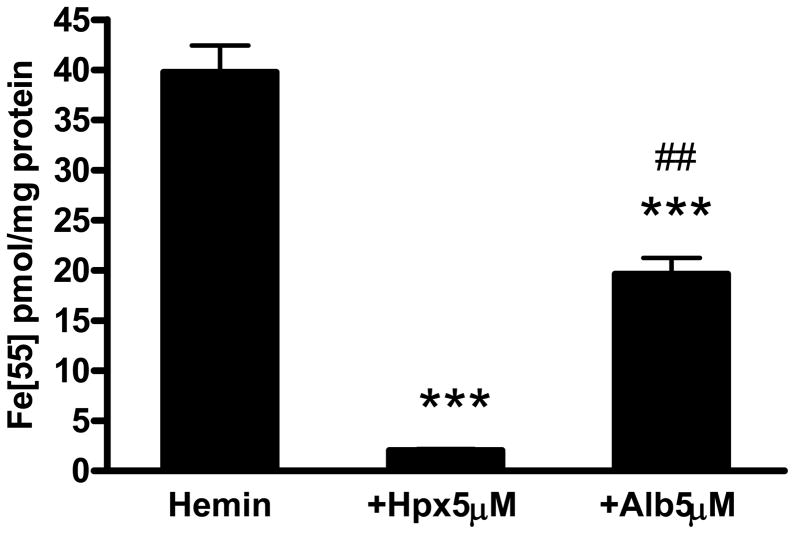
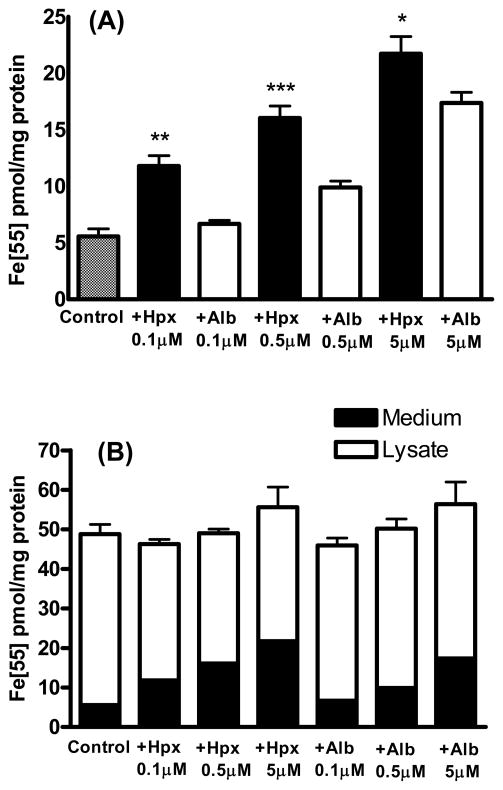
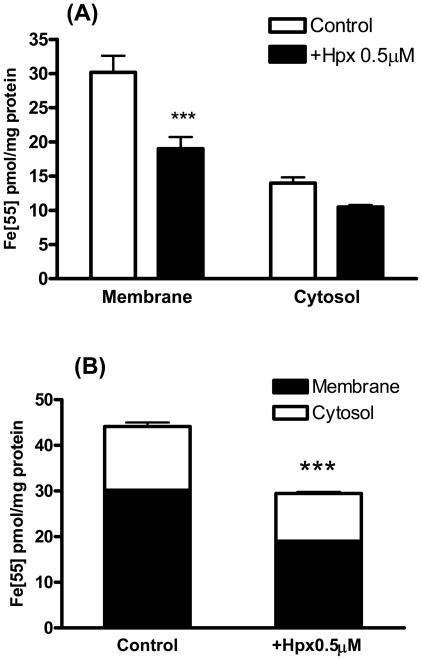
Primary cortical cultures treated for 2 hours with 5 μM 55Fe-hemin accumulated 39.8±2.7 pmoles 55Fe/mg protein (Fig. 1). The 55Fe signal was reduced by over 90% by concomitant treatment with equimolar hemopexin. Equimolar albumin also reduced hemin uptake, but was significantly less effective than hemopexin. Additional cultures were treated with 5 μM 55Fe-hemin for export studies. After medium exchange to remove extracellular isotope, they were incubated in culture medium (MEM10) alone or with 0.1–5 μM hemopexin or albumin (Fig. 2). Consistent with prior observations of Yang et al. in normal rat kidney cells (Yang et al., 2010), hemopexin enhanced the transport of the 55Fe signal from cells into the culture medium. Albumin also facilitated cell hemin loss, but was significantly less effective than hemopexin. In order to determine if hemopexin increased hemin loss from membranes or cytosol, radioactivity was counted separately on these fractions. A significant decrease in the signal was observed only in the membrane fraction (Fig. 3).
Figure 1.
Effect of hemopexin and albumin on hemin accumulation in cortical cultures. Bars represent mean (±S.E.M.) accumulation of 55Fe-hemin by primary cortical cell cultures treated with 5 μM 55Fe-hemin alone or with equimolar hemopexin or albumin (Alb, bovine) for 2 hours. ***P<0.001 v. hemin alone condition, ##P<0.01 v. hemin+hemopexin condition, Bonferroni multiple comparisons test, n = 5–12/condition.
Figure 2.
Hemopexin increases hemin export or extraction from cultured cortical cells. A) Medium 55Fe signal in cultures treated with 5 μM 55Fe-hemin alone as in Fig. 1, then washed and incubated in isotope-free MEM10 alone (Control) or MEM10 containing indicated concentrations of hemopexin (Hpx) or albumin (Alb) for 2 hours. B) Each bar represents the sum of signals in medium and cell lysate, 2 hours after 55Fe-hemin washout. *P<0.05, **P<0.01, **P<0.001 v. corresponding hemin+albumin condition, Bonferroni multiple comparisons test, n=6–15/condition.
Figure 3.
Effect of hemopexin on hemin removal from cytosol and membrane fractions.
A) Cultures were treated with 5 μM 55Fe-hemin for 2 hours. Following isotope washout, they were incubated in MEM10 medium alone (Control) or with 0.5 μM hemopexin (Hpx) for 2 hours. After another wash, cells were harvested and lysed; signal was quantified separately in cytosol and membrane fractions. B) Effect of hemopexin on total (membrane + cytosol) cell hemin. Bars represent mean ± S.E.M., n = 8/condition. ***P<0.001 v. corresponding control condition, Bonferroni multiple comparisons test.
3.2. Hemopexin reduces HO-1 induction and hemin catabolism
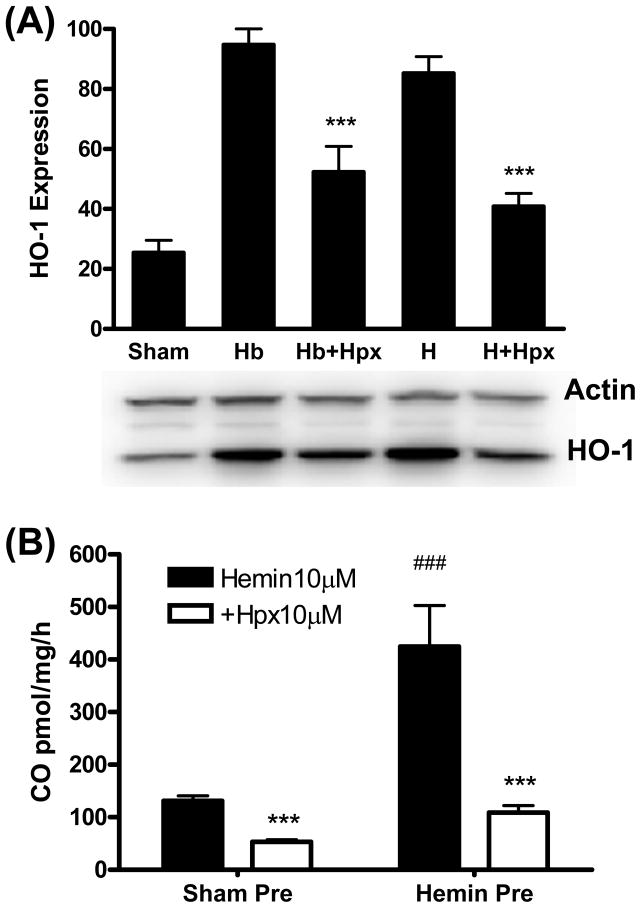
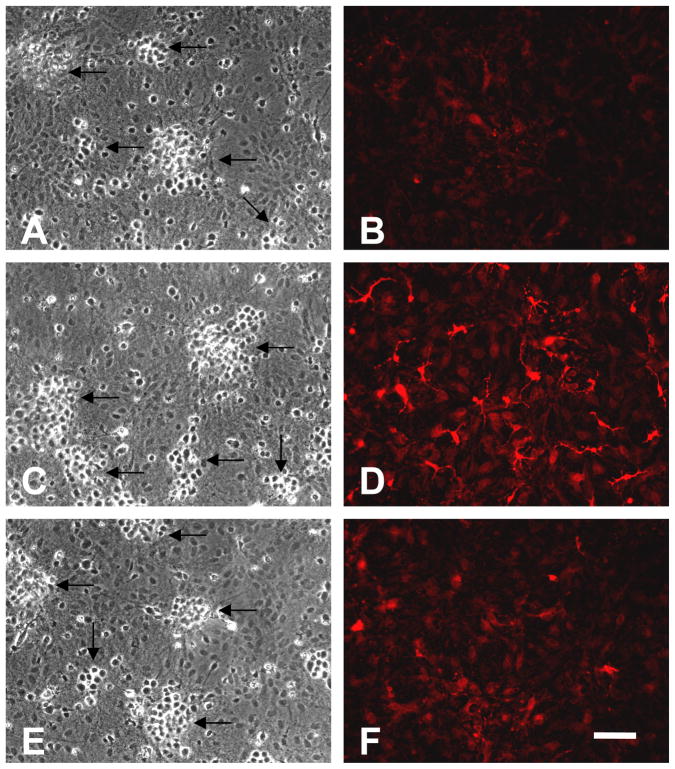
Cultures treated with 1 μM hemin or hemoglobin for 7 hours increased HO-1 expression 3–4-fold compared with controls subjected to medium exchange only (sham, Fig. 4A), in agreement with prior observations (Rogers et al., 2003). Immunostaining demonstrated that this increased expression was present throughout the culture glial monolayer (Fig. 5), as we previously reported (Jaremko et al., 2010). HO-1 induction was significantly reduced by concomitant treatment with equimolar hemopexin. Hemin breakdown assay demonstrated that HO-1 induced by hemin pretreatment was catalytically active in freshly harvested and dissociated cells, with the increase in CO production proportional to the increase in protein expression (Fig. 4B). Medium hemopexin effectively inhibited hemin catabolism in both sham-pretreated and hemin-pretreated cells.
Figure 4.
Hemopexin reduces HO-1 expression and hemin breakdown in cortical cells. A) Mean HO-1 band densities in culture lysates (5/condition) treated with 1 μM hemoglobin (Hb) or hemin (H) for 7 hours, alone or with 1 μM hemopexin (Hpx), expressed as a percentage of the mean value in cultures treated with Hb ( = 100). Sham cultures were incubated in medium (MEM10) only. Bar order is the same as lane order. ***P < 0.001 v. Hb or hemin alone conditions. B) Hemin breakdown in freshly-harvested and dissociated cultured cortical cells pretreated for 7 hours with MEM10 medium only (sham) or with 1 μM hemin. Cells were then placed into septum-sealed gas chromatography vials with fresh DPBS containing 10 μM hemin alone or with equimolar hemopexin, and CO production over subsequent 15 minutes was quantified. ***P < 0.001 v. corresponding hemin alone conditions, ###P < 0.001 v. corresponding sham condition, n = 4–7/condition.
Figure 5.
HO-1 induction by hemin is inhibited by hemopexin. Phase contrast (A,C,E) and fluorescence (B,D,F) photomicrographs of cortical cultures immunostained with anti-HO-1 after they were subjected to: A, B) sham medium exchange only; neurons (arrows) are easily distinguished from glial cells in these mixed cultures by their phase-bright cell bodies which aggregate (Chen-Roetling et al., 2011); C,D) cultures treated with 1 μM hemin alone for 7 hours; HO-1 expression is increased; E,F) cultures treated with 1 μM hemin plus hemopexin. Scale bar = 100 μm.
We and others have reported that HO-2 expression is not inducible in neurons or other CNS cells by hemin alone or in the presence of hemopexin (Chen-Roetling et al., 2009; Leffler et al., 2011; Matz et al., 1997; Rogers et al., 2003). The effect of hemoglobin or hemin treatment on HO-2 expression was therefore not assessed in the present study.
3.3. Hemopexin reduces hemin breakdown by HO-1
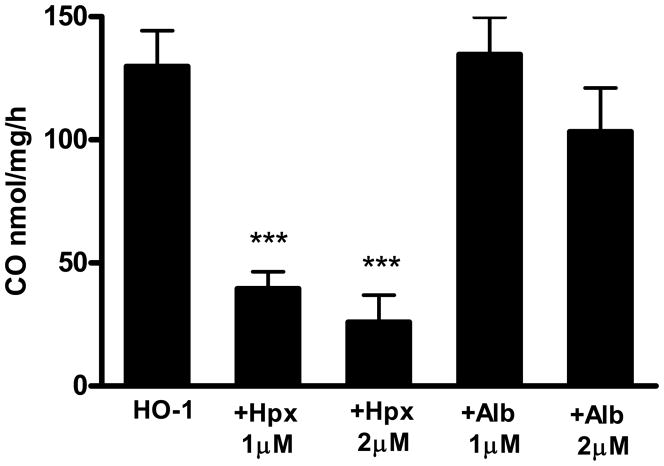
Since hemopexin is expressed in the somata of cortical neurons and some astrocytes (Li et al., 2009), its effect on hemin breakdown was assessed in an in vitro heme oxygenase/cytochrome P450 reductase system. A hemin concentration of 1.56 μM was used since it produced a consistent signal in this assay and was within the range of intracellular hemin concentrations that may be present under pathological conditions (Sassa, 2004). CO production by recombinant HO-1 was 129.8±14.6 nmoles/mg/h (Fig. 6). It was significantly reduced by 1–2 μM hemopexin. The latter effect was compared with that of other proteins with well-characterized but moderate-affinity heme binding sites. Albumin (Kd for hemin 2 x 10−8M, Beaven et al., 1974) is present in some neuronal populations, presumably due to retrograde transport or uptake via endocytosis (Moos, 1995). It had no effect on CO production when tested at the same concentrations as hemopexin. Peroxiredoxin 1, previously known as heme binding protein 23 (Kd 5.5 x 10−8M, Immenschuh et al., 1995) and glutathione S-transferase (Kd 10−7M, Vincent et al., 1988) likewise had no effect on hemin breakdown by recombinant HO-1 (Table 1).
Figure 6.
Hemopexin inhibits hemin breakdown by recombinant HO-1. Carbon monoxide production rate (nanomoles/mg recombinant protein/h) in reaction vials containing 1.25 μg recombinant human HO-1, 0.25μg cytochrome P450 reductase and 1.56 μM hemin, alone or with 1–2 μM human plasma hemopexin (Hpx) or albumin (Alb). Bars represent mean ± S.E.M., n = 3–6/condition, ***P < 0.001 v. HO-1 alone condition, Bonferroni multiple comparisons test.
Table 1.
Hemin breakdown assays were conducted as in Fig. 6, with 1.56 μM hemin alone in the reaction vial or with 1–3 μM human recombinant peroxiredoxin-1 (Pero) or equine liver glutathione-S transferase (GST). Bars represent mean ± S.E.M., n = 4–8/condition.
| HO-1 Activity* | |
|---|---|
| Mean ±SEM | |
| CO nmol/mg/h | |
| HO-1 | 122.8 ± 20.0 |
| +GST1 | 138.0 ± 17.8 |
| +GST2 | 102.1 ± 13.1 |
| +GST3 | 101.7 ± 50.0 |
| +Pero1 | 132.8 ± 18.2 |
| +Pero2 | 161.5 ± 30.5 |
| +Pero3 | 145.1 ± 24.7 |
3.4. Effect of hemopexin on hemin breakdown in adult mouse cortical cells
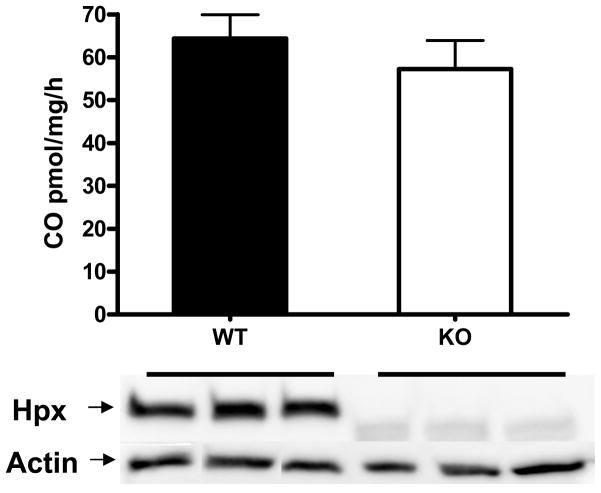
In order to determine if endogenous cellular hemopexin also inhibited HO activity, experiments were conducted using cortical cells from hemopexin knockout and wild-type mice. Dissociated adult murine cortical cells produced CO at a rate of 64.3±5.5 pmoles CO/mg/h when treated with 10 μM hemin (Fig. 7). The hemin breakdown rate was not significantly different in cortical cells from adult hemopexin knockout mice. Immunoblotting of cortical lysates confirmed the presence of hemopexin in wild-type samples and its absence in knockouts.
Figure 7.
Lack of effect of cellular hemopexin on hemin breakdown. Bars represent mean CO production (± S.E.M., 5/conditon) by freshly dissociated adult wild-type (WT) or hemopexin knockout (KO) cortical cells in medium containing 10 μM hemin. Immunoblot demonstrates presence of hemopexin in wild-type cells and its absence in knockouts. Actin is gel-loading control.
3.5. Hemopexin attenuates hemin neurotoxicity
Treatment of cultures with 3 μM hemin plus 3μM albumin for 39 hours resulted in death, as measured by LDH release, of 49.3±6.2% of neurons. In contrast, cultures treated with 3 μM hemin plus equimolar hemopexin sustained loss of only 6.9±4.3% of neurons (P < 0.001, n = 6–7/condition). Consistent with prior observations (Regan et al., 2004), treatment with hemin alone, without any protein, resulted in loss of all neurons, without injury to the glial monolayer.
4. Discussion
These results demonstrate that hemopexin has multiple actions that decrease HO activity in neural cells. The presence of hemopexin in the culture medium reduced hemin uptake, enhanced its export or extraction from cells, attenuated HO-1 induction, and inhibited hemin breakdown. The hemin-hemopexin complex was a very poor substrate for recombinant HO-1 in a cell-free system. In contrast, the catabolism of hemin was not altered when complexed to albumin or other cell proteins with more moderate hemin-binding affinities. Taken together, these observations suggest that if the protection provided by hemopexin in stroke models is dependent on the presence of HO-1 (Li et al., 2009), it is due to either reduced activity or to a non-catalytic effect.
The presence of equimolar hemopexin in the culture medium reduced hemin accumulation in cortical cultures by over 90%. This result is consistent with those of Balla et al. in porcine aortic endothelial cells (Balla et al., 1991), Taketani et al. in hepatocytes (Taketani et al., 1998), and Bui et al. in aortic tissue in vitro (Bui et al., 2004), and likely explains the attenuation of hemin-mediated HO-1 induction by hemopexin. The best-characterized mechanism of cellular heme uptake is via receptor mediated endocytosis of the heme-hemopexin complex by the low density lipoprotein receptor-related protein 1 (LRP1), which is expressed by hepatocytes and to a lesser extent by a variety of cell types including neurons (Ishiguro et al., 1995). However, hemin is lipophilic and will directly accumulate in cell membranes without the need for a protein chaperone (Hebbel and Eaton, 1989; Robinson et al., 2009); this membrane-associated hemin may account for much of the 55Fe-hemin signal detected in the present study. Hemin may also be transported into cells via heme carrier protein-1, which is expressed by both astrocytes and neurons (Dang et al., 2010; Dang et al., 2011). The inhibitory effect of hemopexin on hemin uptake is consistent with greater transport capacity via the latter two mechanisms in neural cells, compared with receptor-mediated uptake.
Transport of the 55Fe-hemin signal from cells to the culture medium was facilitated by hemopexin and to a lesser extent by albumin. Both of these observations are in agreement with those of Yang et al. (Yang et al., 2010) in normal rabbit kidney cells (NRK) overexpressing the heme transport protein feline leukemia virus subgroup C, receptor 1 (FLVCR1). In that model, the effect of hemopexin was attributed to direct interaction of hemopexin with FLVCR1 and consequent regulation of its hemin export activity. In the present study, hemin transport from cells to the medium may be due at least in part to extraction of membrane hemin by hemopexin, as reported by Solar et al. in erythrocyte ghosts (Solar et al., 1989), and by Cannon et al. in protein-free phosphatidylcholine liposomes (Cannon et al., 1984). The weaker but significant effect of albumin may reflect its lower affinity for hemin compared with hemopexin (Beaven et al., 1974; Morgan et al., 1976), and the slower release of hemin-albumin complexes from phospholipid membrane surfaces (Jonas, 1976).
Hemopexin is expressed by murine cortical neurons and some astrocytes (Li et al., 2009). Consistent with these observations, it was readily detected on immunoblots of cortical lysates harvested from wild-type controls in our hemopexin knockout colony. In order to determine if this cellular hemopexin altered hemin breakdown, wild-type and hemopexin cortical tissues were dissociated by trituration, a method used routinely in this laboratory to prepare primary cell cultures. CO production was then quantified in the same manner as that used for cultured cells. In contrast to the inhibitory effect of extracellular hemopexin, CO production in wild-type cells did not differ from that in hemopexin knockouts. These results indicate that cellular hemopexin per se does not alter HO activity in cortical cells subjected to the experimental conditions needed for this hemin breakdown assay (i.e. supraphysiologic hemin concentrations). However, under physiologic conditions, cellular free heme concentrations are very low (~0.03–1.0 μM, Sassa, 2004), and therefore the substrate available for HO-1 is quite limited. It is possible that expression of hemopexin by neurons and other cell types may further reduce its supply and attenuate baseline HO signaling. Unfortunately, such low levels of HO activity cannot be reliably quantified using currently available assays.
Both HO-1 and hemopexin are protective in models of ischemic stroke (Hyun et al., 2010; Li et al., 2009; Panahian et al., 1999); furthermore, the effect of the latter may be dependent on the presence of the former (Li et al., 2009). The observation that hemopexin reduces cellular hemin breakdown is inconsistent with these findings only if catalytic activity is required for HO-mediated protection. Considerable experimental evidence suggests that may not be the case when extracellular heme is in the physiologic range. Under those conditions, the cellular free heme concentration is likely to be near or below the Km values for HO-1 (1 μM), leading Sheftel et al. to hypothesize that substrate is insufficient to attribute the biological activities of HO-1 to heme breakdown per se (Sheftel et al., 2007). In support of this hypothesis, transfection with mutant inactive HO-1 protected cell lines from peroxide-mediated oxidative injury, with efficacy similar to that of the catalytically active enzyme (Hori et al., 2002; Lin et al., 2007). The mechanisms mediating this phenomenon have not been completely defined, but may involve nuclear translocation of HO-1, transcription factor activation, and increased cell catalase and glutathione (Hori et al., 2002; Lin et al., 2007). It is noteworthy that catalytic activity is required to protect cells from supraphysiologic concentrations of hemin (Lin et al., 2007), and may therefore be more relevant to hemorrhagic CNS injuries.
The present results are inconsistent with the hypothesis that the protective effect of hemopexin in stroke models is mediated by enhanced hemin breakdown (Li et al., 2009), and suggest the participation of other mechanisms. In addition to scavenging hemin and thereby preventing its participation in free radical reactions, hemopexin has pleiotropic effects on cell signaling and gene expression that may increase cell survival (Tolosano et al., 2010). Hemopexin-mediated hemin uptake induces ferritin (Davies et al., 1979) while downregulating transferrin receptor-1 (Alam and Smith, 1989); both would be predicted to reduce iron-mediated neurotoxicity, which has been implicated in the pathogenesis of ischemic and hemorrhagic stroke (Hanson et al., 2009; Nakamura et al., 2004). Hemin-hemopexin is also a more efficient inducer of metallothionein-1 than free hemin (Alam and Smith, 1992); overexpression of the latter protects against focal ischemia (van Lookeren Campagne et al., 1999). Hemin-hemopexin, but not free hemin, activates c-Jun N-terminal kinases in Hepa cells, leading to increased expression of cell cycle regulatory proteins p21 and p53, and inhibition of apoptosis (Eskew et al., 1999). Hemopexin per se decreased TLR4 and TLR2 agonist-induced production of TNF and IL-6 in macrophages, consistent with an immunomodulatory role that may directly mitigate the inflammatory response (Liang et al., 2009). Given its manifold cellular roles, it is unlikely that the beneficial effect of hemopexin after ischemic or hemorrhagic stroke will be limited to a single injury cascade or protective response. Further evaluation of its neuroprotective mechanisms in relevant in vivo models seems warranted.
Highlights.
Effect of hemopexin on hemin trafficking and catabolism in cortical cells.
Hemopexin reduced hemin accumulation by 90% and increased export or extraction by fourfold.
Hemin breakdown was reduced by 60–75% in cortical cultures
Protection by hemopexin in stroke is not mediated by increased hemin breakdown
Acknowledgments
This study was supported by a grant from the National Institutes of Health (NS42273) to RFR.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abraham NG, Drummond GS, Lutton JD, Kappas A. The biological significance and physiological role of heme oxygenase. Cell Physiol Biochem. 1996;6:129–168. [Google Scholar]
- Alam J, Smith A. Receptor-mediated transport of heme by hemopexin regulates gene expression in mammalian cells. J Biol Chem. 1989;264:17637–17640. [PubMed] [Google Scholar]
- Alam J, Smith A. Heme-hemopexin-mediated induction of metallothionein gene expression. J Biol Chem. 1992;267:16379–16384. [PubMed] [Google Scholar]
- Balla G, Vercellotti GM, Muller-Eberhard U, Eaton J, Jacob HS. Exposure of endothelial cells to free heme potentiates damage mediated by granulocytes and toxic oxygen species. Lab Invest. 1991;64:648–655. [PubMed] [Google Scholar]
- Beaven GH, Chen SH, d’Albis A, Gratzer WB. A spectroscopic study of the haemin--human-serum-albumin system. Eur J Biochem. 1974;41:539–546. doi: 10.1111/j.1432-1033.1974.tb03295.x. [DOI] [PubMed] [Google Scholar]
- Bui L, Rish K, Jaronczyk K, Bourque S, McLaughlin BE, Brien JF, Marks GS, Smith A, Nakatsu K. The source of heme for vascular heme oxygenase I: heme uptake in rat aorta. Can J Physiol Pharmacol. 2004;82:209–217. doi: 10.1139/y04-014. [DOI] [PubMed] [Google Scholar]
- Cannon JB, Kuo FS, Pasternack RF, Wong NM, Muller-Eberhard U. Kinetics of the interaction of hemin liposomes with heme binding proteins. Biochemistry. 1984;23:3715–3721. doi: 10.1021/bi00311a022. [DOI] [PubMed] [Google Scholar]
- Chen-Roetling J, Chen L, Regan RF. Apotransferrin protects cortical neurons from hemoglobin toxicity. Neuropharmacology. 2011;60:423–431. doi: 10.1016/j.neuropharm.2010.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen-Roetling J, Li Z, Chen M, Awe OO, Regan RF. Heme oxygenase activity and hemoglobin neurotoxicity are attenuated by inhibitors of the MEK/ERK pathway. Neuropharmacology. 2009;56:922–928. doi: 10.1016/j.neuropharm.2009.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Zhang X, Chen-Roetling J, Regan RF. Increased striatal injury and behavioral deficits after intracerebral hemorrhage in hemopexin knockout mice. J Neurosurg. 2011;114:1159–1167. doi: 10.3171/2010.10.JNS10861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Lu H, Dutt K, Smith A, Hunt DM, Hunt RC. Expression of the protective proteins hemopexin and haptoglobin by cells of the neural retina. Exp Eye Res. 1998;67:83–93. doi: 10.1006/exer.1998.0494. [DOI] [PubMed] [Google Scholar]
- Dang TN, Bishop GM, Dringen R, Robinson SR. The putative heme transporter HCP1 is expressed in cultured astrocytes and contributes to the uptake of hemin. Glia. 2010;58:55–65. doi: 10.1002/glia.20901. [DOI] [PubMed] [Google Scholar]
- Dang TN, Robinson SR, Dringen R, Bishop GM. Uptake, metabolism and toxicity of hemin in cultured neurons. Neurochem Int. 2011 doi: 10.1016/j.neuint.2011.03.006. [DOI] [PubMed] [Google Scholar]
- Davies DM, Smith A, Muller-Eberhard U, Morgan WT. Hepatic subcellular metabolism of heme from heme-hemopexin: incorporation of iron into ferritin. Biochem Biophys Res Commun. 1979;91:1504–1511. doi: 10.1016/0006-291x(79)91235-x. [DOI] [PubMed] [Google Scholar]
- Eskew JD, Vanacore RM, Sung L, Morales PJ, Smith A. Cellular protection mechanisms against extracellular heme. heme-hemopexin, but not free heme, activates the N-terminal c-jun kinase. J Biol Chem. 1999;274:638–648. doi: 10.1074/jbc.274.2.638. [DOI] [PubMed] [Google Scholar]
- Hanson LR, Roeytenberg A, Martinez PM, Coppes VG, Sweet DC, Rao RJ, Marti DL, Hoekman JD, Matthews RB, Frey WH, 2nd, Panter SS. Intranasal deferoxamine provides increased brain exposure and significant protection in rat ischemic stroke. J Pharmacol Exp Ther. 2009;330:679–686. doi: 10.1124/jpet.108.149807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Hua Y, Lee JY, Liu W, Keep RF, Wang MM, Xi G. Brain alpha- and beta-globin expression after intracerebral hemorrhage. Transl Stroke Res. 2010;1:48–56. doi: 10.1007/s12975-009-0004-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebbel RP, Eaton JW. Pathobiology of heme interaction with the erythrocyte membrane. Sem Hematol. 1989;26:136–149. [PubMed] [Google Scholar]
- Hori R, Kashiba M, Toma T, Yachie A, Goda N, Makino N, Soejima A, Nagasawa T, Nakabayashi K, Suematsu M. Gene transfection of H25A mutant heme oxygenase-1 protects cells against hydroperoxide-induced cytotoxicity. J Biol Chem. 2002;277:10712–10718. doi: 10.1074/jbc.M107749200. [DOI] [PubMed] [Google Scholar]
- Hyun H, Won YW, Kim KM, Lee J, Lee M, Kim YH. Therapeutic effects of a reducible poly (oligo-D-arginine) carrier with the heme oxygenase-1 gene in the treatment of hypoxic-ischemic brain injury. Biomaterials. 2010;31:9128–9134. doi: 10.1016/j.biomaterials.2010.08.038. [DOI] [PubMed] [Google Scholar]
- Immenschuh S, Iwahara S, Satoh H, Nell C, Katz N, Muller-Eberhard U. Expression of the mRNA of heme-binding protein 23 is coordinated with that of heme oxygenase-1 by heme and heavy metals in primary rat hepatocytes and hepatoma cells. Biochemistry. 1995;34:13407–13411. doi: 10.1021/bi00041a018. [DOI] [PubMed] [Google Scholar]
- Ishiguro M, Imai Y, Kohsaka S. Expression and distribution of low density lipoprotein receptor-related protein mRNA in the rat central nervous system. Brain Res Mol Brain Res. 1995;33:37–46. doi: 10.1016/0169-328x(95)00104-z. [DOI] [PubMed] [Google Scholar]
- Jaremko KM, Chen-Roetling J, Chen L, Regan RF. Accelerated Hemolysis and Neurotoxicity in Neuron-Glia-Blood Clot Co-cultures. J Neurochem. 2010;114:1063–1073. doi: 10.1111/j.1471-4159.2010.06826.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas A. Interaction of phosphatidylcholine with bovine serum albumin. Specificity and properties of the complexes. Biochim Biophys Acta. 1976;427:325–336. doi: 10.1016/0005-2795(76)90308-1. [DOI] [PubMed] [Google Scholar]
- Koh JY, Choi DW. Vulnerability of cultured cortical neurons to damage by excitotoxins: Differential susceptibility of neurons containing NADPH-diaphorase. J Neurosci. 1988;8:2153–2163. doi: 10.1523/JNEUROSCI.08-06-02153.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leffler CW, Parfenova H, Jaggar JH. Carbon monoxide as an endogenous vascular modulator. American Journal of Physiology - Heart and Circulatory Physiology. 2011;301:H1–H11. doi: 10.1152/ajpheart.00230.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li RC, Saleem S, Zhen G, Cao W, Zhuang H, Lee J, Smith A, Altruda F, Tolosano E, Dore S. Heme-hemopexin complex attenuates neuronal cell death and stroke damage. J Cereb Blood Flow Metab. 2009;29:953–964. doi: 10.1038/jcbfm.2009.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X, Lin T, Sun G, Beasley-Topliffe L, Cavaillon JM, Warren HS. Hemopexin down-regulates LPS-induced proinflammatory cytokines from macrophages. J Leukoc Biol. 2009;86:229–235. doi: 10.1189/jlb.1208742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Q, Weis S, Yang G, Weng YH, Helston R, Rish K, Smith A, Bordner J, Polte T, Gaunitz F, Dennery PA. Heme oxygenase-1 protein localizes to the nucleus and activates transcription factors important in oxidative stress. J Biol Chem. 2007;282:20621–20633. doi: 10.1074/jbc.M607954200. [DOI] [PubMed] [Google Scholar]
- Matz PG, Weinstein PR, Sharp FR. Heme oxygenase-1 and heat shock protein-70 induction in glia and neurons throughout rat brain after experimental intracerebral hemorrhage. Neurosurgery. 1997;40:152–160. doi: 10.1097/00006123-199701000-00034. [DOI] [PubMed] [Google Scholar]
- Moos T. Age-dependent uptake and retrograde axonal transport of exogenous albumin and transferrin in rat motor neurons. Brain Res. 1995;672:14–23. doi: 10.1016/0006-8993(94)01329-g. [DOI] [PubMed] [Google Scholar]
- Morgan WT, Liem HH, Sutor RP, Muller-Ebergard U. Transfer of heme from heme-albumin to hemopexin. Biochim Biophys Acta. 1976;444:435–445. doi: 10.1016/0304-4165(76)90387-1. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Keep RF, Hua Y, Schallert T, Hoff JT, Xi G. Deferoxamine-induced attenuation of brain edema and neurological deficits in a rat model of intracerebral hemorrhage. J Neurosurg. 2004;100:672–678. doi: 10.3171/jns.2004.100.4.0672. [DOI] [PubMed] [Google Scholar]
- Panahian N, Yoshiura M, Maines MD. Overexpression of heme oxygenase-1 is neuroprotective in a model of permanent middle cerebral artery occlusion in transgenic mice. J Neurochem. 1999;72:1187–1203. doi: 10.1111/j.1471-4159.1999.721187.x. [DOI] [PubMed] [Google Scholar]
- Regan RF, Chen J, Benvenisti-Zarom L. Heme oxygenase-2 gene deletion attenuates oxidative stress in neurons exposed to extracellular hemin. BMC Neurosci. 2004;5:34. doi: 10.1186/1471-2202-5-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regan RF, Rogers B. Delayed treatment of hemoglobin neurotoxicity. J Neurotrauma. 2003;20:111–120. doi: 10.1089/08977150360517236. [DOI] [PubMed] [Google Scholar]
- Robinson SR, Dang TN, Dringen R, Bishop GM. Hemin toxicity: a preventable source of brain damage following hemorrhagic stroke. Redox Rep. 2009;14:228–235. doi: 10.1179/135100009X12525712409931. [DOI] [PubMed] [Google Scholar]
- Rogers B, Yakopson V, Teng ZP, Guo Y, Regan RF. Heme oxygenase-2 knockout neurons are less vulnerable to hemoglobin toxicity. Free Rad Biol Med. 2003;35:872–881. doi: 10.1016/s0891-5849(03)00431-3. [DOI] [PubMed] [Google Scholar]
- Sassa S. Why heme needs to be degraded to iron, biliverdin IXalpha, and carbon monoxide? Antioxid Redox Signal. 2004;6:819–824. doi: 10.1089/ars.2004.6.819. [DOI] [PubMed] [Google Scholar]
- Sheftel AD, Kim SF, Ponka P. Non-heme induction of heme oxygenase-1 does not alter cellular iron metabolism. J Biol Chem. 2007;282:10480–10486. doi: 10.1074/jbc.M700240200. [DOI] [PubMed] [Google Scholar]
- Smith A, Morgan WT. Haem transport to the liver by haemopexin. Receptor-mediated uptake with recycling of the protein. Biochem J. 1979;182:47–54. doi: 10.1042/bj1820047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solar I, Muller-Eberhard U, Shaklai N. Serum proteins as mediators of hemin efflux from red cell membranes: specificity of hemopexin. FEBS Lett. 1989;256:225–229. doi: 10.1016/0014-5793(89)81753-3. [DOI] [PubMed] [Google Scholar]
- Swerts JP, Soula C, Sagot Y, Guinaudy MJ, Guillemot JC, Ferrara P, Duprat AM, Cochard P. Hemopexin is synthesized in peripheral nerves but not in central nervous system and accumulates after axotomy. J Biol Chem. 1992;267:10596–10600. [PubMed] [Google Scholar]
- Taketani S, Immenschuh S, Go S, Sinclair PR, Stockert RJ, Liem HH, Muller Eberhard U. Hemopexin from four species inhibits the association of heme with cultured hepatoma cells or primary rat hepatocytes exhibiting a small number of species specific hemopexin receptors. Hepatology. 1998;27:808–814. doi: 10.1002/hep.510270324. [DOI] [PubMed] [Google Scholar]
- Tolosano E, Cutufia MA, Hirsch E, Silengo L, Altruda F. Specific expression in brain and liver driven by the hemopexin promoter in transgenic mice. Biochem Biophys Res Commun. 1996;218:694–703. doi: 10.1006/bbrc.1996.0124. [DOI] [PubMed] [Google Scholar]
- Tolosano E, Fagoonee S, Morello N, Vinchi F, Fiorito V. Heme scavenging and the other facets of hemopexin. Antioxid Redox Signal. 2010;12:305–320. doi: 10.1089/ars.2009.2787. [DOI] [PubMed] [Google Scholar]
- Tolosano E, Hirsch E, Patrucco E, Camaschella C, Navone R, Silengo L, Altruda F. Defective recovery and severe renal damage after acute hemolysis in hemopexin-deficient mice. Blood. 1999;94:3906–3914. [PubMed] [Google Scholar]
- van Lookeren Campagne M, Thibodeaux H, van Bruggen N, Cairns B, Gerlai R, Palmer JT, Williams SP, Lowe DG. Evidence for a protective role of metallothionein-1 in focal cerebral ischemia. Proc Natl Acad Sci U S A. 1999;96:12870–12875. doi: 10.1073/pnas.96.22.12870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent SH, Grady RW, Shaklai N, Snider JM, Muller-Eberhard U. The influence of heme-binding proteins in heme-catalyzed oxidations. Arch Biochem Biophys. 1988;265:539–550. doi: 10.1016/0003-9861(88)90159-2. [DOI] [PubMed] [Google Scholar]
- Vreman HJ, Stevenson DK. Heme oxygenase activity as measured by carbon monoxide production. Anal Biochem. 1988;168:31–38. doi: 10.1016/0003-2697(88)90006-1. [DOI] [PubMed] [Google Scholar]
- Yang Z, Philips JD, Doty RT, Giraudi P, Ostrow JD, Tiribelli C, Smith A, Abkowitz JL. Kinetics and specificity of feline leukemia virus subgroup C receptor (FLVCR) export function and its dependence on hemopexin. J Biol Chem. 2010;285:28874–28882. doi: 10.1074/jbc.M110.119131. [DOI] [PMC free article] [PubMed] [Google Scholar]