Abstract
T helper 17 (TH17) cells have been shown to contribute to multiple disease systems. However, the functional phenotype and survival pattern of TH17 cells as well as the underlying mechanisms that control TH17 cells have been poorly investigated in humans, significantly hampering the clinical targeting of these cells. Here, we studied human TH17 cells in the pathological microenvironments of graft-versus-host disease, ulcerative colitis, and cancer; TH17 cell numbers were increased in the chronic phase of these diseases. Human TH17 cells phenotypically resembled terminally differentiated memory T cells but were distinct from central memory, exhausted, and senescent T cells. Despite their phenotypic markers of terminal differentiation, TH17 cells mediated and promoted long-term antitumor immunity in in vivo adoptive transfer experiments. Furthermore, TH17 cells had a high capacity for proliferative self-renewal, potent persistence, and apoptotic resistance in vivo, as well as plasticity—converting into other types of TH cells. These cells expressed a relatively specific gene signature including abundant antiapoptotic genes. We found that hypoxia-inducible factor–1α and Notch collaboratively controlled key antiapoptosis Bcl-2 family gene expression and function in TH17 cells. Together, these data indicate that human TH17 cells may be a long-lived proliferating effector memory T cell population with unique genetic and functional characteristics. Targeting TH17-associated signaling pathway would be therapeutically meaningful for treating patients with autoimmune disease and advanced tumor.
INTRODUCTION
T helper 17 (TH17) cells may contribute to many human diseases. A large body of research has mapped out the transcription factors and cytokine milieu necessary for TH17 development and function (1–4). However, it is poorly understood how human TH17 cells expand and survive in vivo. Human TH17 cells are often found in peripheral tissues and organs (5–8), and it has been assumed that human TH17 cells are effector T cells with a short life span. In support of this notion, mouse TH17 cells may be short-lived and express low levels of CD27 (9), which is associated with memory T cell survival (10).
However, these observations contrast with the activity of TH17 cells in multiple cancer settings. In several types of advanced human cancers, although TH17 cells are a relatively small population compared with other T cell subsets, TH17 cells are associated with potent antitumor immunity and positively predict improved patient survival (8, 11, 12). These data raise the possibility that TH17 cells may have a survival and persistence advantage in humans and may contribute to long-lasting antitumor effects in advanced human cancer. In support of this hypothesis, in the adoptive T cell therapy setting where T cell persistence is critical in achieving tumor eradication, mouse TH17 cells mediate potent tumor regression (13–15).
Here, we studied human TH17 cells in our well-established human system (16–18) and investigated the underlying mechanisms of controlling TH17 cell expansion, apoptosis, and survival. Our studies demonstrated that HIF-1α (hypoxia-inducible factor 1α)/Notch/Bcl-2 signaling cascade is crucial for controlling human TH17 cell survival and apoptosis. Thus, manipulation of this signaling pathway may provide clinical benefit for patients affected by TH17 cells, including patients with autoimmune disease, tumors, and transplantation rejection.
RESULTS
TH17 cell numbers are elevated in sites of chronic disease
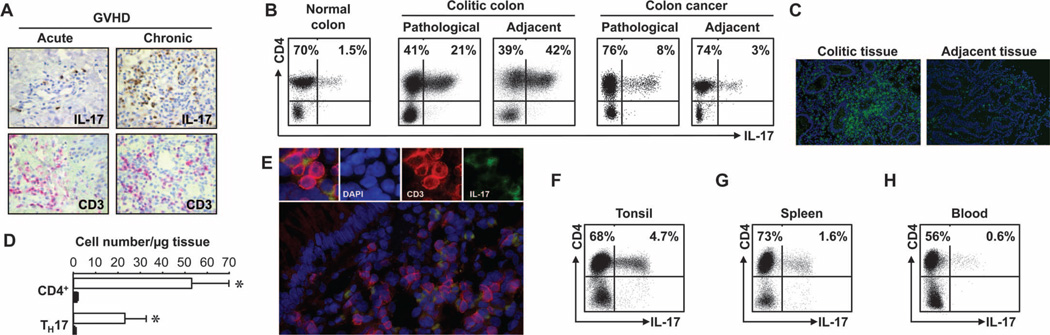
Multiple chronic human diseases, including chronic organ rejections, autoimmune diseases, and cancers, are thought to be affected by TH17 cells. To study TH17 cells in the microenvironments of chronic diseases, we first examined TH17 cells in the diseased sites of acute and chronic graft-versus-host disease (GVHD), ulcerative colitis, and colon cancer. Immunohistochemistry staining revealed high numbers of interleukin-17–positive (IL-17+) (Fig. 1A, upper panel) and CD3+ T cells (Fig. 1A, lower panel) in consecutive oral mucosa tissue sections in patients with chronic, but not acute, GVHD (Fig. 1A and fig. S1A). Flow cytometric analysis demonstrated that these IL-17+ cells were TH17 cells, but not γδ T cells (fig. S1B). In patients with chronic ulcerative colitis, we detected high percentages of IL-17+ T cells in diseased intestinal mucosa and adjacent tissues. These IL-17+ T cells were TH17, not IL-17+CD8+, cells (Fig. 1B). The percentages of TH17 cells were higher in colitic tissues than in normal colon and blood (Fig. 1B and fig. S1, C and D). Because there were more T cells infiltrating colitic lesions than adjacent tissues (Fig. 1, C and D), the absolute numbers of TH17 cells were much higher in colitic lesions than in adjacent tissues (Fig. 1D). High percentages of TH17 cells were also found in colon cancer as demonstrated by flow cytometry analysis (Fig. 1B and fig. S1, C and D) and multiple-color fluorescence staining (Fig. 1E). Increased numbers of TH17 cells were detected in inflammatory tonsil and spleen compared to blood (Fig. 1, F to H, and fig. S1, C and D).
Fig. 1.
High amounts of TH17 cells are found in sites of chronic diseases. Case numbers: blood (31), oral mucosa (6), spleen (5), tonsil (6), colon cancer (21), and colitis (12). (A) High numbers of IL-17+ cells were detected in oral mucosa tissues in patients with GVHD. Consecutive sections of oral mucosa biopsies were stained for IL-17 (brown) or CD3 (red). P < 0.001, acute versus chronic. (B) High percentages of TH17 cells were found in colitic colon and colon cancer tissues. Tissue single cells were stained for TH17 cell markers, analyzed by flow cytometry, and gated on CD3+ cells. (C and D) CD4+ and TH17 cells were found in colitic colon tissues. Colon tissues were stained for CD3 (green) (C). Absolute numbers of CD4+ and TH17 cells were quantified based on flow cytometry analysis. Results are expressed as the absolute numbers of CD4+ or TH17 per microgram tissue ± SEM (D). P < 0.001, colitic lesion (empty bars) versus adjacent tissue (filled bars). (E) Colon tissues were stained for CD3 and IL-17, and analyzed with a fluorescence microscope. Red, CD3; green, IL-17. DAPI, 4′,6-diamidino-2-phenylindole. (F to H) TH17 cells were found in different organs. Single cells were stained for TH17 cell markers and analyzed by flow cytometry. P < 0.001, tonsil and spleen as compared with blood.
Primary TH17 cells exhibit a terminally differentiated phenotype but mediate potent antitumor immunity
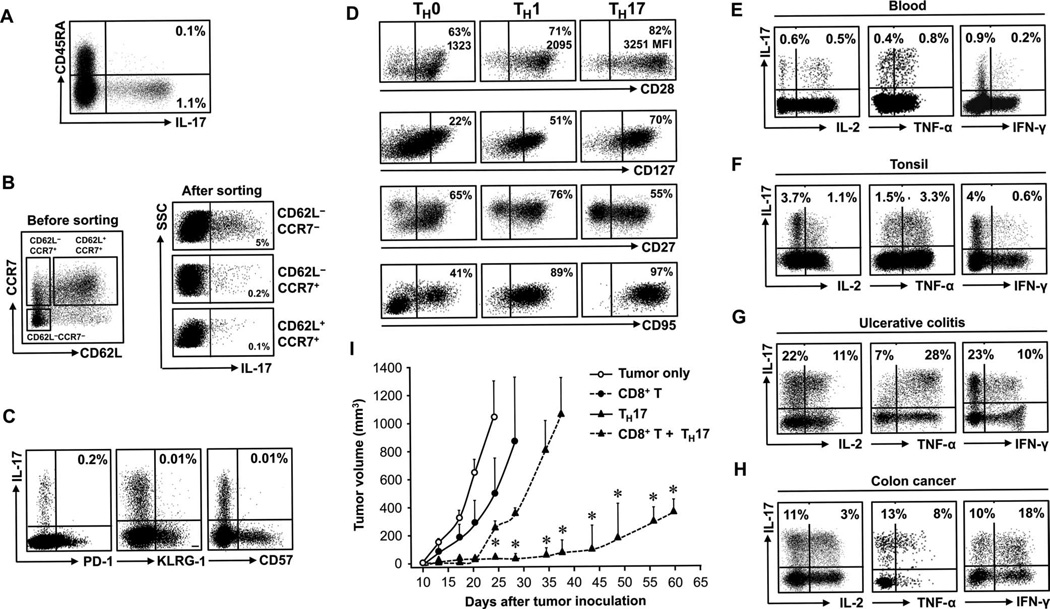
We next examined the phenotype and cytokine profile of primary TH17 cells in the microenvironments of chronic inflammation and cancer and in blood. Blood TH17 cells were in the CD45RA−CD45RO+ population (Fig. 2A). Because intracellular staining affected the detection of certain surface antigens, we sorted CD4+ T cells into CD62L−CCR7−, CD62L−CCR7+, and CD62L+CCR7+ populations and subsequently examined IL-17 expression. Fewer than 0.2% of TH17 cells were found in CD62L−CCR7+ and CD62L+CCR7+ populations. IL-17 expression was primarily confined to CD62L−CCR7− T cells (Fig. 2B). Further phenotypic analysis demonstrated that TH17 cells did not express PD-1, KLRG-1, and CD57 (Fig. 2C).
Fig. 2.
Primary TH17 cells exhibit a terminally differentiated phenotype but mediate potent antitumor immunity. (A to D) Tissue or blood cells were stained for the indicated markers and cytokines and analyzed by flow cytometry. Results are expressed as the percentage of the specific subset of CD4+ T cells. n = 10 to 25. (A) TH17 cells were in the CD45RO+CD45RA− memory T subset. (B) Relationship between TH17 cells and expression of CCR7 and CD62L. Three CD4+ T cell subsets were sorted and stained for IL-17. (C) Relationship between TH17 cells and expression of CD57, KLRG-1, and PD-1. (D) Expression of the described markers on primary TH0, TH1, and TH17 cells. P < 0.001, for CD28 and CD95 expression on TH17 as compared with TH1 and TH0 cells, and P < 0.05, for CD127 and CD27 expression on TH17 as compared with TH1 and TH0 cells. (E to H) Effector cytokine profile of primary TH17 cells in different tissues gated on CD4+ T cells. (I) TH17 cells mediate and promote tumor regression in vivo. Ovarian cancer–bearing NSG mice (16, 17) received PBS, TH17, and/or CD8+ T cells. Mean ± SEM of tumor volumes are shown (n = 4 to 6 mice per group). *P < 0.05.
Next, we compared the expression levels of some survival markers on primary TH0, TH1, and TH17 cells. Primary TH subsets were defined on the basis of specific TH signature cytokine expression in fresh cells with multiple flow gating (fig. S2). Flow analysis revealed that the percentage and the mean fluorescence intensity (MFI) of CD28 were higher in TH17 cells than in TH0 and TH1 cells (Fig. 2D). TH17 cells also expressed higher levels of CD127 than TH0 and TH1 cells. However, we found that TH17 cells expressed moderately lower amounts of CD27 and higher amounts of CD95 than their counterpart TH1 and TH0 cells (Fig. 2D). We further examined the effector function of primary TH17 cells. Primary TH17 cells expressed high levels of IL-2 and TNF-α (tumor necrosis factor–α) and moderate amounts of IFN-γ (interferon-γ) in blood and in the microenvironments of inflammatory tonsil, ulcerative colitic tissue, and colon cancer (Fig. 2, E to H, and fig. S3). The phenotype suggests a terminally differentiated effector phenotype for TH17 cells.
Because TH17 cells exhibit a phenotype of terminal differentiation, we hypothesized that TH17 cells may have poor effector function. To test this hypothesis, we enriched and sorted primary TH17 cells on the basis of a CD4+CD3+CCR6+CD161+CD45RA−CD45RO+ phenotype (figs. S2A and S4A). These primary TH17 cells were 99% CD161+RORγt+ (fig. S4, B and C), produced high amounts of intracellular IL-17 (fig. S4, D and E), and released large amounts of IL-17 (fig. S4F). IL-1 and IL-23 further increased their production of IL-17 (fig. S4F). IL-1– and IL-23–activated primary TH17 cells expressed moderate levels of effector cytokines (fig. S4G). We next generated tumor antigen–associated specific autologous CD8+ T cells. Activated primary TH17 cells (fig. S4) or tumor antigen–associated specific autologous CD8+ T cells were transferred to female nonobese diabetic (NOD)/Shi-scid/IL-2Rγnull (NSG) mice bearing ovarian cancer. As expected, CD8+ T cell transfusion resulted in reduced tumor growth. Unexpectedly, TH17 cells slowed tumor growth as well. Furthermore, the effects of TH17 cells were superior to that of CD8+ effector T cells from the same donors (Fig. 2I).
It is not known whether human TH17 cells affected CD8+ T cell–mediated antitumor immunity. To test this, we cotransfused TH17 and CD8+ T cells to NSG mice. We observed that tumor volume was much smaller in mice that received the cotransfusion than in those that received a single transfusion. More markedly, the immune protection mediated by cotransfusion lasted significantly longer than TH17 or CD8+ cells alone (Fig. 2I). These data indicate that TH17 and CD8+ T cells collaboratively mediate long-term antitumor immunity.
TH17 cells give rise to other TH cell subsets
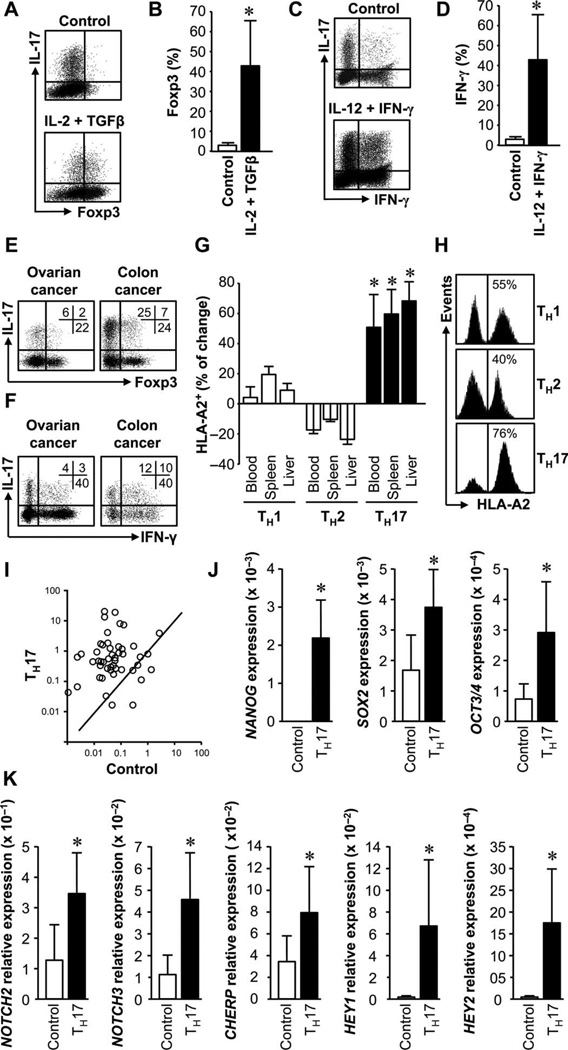
The plasticity of mouse TH17 cells may be one of the functional determinants in TH17 cell biology (19–21). To test human TH17 cell plasticity, we cultured these cells under polarizing conditions. Under the stimulation of IL-2 and TGFγ (transforming growth factor–β), TH17 cells expressed Foxp3 (Fig. 3, A and B). Under TH1 polarization condition, an important fraction of TH17 cells expressed IFN-γ (Fig. 3, C and D). In support of this, Foxp3+IL-17+CD4+ T cells (Fig. 3E) and IFN-γ+IL-17+CD4+ T cells (Fig. 3F) were observed in pathological environments. These Foxp3+IL-17+CD4+ T cells are functional T regulatory (Treg) cells (22). Furthermore, 40 to 60% of the cells retained IL-17 expression in TH1 and Treg polarization conditions (Fig. 3, A to D).
Fig. 3.
TH17 cells have functional and genetic stem cell–like features. (A to D) TH17 cells gave rise to other TH cells. Primary TH17 cells were stimulated with the cytokine milieu for Treg (A and B) or TH1 (C and D) induction for 3 days and analyzed by flow cytometry. Results are expressed as the percentage of Foxp3+ (B) or IFN-g+ cells (D) in TH17 cells. n = 5. *P < 0.05. (E and F) IL-17+Foxp3+(E) and IL-17+IFN-γ+(F) T cells in cancers. Colon (n = 9) and ovarian cancer (n = 25) T cells were analyzed by flow cytometry. (G and H) TH17 cells had superior in vivo persistence. HLA-A2+ TH subsets were mixed with HLA-A2−IL-17−CD4+ T cells and transfused to NSG mice. On day 5, human T cells were analyzed. (I) The percentage of changes between the initial and the recovered ratios of HLA-A2+ and HLA-A2− cells was calculated. Three paired donors. *P < 0.001. (J) Representative histogram shows different T subsets (HLA-A2+) in spleen. (I to K) TH17 cells expressed stem cell genes. SuperArray was performed in primary TH17 and controls (I). Selective stem cell genes were quantified (J and K). n = 8. P < 0.05.
TH17 cells have better persistence in vivo
In vivo persistence is a key feature for effector T cells to mediate antitumor immunity. We compared the in vivo persistence of TH1, TH2, and TH17 cells in the NSG model. We first polarized TH subsets from the same donor (fig. S5). Then, we equally mixed autologous human leukocyte antigen-A2–positive (HLA-A2+) TH17, TH1, or TH2 cells with HLA-A2−CD4+ T cells, transferred these cells into NSG mice, and followed their persistence in vivo. Five days after transfusion, we showed that there were more TH17 than TH1 and TH2 cells in different organs (Fig. 3G) including spleen (Fig. 3H).
We hypothesized that the in vivo persistence of TH17 cells may be associated with a particular gene signature. Gene arrays showed that TH17 cells expressed higher levels of stem cell–associated genes than autologous IL-17− control T cells (Fig. 3I). Real-time polymerase chain reaction (PCR) demonstrated that the levels of NANOG, SOX2, and OCT3/4 (Fig. 3J); Notch signaling genes (Fig. 3K); Wnt/β-catenin signaling genes (fig. S6A); and FOXO3, MYC, and PIM2 (fig. S6B) were higher in TH17 cells than in control.
TH17 cells have increased proliferative capacity
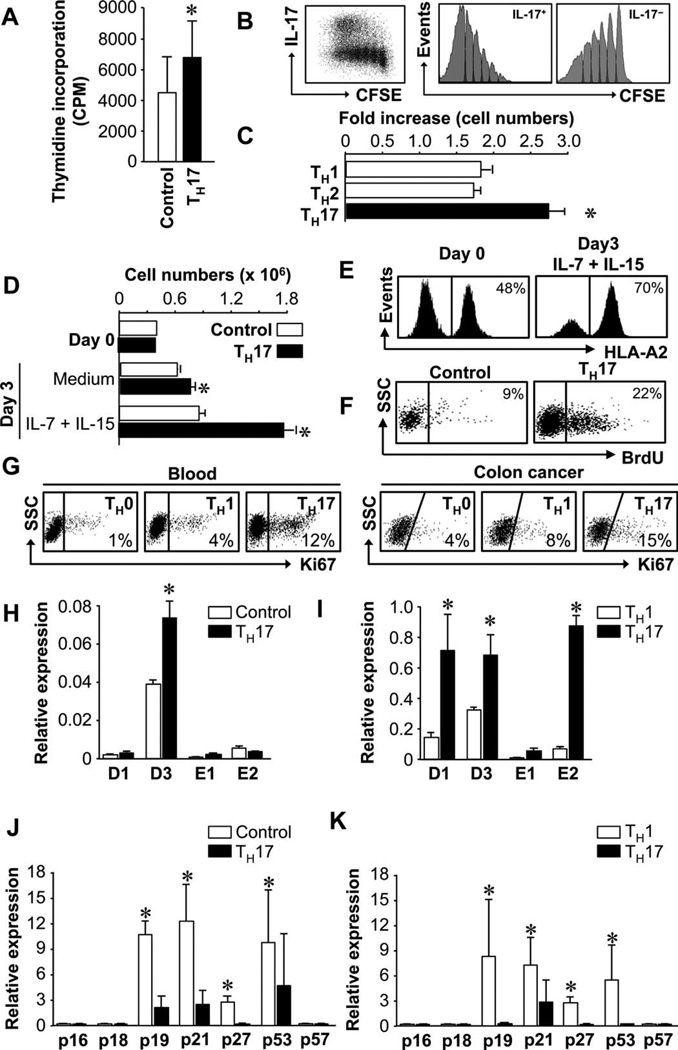
Because TH17 cells exhibit a terminally differentiated phenotype, we hypothesized that TH17 cells had low proliferating potential. Unexpectedly, upon stimulation, there was more thymidine incorporation in the primary TH17 cells than in autologous IL-17− T cells (Fig. 4A). In a similar setting, carboxyfluorescein succinimidyl ester (CFSE)–labeled primary TH17 cells underwent more cell divisions than IL-17− T cells (Fig. 4B). We also examined the expansion potential of polarized T cells. T cell receptor (TCR) engagement induced the proliferation of polarized TH1, TH2, and TH17 cells. However, the absolute numbers of polarized TH17 cells were higher than those of TH1 and TH2 cells (Fig. 4C). We cultured primary TH17 cells and autologous IL-17− T cells with IL-7 and IL-15. TH17 cells were more efficiently expanded than control T cells (Fig. 4D). TH17 and control T cells were separately cultured in this experiment (Fig. 4D) to ensure that all T cells were stimulated in an identical manner. We mixed HLA-A2+ TH17 cells with HLA-A2−IL-17− T cells and cultured these cells with IL-7 plus IL-15. Again, there were more HLA-A2+ TH17 cells than HLA-A2−IL-17− T cells in the culture (Fig. 4E).
Fig. 4.
TH17 cells have increased proliferative capacity. (A to C) TH17 cells had potent expansion capacity. Primary (A and B) and polarized TH subsets (C) were stimulated with TCR for 3 (A and C) or 14 (B) days. Cell proliferation/expansion was detected by [3H]thymidine incorporation (A), CFSE dilution (B), or increased cell numbers (C). n = 3. *P < 0.05. (D and E) Cytokines stimulated TH17 expansion. Primary HLA-A2+ TH17 and HLA-A2−IL-17− control T cells were separately cultured (D) or initially mixed and cocultured (E) for 3 days with IL-7 plus IL-15. The absolute cell numbers (D) or the percentage (E) of TH17 cells was determined by flow cytometry. n = 3. *P < 0.05. (F) TH17 cells were in S phase. NSG mice received TH17 or control cells and BrdU. Cell cycling phase was analyzed on day 5 by flow cytometry to determine BrdU+ human T cells in mouse spleen. n = 2. (G) TH17 cells expressed increased Ki67 expression in blood and colon cancer. Eight to 10 donors. P < 0.05, TH17 as compared with TH1/TH0. (H to K) TH17 cells expressed different amounts of cell cycling genes. Expression of multiple cyclin genes (H and I) and CDK inhibitors (J and K) was quantified in primary (H and J) and polarized TH17 cells (I and K). n = 8. *P < 0.05.
We next investigated the capacity of human TH17 cells to expand in vivo. TH17 cells were transferred into NSG mice with 5-bromo-2′-deoxyuridine (BrdU) administration. Consistent with the in vitro data, there were more TH17 cells than control T cells in S phase (Fig. 4F). There were 15% TH17 cells and 7% control cells in G1–G2 phases, indicating that TH17 cells efficiently entered G1–G2 phase. Furthermore, there were more Ki67+ cells in primary TH17 than TH1 and TH0 cell populations in vivo in blood in healthy humans and in colon cancer tissues (Fig. 4G). In accord with this observation, the expression levels of multiple cyclin genes were higher in primary (Fig. 4H) and polarized (Fig. 4I) TH17 than in the control cells. On the contrary, the expression of multiple cyclin-dependent repressors was lower in primary (Fig. 4J) and polarized (Fig. 4K) TH17 than in the control cells.
TH17 cells are resistant to apoptosis
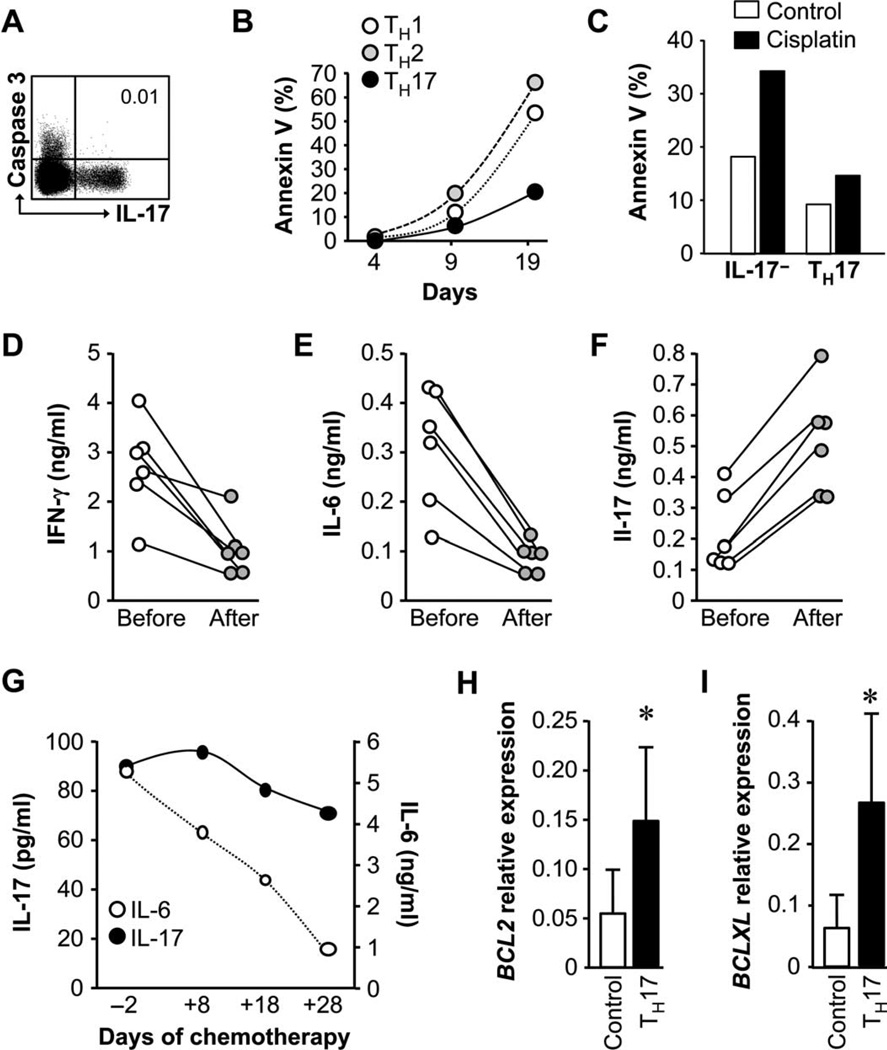
Because TH17 cells exhibited a terminally differentiated phenotype and expressed CD95 (Fig. 2), TH17 cells might be more prone to apoptosis. However, in healthy humans, caspase 3+ T cells were IL-17− T cells (Fig. 5A).
Fig. 5.
TH17 cells are resistant to apoptosis. (A) TH17 cells did not express caspase 3. The expression of caspase 3 in T cells was analyzed by flow cytometry. n = 4. (B) TH17 cells were resistant to apoptosis induced by TCR activation. T subsets were stimulated with anti-CD3, and cell apoptosis was analyzed with annexin V staining. n = 5. P < 0.01, TH17 as compared with TH1/TH2 on day 19. (C) TH17 cells were resistant to apoptosis induced by in vitro cisplatin. Primary TH17 and control cells were treated with cisplatin for 48 hours. Annexin V+ cells were analyzed. n = 4. P < 0.01. (D to F) Chemotherapy increased IL-17 production. Ovarian cancer patients received one cycle of cisplatin therapy. Blood mononuclear cells were activated, and cytokines were measured in the supernatants with enzyme-linked immunosorbent assay (ELISA). n = 6. P < 0.001. (G) Chemotherapy reduced IL-6 but not IL-17 production in ovarian cancer ascites. Ovarian cancer patient was treated with cisplatin. Cytokines were measured in the ascites fluid by ELISA. Left scale shows IL-17 (filled circles), and right scale shows IL-6 (empty circles). (H and I) TH17 cells spontaneously expressed high levels of BCL2 family genes. Real-time PCR was performed in primary TH17 and control cells for BCL2 (H) and BCLXL (I). n = 5. *P < 0.001.
We next cultured polarized TH1, TH2, and TH17 cells and kinetically measured T cell apoptosis after in vitro TCR activation. We consistently observed lower numbers of apoptotic TH17 cells than TH1 and TH2 cells (Fig. 5B). We also examined T cell apoptosis induced by chemotherapeutic agents. When exposed to an optimal concentration of cisplatin, there were less annexin V+ TH17 cells compared to other T cells (Fig. 5C). In patients with ovarian cancer, one cycle of cisplatin treatment in combination with paclitaxel resulted in decreased IFN-γ and IL-6 (Fig. 5, D and E) and increased IL-17 (Fig. 5F) produced by activated blood mononuclear cells. Because IL-17 is primarily derived from TH17 cells in patients with ovarian cancer (8), the data indicate that primary TH17 cells are resistant to chemotherapy-mediated cell death in vivo. In further support, after multiple cycles of chemotherapy in a patient with ovarian cancer, the amounts of IL-6 were reduced, whereas IL-17 production was initially increased and then slightly reduced in malignant ascites (Fig. 5G). These data suggest that apoptosis resistance of primary TH17 cells may not be linked to IL-6 signaling. We further explored whether TH17 apoptosis resistance was associated with the effects of IL-6 and IL-23 during TH17 cell differentiation. We polarized TH17 cells from naïve T cells with antigen-presenting cells (7, 8) (fig. S5A) and blocked IL-6 signaling with anti–IL-6, and IL-23 signaling with small interfering RNA (siRNA)–IL-23 (7). Anti–IL-6 (fig. S7, A and B) and siRNA–IL-23 (fig. S7, C and D) had no effects on T cell viability. Moreover, TH17 cells expressed CD95 (Fig. 2D); however, CD95 engagement did not alter TH17 cell apoptosis. In line with these observations, TH17 cells expressed high amounts of BCL2 and BCLXL (Fig. 5, H and I).
HIF-1α regulates TH17 cell apoptosis and persistence
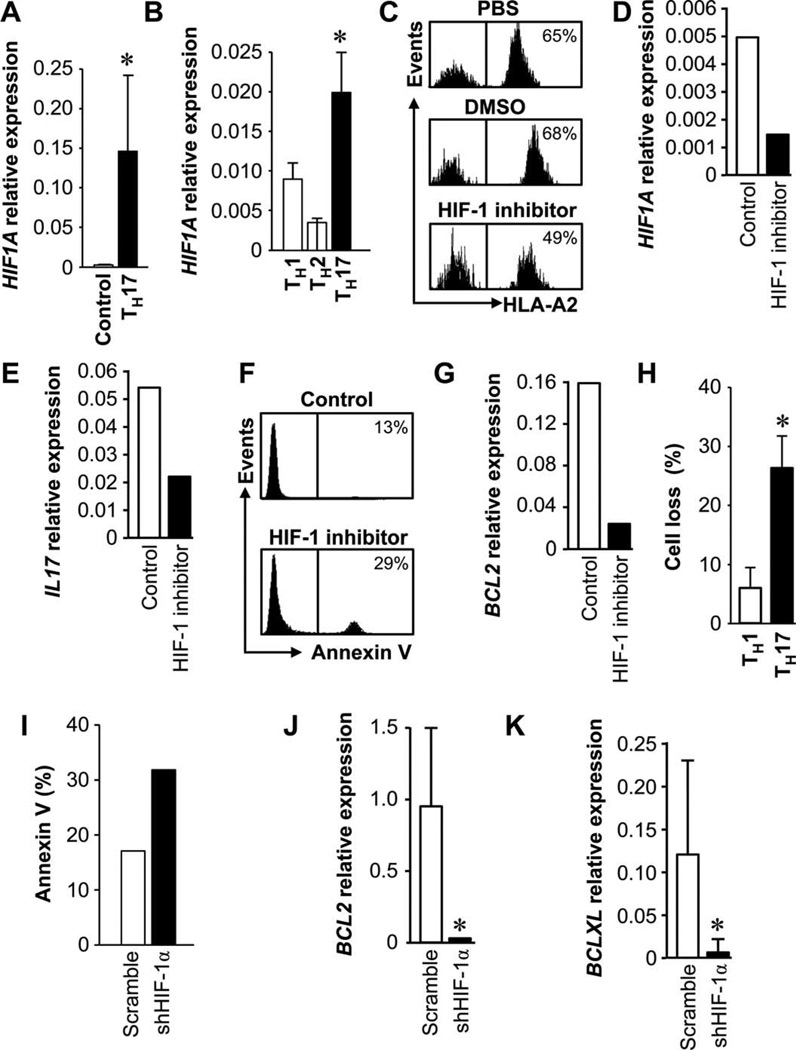
We next examined why TH17 cells were less apoptotic and efficiently persisted in vivo. Low oxygen pressures exist in many solid tissues. One key element in cellular adaptation for survival to hypoxia is induced expression of HIF-1. We investigated whether apoptosis resistance and persistence of TH17 cells are associated with HIF-1α. As expected, primary (Fig. 6A) and polarized TH17 cells (Fig. 6B) expressed higher amounts of HIF-1α compared to IL-17− T, TH1, and TH2 cells.
Fig. 6.
HIF-1α regulates TH17 cell apoptosis and persistence. (A and B) TH17 cells expressed HIF1A. HIF1A expression was quantified by real-time PCR in primary TH17 (A) and polarized T cell subsets (B). n = 5 to 8. *P < 0.05. (C to E) HIF-1 blockade reduced TH17 persistence in vivo. Primary HLA-A2+ TH17 cells and HLA-A2−IL-17−CD4+ T cells were mixed and pretreated with PBS, DMSO, and the HIF-1 inhibitor echinomycin for 48 hours and transfused to NSG mice. Human T cells were stained and analyzed for HLA-A2 and human CD5 (C), and human HIF1A (D) and IL17A (E) were quantified in the spleen. n = 5. (F and G) HIF-1 blockade increased TH17 apoptosis in vivo. Primary TH17 cells were pretreated with HIF-1 inhibitor or DMSO and transferred to NSG mice. After 2 days, annexin V expression (F) and BCL2 gene expression (G) were analyzed in human T cells in mouse spleen. n = 6. P < 0.05. (H) HIF-1 blockade increased TH17 apoptosis in vitro. Enriched primary TH17 cells were transfected with lentiviral vector encoding shHIF-1α and then activated with TCR for 3 days. TH17 and TH1 cell numbers were counted, and the percentage of cell loss was calculated. n = 3. P < 0.05. (I to K) HIF-1 blockade increased TH17 apoptosis induced by chemotherapy. Primary TH17 cells were transfected with lentiviral vector encoding shHIF-1 or scramble and cultured with cisplatin for 48 hours. Cell apoptosis was analyzed with annexin V expression (I). BCL2 (J) and BCLXL (K) genes were quantified. n = 7. P < 0.01.
We tested the role of HIF-1α in TH17 cell persistence in vivo in NSG mice. We equally mixed IL-17+HLA-A2+ TH17 cells and IL-17−HLA-A2−CD4+ T cells and treated these cells with phosphate-buffered saline (PBS), dimethyl sulfoxide (DMSO), and the HIF-1 inhibitor echinomycin (fig. S8A) (23). The mixed cells were transfused into NSG mice. In the HIF-1 inhibitor–treated group, the number of IL-17+HLA-A2+ TH17 cells recovered from mouse spleen was reduced by 20% within 36 hours (Fig. 6C). These data suggest that HIF-1 blockade reduces TH17 cell persistence in vivo. We confirmed that the recovered human T cells from mouse spleen expressed limited HIF1A (Fig. 6D) and reduced IL17A (Fig. 6E), whereas the expression of human IFNG and CD3 was not changed (fig. S9, A and B).
We further dissected whether HIF-1 blockade affected TH17 cell apoptosis in vivo. TH17 cells were initially treated with DMSO or HIF-1 inhibitor and subsequently transfused into NSG mice. After 48 hours, we showed that there were more annexin V+ TH17 cells in the HIF-1 inhibitor–treated group than in the control group (Fig. 6F). Furthermore, HIF-1 blockade reduced BCL2 expression in TH17 cells (Fig. 6G). Thus, HIF-1 blockade increases TH17 cell apoptosis in vivo.
We further examined whether HIF-1 blockade had an impact on TH17 cell apoptosis during T cell activation and chemotherapy (Fig. 5). TH17 and TH1 cells were activated in the presence of HIF-1 inhibitor for 3 days. The number of TH17 cells was reduced by 28%, whereas there was only 5% reduction for TH1 cells (Fig. 6H).
We genetically blocked HIF-1α with small hairpin HIF-1 (shHIF-1) (fig. S8B) and examined TH17 cell apoptosis in response to cisplatin treatment. Genetic HIF-1 blockade increased the number of annexin V+ TH17 cells compared to scramble controls (Fig. 6I). In line with this observation, the expression of BCL2 (Fig. 6J) and BCLXL (Fig. 6K) was inhibited by genetic HIF-1 blockade. These data indicate that HIF-1 is crucial for controlling the survival/apoptosis and persistence of TH17 cells in multiple experimental settings.
HIF-1α is linked to Notch and Bcl-2 family and regulates TH17 cell biology
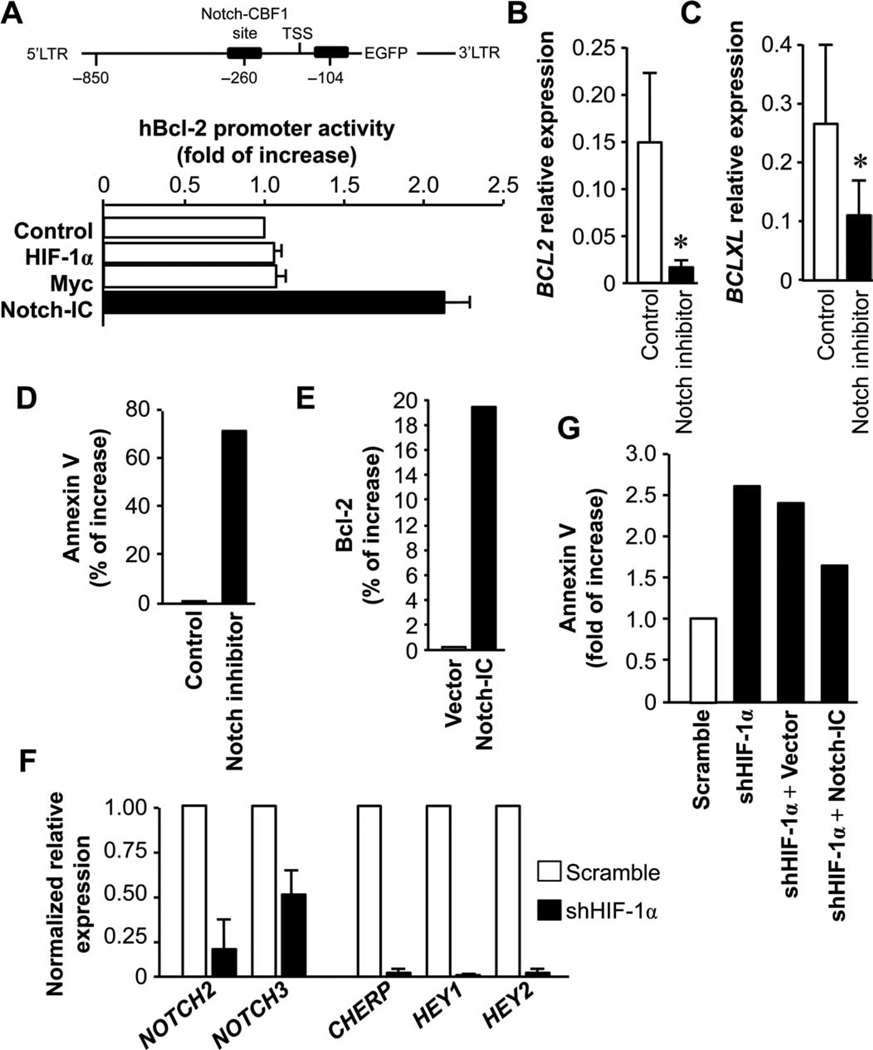
We further hypothesized that HIF-1α targeted the Bcl-2 family and in turn controlled TH17 cell survival and apoptosis. Unexpectedly, in the promoter binding and activity assays, HIF-1α and Myc (control) were not capable of activating the BCL2 proximal promoter (Fig. 7A, lower panel). Because TH17 cells expressed high amounts of Notch signaling genes (Fig. 3K), we next examined whether the Notch pathway was involved in the regulation of Bcl-2 family gene expression and function. The intracellular active domain of Notch (Notch-IC) stimulated BCL2 promoter activities (Fig. 7A, lower panel). As confirmation, Notch-IC activated key Notch signaling genes (fig. S10). In line with this, there were multiple Notch binding sites in the BCL2 promoter area (Fig. 7A, upper panel). Furthermore, Notch inhibition resulted in reduced expression of BCL2 and BCLXL (Fig. 7, B and C) and more annexin V+ TH17 cells (Fig. 7D). We confirmed that Notch activation induced the expression of Bcl-2 on TH17 cells (Fig. 7E).
Fig. 7.
Notch and Bcl-2 family regulate the stem cell-like feature of the TH17 cells. (A) Notch bound to and activated the Bcl-2 promoter. Black boxes show Notch binding sites in the BCL2 promoter (upper panel). Human embryonic kidney (HEK) 293 cells were cotransfected with the hBCL2-EGFP (enhanced green fluorescent protein) promoter with the indicated plasmids for 38 hours. The intensity of EGFP was measured with flow cytometry. LTR, long terminal repeat; TSS, transcription start site. (B and C) Notch blockade suppressed BCL2 and BCLXL expression. Primary T cells were treated with Notch inhibitor for 24 hours. BCL2 (B) and BCLXL (C) expression was quantified by real-time PCR. n = 3. P < 0.05. (D) Notch blockade increased TH17 cell apoptosis. Primary TH17 cells were cultured with the Notch inhibitor for 3 days. Annexin V expression was analyzed by flow cytometry. n = 3. (E) Notch activation increased BCL2 expression on TH17 cells. Primary TH17 cells were transfected with Notch-IC. After 24 hours, Bcl-2 expression was analyzed by flow cytometry. n = 3. (F) HIF blockade reduced Notch signaling gene expression in TH17 cells. Primary TH17 cells were transfected with shHIF-1α. After 24 hours, Notch signaling gene expression was quantified. n = 3. (G) Notch activation rescued TH17 cell apoptosis induced by HIF blockade. Primary TH17 cells were transfected with lentiviral vectors encoding shHIF-1α and/or Notch-IC. After 24 hours, annexin V expression was analyzed by flow cytometry. n = 3.
Because both HIF-1 (Fig. 6) and Notch (Fig. 7, A to E) signaling pathways regulate TH17 cell survival and apoptosis, we further examined the mechanistic relationship between these two pathways. It is possible that HIF-1 might directly activate and maintain Notch signaling gene expression and in turn regulate Bcl-2 family gene expression and function through Notch signaling pathway. In support of this, genetic blockade of HIF-1α reduced the expression of key Notch signaling genes in TH17 cells (Fig. 7F). Furthermore, althoughHIF-1 blockade induced TH17 cell apoptosis (Figs. 6 and 7G), when TH17 cells were cotransfected with shHIF-1 and Notch-IC, Notch activation partially but importantly reduced TH17 cell apoptosis mediated by HIF-1 blockade (Fig. 7G). The results indicate that Notch activation may independently regulate TH17 cell survival and apoptosis, whereas HIF-1 may regulate TH17 cells through activating and maintaining the Notch signaling pathway.
DISCUSSION
Here, we have reported several findings: (i) human TH17 cells are long-lived cells with a high capacity for expansion and are resistant to apoptosis, (ii) HIF-1α/Notch/Bcl-2 is a key signaling pathway controlling the TH17 cell survival and apoptosis pattern, and (iii) human TH17 cells persist in vivo and mediate or promote long-term antitumor immunity.
Regardless of their tissue origins, TH17 cells are confined to the memory T cell compartment with a phenotype of CD28+CD127+PD-1−Foxp3−KLRG-1−CD57−IL-10−. This phenotype makes them unlikely candidates for being exhausted PD-1+ T cells, suppressive Foxp3+ or IL-10+ T cells, or senescent CD28−CD57+KLRG-1+ T cells. However, TH17 cells express high amounts of CD95 and lower amounts of CD27 and produce effector cytokines (8). Thus, TH17 cells exhibit a terminally differentiated effector cell phenotype.
Although TH17 cells have a terminal differentiation phenotype, they express high levels of Ki67 and efficiently expand. TH17 cells are resistant to apoptosis induced by different stimuli, have better persistence in vivo, and mediate potent antitumor immunity. Under specific conditions, in line with mouse studies (19–21), TH17 cells can be differentiated into TH1 and Treg cells, indicating their high plasticity. These characteristics endow unique features for human TH17 cells. The long-lived capacity can help maintain a constant repertoire of memory TH17 cells for a human lifetime despite the finite life span of individual effector cells and reduced thymus function.
Genetic and molecular experiments have demonstrated that BCL family genes control TH17 cell survival and apoptosis resistance. Human TH17 cells express high levels of Bcl-2 family members. In support of our observations on human TH17 cells, high amounts of BCL2 expression are associated with long-term survival in viral-specific CD8+ T cells in mice (24) and with memory CD8+ T cell renewal in a mouse bone marrow transplantation model (25).
We have further explored the molecular mechanisms controlling the expression and function of the Bcl-2 family in TH17 cells. It has been proposed that hypoxic environment is required for cancer stem cell function and HIF-1 is important for maintaining an active niche for long-term hematopoietic stem cells (HSCs) (26). Hypoxia is a common phenomenon in inflammatory and tumor tissues (27, 28). Human tissue TH17 cells express high amounts of HIF1A. Our gain- and loss-of-function experiments demonstrate that the HIF-1α signaling pathway is crucial for the expression of Bcl-2 family members. However, HIF-1α does not directly target the BCL2 promoter and control its expression; instead, the promoter activity assay revealed that Notch, but not HIF-1α, activates BCL2 promoter. There were multiple binding sites for Notch binding on the BCL2 promoter. Furthermore, similar to HIF-1α, Notch controlled the expression and functions of the Bcl-2 family in TH17 cells and affected TH17 cell survival and apoptosis. The data point toward an interaction between HIF-1α and Notch signaling pathways in regulating TH17 cell biology. In support of this, we have uncovered that HIF-1α controlled Notch signaling gene expression. However, genetic HIF-1α blockade did not disable the effects of Notch activation on the expression and function of the Bcl-2 family on TH17 cells. These data suggest that Notch signaling may independently regulate TH17 cell function. Therefore, HIF-1α activation is important for maintaining TH17 cell survival and apoptosis through activating the Notch signaling pathway, whereas the collaboration between HIF-1α and Notch signaling pathways promotes and maintains TH17 functional integrity.
The next question is to identify the key downstream gene targets of the HIF/Notch/Bcl-2 signaling pathway, which may be important to determine TH17 cell biology. TH17 cells express high levels of cyclins and reduced CDK repressors. CDK repressors contribute to multiple types of cellular senescence and exhaustion, and suppression of p16Ink4a and p19Arf is essential for HSC self-renewal (29). TH17 cells also express high amounts of Wnt/β-catenin–associated genes. Certain mouse CD8+ memory T cells have increased activity ofWnt/β-catenin pathway and mediate potent antitumor immunity (30). Further experiments are warranted to examine the importance of the Wnt/β-catenin pathway in human TH17 cell biology.
TH17 cells express high amounts of CD95, which may be a stem cell–associated gene (31). Indeed, CD95 engagement does not induce TH17 cell apoptosis. TH17 cells express relatively lower amounts of CD27. This observation is consistent with levels reported on mouse TH17 cells (9). Given the importance of CD27 in T cell survival (10), it is thought that mouse TH17 cells are a short-lived population (9). In humans, after activation, human primary TH17 cells quickly acquire high levels of CD27 expression. CD27 expression would not be a limiting factor for TH17 cell survival. Furthermore, TH17 cells express high amounts of the IL-7 receptorα, CD127. IL-7 and IL-15 induce TH17 cell expansion. Because these two cytokines maintain and promote the memory CD8+ T cell pool, the data suggest that TH17 cells share some properties with memory CD8+ T cells.
The genetic and functional features of human TH17 cells we described here may be therapeutically important in T cell–based immunotherapy for cancer and in molecule-based immunotherapy for autoimmune disease. Mouse TH17 cells induce tumor eradication (13, 14, 32), and ICOS (inducible costimulator)–expanded human TH17 cells mediate antitumor activity (33). In line with these reports, we have demonstrated that human TH17 cells execute/promote potent long-term antitumor effects in vivo and are positively associated with patient survival in human ovarian cancer (8). Experimental and clinical evidence indicates that expansion potential, persistence, survival, and capacity for apoptosis are key factors that determine the therapeutic efficacy of adoptive CD8+ cell therapy (15). We analogically reason that potent antitumor immunity mediated and promoted by TH17 cells may be partially due to their important functional features including high expansion potential, resistance to apoptosis, long-lived capacity, and high plasticity. However, the in vivo TH17 cell dynamics and evolution and the relative contribution of each functional feature to human diseases remain to be dissected. The importance of HIF/Notch/Bcl-2 signaling pathway in TH17 cell biology suggests that enforced HIF/Notch/Bcl-2 expression and activation may further promote this interesting feature and improve TH17-mediated antitumor immunity. Thus, human TH17 cells may be used to treat patients with advanced cancer in combination with CD8+ T cell therapy or other immune therapeutic regimens. In a similar vein, disruption of HIF/Notch/Bcl-2 expression and activation may promote TH17 cell apoptosis and in turn disable the pathologic effects of TH17 cells in patients with GVHD and autoimmune diseases.
In conclusion, we have provided evidence at the genetic, molecular, and functional level that human TH17 cells are long-lived cells. This property may be critically important for controlling TH17 cell biology. We suggest that manipulation of TH17 cell apoptosis and survival may be therapeutically interesting for treating patients with chronic diseases affected by TH17 cells.
Supplementary Material
Acknowledgments
We thank N. Restifo [NIH/National Cancer Institute (NCI)] for discussion.
Funding: Supported in part by research grants from the NIH/NCI R01, the Ovarian Cancer Research Foundation (W.Z.), P50CA97248, and the NIH through the University of Michigan’s Cancer Center Support Grant (P30CA46592).
Footnotes
SUPPLEMENTARY MATERIAL
www.sciencetranslationalmedicine.org/cgi/content/full/3/104/104ra100/DC1
Materials and Methods
Fig. S1. TH17 cells in different tissues/organs.
Fig. S2. Flow-based gating for primary TH cell subsets.
Fig. S3. Cytokine profile of primary TH17 cells.
Fig. S4. Flow-based gating and sorting for TH17 cells.
Fig. S5. Polarized T cell subsets.
Fig. S6. TH17 cells express high levels of stem cell genes.
Fig. S7. Effects of IL-6 and IL-23 on TH17 cell apoptosis.
Fig. S8. HIF-1 blockade with echinomycin and shHIF-1α.
Fig. S9. The HIF-1 inhibitor echinomycin had no effects on the human IFNG and CD3 expression in vivo.
Fig. S10. Notch-IC activated Notch signaling gene expression.
References
Author contributions: Y.L., E.Z., Y.W., L.V., W.S., J.M., A.K., and A.L. performed the experiments, interpreted the data, and assisted with the paper. I.K. and W.Z. designed the research, interpreted the data, and wrote the paper.
Competing interests: The authors declare that they have no competing interests.
REFERENCES AND NOTES
- 1.Dong C. Diversification of T-helper-cell lineages: Finding the family root of IL-17-producing cells. Nat. Rev. Immunol. 2006;6:329–333. doi: 10.1038/nri1807. [DOI] [PubMed] [Google Scholar]
- 2.Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT. Interleukin 17–producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat. Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 3.Kolls JK, Lindén A. Interleukin-17 family members and inflammation. Immunity. 2004;21:467–476. doi: 10.1016/j.immuni.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 4.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFβ in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 5.Kleinschek MA, Boniface K, Sadekova S, Grein J, Murphy EE, Turner SP, Raskin L, Desai B, Faubion WA, de Waal Malefyt R, Pierce RH, McClanahan T, Kastelein RA. Circulating and gut-resident human Th17 cells express CD161 and promote intestinal inflammation. J. Exp. Med. 2009;206:525–534. doi: 10.1084/jem.20081712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zou W, Restifo NP. TH17 cells in tumour immunity and immunotherapy. Nat. Rev. Immunol. 2010;10:248–256. doi: 10.1038/nri2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kryczek I, Bruce AT, Gudjonsson JE, Johnston A, Aphale A, Vatan L, Szeliga W, Wang Y, Liu Y, Welling TH, Elder JT, Zou W. Induction of IL-17+ T cell trafficking and development by IFN-γ: Mechanism and pathological relevance in psoriasis. J. Immunol. 2008;181:4733–4741. doi: 10.4049/jimmunol.181.7.4733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kryczek I, Banerjee M, Cheng P, Vatan L, Szeliga W, Wei S, Huang E, Finlayson E, Simeone D, Welling TH, Chang A, Coukos G, Liu R, Zou W. Phenotype, distribution, generation, and functional and clinical relevance of Th17 cells in the human tumor environments. Blood. 2009;114:1141–1149. doi: 10.1182/blood-2009-03-208249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pepper M, Linehan JL, Pagán AJ, Zell T, Dileepan T, Cleary PP, Jenkins MK. Different routes of bacterial infection induce long-lived TH1 memory cells and short-lived TH17 cells. Nat. Immunol. 2010;11:83–89. doi: 10.1038/ni.1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hendriks J, Gravestein LA, Tesselaar K, van Lier RA, Schumacher TN, Borst J. CD27 is required for generation and long-term maintenance of T cell immunity. Nat. Immunol. 2000;1:433–440. doi: 10.1038/80877. [DOI] [PubMed] [Google Scholar]
- 11.Ye ZJ, Zhou Q, Gu YY, Qin SM, Ma WL, Xin JB, Tao XN, Shi HZ. Generation and differentiation of IL-17–producing CD4+ T cells in malignant pleural effusion. J. Immunol. 2010;185:6348–6354. doi: 10.4049/jimmunol.1001728. [DOI] [PubMed] [Google Scholar]
- 12.Chen JG, Xia JC, Liang XT, Pan K, Wang W, Lv L, Zhao JJ, Wang QJ, Li YQ, Chen SP, He J, Huang LX, Ke ML, Chen YB, Ma HQ, Zeng ZW, Zhou ZW, Chang AE, Li Q. Intratumoral expression of IL-17 and its prognostic role in gastric adenocarcinoma patients. Int. J. Biol. Sci. 2011;7:53–60. doi: 10.7150/ijbs.7.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hinrichs CS, Kaiser A, Paulos CM, Cassard L, Sanchez-Perez L, Heemskerk B, Wrzesinski C, Borman ZA, Muranski P, Restifo NP. Type 17 CD8+ T cells display enhanced antitumor immunity. Blood. 2009;114:596–599. doi: 10.1182/blood-2009-02-203935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martin-Orozco N, Muranski P, Chung Y, Yang XO, Yamazaki T, Lu S, Hwu P, Restifo NP, Overwijk WW, Dong C. T helper 17 cells promote cytotoxic T cell activation in tumor immunity. Immunity. 2009;31:787–798. doi: 10.1016/j.immuni.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gattinoni L, Powell DJ, Jr, Rosenberg SA, Restifo NP. Adoptive immunotherapy for cancer: Building on success. Nat. Rev. Immunol. 2006;6:383–393. doi: 10.1038/nri1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, Zhu Y, Wei S, Kryczek I, Daniel B, Gordon A, Myers L, Lackner A, Disis ML, Knutson KL, Chen L, Zou W. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat. Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 17.Curiel TJ, Wei S, Dong H, Alvarez X, Cheng P, Mottram P, Krzysiek R, Knutson KL, Daniel B, Zimmermann MC, David O, Burow M, Gordon A, Dhurandhar N, Myers L, Berggren R, Hemminki A, Alvarez RD, Emilie D, Curiel DT, Chen L, Zou W. Blockade of B7-H1 improves myeloid dendritic cell–mediated antitumor immunity. Nat. Med. 2003;9:562–567. doi: 10.1038/nm863. [DOI] [PubMed] [Google Scholar]
- 18.Kryczek I, Zou L, Rodriguez P, Zhu G, Wei S, Mottram P, Brumlik M, Cheng P, Curiel T, Myers L, Lackner A, Alvarez X, Ochoa A, Chen L, Zou W. B7-H4 expression identifies a novel suppressive macrophage population in human ovarian carcinoma. J. Exp. Med. 2006;203:871–881. doi: 10.1084/jem.20050930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murphy KM, Stockinger B. Effector T cell plasticity: Flexibility in the face of changing circumstances. Nat. Immunol. 2010;11:674–680. doi: 10.1038/ni.1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee YK, Turner H, Maynard CL, Oliver JR, Chen D, Elson CO, Weaver CT. Late developmental plasticity in the T helper 17 lineage. Immunity. 2009;30:92–107. doi: 10.1016/j.immuni.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martin-Orozco N, Chung Y, Chang SH, Wang YH, Dong C. Th17 cells promote pancreatic inflammation but only induce diabetes efficiently in lymphopenic hosts after conversion into Th1 cells. Eur. J. Immunol. 2009;39:216–224. doi: 10.1002/eji.200838475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kryczek I, Wu K, Zhao E, Wei S, Vatan L, Szeliga W, Huang E, Greenson J, Chang A, Roliński J, Radwan P, Fang J, Wang G, Zou W. IL-17+ regulatory T cells in the micro-environments of chronic inflammation and cancer. J. Immunol. 2011;186:4388–4395. doi: 10.4049/jimmunol.1003251. [DOI] [PubMed] [Google Scholar]
- 23.Wang Y, Liu Y, Malek SN, Zheng P, Liu Y. Targeting HIF1α eliminates cancer stem cells in hematological malignancies. Cell Stem Cell. 2011;8:399–411. doi: 10.1016/j.stem.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grayson JM, Zajac AJ, Altman JD, Ahmed R. Cutting edge: Increased expression of Bcl-2 in antigen-specific memory CD8+ T cells. J. Immunol. 2000;164:3950–3954. doi: 10.4049/jimmunol.164.8.3950. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Y, Joe G, Hexner E, Zhu J, Emerson SG. Host-reactive CD8+ memory stem cells in graft-versus-host disease. Nat. Med. 2005;11:1299–1305. doi: 10.1038/nm1326. [DOI] [PubMed] [Google Scholar]
- 26.Takubo K, Goda N, Yamada W, Iriuchishima H, Ikeda E, Kubota Y, Shima H, Johnson RS, Hirao A, Suematsu M, Suda T. Regulation of the HIF-1α level is essential for hematopoietic stem cells. Cell Stem Cell. 2010;7:391–402. doi: 10.1016/j.stem.2010.06.020. [DOI] [PubMed] [Google Scholar]
- 27.Semenza GL. Targeting HIF-1 for cancer therapy. Nat. Rev. Cancer. 2003;3:721–732. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- 28.Kryczek I, Lange A, Mottram P, Alvarez X, Cheng P, Hogan M, Moons L, Wei S, Zou L, Machelon V, Emilie D, Terrassa M, Lackner A, Curiel TJ, Carmeliet P, Zou W. CXCL12 and vascular endothelial growth factor synergistically induce neoangiogenesis in human ovarian cancers. Cancer Res. 2005;65:465–472. [PubMed] [Google Scholar]
- 29.Bracken AP, Kleine-Kohlbrecher D, Dietrich N, Pasini D, Gargiulo G, Beekman C, Theilgaard-Mönch K, Minucci S, Porse BT, Marine JC, Hansen KH, Helin K. The Polycomb group proteins bind throughout the INK4A-ARF locus and are disassociated in senescent cells. Genes Dev. 2007;21:525–530. doi: 10.1101/gad.415507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gattinoni L, Zhong XS, Palmer DC, Ji Y, Hinrichs CS, Yu Z, Wrzesinski C, Boni A, Cassard L, Garvin LM, Paulos CM, Muranski P, Restifo NP. Wnt signaling arrests effector T cell differentiation and generates CD8+ memory stem cells. Nat. Med. 2009;15:808–813. doi: 10.1038/nm.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Corsini NS, Sancho-Martinez I, Laudenklos S, Glagow D, Kumar S, Letellier E, Koch P, Teodorczyk M, Kleber S, Klussmann S, Wiestler B, Brüstle O, Mueller W, Gieffers C, Hill O, Thiemann M, Seedorf M, Gretz N, Sprengel R, Celikel T, Martin-Villalba A. The death receptor CD95 activates adult neural stem cells for working memory formation and brain repair. Cell Stem Cell. 2009;5:178–190. doi: 10.1016/j.stem.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 32.Muranski P, Boni A, Antony PA, Cassard L, Irvine KR, Kaiser A, Paulos CM, Palmer DC, Touloukian CE, Ptak K, Gattinoni L, Wrzesinski C, Hinrichs CS, Kerstann KW, Feigenbaum L, Chan CC, Restifo NP. Tumor-specific Th17-polarized cells eradicate large established melanoma. Blood. 2008;112:362–373. doi: 10.1182/blood-2007-11-120998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paulos CM, Carpenito C, Plesa G, Suhoski MM, Varela-Rohena A, Golovina TN, Carroll RG, Riley JL, June CH. The inducible costimulator (ICOS) is critical for the development of human TH17 cells. Sci. Transl. Med. 2010;2 doi: 10.1126/scitranslmed.3000448. 55ra78. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.