ABSTRACT
Rarely, if ever, has a single bacterial cell been confirmed to simultaneously host two fundamentally different predators. Two such predators are viruses and the predatory prokaryotes known as Bdellovibrio and like organisms. Viruses or bacteriophage are particles requiring prey cells in an active metabolic state to complete their life cycle. The Bdellovibrio and like organisms, unlike viruses, are bacteria that can efficiently infect and grow in prey which are in stationary phase. In this study, electron microscopic examination revealed an unprecedented coinfection by the two agents of Vibrio vulnificus, introducing a new bacterial predation paradigm. Rather than the viruses and Bdellovibrio and like organisms competing for a single prey cell, both can survive in the same cell and successfully reproduce themselves. This is an especially valuable mechanism when the prey is in short supply, and the survival of the predators may be at stake.
IMPORTANCE
This article describes the coinfection of a prokaryotic prey or host cell by both a bacteriophage (phage) and the predatory bacterium of the group Bdellovibrio and like organisms (BALOs). Such coinfection has not been previously reported and therefore introduces a new paradigm for predation of bacteria. This finding invites new studies on the interactions of BALOs, phage, and prey in predation. Predation is an important mechanism in nature for helping to keep bacterial populations in check and also plays a major role in the cycling of nutrients through the microbial loop. How dual infection of phage and BALOs imposes on these and other functions of predation is fertile ground for future studies and serves as a keystone reference on bacterial predation and mortality.
Observation
A case of two fundamentally different microbial predators simultaneously infecting a single eukaryotic or bacterial cell has not been documented to our knowledge. Major bacterial predators include viruses (bacteriophages) and a group of bacterial species collectively known as Bdellovibrio and like organisms (BALOs). Both result in the killing of the host or prey cell and the recycling of cellular material through the microbial loop. Viruses are most often referred to as parasites and the cells they infect as their hosts. However, they may have attributes of both parasites, which typically feed off and coexist with their host for extended periods, and predators, which rapidly kill their target organism for food. In this report, we refer to bacteriophage as predators, as they are being studied with the predatory Bdellovibrio and like organisms.
Although BALOs and bacteriophages occur in the same environments and may prey on the same bacterial species, coinfection by these two agents has not been considered. Here, we report a unique case of such coinfection.
The prey bacterium used in infection experiments was Vibrio vulnificus FLA042, a capsulated spontaneous rifampin-resistant mutant of a virulent environmental strain MLT403 which can cause septicemia and wound infection in humans. The test predator strains were cluster IX of Bacteriovorax (a saltwater genus of BALOs) and bacteriophage CK 2, both of which have been characterized in previous studies as the most efficient predators in controlling V. vulnificus compared to many of their counterpart strains (1, 2).
Equal numbers of Bacteriovorax cluster IX and bacteriophage CK2 (final concentration of 1 × 108 PFU ml−1) were inoculated into a microcosm containing V. vulnificus suspended in 200 ml sterilized natural seawater at predator-prey ratios of 1:1. The microcosm suspensions were shaken at 27°C. Fifty milliliters of sample was removed after 30 min, 1 h, and 4 h and fixed for electron microscopic examination (3). Briefly, the cells were centrifuged for 20 min at 11,952 × g, resuspended in 1 ml of 0.1 M sodium phosphate buffer (pH 7), and centrifuged for 15 min at 10,600 × g. The pellet was resuspended in 2 ml of 0.1 M cacodylate buffer containing 2% glutaraldehyde and 1% formaldehyde, both diluted from 25% (vol/vol) and 16% (vol/vol) stock solutions, respectively. After 60 min at 4°C and centrifugation at 10,600 × g, the pellet was overlaid with cacodylate buffer, and an aliquot of sample was stained with uranyl acetate. Negatively stained samples in which predator and prey infections were clearly observed were selected for embedding in epoxy. Cells were postfixed in OsO4, washed, dehydrated, and embedded in resin. Ultrathin sections were counterstained with uranyl acetate and lead citrate and examined with a Hitachi H-7600 transmission electron microscope.
For scanning electron microscopy, fixed cells were also deposited onto 0.2-µm-pore-size polycarbonate membrane filters and processed with the aid of a Pelco BioWave Pro laboratory microwave (Ted Pella, Redding, CA). Samples were washed 3 times with 0.1 M cacodylate buffer (pH 7.24); postfixed with 2% buffered osmium tetroxide; water washed; dehydrated in a graded ethanol series 25%, 50%, 75%, 95%, 100%; and critical point dried (Autosamdri-815, Tousimis Research Corp, Rockville, MD). Dried samples were mounted on carbon adhesive tabs on aluminum specimen mounts and Au/Pd sputter coated (DeskV Denton Vacuum, Moorestown, NJ). High-resolution digital micrographs were acquired with a field emission scanning electron microscope (S-4000; Hitachi High Technologies America, Inc., Schaumburg, IL).
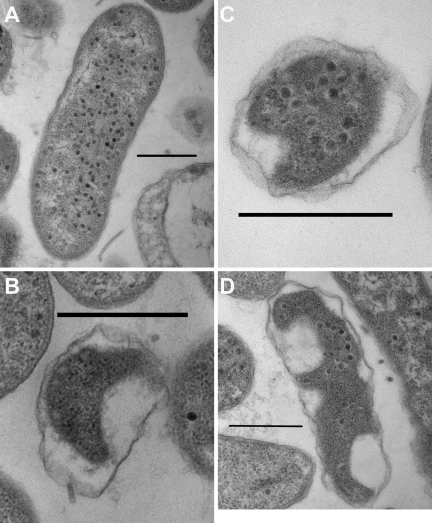
When virus strain CK2 and BALO member Bacteriovorax were spiked simultaneously into a seawater microcosm containing the prey bacterium, Vibrio vulnificus, we observed by scanning and transmission electron microscopy that single cells of V. vulnificus were infected by either bacteriophages (Fig. 1A) or BALOs (Fig. 1B) and some were coinfected with both types of the predators (Fig. 1C, D, and 2). This finding reveals a new form of predation and parasitism in bacteria and raises important questions about their predation biology and ecology.
FIG 1 .
Micrographs of thin sections of V. vulnificus prey cells infected by phage only (A), BALO only (B), and BALO and phage (C). (D) Another example of a coinfected cell; in this case, BALO did not change the shape of the prey cell. Panels B, C, and D show the bdelloplast, the post-BALO infection structure with the predator residing inside the prey cell. Bars represent 500 nm.
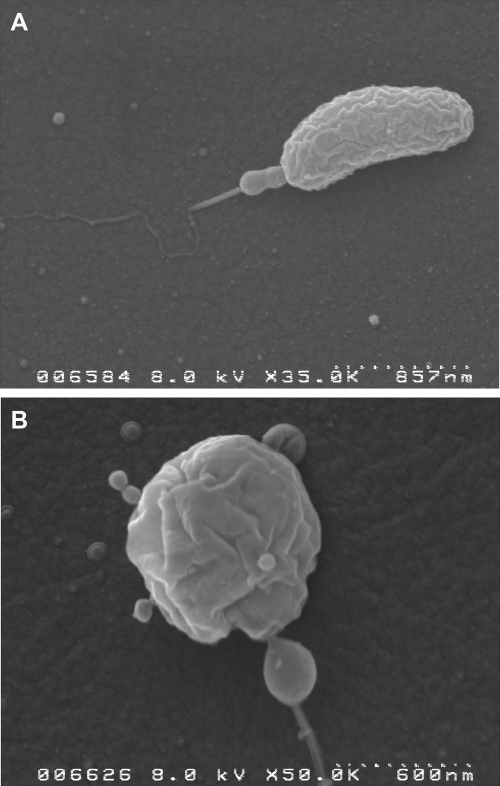
FIG 2 .
Scanning electron micrographs showing BALO cells and phages attached to a prey cell (A) and a bdelloplast (B).
One such question relates to the dynamics and order of invasion by the two predators. Both bacteriophage and BALOs require an intact cell as a nutrient resource for their growth and multiplication, yet their infection requirements and impacts on the cell differ in critical ways. Phage adsorption requires specific host cell surface structures, and viral replication is dependent on active host metabolic machinery, both of which may be altered by BALO infection. Once BALOs invade their prey, the prey is killed within 15 min (4), and the prey cell wall is partially digested to form a bdelloplast (5).
Although no prior information on coinfection of these agents has been reported, possible mechanisms can be hypothesized based on what is known about their respective predation cycles. Since viral particles were observed within the bdelloplast, we reason that the phage infected the host first and began to replicate utilizing the host’s metabolic machinery. Shortly afterward, a BALO cell penetrated the cell wall, shutting down the host’s metabolic functions, then lodged in the periplasmic space and fed on the prey’s cellular content. When the metabolic functions of the host cell were halted, further viral replication was greatly reduced, if not terminated. The BALO cell would continue its growth and replication, culminating in lysis of the prey, releasing both the phage and BALO progeny.
The dynamics of predator infection are further complicated by various inherent properties of viral and BALO predation and environmental factors. Phages are restricted to infecting specific host bacteria, whereas BALOs typically prey on a variety of Gram-negative bacteria, although some strains show preferences (1). Viruses typically replicate more efficiently in rapidly growing cells (6), whereas BALOs grow better on bacteria in stationary growth (7). In the competition for food, these properties give BALOs the advantage where a particular prey species is in short supply or slow growing. This type of competition can be categorized as exploitation (8).
Coinfection by bacteriophage and BALO within a single cell has benefits and consequences for both predators. The osmotically stable bdelloplast provides protection for phage and BALOs against unfavorable conditions. The two predators also compete for host resources. These events introduce a unique relationship between predators which can be described as competitive alliance.
This observation of coinfection presents a new paradigm for predator competition and will drive opportunities for further research. Other questions generated by this finding include how universal coinfection is and what conditions favor it in the environment. How does coinfection affect viral release of bacterial dissolved organic matter (DOM) in biogeochemical cycles? What are the tradeoffs for the two predators to infect the same cell? Could the enclosed bdelloplast chamber provide conditions for possible lateral gene transfer from prey to BALO aided by virus? These are significant areas to be pursued.
ACKNOWLEDGMENTS
This research was funded by NSF grant no. HRD-0932137.
We thank Paul Gulig for providing phage and bacterial cultures. We also thank for their assistance Jill W. Verlander and Sharon W. Matthews at the University of Florida, College of Medicine Electron Microscopy Facility, and Karen Kelley, CEMT (coordinator/manager), University of Florida, Interdisciplinary Center for Biotechnology Research, Electron Microscopy and Bio-Imaging Lab.
Footnotes
Citation Chen H, Williams HN. 2012. Sharing of prey: coinfection of a bacterium by a virus and a prokaryotic predator. mBio 3(2):e00051-12. doi:10.1128/mBio.00051-12.
REFERENCES
- 1. Chen H, Athar R, Zheng G, Williams HN. 2011. Prey bacteria shape the community structure of their predators. ISME J. 5:1314–1322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Martin JE. 2005. Use of bacteriophages to decontaminate naturally and experimentally infected oysters of Vibrio vulnificus. University of; Florida, Gainsville, FL [Google Scholar]
- 3. Koval SF, Bayer ME. 1997. Bacterial capsules: no barrier against Bdellovibrio. Microbiology 143(Pt 3):749–753 [DOI] [PubMed] [Google Scholar]
- 4. Thomashow MF, Rittenberg SC. 1978. Intraperiplasmic growth of Bdellovibrio bacteriovorus 109J: solubilization of Escherichia coli peptidoglycan. J. Bacteriol. 135:998–1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Starr MP, Baigent NL. 1966. Parasitic interaction of Bdellovibrio bacteriovorus with other bacteria. J. Bacteriol. 91:2006–2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Robb F, Hill R. 2000. Bacterial viruses and hosts: influence of culturable state, p 199–208 In Colwell RR, Grimes DJ, Nonculturable microorganisms in the environment. ASM Press, Washington, DC [Google Scholar]
- 7. Williams H, Piñeiro S. 2007. Ecology of the predatory Bdellovibrio and like organisms, p 213–248 In Jurkevitch E, Predatory prokaryotes, vol 4 Springer Verlag, Berlin/Heidelberg, Germany [Google Scholar]
- 8. Mideo N. 2009. Parasite adaptations to within-host competition. Trends Parasitol. 25:261–268 [DOI] [PubMed] [Google Scholar]