Abstract
Background:
Hirayama disease (HD) is benign focal amyotrophy of the distal upper limbs, often misdiagnosed as motor neuron disease. Routine magnetic resonance imaging (MRI) is often reported normal.
Objective:
To study the clinicoradiological profile of hand wasting in young males.
Materials and Methods:
Patients presenting with insidious-onset hand wasting from March 2008 to May 2011 were evaluated electrophysiologically. Cervical MRI in neutral position was done in 11 patients and flexion contrast imaging was done in 10 patients.
Results:
All patients were males less than 25 years of age, with median age 23 years, except one patient who was 50 years old. Duration of illness was 3 months to 3 years. All (100%) had oblique amyotrophy, four (36%) cold paresis, 10 (91%) minipolymyoclonus and three (27%) had fasciculations. Regional reflexes were variably absent. Two patients (18%) had brisk reflexes of lower limbs with flexor plantars. Electromyography (EMG) showed chronic denervation in the C7-T1 myotomes. Neutral position MRI showed loss of cervical lordosis in 10/11 (91%), localized lower cervical cord atrophy in 9/11 (82%), asymmetric cord flattening in 11/11 (100%) and intramedullary hyperintensity in 2/11 (18%); flexion study showed loss of dural attachment, anterior displacement of dorsal dura, epidural flow voids in 9/10 (90%) and enhancing epidural crescent in 10/10 (100%). Clinical profile, imaging and electrophysiological findings of the patient aged 50 years will be described in detail as presentation at this age is exceptional. Collar therapy slowed progression in most cases.
Conclusion:
Clinical features of HD corroborated well with electrophysiological diagnosis of anterior horn cell disease of lower cervical cord. While dynamic contrast MRI is characteristic, routine studies have a high predictive value for diagnosis. Prompt diagnosis is important to institute early collar therapy.
Keywords: Collar therapy, flexion magnetic resonance imaging, hand wasting, Hirayama disease, monomelic amyotrophy
Introduction
Hirayama disease (HD) is a rare disease affecting primarily young men in the second to third decades of life.[1,2] It has been called differently as juvenile muscular atrophy of the distal upper extremity (JMADUE),[3] monomelic amyotrophy (MMA, Gourie-Devi et al.)[4] and juvenile asymmetric segmental spinal muscular atrophy (JASSMA, Pradhan and Gupta).[5]
JMADUE (HD) was first reported in 1959 as “juvenile muscular atrophy of unilateral upper extremity.” Since then, similar patients in their teens or 20 s have been described under a variety of names, not only in Japan but also in other Asian countries as well as in Europe and North America.[6]
HD is characterized by the insidious onset of unilateral or asymmetric atrophy of the hand and forearm with sparing of brachioradialis, giving the characteristic appearance of oblique amyotrophy involving the C7, C8 and T1 myotomes.[7] It is thought to be a kind of cervical myelopathy related to flexion movements of the neck.[8] HD differs from classical types of motor neuron diseases (MND) because of its nonprogressive course and pathologic findings of chronic microcirculatory changes in the territory of the anterior spinal artery supplying the anterior horns of the lower cervical cord.[1,9,10]
The pathogenetic mechanism of this disease is attributed to forward displacement of the posterior wall of the lower cervical dural canal when the neck is in flexion, which causes marked, often asymmetric, flattening of the lower cervical cord.[1,10–12] Since HD differs from MND, or spinal muscular atrophy, this disease entity should be more widely recognized as prognosis in this condition is benign and early detection and effective treatments may be considered.[6]
A spectrum of diagnostic magnetic resonance imaging (MRI) features has been described in the literature. Pradhan and Gupta from India reported the characteristic MRI features, as they are known today, in 1997.[5] The present study reviews the clinical and MRI features in HD in the neutral and flexion positions as well as documents subjective response to cervical collar therapy. Early diagnosis of HD, also called MMA, based on clinical and imaging characteristics, is important so that timely intervention with cervical collar can be instituted.
Materials and Methods
The study was conducted at two tertiary care hospitals in India. The first four patients underwent clinical evaluation, electrophysiology and neuroimaging beginning in March 2008 at Pune. Subsequently, other patients underwent clinical evaluation and electrophysiological examination at Kolkata by the first author (KMH), and neuroimaging was done at Bangalore by the second author (HS, Neuroradiologist). All the cases have been followed-up by the first author (KMH).
Twelve patients with a clinical suspicion of HD were seen from March 2008 to May 2011. One patient after initial evaluation was lost to follow-up and has not been included in the final analysis. The pre- and postcontrast MRI were done in neutral and flexion positions of cervical spine and imaging findings were correlated with the clinical presentation and electrophysiology findings. Another patient (patient number 12) declined to undergo dynamic contrast study.
The criteria for patient selection[1] were: (a) weakness and wasting predominantly in C7, C8 and T1 myotomes in one upper limb or asymmetrically in both upper limbs, (b) insidious onset in the teens or in the early 20 s, (c) progression for 1–3 years followed by arrest of disease or relatively benign course, (d) irregular coarse tremors (minipolymyoclonus) in the fingers of the affected hand(s), (e) mild transient worsening of symptoms on exposure to cold, (f) EMG evidence of chronic denervation in the clinically or subclinically affected muscles and (g) absence of substantial sensory loss/reflex abnormalities, cranial nerve, pyramidal tract signs in lower limbs, sphincter or cerebellar deficits.
Electrophysiological study included nerve conduction study (NCS) and EMG. Motor and sensory nerves conduction studies were carried out in the median and ulnar nerves of both upper limbs. Needle EMG studies were done in muscles of the C5-T1 myotomes.
MRI was performed on a 1.5 Tesla MRI (Siemens Symphony or Avanto). The MRI protocol included imaging in neutral position followed by imaging in hyperflexion. The sequences performed in neutral position included Sagittal SE T1W, TSE T2, Gradient Echo T2, MR myelogram in sagittal and coronal planes. The sequences performed in hyperflexion of cervical spine included Sagittal SE T1W with and without fat saturation, TSE T2, Gradient Echo T2 and MR myelogram in sagittal and coronal planes. Postcontrast SE T1W was done in transverse plane. Maximal possible hyperflexion of neck was achieved by asking the subject to first move the head as forwards as possible and then to touch the chin to the chest. The shoulders were pushed as far caudal as possible. The position was maintained by supporting the neck and shoulders with MR-compatible foam pads. The gadolinium-based MR contrast agent was used in the dose of 0.5 mmol/kg and was administered as bolus intravenously.
The following features were evaluated: (a) localized lower cervical cord atrophy, (b) asymmetric cord flattening, (c) abnormal cervical curvature, (d) loss of attachment between the posterior dural sac and subjacent lamina, (e) anterior shifting of the posterior wall of the cervical dural canal, (f) enhancing epidural component with flow voids and (g) intramedullary signal hyperintensity. The foregoing features were identified based on the definition mentioned in the literature as below.
Lower cervical cord was defined as the cord between C4 and C7. Localized cord atrophy was defined as a decrease in cord size in comparison with the normal cord above and that below the affected level on sagittal MR images and confirmed on transverse MR images.[13] Asymmetric cord flattening was evaluated on transverse MR images. Cord flattening was defined as flattening without a narrowed or obliterated adjacent subarachnoid space. An elliptic spinal cord was considered normal, a pear-shaped spinal cord was considered asymmetric cord flattening and a triangular spinal cord was considered symmetric cord flattening[13]
Cervical curvature was classified according to the principles suggested by Batzdorf and Batzdorff.[14] Cervical curvature was measured according to the relationship of the dorsal aspect of the vertebral bodies C3 through C6 to a line drawn from the dorsocaudal aspect of the body of C2 vertebra to the dorsocaudal aspect of the body of C7 vertebra. By definition, normal lordotic cervical curvature is curvature in which no part of the dorsal aspect of the vertebral bodies C3 through C6 crosses the line from C2 through C7. An abnormal (straight or kyphotic) curvature is curvature in which part or all of the dorsal aspects of the vertebral bodies C3 through C6 meet or cross through the line from C2 through C7.
For evaluating the loss of attachment between the posterior dural sac and subjacent lamina, the lamina was defined as the part of vertebra between junctions of laminae medially and laterally by a tangential line along the medial aspect of the pedicle. This was divided equally into three parts. More than one-third loss of attachment between the posterior dural sac and the subjacent lamina was considered significant.[13]
On flexion studies, anterior displacement of dural sac and appearance of epidural flow voids with enhancing epidural component posterior to the thecal sac was noted. Noncompressive intramedullary high signal intensity was considered if a patent subarachnoid space and intramedullary high signal intensity were noted.[13]
All patients in the study were advised use of cervical collar on a daily basis until arrest of the disease process. Follow-up was available for 10 patients.
Results
Twelve patients fulfilled the clinical criteria. One patient was lost to follow-up, and another patient (patient no. 12) could not undergo the flexion contrast study. Thus, neutral position MRI was done in all 11 patients and flexion contrast MRI was done in 10 patients.
Clinical features
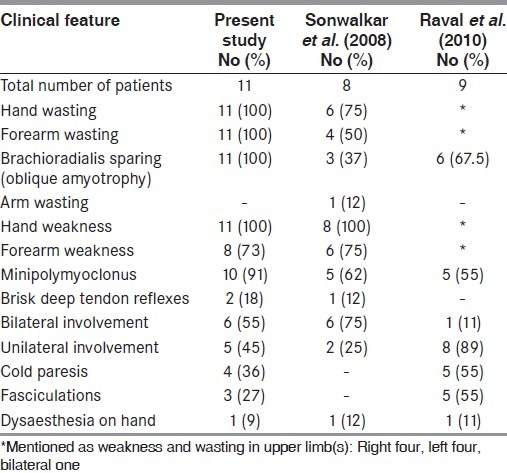
All the patients were males, with median age 23 years (range 19–50 years) at the time of clinical examination. Majority of the patients belonged to north and west India, while others were from south and north-east India. All patients were less than 25 years of age, except one patient who was 50 years old. The duration of illness at the time of presentation was 3 months to 3 years. Four patients had symptoms for less than 1 year, five had symptoms between 1 and 2 years and two had symptoms between 2 and 3 years.
All patients presented with insidious-onset progressive weakness and lower motor neuron type of wasting of one or both hands. Two (18%) patients presented with right-sided, four (36%) with left-sided and another five (46%) presented with bilateral asymmetric weakness and wasting. None of the patients had neck pain or radicular symptoms.
All (100%) had oblique amyotrophy, four (36%) cold paresis, 10 (91%) minipolymyoclonus and three (27%) had fasciculations [Table 1]. None had Horner's syndrome. Regional reflexes were variably absent. Two patients had brisk reflexes of lower limbs. Plantars were flexors and sensory examination was normal in all patients.
Table 1.
Clinical findings
Electrophysiology
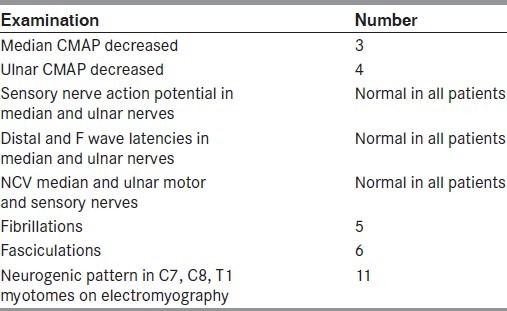
NCS and EMG were done in all the patients within 1 week of the clinical examination. Median and ulnar compound muscle action potentials (CMAPs) were reduced in the same three patients, and ulnar CMAP was reduced in another patient. However, distal latencies and F-wave latencies were within the normal range. Conduction velocities were normal in all nerves. Sensory NCS was normal in all the patients. EMG revealed features of active denervation in the form of fibrillations in five patients and fasciculations in six patients, and features of chronic denervation in the form of neurogenic changes in C7, C8 and T1 myotomes in all the patients [Table 2]. EMG of C5, C6 myotomes, namely deltoid, biceps brachii and brachioradialis, was normal.
Table 2.
Electrophysiological examination
MRI findings
Localised lower cervical cord atrophy was seen in 9/11 (82%) of the suspected cases of HD. Asymmetric cord flattening was noted in all 11 (100%) cases. Loss of cervical lordosis was seen in 10/11 (91%), loss of dural attachment in 9/10 (90%), anterior displacement of dorsal dura on flexion in 9/10 (90%) and epidural flow voids were seen in 9/10 (90%) cases. Enhancing epidural crescent in flexion was seen in all 10/10 (100%) cases who underwent dynamic study. Intramedullary hyperintensity was seen in 2/11 (18%) cases [Table 3]. Salient MRI features are shown in Figures 1–3.
Table 3.
MRI findings
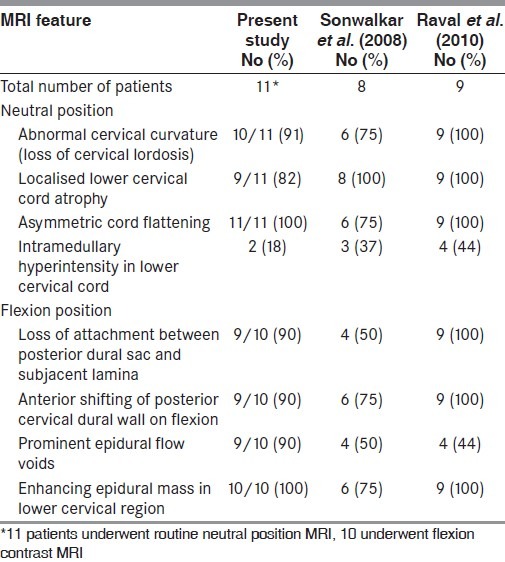
Figure 1.
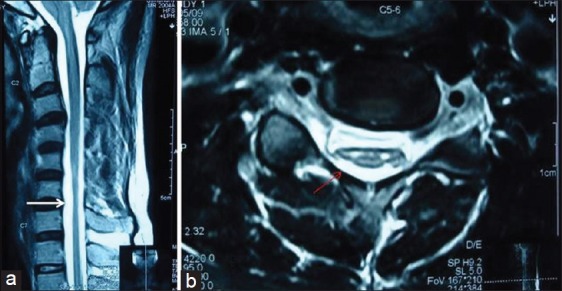
Patient no. 3 – A 25 - year - old male with asymmetric weakness and wasting both hands and forearms for 2 years and brisk deep tendon jerks – neutral position sagittal T2WI magnetic resonance imaging (MRI) showing loss of cervical lordosis and localised lower cervical cord atrophy (white arrow) (a), T2WI axial MRI showing anteroposterior triangular cord flattening with loss of attachment of dura from subjacent lamina (thin red arrow) and intramedullary hyperintensity (b)
Figure 3.
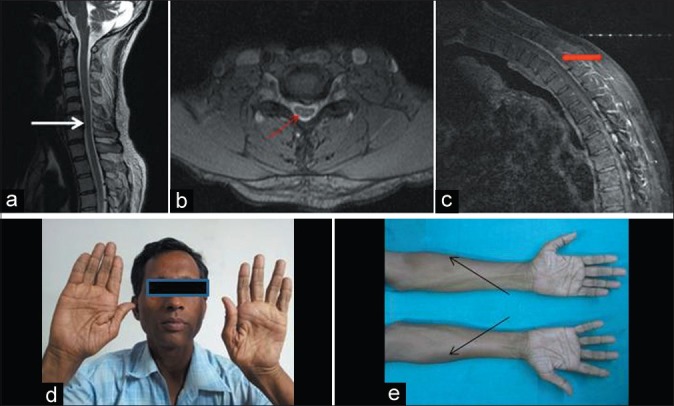
Patient no. 8 – A 50-year-old male with weakness and wasting both hands and forearms for 2 years – neutral position sagittal T2WI magnetic resonance imaging (MRI) showing loss of cervical lordosis and localised lower cervical cord atrophy (white arrow) (a), axial T2WI MRI showing anteroposterior cord flattening with loss of attachment of dura from subjacent lamina (thin red arrow) (b), flexion contrast MRI showing posterior epidural thin crescentic enhancing region (thick red arrow) (c), wasting of both hands (d) and forearms with oblique amyotrophy (black arrows) (e)
Figure 2.
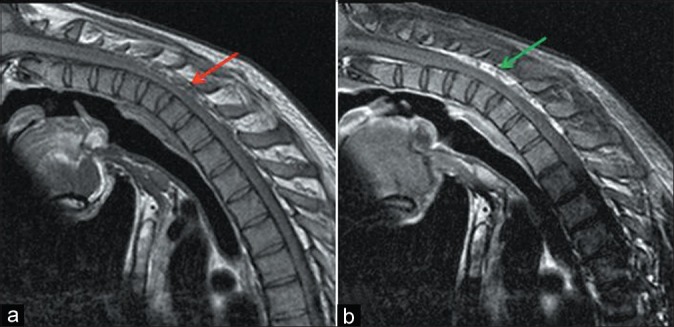
Patient no. 7 – A 23-year-old male with weakness and wasting left hand and forearm – flexion T1WI magnetic resonance imaging (MRI) showing dorsal epidural voids (red arrow) (a), flexion contrast MRI showing epidural crescentic enhancing region (green arrow) (b)
Patient aged 50 years
Clinical profile, imaging and electrophysiological findings of the patient aged 50 years are being described in detail as presentation at this age is unusual. This patient, an ex-soldier from eastern India, presented with insidious-onset weakness of the left hand for 2 years and that of the right hand for 1.5 years. He had cold paresis in both the hands in his youth. He denied weakness or wasting of hands at the time. On examination, he had wasting both hands and forearms, with oblique amyotrophy and minipolymyoclonus in both hands. There were no sensory or long tract signs. CMAPs were decreased in both median and ulnar nerves. Sensory NCS was normal. EMG was neurogenic in both abductor pollicis brevis and extensor digitorum communis, and left abductor digiti minimi and flexor carpi ulnaris. EMG of brachioradialis was normal. MRI showed loss of cervical lordosis, localised lower cervical cord atrophy, asymmetric cord flattening, loss of dural attachment, anterior displacement of dorsal dura, epidural flow voids and enhancing epidural crescent in flexion [Figure 3].
Discussion
HD is a benign disorder with a stationary stage after a progressive course for up to 6 or fewer years. It occurs mainly in young males between the ages of 15 and 25 years.[6,10] All our patients were younger than 25 years, except one patient who was 50 years old with a disease duration of 2 years. It is circumstantially unlikely that the patient would have had significant disability earlier in life as he was able to handle weapons with ease as a soldier. Such late-onset HD has not been reported earlier. We postulate that this patient perhaps had milder disease in his youth, with secondary worsening later in life. That this patient had HD is proved by electrophysiological examination and MRI findings [Figure 3].
The clinical features include insidious onset, predominantly unilateral upper extremity weakness and atrophy, cold paresis and no sensory or pyramidal tract involvement.[2,8,10,15] The amyotrophy is unilateral in most patients, asymmetrically bilateral in some and rarely symmetric in others.[15] Six of our patients presented with unilateral and five patients presented with bilateral asymmetric weakness and wasting of the hands and forearms. The clinical findings in our study are more elaborate, with a greater proportion of the patients showing the characteristic abnormalities of HD than in the studies by Sonwalkar et al.[2] and Raval et al.[15] This is perhaps due to the fact that this study was conducted by a neurologist, wherein greater emphasis was placed on the pattern of clinical involvement and to correlate the same with electrophysiology and dynamic MRI of cervical spine.
Although Hirayama et al. first reported this disease in 1959, pathologic study was not possible until 1982 because of its benign course.[9,16] At pathologic examination, shrinkage, necrosis and gliosis were found in the anterior horns of the spinal cord from C-5 to T-1, particularly marked at C-7 and C-8.[9] However, the underlying pathogenesis of the disease was not known until 1987 when Kikuchi et al. first proposed tight dural canal as the underlying predisposing factor.[10,17] Atopy and elevated serum IgE level have been postulated to be precipitating factors in HD.[18,19]
An imbalanced growth resulting in disproportional length between the patient's vertebral column and spinal canal contents has been suggested as the cause of a tight dural sac and anterior displacement of posterior dural wall when the neck is flexed.[5,10,17,20] The different growth rates between male and female patients have been proposed by Toma et al. to be the factor related to the male preponderance of HD.[21] The disproportionate shortening of the dural sac is perhaps accentuated during the juvenile growth spurt, explaining the preponderance in adolescence.[21]
In normal spine, the spinal dura mater is a loose sheath consisting of several transverse folds that is anchored in the vertebral canal by nerve roots and by its attachment to the periosteum at foramen magnum and dorsal surfaces of C2-C3 vertebrae above and at coccyx below, which compensates for increased length of cervical canal in flexion.[1,8,9] The difference in length between extension and flexion from T-1 to top of atlas is 1.5 cm at the anterior wall and 5 cm at the posterior wall.[22] In health, the dura remains in close contact with the walls of the spinal canal without anterior displacement in flexion.[8]
However, in patients with HD, on neck flexion, the tight dural sac cannot compensate for the increased length of posterior wall, which causes anterior shifting of the posterior dural wall and consequent compression of the cord against the posterior margin of the adjacent vertebral bodies. This compression may cause microcirculatory disturbances in the territory of anterior spinal artery in the lower cervical spinal cord.[1,9] The chronic circulatory disturbance resulting from repeated or sustained flexion of neck may produce necrosis of the anterior horns resulting in gliosis and localized cord atrophy at the lower cervical region.[1,23] Vulnerability of the anterior horn to ischemia accounts for the atrophy that follows.[1] Patients with severe cervical cord compression in flexion may also develop extensive cord injury beyond the anterior horns.[24] Two patients in our study had brisk deep tendon jerks likely due to cord injury beyond the anterior horns.
In patients with HD, conventional radiographic studies of the cervical spine may show loss of cervical lordosis.[20] Myelography is difficult to perform, because it is not easy to retain the contrast medium in the cervical subarachnoid space when the neck is flexed.[8] MRI with flexion contrast study is the gold standard of diagnosis.
The findings reported more frequently are asymmetrical or symmetrical atrophy of lower cervical cord, prominence and enhancement of posterior epidural venous plexus on flexion studies and anterior shifting of posterior dural sac on flexion. Loss of attachment between the posterior dural sac and subjacent lamina on neutral position, anterior shifting of the posterior wall of cervical dural canal, enhancing epidural crescentic mass in the lower cervical and thoracic region and prominent posterior epidural flow voids suggestive of dilated epidural venous plexus on flexion studies are reported as highly suggestive for the diagnosis of HD.[1,5,8,13,20,23]
Among the imaging features discussed above, localized lower cervical cord atrophy, asymmetric cord flattening and loss of attachment have an accuracy of 80% in identification of the disease; loss of attachment has been proposed as the most valuable finding for diagnosing HD in the neutral position.[13,25] In our study, localised lower cervical cord atrophy and loss of cervical lordosis were seen in over 80% of the cases of HD. In earlier studies, Pradhan and Gupta found focal cord atrophy in neutral neck position on MRI in 100% of their cases,[5] while Hirayama and Tokumaru have reported this in only 50% of their cases.[7] Asymmetric cord flattening was noted in all our cases. Loss of dural attachment, anterior displacement of dorsal dura and epidural flow voids on flexion were seen in 90% of the cases. Enhancing epidural crescent in flexion was seen in all cases who underwent dynamic study. Our findings are in greater conformity with the findings of Raval et al.[15] than that of Sonwalkar et al.[2] Such variation is likely due to the difference in patient characteristics and extent of disease.
Compression of spinal cord by tight dura is probably the most important pathogenetic factor. Hirayama et al. in their studies on 73 patients revealed that dynamic compression of the lower cervical cord due to forward displacement of the posterior cervical dural sac and spinal cord on neck flexion was confined to an early and progressive stage of the disease.[10] However, Pradhan and Gupta from their study on 35 patients showed that both spinal cord and posterior dura mater move forward independently under a longitudinal stretch, and that the forward displacement of the dura mater was not responsible for the cord compression.[26]
Contrariwise, in one study, forward shifting of the posterior cervical dural sac without associated cord compression was depicted in 46% of the normal subjects upon flexion; the intrinsic compensatory mechanism is perhaps inadequate in patients of HD leading to cord compression. The authors concluded that depicting of forward shifting of posterior dural sac alone on flexion cannot reliably diagnose HD.[27] Pradhan and Gupta have observed forward movement of the spinal cord even in normal individuals during flexion.[26] An absence of forward displacement in a later and nonprogressive stage of the disease suggests that the dynamic compression has pathogenic significance.[10] Intriguingly, the patient aged 50 years in this study had demonstrable forward dural shift [Figure 3], which is in conformity with clinically progressive stage of the disease in this patient at this age.
Venous congestion in flexion might also play an additional role in determining spinal cord ischemic changes.[28] The proposed mechanisms for venous engorgement seen on flexion studies is due to increased flow to posterior internal vertebral venous plexus resulting from the negative pressure in posterior spinal canal as a result of anterior shifting of dural canal and decrease in drainage of jugular veins impeding venous return of internal vertebral venous plexus.[25] Additionally, the compressed anterior internal vertebral venous plexus caused by anterior displacement of dural canal increases the burden of posterior internal vertebral venous plexus leading to its distension.[8,25] Pradhan and Gupta have postulated that the overstretched posterior dura mater that forcefully moves forward during neck flexion results in negative space behind, which appears on MRI as congested epidural space.[26] Posterior dura mater came forward in all their patients, resulting in a dilated crescent-shaped epidural space.[26] Dilated crescent-shaped epidural space was seen in all our patients who underwent dynamic study.
Even though the forward motion of the posterior dura mater was known earlier, the MRI features of the disease were first described in detail by Pradhan and Gupta in 1997,[5] and the same findings were reproduced by Hirayama and Tokumaru in 2000.[7] Pradhan and Gupta had elaborately demonstrated forward displacement and flattening of cord against bodies of lower cervical vertebrae in flexed neck position.[26] We feel that asymmetric cord flattening on transverse neutral MRI is the most important clue to diagnosis of HD; anterior displacement of dura and enhancing posterior epidural compartment have high predictivity for diagnosis of HD.
HD patients have an increased range of cervical spine flexion, which contributes to the pathophysiological changes.[29] Although HD is self-limiting, early diagnosis and therapeutic intervention in the form of cervical collar therapy to prevent neck flexion may minimize the functional disability of young patients.[8,10,30] Cervical collar therapy induces a premature arrest of this disease. Improvement is expected in patients who have a shorter duration of illness and have mild cord atrophy in neutral neck position.[30]
Tokumaru and Hirayama, in 1992, reported the use of cervical collar therapy for nonprogressive juvenile spinal muscular atrophy of the distal upper limb.[31] Pradhan and Gupta in 2001 recommended cervical collar during the acute progressive stage of the disease.[26] This was followed by a report by Tokumaru and Hirayama in 2001 on the role of cervical collar therapy in 38 cases of HD.[30] They found that all the patients in the treatment group showed no further progression after introduction of cervical collar treatment. Because the progressive stage is expected to cease in a few years, application of a cervical collar for 3–4 years generally has been advocated.[30] In our study, five patients who were compliant with cervical collar use had subjective feeling of arrest of disease progression after 2–3 months, while others without collar use felt slowing of progression only after 12–18 months of presentation. However, as power and wasting were not objectively measured and monitored, no firm conclusions from this study can be drawn on the role of collar therapy in HD.
While application of cervical collar is believed to prevent progression of the disease in early stages, duraplasty, anterior cervical decompression and reconstructions with tendon transfers have yielded encouraging results in selected patients.[30,32,33]
Although a diagnosis of HD is straightforward at flexion MR imaging, the challenge for radiologists is how to identify this condition on routine nonflexion MR studies.[8] To ensure that this diagnosis is not missed in patients presenting with focal hand wasting, flexion contrast MRI should be done if the routine MRI otherwise looks normal and if MND has been excluded. Several conditions like syringomyelia, MND, cervical spondylotic myelopathy, spinal cord tumor and traumatic myelopathy may cause localized amyotrophy of the distal arm, and these should be excluded first by imaging modalities.[21]
From our cases, we found that asymmetry is one of the most characteristic findings of this disease, both clinically and radiologically. Thus, in cases of adolescent onset of distal upper limb weakness, the finding of asymmetric cord atrophy on routine nonflexion MR studies, especially at the lower cervical cord, should raise the suspicion of HD. When this finding is seen, flexion MR study should be performed to confirm the diagnosis.[2,8]
NCS of median and ulnar nerves, apart from decreased CMAP in some cases, was normal. EMG of the upper cervical myotomes was normal while that of the lower cervical myotomes showed active and/or chronic denervation in all patients. While the clinical–electrophysiological profile was suggestive of anterior horn cell disease, dynamic flexion MRI of the cervical spine supports the theory of forward dural shift, causing lower cervical cord compression and ischemia of anterior horns. Our study supports the hypothesis that HD differs from classical MND or its variants involving the distal upper limbs in young males by being a chronic ischemic myelopathy rather than a degenerative condition.
Monomelic amyotrophy has been traditionally subclassified under the idiopathic group of MND seen in India.[34] Young patients with unilateral upper limb atrophy have been classically labeled brachial MMA, which represent cases of HD. Gourie-Devi et al. found in their study, from 1977 through 1981, 13 patients with single upper limb atrophy. The clinical description of these patients with single upper limb atrophy reported by Gourie-Devi et al. is similar to the clinical profile of HD described by Hirayama et al.[4,10] Recently, Pradhan et al. in their series of 106 patients of HD seen from 1992 to 2008 reported around 10% of all the patients to have bilaterally symmetric involvement, a severe form of classic HD, which remains undiagnosed due to a common notion that it is a unilateral or grossly asymmetric disease. These cases of brachial monomelic amyotrophy represent HD.[35] Need for dedicated neuroimaging of the cervical spine in young adolescents with hand wasting cannot be overemphasized as early institution of cervical collar therapy may be beneficial in patients of HD.
Conclusion
HD, a rare disease affecting young men in the second to third decades of life, is characterized by insidious onset and slowly progressive course followed few years later by static phase of unilateral or asymmetric atrophy of the hand(s) and forearm(s) with sparing of the brachioradialis, characterized as oblique amyotrophy. It is thought to be a cervical flexion myelopathy related to repeated movements of the neck causing chronic microcirculatory changes in the territory of the anterior spinal artery supplying the anterior horns of the lower cervical cord. While dynamic contrast MRI is characteristic of HD, routine MRI has a high predictive value for diagnosis. Prompt diagnosis is important to institute early cervical collar therapy. Young adolescents with focal upper limb wasting should be evaluated to exclude HD or MMA, a benign condition amenable to collar therapy.
Footnotes
Source of Support: Nil
Conflict of Interest: Nil
References
- 1.Hirayama K. Non-progressive juvenile spinal muscular atrophy of the distal upper limb [Hirayama's disease] In: De Jong JM, editor. Handbook of clinical neurology. Vol. 15. Amsterdam, the Netherlands: Elsevier; 1991. pp. 107–20. [Google Scholar]
- 2.Sonwalkar HA, Shah RS, Khan FK, Gupta AK, Bodhey NK, Vottath S, et al. Imaging features in Hirayama disease. Neurol India. 2008;56:22–6. doi: 10.4103/0028-3886.39307. [DOI] [PubMed] [Google Scholar]
- 3.Biondi A, Dormont D, Weitzner I, Jr, Bouche P, Chaine P, Bories J. MR imaging of the cervical cord in juvenile amyotrophy of distal upper extremity. AJNR Am J Neuroradiol. 1989;10:263–8. [PMC free article] [PubMed] [Google Scholar]
- 4.Gourie-Devi M, Suresh TG, Shankar SK. Monomelic amyotrophy. Arch Neurol. 1984;41:388–94. doi: 10.1001/archneur.1984.04050160050015. [DOI] [PubMed] [Google Scholar]
- 5.Pradhan S, Gupta RK. Magnetic resonance imaging in juvenile asymmetric segmental spinal muscular atrophy. J Neurol Sci. 1997;146:133–8. doi: 10.1016/s0022-510x(96)00296-1. [DOI] [PubMed] [Google Scholar]
- 6.Tashiro K, Kikuchi S, Itoyama Y, Tokumaru Y, Sobue G, Mukai E, et al. Nationwide survey of juvenile muscular atrophy of distal upper extremity (Hirayama disease) in Japan. Amyotroph Lateral Scler. 2006;7:38–45. doi: 10.1080/14660820500396877. [DOI] [PubMed] [Google Scholar]
- 7.Hirayama K, Tokumaru Y. Cervical dural sac and spinal cord in juvenile muscular atrophy of distal upper extremity. Neurology. 2000;54:1922–6. doi: 10.1212/wnl.54.10.1922. [DOI] [PubMed] [Google Scholar]
- 8.Chen CJ, Chen CM, Wu CL, Ro LS, Chen ST, Lee TH. Hirayama Disease: MR Diagnosis. AJNR Am J Neuroradiol. 1998;19:365–8. [PMC free article] [PubMed] [Google Scholar]
- 9.Hirayama K, Tomonaga M, Kitano K, Yamada T, Kojima S, Arai K. Focal cervical poliopathy causing juvenile muscular atrophy of distal upper extremity: A pathological study. J Neurol Neurosurg Psychiatry. 1987;50:285–90. doi: 10.1136/jnnp.50.3.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hirayama K. Juvenile muscular atrophy of unilateral upper extremity (Hirayama disease) – half-century progress and establishment since its discovery. Brain Nerve. 2008;60:17–29. [PubMed] [Google Scholar]
- 11.Kikuchi S, Tashiro K, Kitagawa K, Iwasaki Y, Abe H. A mechanism of juvenile muscular atrophy localized in the hand and forearm (Hirayama's disease): Flexion myelopathy with tight dural canal in flexion [in Japanese] Clin Neurol (Tokyo) 1987;27:412–9. [PubMed] [Google Scholar]
- 12.Tokumaru Y, Hirayama K. Anterior shift of posterior lower cervical dura mater in patients with juvenile muscular atrophy of unilateral upper extremity. Clin Neurol (Tokyo) 1989;29:1237–43. [PubMed] [Google Scholar]
- 13.Chen CJ, Hsu HL, Tseng YC, Lyu RK, Chen CM, Huang YC, et al. Hirayama flexion myelopathy: Neutral-position MR imaging findings—importance of loss of attachment. Radiology. 2004;231:39–44. doi: 10.1148/radiol.2311030004. [DOI] [PubMed] [Google Scholar]
- 14.Batzdorf U, Batzdorff A. Analysis of cervical spine curvature in patients with cervical spondylosis. Neurosurgery. 1988;22:827–36. doi: 10.1227/00006123-198805000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Raval M, Kumari R, Dung-Dung AA, Guglani B, Gupta N, Gupta R. MRI findings in Hirayama disease. Neuroradiology. 2010;20:245–9. doi: 10.4103/0971-3026.73528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hirayama K, Toyokura Y, Tsubaki T. Juvenile muscular atrophy of unilateral upper extremity: A new clinical entity. Psychiatr Neurol Jpn. 1959;61:2190–7. doi: 10.1212/wnl.13.5.373. [DOI] [PubMed] [Google Scholar]
- 17.Kikuchi S, Tashiro K, Kitagawa K, Iwasaki Y, Abe H. A mechanism of juvenile muscular atrophy localized in the hand and forearm (Hirayama's disease): Flexion myelopathy with tight dural canal in flexion. Rinsho Shinkeigaku. 1987;27:412–9. [PubMed] [Google Scholar]
- 18.Kira J, Ochi H. Juvenile muscular atrophy of the distal upper limb (Hirayama disease) associated with atopy. J Neurol Neurosurg Psychiatry. 2001;70:798–801. doi: 10.1136/jnnp.70.6.798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen TH, Hung CH, Hsieh TJ, Lu SR, Yang SN, Jong YJ. Symmetric atrophy of bilateral distal upper extremities and hyperIgEaemia in a male adolescent with Hirayama disease. J Child Neurol. 2010;25:371–4. doi: 10.1177/0883073809336876. [DOI] [PubMed] [Google Scholar]
- 20.Mukai E, Sobue I, Muto T, Takahashi A, Goto S. Abnormal radiological findings on juvenile-type distal and segmental muscular atrophy of upper extremities. Rinsho Shinkeigaku. 1985;25:620–6. [PubMed] [Google Scholar]
- 21.Toma S, Shiozawa Z. Amyotrophic cervical myelopathy in adolescence. J Neurol Neurosurg Psychiatry. 1995;58:56–64. doi: 10.1136/jnnp.58.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bland JH. Basic anatomy. In: Bland JH, editor. Disorders of the Cervical Spine: Diagnosis and Medical Management. 2nd ed. Philadelphia, Pa: Saunders; 1994. pp. 41–70. [Google Scholar]
- 23.Schroder R, Keller E, Flacke S, Schmidt S, Pohl C, Klockgether T, et al. MRI findings in Hirayama's disease: Flexion-induced cervical myelopathy or intrinsic motor neuron disease? J Neurol. 1999;246:1069–74. doi: 10.1007/s004150050514. [DOI] [PubMed] [Google Scholar]
- 24.Sakai K, Ono K, Okamoto Y, Murakami H, Yamada M. Cervical flexion myelopathy in a patient showing apparent long tract signs: A severe form of Hirayama disease. Joint Bone Spine. 2011;78:316–8. doi: 10.1016/j.jbspin.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 25.Mukai E, Matsuo T, Muto T, Takahashi A, Sobue I. Magnetic resonance imaging of juvenile-type distal and segmental muscular atrophy of upper extremities. Clin Neurol (Tokyo) 1987;27:99–107. [PubMed] [Google Scholar]
- 26.Pradhan S, Gupta RK. Cervical dural sac and spinal cord in juvenile muscular atrophy of distal upper extremity. Neurology. 2001;56:575. doi: 10.1212/wnl.56.4.575. [DOI] [PubMed] [Google Scholar]
- 27.Lai V, Wong YC, Poon WL, Yuen MK, Fu YP, Wong OW. Forward shifting of posterior dural sac during flexion cervical magnetic resonance imaging in Hirayama disease: An initial study on normal subjects compared to patients with Hirayama disease. Eur J Radiol. 2011;80:724–8. doi: 10.1016/j.ejrad.2010.07.021. [DOI] [PubMed] [Google Scholar]
- 28.Ciceri EF, Chiapparini L, Erbetta A, Longhi L, Cicardi B, Milani N, et al. Angiographically proven cervical venous engorgement: A possible concurrent cause in the pathophysiology of Hirayama's myelopathy. Neurol Sci. 2010;31:845–8. doi: 10.1007/s10072-010-0405-3. [DOI] [PubMed] [Google Scholar]
- 29.Xu X, Han H, Gao H, Hou C, Fan D, Fu Y, et al. The increased range of cervical flexed motion detected by radiographs in Hirayama disease. Eur J Radiol. 2011;78:82–6. doi: 10.1016/j.ejrad.2010.08.012. [DOI] [PubMed] [Google Scholar]
- 30.Tokumaru Y, Hirayama K. Cervical collar therapy for juvenile muscular atrophy of distal upper extremity (Hirayama disease): Results from 38 cases. Rinsho Shinkeigaku. 2001;41:173–8. [PubMed] [Google Scholar]
- 31.Tokumaru Y, Hirayama K. A cervical collar therapy for nonprogressive juvenile spinal muscular atrophy of the distal upper limb (Hirayama's disease) Clin Neurol (Tokyo) 1992;32:1102–6. [PubMed] [Google Scholar]
- 32.Chiba S, Yonekura K, Nonaka M, Imai T, Matumoto H, Wada T. Advanced Hirayama disease with successful improvement of activities of daily living by operative reconstruction. Intern Med. 2004;43:79–81. doi: 10.2169/internalmedicine.43.79. [DOI] [PubMed] [Google Scholar]
- 33.Lin MS, Kung WM, Chiu WT, Lyu RK, Chen CJ, Chen TY. Hirayama disease. J Neurosurg Spine. 2010;12:629–34. doi: 10.3171/2009.12.SPINE09431. [DOI] [PubMed] [Google Scholar]
- 34.Velmurugendran CU, Srinivasan AV. Motor neuron disease in tropics. In: Chopra JS, Sawhney IM, editors. Neurology in tropics. New Delhi: Elsevier, Division of Reed Elsevier India; 2004. pp. 437–44. [Google Scholar]
- 35.Pradhan S. Bilaterally symmetric form of Hirayama disease. Neurology. 2009;72:2083–9. doi: 10.1212/WNL.0b013e3181aa5364. [DOI] [PubMed] [Google Scholar]


