Abstract
Objective:
To investigate medical decision-making capacity (MDC) in patients with acute traumatic brain injury (TBI) across a range of injury severity.
Methods:
We evaluated MDC cross-sectionally 1 month after injury in 40 healthy controls and 86 patients with TBI stratified by injury severity (28 mild [mTBI], 15 complicated mild [cmTBI], 43 moderate/severe [msevTBI]). We compared group performance on the Capacity to Consent to Treatment Instrument and its 5 consent standards (expressing choice, reasonable choice, appreciation, reasoning, understanding). Capacity impairment ratings (no impairment, mild/moderate impairment, severe impairment) on the consent standards were also assigned to each participant with TBI using cut scores referenced to control performance.
Results:
One month after injury, the mTBI group performed equivalently to controls on all consent standards. In contrast, the cmTBI group was impaired relative to controls on the understanding standard. No differences emerged between the mTBI and cmTBI groups. The msevTBI group was impaired on almost all standards relative to both control and mTBI groups, and on the understanding standard relative to the cmTBI group. Capacity compromise (mild/moderate or severe impairment ratings) on the 3 clinically complex standards (understanding, reasoning, appreciation) occurred in 10%–30% of patients with mTBI, 50% of patients with cmTBI, and 50%–80% of patients with msevTBI.
Conclusions:
One month following injury, MDC is largely intact in patients with mTBI, but is impaired in patients with cmTBI and msevTBI. Impaired MDC is prevalent in acute TBI and is strongly related to injury severity.
Clinical and ethical issues concerning a patient's medical decision-making capacity (MDC) frequently arise following traumatic brain injury (TBI). Patients with TBI often abruptly experience impaired MDC at time of injury, and this impairment can continue during rehabilitation and afterwards.1 Such patients simultaneously face complex, ongoing medical decisions concerning shunting, orthopedic surgery, rehabilitation programming, and neuropsychiatric treatment.1,2 Thus, following TBI, clinicians must rapidly determine whether a patient retains MDC, and if not, assess over time whether the patient has regained MDC.
The scientific literature provides clinicians little guidance in this regard. Previous studies by our group using a small sample of controls and patients with moderate/severe TBI (msevTBI)1,3 found impairments at time of injury in complex consent abilities of understanding, reasoning, and appreciation, with partial recovery of these abilities at 6 months. Cognitive abilities of verbal memory, working memory, and executive function were associated with acute impairment and partial recovery of MDC in this msevTBI group.3
The present study expands on this prior work by investigating MDC in patients with TBI with a wider range of injury severity (mild TBI [mTBI], complicated mild TBI [cmTBI], and msevTBI). We hypothesized that 1 month following acute injury, mTBI and complicated mild TBI groups would perform below controls, but better than the msevTBI group, on the 3 complex consent abilities. We further hypothesized that the cmTBI group would perform below the mTBI group on these 3 abilities. Finally, we hypothesized that the msevTBI group would show deficits on all consent standards relative to other groups.
METHODS
Participants.
Healthy adult controls and patients with recent TBI were prospectively enrolled between October 2007 and March 2011 as part of an ongoing NIH-funded longitudinal study investigating MDC in patients with TBI (Recovery of Lost Abilities in Medical Decision-Making Study [RECLAIMED]). The number of accrued participants with complete baseline data on the Capacity to Consent to Treatment Instrument (CCTI) determined the sample size for this study.
Eighty-six patients with TBI were recruited through the Emergency Department, Neurosurgery inpatient service, and Spain Rehabilitation Center at the School of Medicine, University of Alabama at Birmingham (UAB). Mean time from date of brain injury to date of study evaluation (baseline) was 32.4 days (SD = 12.2 days). All TBI participants were assigned a study diagnosis and injury severity level by a board-certified rehabilitation neuropsychologist (T.A.N.) using diagnostic criteria from the TBI Model Systems.4 These criteria define TBI as damage to brain tissue caused by an external mechanical force as evidenced by loss of consciousness due to brain trauma, post-traumatic amnesia (PTA), or objective neurologic findings that can be reasonably attributed to TBI on physical examination or mental status criteria established by the examination.5 All participants in the study had closed head injuries; individuals with penetrating brain injuries (e.g., gunshot wound) were excluded.
mTBI was characterized by one or more of the following criteria: 1) an initial Glasgow Coma Scale (GCS) score of 13 or higher,6 2) PTA, if extant, not exceeding 24 hours, or 3) any loss of consciousness (LOC) not to exceed 30 minutes. In addition, mTBI patients had no evidence of structural brain changes on conventional CT scans.
cmTBI was characterized like mTBI except that patients also had evidence of structural brain changes (such as contusions, subdural hematoma, or diffuse axonal injury) on cranial MRI scan or CT scan.
msevTBI was characterized by one or more of the following criteria: 1) initial GCS score of 12 or less7,8 or 2) PTA lasting more than 24 hours,6,9,10 and 3) evidence of structural brain changes on cranial MRI scan or CT scan. Initial GCS scores were not available for intubated msevTBI patients.
Patients were excluded from the study if they had a history of alcohol or drug abuse (established by patient or family report) or a history of pre-existing CNS neurologic diagnosis (including prior msevTBI), severe psychiatric disturbance (e.g., bipolar disorder, schizophrenia, major depression [but allowing mild depression]), or a developmental disorder. Individuals with a history of a prior mTBI were not excluded as long as the prior injury occurred at least 1 year prior to enrollment.
Forty control participants were recruited through local advertisements. Controls were selected to match TBI participants on demographic variables of age, gender, ethnicity, and years of education. Controls were without diseases or conditions that could potentially affect cognition, including psychiatric disturbance (except mild depression), substance abuse, cerebrovascular disease, or other neurologic diseases. None of the control participants were taking medications known to affect cognition.
Procedures.
All participants completed the study's consent capacity measure and a comprehensive battery of neuropsychological and psychological measures. Results from the neuropsychological test battery will be reported in a separate article.
Standard protocol approvals, registrations, and patient consents.
Written informed consent was obtained from each participant or in some cases their legal representative or a family member. The UAB institutional review board approved this study.
Measures.
Consent capacity measure.
Treatment consent capacity was assessed using the CCTI,11 a conceptually based, reliable, and valid psychometric instrument for assessing MDC in both healthy and cognitively impaired adults.11,12 The CCTI comprises 2 clinical vignettes that each present a hypothetical medical problem (A: neoplasm and B: cardiovascular disease) and symptoms, and 2 treatment alternatives with associated risks and benefits. Participants then answer standardized questions designed to assess each of the following 4 core consent standards derived from legal and medical literature11,13:
S1: simply expressing a treatment choice (expressing choice)
S3: appreciating the personal consequences of a treatment choice (appreciation)
S4: providing rational reasons for a treatment choice (reasoning)
S5: understanding the treatment situation, available treatment choices, and respective risks/benefits of the treatment choices (understanding)
A fifth standard [S2] measures the ability to make a reasonable treatment choice.11 [S2] is not a clinically accepted consent standard because of concerns about the potential arbitrariness of the operative term “reasonable.” Therefore, we treat [S2] (reasonable choice) as experimental and use brackets to distinguish it from the 4 core consent standards.
The 2 CCTI vignettes were presented in both oral and written formats to all participants. After presentation of a vignette, the written format was removed and participants responded to oral questions tapping the 5 CCTI standards. CCTI administration and scoring were performed by trained research assistants according to detailed and well-operationalized criteria.11 Each participant's responses to the CCTI questions were audiotaped and subsequently transcribed to ensure a high level of scoring accuracy. Research assistants were blinded to participants' group status and the study investigators were not involved in either administration or scoring of the CCTI.
Data analysis.
Demographic variables were analyzed using one-way analysis of variance (age, years of education, GCS score, and Galveston Orientation and Amnesia Test [GOAT] score14) or χ2 tests (gender and race). Group comparisons on the CCTI consent standards were performed using one-way analysis of variance (S3–S5), Kruskal-Wallis (S1), or χ2 tests ([S2]).
We also examined capacity impairment ratings (no impairment, mild/moderate impairment, or severe impairment) for each patient with TBI using psychometric cut scores derived from our control group performance. For S3, S4, and S5, a no impairment rating was defined as a score <1.5 SD below the control group mean on that standard; a mild/moderate impairment rating was defined as a score >1.5 SD but ≤2.5 SD below the control group mean; and a severe impairment rating was defined as a score >2.5 SD below the control group mean. For the interval level variable (S1), which has a maximum possible score of 4, a no impairment rating was defined as a score of 4, mild/moderate impairment rating as a score of 3, and a severe impairment rating as a score of ≤2. For [S2], a dichotomous variable, the only 2 possible impairment ratings were no impairment (1) and severe impairment (0).
Assignment of psychometrically derived impairment ratings is useful in categorizing level of decisional impairment and has been used successfully in previous capacity studies.11,15,16 While these impairment ratings have scientific and clinical value, they are used experimentally for scientific purposes and are not representative of participants' legal or clinical competency status.
All statistical analyses were performed using SPSS 13.0.17 The significance level for all analyses was p < 0.05. All significant omnibus analyses were followed up with Games-Howell post hoc tests (for S3–S5), and a Mann-Whitney U test (S1) with a Holm adjustment to adjust for multiple comparisons.
RESULTS
Demographics.
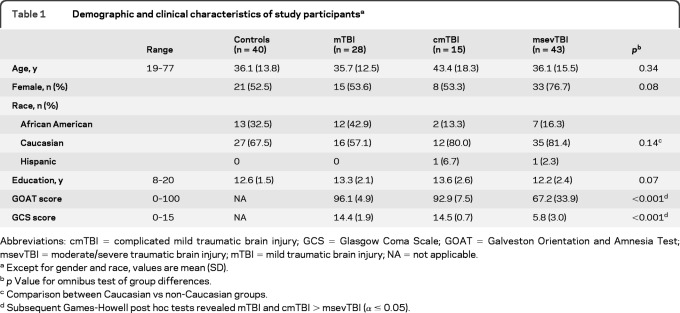
Table 1 presents demographic variables for all participants and injury severity data for TBI participants. Groups did not differ from each other in age, years of education, or gender or racial distributions. As expected, the msevTBI group had significantly lower GCS and GOAT scores than both the mTBI and cmTBI groups.
Table 1.
Demographic and clinical characteristics of study participantsa
Abbreviations: cmTBI = complicated mild traumatic brain injury; GCS = Glasgow Coma Scale; GOAT = Galveston Orientation and Amnesia Test; msevTBI = moderate/severe traumatic brain injury; mTBI = mild traumatic brain injury; NA = not applicable.
Except for gender and race, values are mean (SD).
p Value for omnibus test of group differences.
Comparison between Caucasian vs non-Caucasian groups.
Subsequent Games-Howell post hoc tests revealed mTBI and cmTBI > msevTBI (α ≤ 0.05).
Capacity performance results.
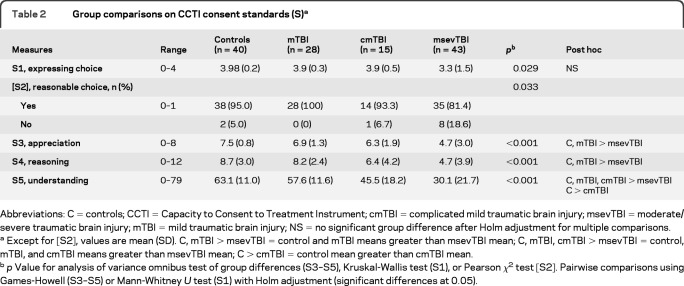
Table 2 displays CCTI consent standard performance across groups. The control and mTBI groups did not differ on any consent standards. This finding was unexpected, as we had hypothesized that 1 month after injury the mTBI group would perform below controls on the 3 clinically complex standards of appreciation (S3), reasoning (S4), and understanding (S5). Partially consistent with hypothesis, the cmTBI group performed below controls on the understanding standard, but not on the appreciation or reasoning standards.
Table 2.
Group comparisons on CCTI consent standards (S)a
Abbreviations: C = controls; CCTI = Capacity to Consent to Treatment Instrument; cmTBI = complicated mild traumatic brain injury; msevTBI = moderate/severe traumatic brain injury; mTBI = mild traumatic brain injury; NS = no significant group difference after Holm adjustment for multiple comparisons.
Except for [S2], values are mean (SD). C, mTBI > msevTBI = control and mTBI means greater than msevTBI mean; C, mTBI, cmTBI > msevTBI = control, mTBI, and cmTBI means greater than msevTBI mean; C > cmTBI = control mean greater than cmTBI mean.
p Value for analysis of variance omnibus test of group differences (S3–S5), Kruskal-Wallis test (S1), or Pearson χ2 test [S2]. Pairwise comparisons using Games-Howell (S3–S5) or Mann-Whitney U test (S1) with Holm adjustment (significant differences at 0.05).
As anticipated, controls performed better than the msevTBI group on the 3 complex consent standards. These latter results replicated baseline findings from our prior study.1 In addition, the mTBI group performed better than the msev group on these 3 standards. In contrast, the cmTBI group performed better than the msevTBI group only on the understanding standard.
The mTBI and cmTBI groups did not differ statistically on any consent standard, although the cmTBI group raw scores fell below mTBI group scores on the 3 complex standards.
After correcting for multiple comparisons, there were no statistically significant group differences on the elementary standard of expressing choice (S1), although the control and msevTBI group difference showed a very strong trend suggesting probable impairment (p = 0.054). For reasonable choice [S2], there was an omnibus effect with the msevTBI group having the greatest number of impaired responses.
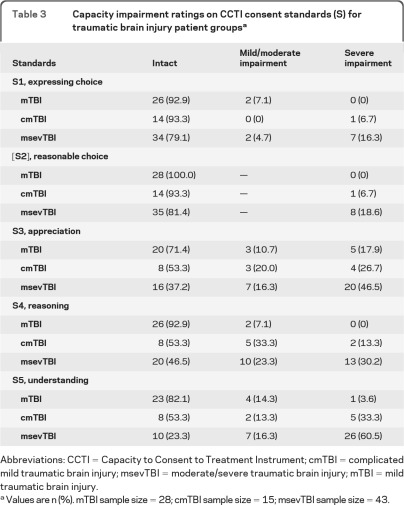
Capacity impairment ratings.
Table 3 presents individual capacity impairment ratings (intact, mild/moderate impairment, severe impairment) by TBI subgroup across the 5 CCTI consent standards. mTBI patients showed high proportions of intact ratings across the standards (>90% for S1, [S2], and S4, >80% for S5, and >70% for S3). In contrast, cmTBI group patients showed high proportions of intact ratings for simple standards (>90% for S1 and [S2]), but much lower proportions of intact ratings for the 3 complex standards (53% for S3, S4, and S5). Not surprisingly, the msevTBI group showed the lowest proportions of intact ratings across the 5 standards (79% for S1, 81% for [S2], 37% for S3, 47% for S4, and 23% for S5). These findings were consistent with our hypotheses that TBI patients' performance and impairment ratings would be associated with injury severity.
Table 3.
Capacity impairment ratings on CCTI consent standards (S) for traumatic brain injury patient groupsa
Abbreviations: CCTI = Capacity to Consent to Treatment Instrument; cmTBI = complicated mild traumatic brain injury; msevTBI = moderate/severe traumatic brain injury; mTBI = mild traumatic brain injury.
Values are n (%). mTBI sample size = 28; cmTBI sample size = 15; msevTBI sample size = 43.
DISCUSSION
This cross-sectional study investigated medical decision-making capacity (MDC) as measured by a standardized psychometric instrument in patients with acute TBI across a range of injury severity. Our present study results demonstrate that impairment of MDC is prevalent in acute TBI and is strongly related to injury severity. Specifically, 1 month after injury, MDC was largely intact for our mTBI group compared to controls, but significantly impaired for both cmTBI and msevTBI groups.
As noted, mTBI patients performed equivalently to controls on all 5 consent standards. This finding suggests that 1 month following injury, consent capacity has returned to normal levels for many patients with mTBI, assuming initial impairment after injury. This finding reflects the broader neuropsychological literature on cognitive recovery in patients with mTBI, which has shown that mTBI patients as a group perform equivalently with controls on most cognitive testing shortly after injury.18–21
At the same time, our study showed some heterogeneity in the MDC of mTBI patients in the acute injury period. Specifically, our categorical impairment ratings showed individual variability in the capacity performance of mTBI patients. Approximately 30% of mTBI patients demonstrated capacity compromise (combined mild/moderate and severe impairment ratings) on appreciation, and another 20% showed compromise on understanding. These capacity ratings thus captured MDC impairment in some individuals with mTBI that were otherwise obscured by group mean scores. There appear to be a subset of individuals with mTBI in this study who continue to have impaired consent abilities a month or more following their injury. It should be noted that multiple studies have shown similar individual heterogeneity in recovery of cognitive abilities over time in mTBI, with a subset of patients demonstrating cognitive impairments a month or longer after their injury.22–24 Such persisting impairment may relate to a number of factors, including age, education, gender, medical issues, premorbid psychiatric status, substance abuse history, and premorbid cognitive status.25–27
In contrast to mTBI, patients with cmTBI 1 month following injury showed impairment on the understanding standard relative to controls. In addition, capacity impairment ratings for this group reflected a different pattern, with nearly half of cmTBI patients showing some level of compromise (mild/moderate or severe ratings) on the 3 complex standards. These findings suggest that structural brain changes found in complicated mild injuries contribute to more significant impairments in decisional capacity that have not resolved a month after injury. This study is thus consistent with literature demonstrating slower recovery rates and worse outcomes in patients with cmTBI compared to patients with uncomplicated mild TBIs.28,29
MDC was most impaired in patients with msevTBI. Relative to the control and mTBI groups, the msevTBI group was impaired on the 3 complex consent standards (S3–S5), and was impaired relative to the cmTBI group on the understanding standard. In addition, the msevTBI group was impaired relative to other groups on the simple standard of expressing choice. Capacity impairment ratings in the msevTBI group showed a similar pattern, with 77% capacity compromise for understanding, 63% for appreciation, and 53% for reasoning, but also 21% for expressing a choice, and 19% for making the reasonable choice.
As previously noted, the msevTBI results replicated and cross-validated results from a previous study investigating MDC in a different sample of msevTBI patients.1 In addition, the present study extended earlier findings by showing msevTBI group impairment on simple consent standards as well (S1 and [S2]). It is likely that the larger msevTBI patient sample in the present study resulted in greater power to detect differences on these elementary standards. In addition, the current sample was likely more severely injured and cognitively impaired than the earlier sample, as evidenced by lower GOAT and GCS scores.
An interesting aspect of the present study was the opportunity to compare the decisional capacity of participants with mTBI vs cmTBI. Across the board, the raw scores of cmTBI patients fell below patients with mTBI on the different CCTI standards. However, the group differences did not reach significance, in substantial part due to the cmTBI group's small sample size and large standard deviations. We anticipate that CCTI score mean differences between the 2 mild groups will reach significance with larger sample sizes.
With respect to individual capacity impairment ratings, however, the 2 mild TBI groups showed clear separation. For example, on appreciation, capacity compromise for cmTBI patients was 47% vs 29% for mTBI patients, a difference of 18%. On understanding, capacity compromise was 47% for cmTBI patients vs 18% for mTBI patients, a difference of 29%. The greatest differential emerged on reasoning, where capacity compromise was 47% for cmTBI but only 7% for mTBI, a difference of 40%. Thus despite having very similar GCS and GOAT scores, mTBI and cmTBI groups had distinctly different capacity impairment rating profiles at 1 month postinjury. This finding highlights the importance of injury severity in understanding initial impairment of decisional capacity in TBI.
The findings also raise questions about how mTBI is defined. In our study, mTBI and cmTBI group were distinguished using conventional cranial CT and MRI scan findings. It is possible that some of the 30% of mTBI cases with impaired capacity ratings in our study might have met criteria for cmTBI if different neuroimaging studies had been performed. For example, recent studies using CT and MRI perfusion, MRS, and DTI show abnormalities in mTBI patients who have no abnormalities on conventional imaging.30–33 Other markers such as reaction time, eye tracking, dynamic balance, and vestibular function may also distinguish mTBI and cmTBI.34
Our finding that one-third of the patients with mTBI had compromised capacity ratings suggests that MDC in TBI must be carefully considered in all patients, including mTBI patients with normal findings on conventional neuroimaging. Approaches in clinic to ensure adequate capacity to consent can include using multiple modalities to convey treatment information (i.e., both auditory and visual), having the patient with TBI explain treatment information presented to them, or administering formal capacity measures like the CCTI.
There were several limitations to the study. First, our sample of cmTBI cases was relatively small and underpowered to find differences between mTBI and cmTBI groups. Second, the impairment ratings were used experimentally for scientific purposes and are not representative of participants' actual legal or clinical competency status. Third, individuals may respond differently to a hypothetical vignette than to real-life, personal medical situations. For example, real-life medical problems may trigger emotional aspects of MDC not captured by hypothetical vignettes. Finally, while our study population was comparable to other TBI patient populations, participants who agree to be in research studies may not completely reflect the general population. Cross-validation of findings in other mild, complicated mild, and moderate/severe TBI patient samples would further establish external validity of the study findings.
Supplementary Material
GLOSSARY
- CCTI
Capacity to Consent to Treatment Instrument
- cmTBI
complicated mild traumatic brain injury
- GCS
Glasgow Coma Scale
- GOAT
Galveston Orientation and Amnesia Test
- LOC
loss of consciousness
- MDC
medical decision-making capacity
- msevTBI
moderate/severe traumatic brain injury
- mTBI
mild traumatic brain injury
- PTA
post-traumatic amnesia
- TBI
traumatic brain injury
- UAB
University of Alabama at Birmingham
Footnotes
Editorial, page 1454
AUTHOR CONTRIBUTIONS
Dr. Triebel participated in the drafting/revising the manuscript for content, study concept or design, and analyses and interpretation of the data. Dr. Martin participated in the study concept or design, revising the manuscript for content, and assisted with interpretation of data. Dr. Novack participated in the study concept or design, drafting/revising manuscript content, and interpretation of data. Dr. Dreer participated in the study concept or design. Ms. Turner participated in the analysis or interpretation of data, study coordination, and acquisition of data. Dr. Pritchard participated in the study concept or design. Dr. Raman participated in the analysis of data. Dr. Marson participated in drafting/revising the manuscript, study concept and design, interpretation of data, study supervision, and obtaining funding.
ACKNOWLEDGMENT
The authors thank the following contributors: Sandra Caldwell, MA (UAB Department of Physical Medicine and Rehabilitation, data collection); UAB Neuropsychology Laboratory Staff (data collection); and Sarah Nafziger, MD (UAB Department of Emergency Medicine, referring study participants).
DISCLOSURE
Dr. Triebel receives research support from the NIH (NICHD 1R01 HD053074 and 2R01 AG021927-06A1) and has received research support from the National Institute on Aging (P30 AG031054 and R01 AG021927). Dr. Martin receives research support from the NIH (NICHD 1R01 HD053074, 2R01 AG021927-06A1, and CDC MM-1042) and has received research support from the NIA (1P50 AG16582 and 1R01 AG021927). Dr. Novack receives research support from the NIH for NICHD 1R01 HD053074. Dr. Dreer receives research support from the National Eye Institute (K23 EY017327), the EyeSight Foundation of Alabama, and Research to Prevent Blindness, and has received research support from the National Institute on Aging (P30AG031054). Ms. Turner receives research support from the NIH for NICHD 1R01 HD053074. Dr. Pritchard receives research support from the NIH for NICHD 1R01 HD053074 and from Medtronic Spine LLC, and has served as a training and educational consultant for Medtronic/Kyphon. Dr. Marson serves as a consultant to Medivation, Inc. and for the American Bar Association and American Psychological Association; receives research support from the NIH (NICHD 1R01 HD053074 and NIA 2R01 AG021927-06A1); has received research support from the NIH (NIA AG021927, AG16582, NIA Director's Supplementary Reserve, AG24904, and AG10483); and receives royalty payments as coinventor of CCTI assessment instrument, which is owned by UAB Research Foundation (since 1996). Go to Neurology.org for full disclosures.
REFERENCES
- 1. Marson DC, Dreer LE, Krzywanski S, Huthwaite JS, DeVivo MJ, Novack TA. Impairment and partial recovery of medical decision-making capacity in traumatic brain injury: a 6-month longitudinal study. Arch Phys Med Rehabil 2005; 86: 889– 895 [DOI] [PubMed] [Google Scholar]
- 2. Rosenthal M, Ricker J. Traumatic brain injury. In: Frank RG, Elliot TR. eds. Handbook of Rehabilitation Psychology. Washington, DC: American Psychological Association; 2000: 49–74 [Google Scholar]
- 3. Dreer LE, DeVivo MJ, Novack TA, Krzywanski S, Marson DC. Cognitive predictors of medical decision-making capacity in traumatic brain injury. Rehabil Psychol 2008; 53: 486– 497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bushnik T. Traumatic brain injury model systems of care 2002–2007. Arch Phys Med Rehabil 2008; 89: 894– 895 [DOI] [PubMed] [Google Scholar]
- 5. Dahmer ER, Shilling MA, Hamilton BB, et al. A model systems database for traumatic brain injury. J Head Trauma Rehabil 1993; 8: 12– 25 [Google Scholar]
- 6. American Congress of Rehabilitation Medicine. Definition of mild traumatic brain injury. J Head Trauma Rehabil 1993; 8: 86– 87 [Google Scholar]
- 7. Miller JD. Head injury. J Neurol Neurosurg Psychiatry 1993; 56: 440– 447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pentland B, Wittle I. Acute management of brain injury. In: Rosenthal M, Kreutzer JS, Griffith ER, et al. eds. Rehabilitation of the Adult and Child with Traumatic Brain Injury, 3rd ed Philadelphia: Davis; 1999: 42–52 [Google Scholar]
- 9. Russell WR, Smith A. Post-traumatic amnesia in closed head injury. Arch Neurol 1961; 5: 4– 17 [DOI] [PubMed] [Google Scholar]
- 10. McKinlay W, Watkins A. Cognitive and behavioral effects of brain injury. In: Rosenthal M, Kreutzer JS, Griffith ER, et al., eds. Rehabilitation of the Adult and Child with Traumatic Brain Injury, 3rd ed Philadelphia: Davis; 1999: 74–86 [Google Scholar]
- 11. Marson DC, Ingram KK, Cody HA, Harrell LE. Assessing the competency of patients with Alzheimer's disease under different legal standards. A prototype instrument. Arch Neurol 1995; 52: 949– 954 [DOI] [PubMed] [Google Scholar]
- 12. Dymek MP, Marson DC, Harrell L. Factor structure of capacity to consent to medical treatment in patients with Alzheimer's disease: an exploratory study. J Forens Neuropsychol 1999; 1: 27– 48 [Google Scholar]
- 13. Appelbaum P, Grisso T. Assessing patients' capacities to consent to treatment. N Engl J Med 1988; 319: 1635– 1638 [DOI] [PubMed] [Google Scholar]
- 14. Levin HS, O'Donnell VM, Grossman RG. The Galveston Orientation and Amnesia Test: a practical scale to assess cognition after head injury. J Nerv Ment Dis 1979; 167: 675– 684 [DOI] [PubMed] [Google Scholar]
- 15. Okonkwo O, Griffith HR, Belue K, et al. Medical decision-making capacity in patients with mild cognitive impairment. Neurology 2007; 69: 1528– 1535 [DOI] [PubMed] [Google Scholar]
- 16. Triebel KL, Martin RC, Nabors LB, Marson DC. Medical decision-making capacity in patients with malignant glioma. Neurology 2009; 73: 2086– 2092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Statistical Package For The Social Sciences (SPSS) [computer program], version 13.0. Chicago: SPSS; 2004 [Google Scholar]
- 18. Levin HS, Mattis S, Ruff RM, et al. Neurobehavioral outcome following minor head injury: a three-center study. J Neurosurg 1987; 66: 234– 243 [DOI] [PubMed] [Google Scholar]
- 19. McCrea M, Guskiewicz KM, Marshall SW, et al. Acute effects and recovery time following concussion in collegiate football players: the NCAA Concussion Study. JAMA 2003; 290: 2556– 2563 [DOI] [PubMed] [Google Scholar]
- 20. McCrea MA. Mild Traumatic Brain Injury and Postconcussion Syndrome: The New Evidence Base for Diagnosis and Treatment. New York: Oxford University Press; 2007 [Google Scholar]
- 21. Dikmen SS, Corrigan JD, Levin HS, Machamer J, Stiers W, Weisskopf MG. Cognitive outcome following traumatic brain injury. J Head Trauma Rehabil 2009; 24: 430– 438 [DOI] [PubMed] [Google Scholar]
- 22. Sterr A, Herron KA, Hayward C, Montaldi D. Are mild head injuries as mild as we think? Neurobehavioral concomitants of chronic post-concussion syndrome. BMC Neurol 2006; 6: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Vanderploeg RD, Curtiss G, Belanger HG. Long-term neuropsychological outcomes following mild traumatic brain injury. J Int Neuropsychol Soc 2005; 11: 228– 236 [DOI] [PubMed] [Google Scholar]
- 24. Lee H, Wintermark M, Gean AD, Ghajar J, Manley GT, Mukherjee P. Focal lesions in acute mild traumatic brain injury and neurocognitive outcome: CT versus 3T MRI. J Neurotrauma 2008; 25: 1049– 1056 [DOI] [PubMed] [Google Scholar]
- 25. Iverson GL. Outcome from mild traumatic brain injury. Curr Opin Psychiatry 2005; 18: 301– 317 [DOI] [PubMed] [Google Scholar]
- 26. Kraus J, Hsu P, Schaffer K, et al. Preinjury factors and 3-month outcomes following emergency department diagnosis of mild traumatic brain injury. J Head Trauma Rehabil 2009; 24: 344– 354 [DOI] [PubMed] [Google Scholar]
- 27. Ponsford J, Willmott C, Rothwell A, et al. Factors influencing outcome following mild traumatic brain injury in adults. J Int Neuropsychol Soc 2000; 6: 568– 579 [DOI] [PubMed] [Google Scholar]
- 28. Iverson GL. Complicated vs uncomplicated mild traumatic brain injury: acute neuropsychological outcome. Brain Inj 2006; 20: 1335– 1344 [DOI] [PubMed] [Google Scholar]
- 29. Borgaro SR, Prigatano GP, Kwasnica C, Rexer JL. Cognitive and affective sequelae in complicated and uncomplicated mild traumatic brain injury. Brain Inj 2003; 17: 189– 198 [DOI] [PubMed] [Google Scholar]
- 30. MacDonald CL, Johnson AM, Cooper D, et al. Detection of blast-related traumatic brain injury in U.S. military personnel. N Engl J Med 2011; 364: 2091– 2100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Metting Z, Rodiger LA, Stewart RE, Oudkerk M, De Keyser J, van der Naalt J. Perfusion computed tomography in the acute phase of mild head injury: regional dysfunction and prognostic value. Ann Neurol 2009; 66: 809– 816 [DOI] [PubMed] [Google Scholar]
- 32. Metting Z, Rodiger LA, de Jong BM, Stewart RE, Kremer BP, van der Naalt J. Acute cerebral perfusion CT abnormalities associated with posttraumatic amnesia in mild head injury. J Neurotrauma 2010; 27: 2183– 2189 [DOI] [PubMed] [Google Scholar]
- 33. Ge Y, Patel MB, Chen Q, et al. Assessment of thalamic perfusion in patients with mild traumatic brain injury by true FISP arterial spin labelling MR imaging at 3T. Brain Inj 2009; 23: 666– 674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. de Guise E, Lepage JF, Tinawi S, et al. Comprehensive clinical picture of patients with complicated vs uncomplicated mild traumatic brain injury. Clin Neuropsychol 2010; 24: 1113– 1130 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.