Abstract
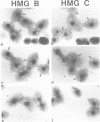
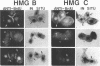
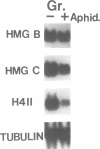
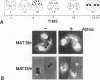
Two abundant high-mobility-group (HMG)-like proteins, HMG B and HMG C, exist in the ciliated protozoan, Tetrahymena thermophila. Of these, HMG C is specific to transcriptionally active macronuclei, while HMG B is found in macronuclei and in transcriptionally inactive micronuclei [1]. Using Northern and in situ analyses, we show that the genes encoding HMG B and HMG C are not expressed uniformly throughout the vegetative cycle or during the sexual process, conjugation. Elevated expression of both genes is observed during macronuclear S phase of the vegetative cycle and during endoreplication of developing new macronuclei in later stages of conjugation. Interruption of any of these macronuclear DNA replications by aphidicolin leads to a rapid drop in the message levels of HMG B and HMG C. These results resemble what is typically observed for replication-dependent nucleosomal histones and differ from the apparent lack of cell cycle regulation observed for HMG genes in vertebrates. A specific-induction of HMG B mRNA is also observed early in conjugation and during this interval, inhibition of micronuclear DNA synthesis by aphidicolin does not affect the message level of HMG B. Thus, during conjugation, expression of HMG B shows both replication-dependent and independent regulation. Results similar to these with HMG B are obtained with histone H4II gene, a gene which is also expressed during micro- and macronuclear S phases during the vegetative cycle. These results demonstrate surprising complexity in the expression of HMG genes in Tetrahymena and lend support to the hypothesis that cell cycle regulation plays an important role in directing HMG-like proteins to the appropriate nucleus [2]. Interestingly, expression of neither HMG gene is perfectly synchronized with that of histone H4II gene during the developmental program suggesting that important differences exist between vegetatively growing (cell cycle control) and conjugating (developmental control) cells.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allis C. D., Colavito-Shepanski M., Gorovsky M. A. Scheduled and unscheduled DNA synthesis during development in conjugating Tetrahymena. Dev Biol. 1987 Dec;124(2):469–480. doi: 10.1016/0012-1606(87)90500-8. [DOI] [PubMed] [Google Scholar]
- Allis C. D., Dennison D. K. Identification and purification of young macronuclear anlagen from conjugating cells of Tetrahymena thermophila. Dev Biol. 1982 Oct;93(2):519–533. doi: 10.1016/0012-1606(82)90139-7. [DOI] [PubMed] [Google Scholar]
- Bruns P. J., Brussard T. B. Pair formation in tetrahymena pyriformis, an inducible developmental system. J Exp Zool. 1974 Jun;188(3):337–344. doi: 10.1002/jez.1401880309. [DOI] [PubMed] [Google Scholar]
- Bustin M., Lehn D. A., Landsman D. Structural features of the HMG chromosomal proteins and their genes. Biochim Biophys Acta. 1990 Jul 30;1049(3):231–243. doi: 10.1016/0167-4781(90)90092-g. [DOI] [PubMed] [Google Scholar]
- Bustin M., Soares N., Landsman D., Srikantha T., Collins J. M. Cell cycle regulated synthesis of an abundant transcript for human chromosomal protein HMG-17. Nucleic Acids Res. 1987 Apr 24;15(8):3549–3561. doi: 10.1093/nar/15.8.3549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan R. C., Shalke G., Gorovsky M. A. Developmental rearrangements associated with a single type of expressed alpha-tubulin gene in Tetrahymena. Cell. 1984 Feb;36(2):441–445. doi: 10.1016/0092-8674(84)90237-x. [DOI] [PubMed] [Google Scholar]
- Cox K. H., DeLeon D. V., Angerer L. M., Angerer R. C. Detection of mrnas in sea urchin embryos by in situ hybridization using asymmetric RNA probes. Dev Biol. 1984 Feb;101(2):485–502. doi: 10.1016/0012-1606(84)90162-3. [DOI] [PubMed] [Google Scholar]
- Ghosh P. K., Reddy V. B., Piatak M., Lebowitz P., Weissman S. M. Determination of RNA sequences by primer directed synthesis and sequencing of their cDNA transcripts. Methods Enzymol. 1980;65(1):580–595. doi: 10.1016/s0076-6879(80)65061-7. [DOI] [PubMed] [Google Scholar]
- Gorovsky M. A., Yao M. C., Keevert J. B., Pleger G. L. Isolation of micro- and macronuclei of Tetrahymena pyriformis. Methods Cell Biol. 1975;9(0):311–327. doi: 10.1016/s0091-679x(08)60080-1. [DOI] [PubMed] [Google Scholar]
- Horowitz S., Bowen J. K., Bannon G. A., Gorovsky M. A. Unusual features of transcribed and translated regions of the histone H4 gene family of Tetrahymena thermophila. Nucleic Acids Res. 1987 Jan 12;15(1):141–160. doi: 10.1093/nar/15.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K. R., Disney J. E., Wyatt C. R., Reeves R. Expression of mRNAs encoding mammalian chromosomal proteins HMG-I and HMG-Y during cellular proliferation. Exp Cell Res. 1990 Mar;187(1):69–76. doi: 10.1016/0014-4827(90)90118-t. [DOI] [PubMed] [Google Scholar]
- Kolodrubetz D., Burgum A. Duplicated NHP6 genes of Saccharomyces cerevisiae encode proteins homologous to bovine high mobility group protein 1. J Biol Chem. 1990 Feb 25;265(6):3234–3239. [PubMed] [Google Scholar]
- Lee K. L., Pentecost B. T., D'Anna J. A., Tobey R. A., Gurley L. R., Dixon G. H. Characterization of cDNA sequences corresponding to three distinct HMG-1 mRNA species in line CHO Chinese hamster cells and cell cycle expression of the HMG-1 gene. Nucleic Acids Res. 1987 Jul 10;15(13):5051–5068. doi: 10.1093/nar/15.13.5051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilley D. M. DNA--protein interactions. HMG has DNA wrapped up. Nature. 1992 May 28;357(6376):282–283. doi: 10.1038/357282a0. [DOI] [PubMed] [Google Scholar]
- Love H. D., Jr, Allen-Nash A., Zhao Q. A., Bannon G. A. mRNA stability plays a major role in regulating the temperature-specific expression of a Tetrahymena thermophila surface protein. Mol Cell Biol. 1988 Jan;8(1):427–432. doi: 10.1128/mcb.8.1.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lycan D. E., Osley M. A., Hereford L. M. Role of transcriptional and posttranscriptional regulation in expression of histone genes in Saccharomyces cerevisiae. Mol Cell Biol. 1987 Feb;7(2):614–621. doi: 10.1128/mcb.7.2.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martindale D. W., Allis C. D., Bruns P. J. RNA and protein synthesis during meiotic prophase in Tetrahymena thermophila. J Protozool. 1985 Nov;32(4):644–649. doi: 10.1111/j.1550-7408.1985.tb03094.x. [DOI] [PubMed] [Google Scholar]
- Ner S. S. HMGs everywhere. Curr Biol. 1992 Apr;2(4):208–210. doi: 10.1016/0960-9822(92)90541-h. [DOI] [PubMed] [Google Scholar]
- Osley M. A. The regulation of histone synthesis in the cell cycle. Annu Rev Biochem. 1991;60:827–861. doi: 10.1146/annurev.bi.60.070191.004143. [DOI] [PubMed] [Google Scholar]
- Pash J. M., Bhorjee J. S., Patterson B. M., Bustin M. Persistence of chromosomal proteins HMG-14/-17 in myotubes following differentiation-dependent reduction of HMG mRNA. J Biol Chem. 1990 Mar 15;265(8):4197–4199. [PubMed] [Google Scholar]
- Schulman I. G., Cook R. G., Richman R., Allis C. D. Tetrahymena contain two distinct and unusual high mobility group (HMG)-like proteins. J Cell Biol. 1987 Jun;104(6):1485–1494. doi: 10.1083/jcb.104.6.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulman I. G., Wang T. T., Stargell L. A., Gorovsky M. A., Allis C. D. Cell-cell interactions trigger the rapid induction of a specific high mobility group-like protein during early stages of conjugation in Tetrahymena. Dev Biol. 1991 Feb;143(2):248–257. doi: 10.1016/0012-1606(91)90075-e. [DOI] [PubMed] [Google Scholar]
- Schulman I. G., Wang T., Wu M., Bowen J., Cook R. G., Gorovsky M. A., Allis C. D. Macronuclei and micronuclei in Tetrahymena thermophila contain high-mobility-group-like chromosomal proteins containing a highly conserved eleven-amino-acid putative DNA-binding sequence. Mol Cell Biol. 1991 Jan;11(1):166–174. doi: 10.1128/mcb.11.1.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schümperli D. Cell-cycle regulation of histone gene expression. Cell. 1986 May 23;45(4):471–472. doi: 10.1016/0092-8674(86)90277-1. [DOI] [PubMed] [Google Scholar]
- Stargell L. A., Karrer K. M., Gorovsky M. A. Transcriptional regulation of gene expression in Tetrahymena thermophila. Nucleic Acids Res. 1990 Nov 25;18(22):6637–6639. doi: 10.1093/nar/18.22.6637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner C. R., Hamana K., Elgin S. C. A high-mobility-group protein and its cDNAs from Drosophila melanogaster. Mol Cell Biol. 1992 May;12(5):1915–1923. doi: 10.1128/mcb.12.5.1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White E. M., Gorovsky M. A. Localization and expression of mRNA for a macronuclear-specific histone H2A variant (hv1) during the cell cycle and conjugation of Tetrahymena thermophila. Mol Cell Biol. 1988 Nov;8(11):4780–4786. doi: 10.1128/mcb.8.11.4780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodard J., Kaneshiro E., Gorovsky M. A. Cytochemical studies on the problem of macronuclear subnuclei in tetrahymena. Genetics. 1972 Feb;70(2):251–260. doi: 10.1093/genetics/70.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M., Allis C. D., Gorovsky M. A. Cell-cycle regulation as a mechanism for targeting proteins to specific DNA sequences in Tetrahymena thermophila. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2205–2209. doi: 10.1073/pnas.85.7.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu S. M., Gorovsky M. A. In situ dot blots: quantitation of mRNA in intact cells. Nucleic Acids Res. 1986 Oct 10;14(19):7597–7615. doi: 10.1093/nar/14.19.7597. [DOI] [PMC free article] [PubMed] [Google Scholar]