Abstract
Background
Human African Trypanosomiasis (HAT) is caused by two species of the tsetse fly vectored protozoan hemoflagellates belonging to Trypanosma brucei, namely T.b gambiense which predominates in Western Africa and follows a chronic disease course and T.b rhodensiense which is more prevalent in Southern and Eastern Africa, Malawi included, and follows a more acute and aggressive disease course. Previous studies in the Democratic Republic of Congo, Angola, Uganda and Sudan have demonstrated that the prevalence rates of T.b rhodensiense infection have reached epidemic proportions.
Objectives
To describe the epidemiology of Trypanosomiasis in Rumphi District over the past ten years.
Methodology
A total of 163 records from January 2000 to December 2006 were retrospectively studied.
Results
There were more males than females (121 vs. 40) with the 20 – 29 years age bracket having the highest number of cases (26.3%, n=160). Stage 2 HAT was the commonest stage at presentation (58.2%, n=158) with the patients in the same being 3.5 times more likely to die than those with stage 1 HAT. Case fatality rates for late and early stage disease were 21.5% (n = 92) and 7.2% (n = 66) respectively with 84.6% having been cured (n=162). Convulsions were associated with fatal disease outcome and the majority of cases (97.2%, n=103) lived within 5 kilometres of the Vwaza game reserve boundary.
Conclusion
More men have been infected than women, with a high involvement in the 20 – 29 age brackets. A dramatic increase with active case finding indicates a high under-detection of the disease with late stage HAT being predominant at presentation. Though it has been found that cases with late stage disease have an increased likelihood of dying compared to those in early stage HAT, the high proportion of successful treatment indicates that the disease still carries a high degree of favourable outcome with treatment. It has also been demonstrated in this study that more than 95% of trypanosomiasis cases live within 5 km of game reserve boundary. Disease interventions should be implemented in areas within 5km of marshland game reserve boundary as priority areas.
Introduction
Human African Trypanosomiasis (HAT) or sleeping sickness is an important but equally neglected tropical disease in sub-Saharan Africa with an estimated 60 million people at risk in nearly 200 separate active foci from 36 sub-Saharan countries1. The location of endemic foci of HAT follows the distribution of tsetse flies2. In Malawi, foci areas are in the districts of Rumphi, Nkhotakota, Ntchisi and Kasungu. The disease is caused by protozoan hemoflagellates belonging to Trypanosoma brucei with two species causing distinct disease patterns in humans namely Trypanosma brucei gambiense and Trypanosma brucei rhodensiense1. T.b gambiense is found in central, west and some parts of eastern Africa, whereas T.b rhodesiense is found in southern and eastern Africa1. T.b rhodensiense infection is usually acute, causing severe symptoms and death within a few days or weeks. However, studies in Malawi have shown T.b rhodensiense with a chronic disease profile3. T.b gambiense infections tend to progress more slowly (over several years) and causes less severe symptoms1.
Human infection occurs sporadically in individuals who come into contact with the zoonotic cycle such as poachers, hunters, firewood collectors and tourists4. Inoculation of the protozoa by the vector (Glossina sp) into the blood stream is followed by an initial stage of proliferation at the site of the bite, causing local inflammation (chancre) with subsequent invasion of the hemolymphatic system marking what is known as the early stage HAT, also known as stage 1 HAT. Concomitant involvement of the central nervous system leads to meningocephalitic stage of the disease, also referred to as late stage or stage 2 HAT. Without treatment, the disease progresses to coma and death3. Stage 1 is treated with suramin, but stage 2 can only be treated with melarsoprol, a toxic arsenic derivative that is associated with the development of a severe post-treatment reactive encephalopathy (PTRE)5. This severe adverse reaction can occur in up to 10% of cases 6,7, and may be fatal in 50% of the cases8.
HAT has been described as a major public health problem in sub-Saharan Africa, where it affects mainly the rural poor populations. The most recent prevalence estimates from the World Health Organization are 50,000–70,000 cases, based on a total number of 17,500 new cases reported per year in Africa9. The disease has been reported to have reached epidemic proportions in four countries, with a prevalence of over 20% in some areas of: Sudan, Uganda, the Democratic Republic of Congo, and Angola4,10,11,12. It is well appreciated that cases tend to cluster in close proximity to animal reservoirs such as game parks. Studies on the effect of distance to health care in relation to stage of presentation have previously been carried out and have shown that the closer the patient lives to the reporting health facility, the greater the likelihood to that they will be detected with the early stage of infection13. It has also been demonstrated that the prognosis of late stage disease is poorer than in early stage disease14.
WHO estimates that current HAT control activities reach only 10% of persons at risk15. With the results from previous studies in other countries suggesting resurgences and epidemics of HAT, it is important to describe the local epidemiology of HAT in order to estimate the burden of disease attributable to HAT in Malawi and to target interventions for the communities at risk. This study was therefore designed to describe the epidemiology of HAT in Rumphi District which is one of the recognized Trypanosomiasis rhodensiense endemic areas in Malawi.
Methods
This was a retrospective study which reviewed hospital records from the laboratory and registry offices, of Trypanosomiasis cases from January 1997 to December 2006 at Rumphi District Hospital, which is located in the northern Region of Malawi, where Vwaza game reserve and Nyika National Parks are located. The populaces of the district are mainly subsistence farmers and tobacco growing is a major agricultural activity.
Clinical records of all confirmed cases of Trypanosomiasis that were recorded at the district hospital were retrieved for analysis. In all the cases, blood and cerebrospinal fluid (CSF) samples were taken for diagnosing and staging of Trypanosomiasis infection. The diagnosis of Trypanosomiasis infection was confirmed by demonstration of parasites in the blood or CSF. The presence of parasites in CSF denoted stage 2 and presence of parasites in the blood only denoted stage 1 infection.
Data Collection
Data on patient age, gender, village of residence, stage of disease at presentation and disease outcome was extracted from clinical records and double entered into a Microsoft Access database. Geographical coordinates for villages of residence for all the cases were collected using a portable Global Positioning System (Garmin etrex, Garmin International, Inc USA © 2004 Garmin Ltd).
Data Analysis
Analysis was done using Intercooled STATA version 9.0 (StataCorp LP, 4905 Lakeway Drive, College Station, TX 77845 USA) and maps to depict spatial characteristics of interest in the study were created in ArcView GIS Version 3.1 (ArcView GIS Version 3.1 © 1992–1998, Environmental Systems Research Institute, Inc). Graphs were produced using Microsoft Excel software.
The associations between signs and symptoms with stage of presentation and disease outcome were tested using the Pearson Chi-Square test or the Fisher's Exact test were appropriate at the 5% level of significance. In order to examine the relationship of spatial distribution of cases and disease stage at presentation with respect to distance from the treating unit, the Mantel-Haenszel χ2 test was used. Logistic regression was used to assess the factors associated with the odds of dying from Trypanosomiasis.
Ethical Consideration
Ethical approval and oversight was granted by the College of Medicine Research and Ethics Committee (COMREC).
Results
Demographic characteristics of Trypanosomiasis cases
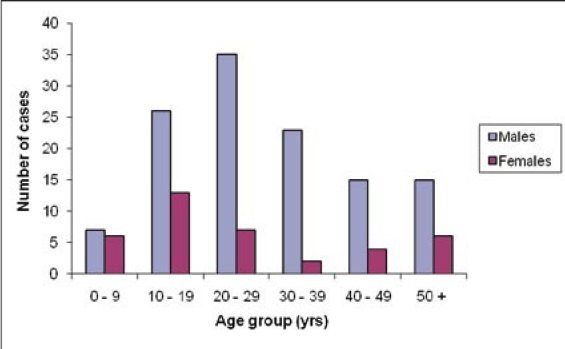
In this study, 163 records of confirmed Trypanosomiasis cases occurring over a period of 7 years were available. The age and sex distribution of the cases is shown in Figure 1. Of note, there were more males than females (3:1). The overall median age was 25 years (range 0 – 70 years) with adults accounting for 67.3% of the cases.
Figure 1.
Age and sex distribution of HAT cases by sex at Rumphi District Hospital (2000 – 2006)
Temporal trends in the number of Trypanosomiasis cases
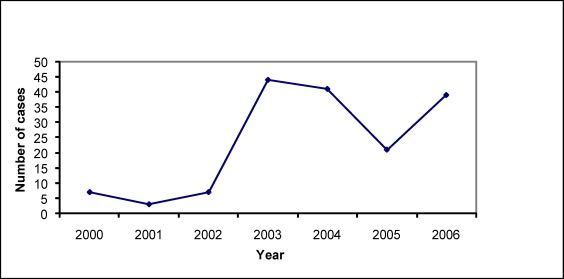
The trends in the number of confirmed cases, being attended to by the hospital, showed a general rise over the 7 year period under study, with a marked increase in 2003 (Figure 2).
Figure 2.
Trend of Trypanosomiasis cases attended to at Rumphi District Hospital (2000 – 2006)
Clinical characteristics of Trypanosomiasis cases
There was a predominance of Stage 2 HAT cases at presentation, accounting for 58.2% of the cases. Treatment outcomes were considerably good despite the fact that most of the cases presented in the late stage of disease. In this cohort of patients, mortality was 15.4% with 80% of the deaths having had late stage disease. Case fatality rates for late and early stage disease were 21.5% (n = 92) and 7.2% (n = 66) respectively with 84.6% having been cured (n=162. Fever was the most reported symptom in both stages (Early stage = 50%; Late stage = 40.7%). However, there was no statistically significant difference in the proportion of patients with fever as a symptom between the two clinical stages and no associations were found between any of the remaining documented symptoms or signs with stage at presentation (Table 1).
Table 1.
Relationship between symptoms and signs with stage at presentation
| Symptoms and Signs | Early (frequency) |
Late (frequency) |
p value (Fisher's exact test) |
| Dizziness | 3 | 3 | 0.68 |
| Convulsions | 1 | 2 | 0.56 |
| Headache | 15 | 8 | 0.60 |
| Palpitations | 4 | 0 | 0.14 |
| Poor vision | 3 | 1 | 0.64 |
| Abnormal behaviour | 2 | 2 | 1.00 |
| Vomiting | 4 | 0 | 0.14 |
| Abdominal pain | 6 | 5 | 0.75 |
| General body pains | 13 | 8 | 1.00 |
| General body weakness |
8 | 6 | 1.00 |
| Yellow eyes | 0 | 2 | 0.16 |
| Abnormally sleepy | 8 | 7 | 0.57 |
| Oral sores | 2 | 1 | 1.00 |
| Speech deficit | 1 | 4 | 0.15 |
| Low consciousness | 3 | 3 | 0.68 |
| Cough | 5 | 2 | 0.69 |
| Chest pains | 4 | 3 | 1.00 |
| Fever | 20 | 11 | 0.62 |
| Weight loss | 4 | 2 | 1.00 |
| Swollen face | 1 | 0 | 1.00 |
| Anaemia | 4 | 6 | 0.29 |
| Lymphadenopathy | 6 | 4 | 1.00 |
| Hepatomegaly | 2 | 1 | 1.00 |
| Splenomegaly | 2 | 4 | 0.21 |
| Edema | 3 | 5 | 0.25 |
| Neck stiffness | 3 | 3 | 0.68 |
| Tachycardia | 1 | 0 | 1.00 |
| Jaundice | 0 | 2 | 0.16 |
Factors associated with outcome
Treatment outcome was significantly associated with stage at presentation (p=0.01) and presence of convulsions (p=0.005). Multivaraite analyses indicated that after adjusting for the presence of convulsions, individuals who presented with Stage 2 HAT were more likely to die than those with Stage 1 HAT (odds ratio (OR) = 3.51, 95% Confidence Interval (CI) 1.24 – 9.88). Fever was not associated with either outcome (45.5% amongst those that survived, 50% in those that died, (Table 2).
Table 2.
Relationship between symptoms and signs with outcome
| Symptoms and Signs | Cured (frequency) |
Died (frequency) |
p value (Fisher's exact test) |
| Dizziness | 3 | 3 | 0.07 |
| Convulsions | 0 | 3 | 0.01 |
| Headache | 21 | 2 | 0.20 |
| Palpitations | 4 | 0 | 1.00 |
| Poor vision | 3 | 1 | 0.56 |
| Abnormal behaviour | 3 | 1 | 0.56 |
| Vomiting | 3 | 1 | 0.56 |
| Abdominal pain | 11 | 0 | 0.19 |
| General body pains | 19 | 2 | 0.31 |
| General body weakness |
10 | 4 | 0.26 |
| Yellow eyes | 1 | 1 | 0.33 |
| Abnormally sleepy | 10 | 5 | 0.12 |
| Oral sores | 2 | 1 | 0.45 |
| Speech deficit | 4 | 1 | 1.00 |
| Low consciousness | 4 | 2 | 0.29 |
| Cough | 6 | 1 | 1.00 |
| Chest pains | 5 | 2 | 0.60 |
| Fever | 25 | 6 | 1.00 |
| Weight loss | 6 | 0 | 0.58 |
| Swollen face | 1 | 0 | 1.00 |
| Anaemia | 8 | 2 | 1.00 |
| Lymphadenopathy | 10 | 0 | 0.19 |
| Hepatomegaly | 3 | 0 | 1.00 |
| Splenomegaly | 6 | 0 | 0.58 |
| Edema | 5 | 3 | 0.15 |
| Neck stiffness | 4 | 2 | 0.29 |
| Tachycardia | 1 | 0 | 1.00 |
| Jaundice | 2 | 0 | 1.00 |
Spatial distribution of Trypanosomiasis cases
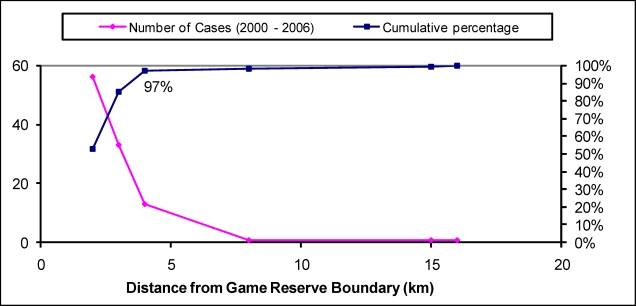
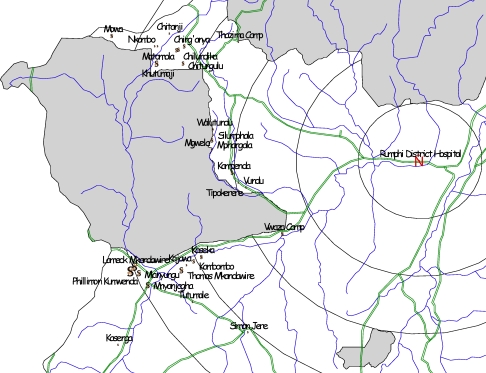
Most of the cases, 97.2 % (n = 103) came from within 5km of the game reserve boundary (Figure 3). The vast majority of cases were clustered along the Vwaza Game Reserve boundary compared to Nyika Game Reserve as demonstrated by Geo-referencing (Figure 4). We did not find an association between distance from diagnostic facility and stage at presentation (p-value = 0.60) or outcome (p-value = 0.32).
Figure 3.
Distance of villages of residence of trypanosomiasis cases from game reserve boundary (2000 – 2006)
Figure 4.
Spatial distribution of Trypanosomiasis cases with respect to Rumphi District Hospital and game reserve boundary.
Discussion
This data has shown that more men are infected with Trypanosomiasis in Rumphi district. This could be attributed to greater exposure to the parasite by men because of the nature of the activities which they engage in which include poaching, honey gathering and encroachment into the game reserve to clear land for tobacco farming, which is a major economic activity in this area. It is within the same vein that the greater proportions of cases are adults owing to activities that bring them into contact with the infected vectors.
We also found that in general there was an increase in the number of cases of Trypanosomiasis over the 7 year period understudy. However, there was a marked increase that was noted in 2003 by 600%. This could be attributed to active case finding that was done that year with support from the East African Network for Trypanosomiasis (EANET) as part of a Trypanosomiasis surveillance programme. Such a change concurs well with findings from a previous study conducted in Uganda of under-detection of T.b. rhodensiense infection16.
With more than 50% of the cases having been diagnosed with late stage disease, the study findings run in tandem with findings of a study conducted in Uganda14 in which approximately half the Trypanosomiasis patients presented with late stage disease. This finding could be due to weak surveillance. It could also be attributed to possible causative factors described as either patient factors or the health system. Some of these factors could include poverty of endemic countries, poverty of patients and low sensitivity of the diagnostic techniques13. In Rumphi, distance from the health facility does not seem to influence stage at presentation. As such, other parameters could be responsible for this observation, such as patient perceptions toward the seriousness of the disease, quality of health care or access to health education. These parameters were not assessed in the current study.
There was a greater chance of dying with late stage disease than early stage. This observation is in agreement with observations from a previous study that showed that the prognosis of late stage sleeping sickness presentation is significantly poorer that in the one in the early stage of the disease14. There were marked successful outcomes in treatment. These outcomes are likely due to the efficacy of the treatment and management at the hospital. Fever was the most reported symptom in both stage 1 and 2 HAT. However, there was no statistically significant association to warrant it as a clinical predictor of disease stage. There was a significant association between convulsions and fatal outcome. As such, convulsions could serve as a prognostic indicator of HAT.
The vast majority of cases were in close proximity to Vwaza game reserve which is a marsh land compared to Nyika game reserve which is mountain ecology. Such spatial distribution of the cases agrees with observations in a study done in 200516 where proximity to swampland was predictive of T.b rhodensiense infection. This observation could be attributed to an ecology dependant factor for the reservoir vector which is favoured by marsh land. This however, was not assessed in the current study.
The greatest limitation encountered in the study was the unavailability of records dating from 1997 to 1999. Other limitations included the poor recording of clinical parameters with lack of specific definitions for signs and symptoms and the inability to trace the areas of residence of some of the cases. Despite the limitations, this study managed to attain a satisfactory number of cases as well as having clear definitions for disease stage at presentation. Demographic and clinical outcome data was clearly documented from the available records. Additionally, this is the first retrospective study that has described in detail the epidemiology of Trypanosomiasis in a recognized endemic area in Malawi.
Conclusion
This study indicates that over the past seven years, more men have been infected than women, with a high involvement in the 20 – 29 age brackets. A dramatic increase with active case finding indicates a high under-detection of the disease with late stage HAT being predominant at presentation. Though it has been found that cases with late stage disease have an increased likelihood of dying compared to those in early stage HAT, the high proportion of successful treatment indicates that the disease still carries a high degree of favourable outcome with treatment. It has also been demonstrated in this study that more than 95% of trypanosomiasis cases live within 5 km of game reserve boundary.
In light of the findings in this study, it is recommended that disease interventions should be preferentially targeted to communities who live within 5 km of marsh land game reserve boundary. There is also a need to implement interventions that would lead to earlier presentation to the health facility in order to reduce the marked proportion of late stage presentations which are associated with more than a threefold chance of dying. Such interventions could include increased community sensitization campaigns and stronger disease surveillance.
Acknowledgements
Acknowledgements with gratitude go to the following: EANET for funding, Professor John Chisi and Dr Bagrey Ngwira for guidance and mentoring throughout the course of the study, Dr Linda Kalilani-Phiri for supervision through the course of the study and the writing of the manuscript for publication, Dr Anna Molesworth for assistance rendered with geographic information systems, Mr. A Kumitawa, for statistical analysis, Mr Chirongo of Rumphi District Hospital for his assistance in the field work, the DHO and all members of staff of Rumphi District Hospital.
References
- 1.World Health Organization, author. Control and surveillance of African trypanosomiasis: report of a WHO expert committee. Geneva: World Health Organization; 1998. [PubMed] [Google Scholar]
- 2.Barrett MP, Burchmore RJ, Stich A, Lazzari JO, Frasch AC, Cazzulo JJ, Krishna S. The trypanosomiases. Lancet. 2003;362:1469–1480. doi: 10.1016/S0140-6736(03)14694-6. [DOI] [PubMed] [Google Scholar]
- 3.MacLean L, Chisi JE, Odiit M, Gibson W, Ferris V, Picozzi K, Sternberg J M. Severity of human African trypanosomiasis in East Africa is associated with geographic location, parasite genotype, and host inflammatory cytokine response profile. Infect Immun. 2004:7040–7044. doi: 10.1128/IAI.72.12.7040-7044.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith DH, Pepin J, Stich AHR. Human African trypanosomiasis: an emerging public health crisis. Brit Med Bull. 1998;54:341–355. doi: 10.1093/oxfordjournals.bmb.a011692. [DOI] [PubMed] [Google Scholar]
- 5.de Atouguia JLM, Kennedy PGE. Neurological aspects of human African trypanosomiasis. In: Davies LE, Kennedy PGE, editors. Infectious diseases of the nervous system. Oxford: Butterworth-Heinemann; 2000. pp. 321–372. [Google Scholar]
- 6.Adams JH, Haller L, Boa FY, Doua F, Dago A, Konian K. Human African trypanosomiasis (T.b gambiense): a study of 16 fatal cases of sleeping sickness with some observations on acute reactive arsenical encephalopathy. Neuropathol Appl Neurobiol. 1986;12:81–94. doi: 10.1111/j.1365-2990.1986.tb00682.x. [DOI] [PubMed] [Google Scholar]
- 7.Pepin J, Milord F. African trypanosomiasis and drug-induced encephalopathy: risk factors and pathogenesis. Trans R Soc Trop Med Hyg. 1991;85:222–224. doi: 10.1016/0035-9203(91)90032-t. [DOI] [PubMed] [Google Scholar]
- 8.Pepin J, Milord F. The treatment of human African trypanosomiasis. Adv Parasitol. 1994;33:1–47. doi: 10.1016/s0065-308x(08)60410-8. [DOI] [PubMed] [Google Scholar]
- 9.World Health Organization, author. Human African trypanosomiasis (sleeping sickness): epidemiological update. Wkly Epidemiol Rec. 2006;81:71–80. [PubMed] [Google Scholar]
- 10.Moore A, Richer M. Re-emergence of epidemic sleeping sickness in southern Sudan. Trop Med Int Health. 2001;6:342–347. doi: 10.1046/j.1365-3156.2001.00714.x. [DOI] [PubMed] [Google Scholar]
- 11.Stanghellini A, Josenando T. The situation of sleeping sickness in Angola: a calamity. Trop Med Int Health. 2001;6:330–334. doi: 10.1046/j.1365-3156.2001.00724.x. [DOI] [PubMed] [Google Scholar]
- 12.Van Nieuwenhove S, Betu-Ku-Mesu VK, Diabakana PM, Declercq J, Miaka C. Sleeping sickness resurgence in the DRC: the past decade. Trop Med Int Health. 2001;6:335–341. doi: 10.1046/j.1365-3156.2001.00731.x. [DOI] [PubMed] [Google Scholar]
- 13.Odiit M, Coleman PG, McDermott JJ, Fevre EM, Welburn SC, Woolhouse MEJ. Spatial and temporal factors for the early detection of Trypanosoma brucei rhodesiense sleeping sickness in Tororo and Busia districts, Uganda. Trans R Soc Trop Med Hyg. 2004;98:569–576. doi: 10.1016/j.trstmh.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 14.Odiit M, Kansiime F, Enyaru JCK. Duration of symptoms and case fatality of sleeping sickness caused by Trypanosoma brucei rhodesiense sleeping sickness in Tororo, Uganda. East Afr Med J. 1997;74:792–795. [PubMed] [Google Scholar]
- 15.Lutumba P, Robays J, Miaka C, Kande V, Molisho D, Declercq J, et al. Trypanosomiasis control, Democratic Republic of Congo, 1993–2003. Emerg Infect Dis. 2005;11:1382–1388. doi: 10.3201/eid1109.041020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Odiit M, Coleman PG, Liu WC, McDermott JJ, Fèvre EM, Welburn SC, Woolhouse MEJ. Quantifying the level of under-detection of Trypanosoma brucei rhodesiense sleeping sickness cases. Trop Med Int Health. 2005;10(9):840–849. doi: 10.1111/j.1365-3156.2005.01470.x. [DOI] [PubMed] [Google Scholar]