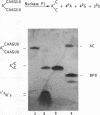
Abstract
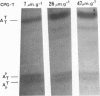
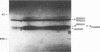
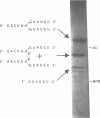
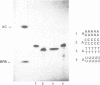
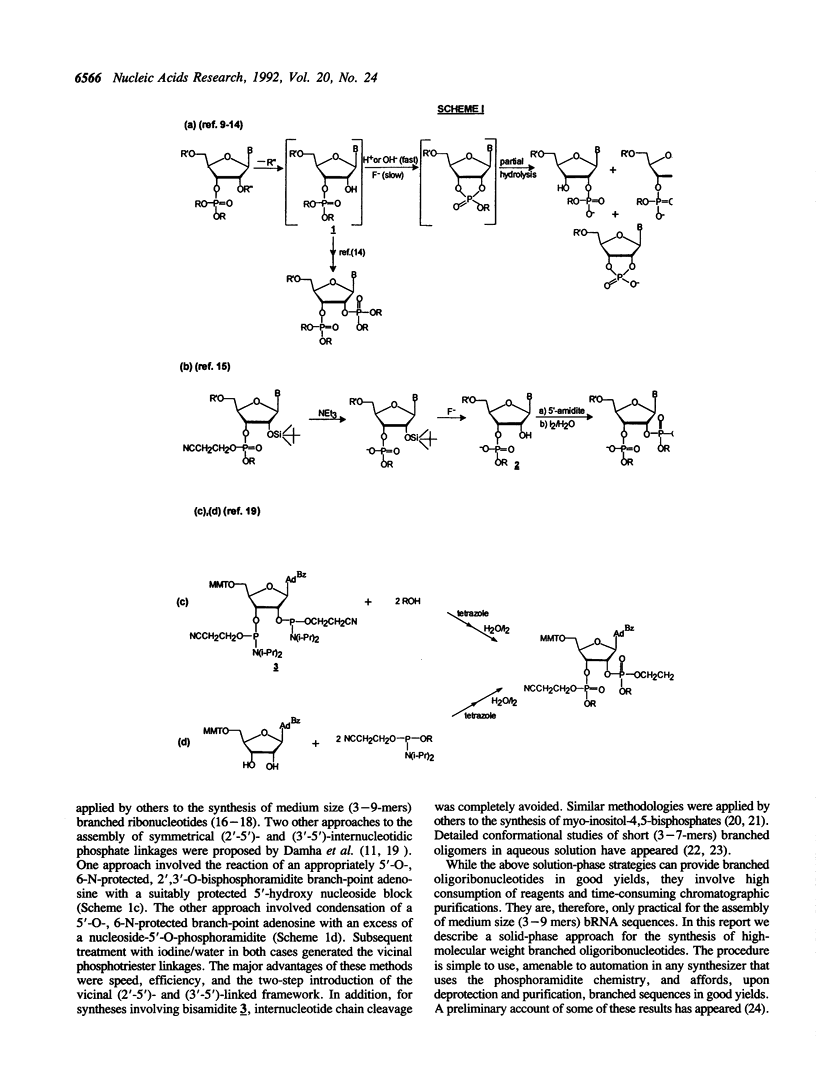
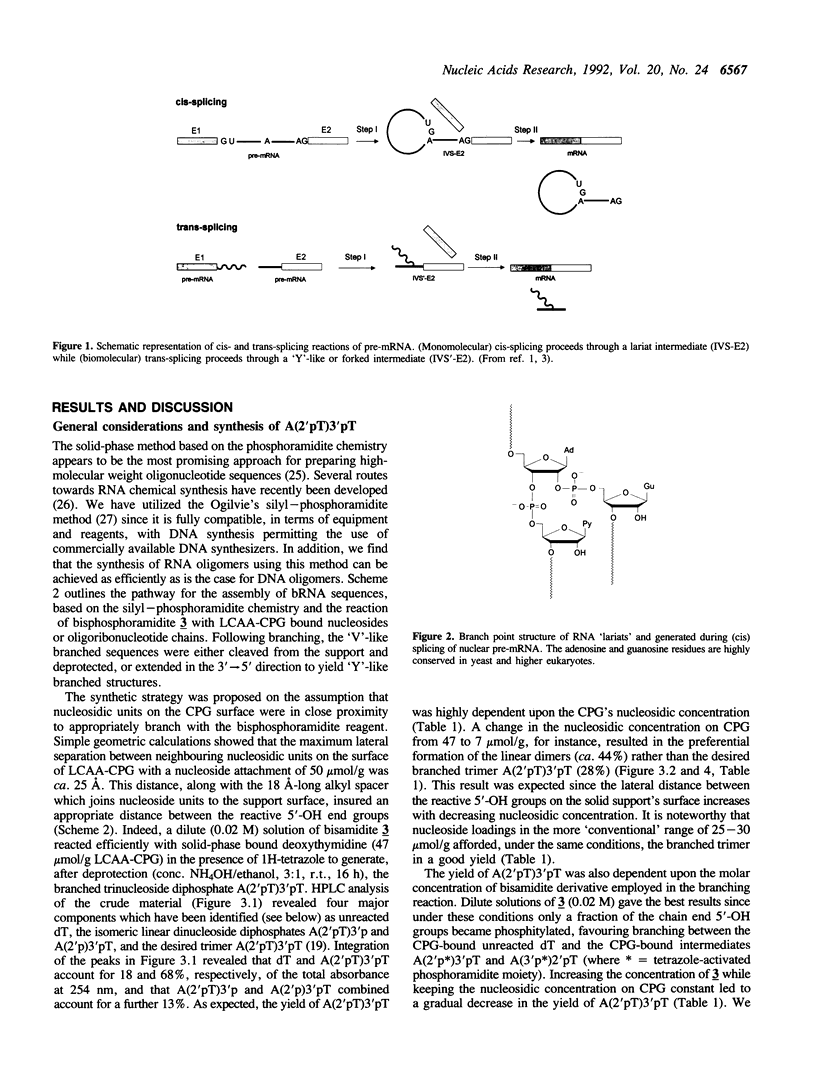
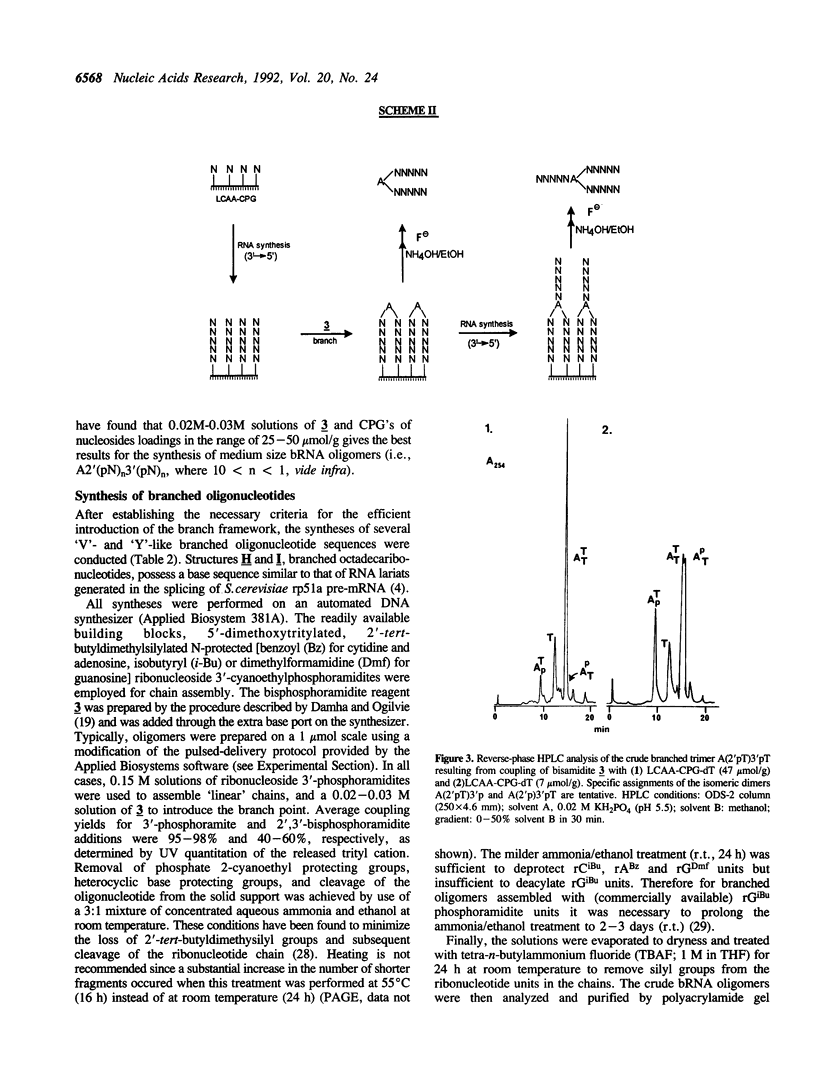
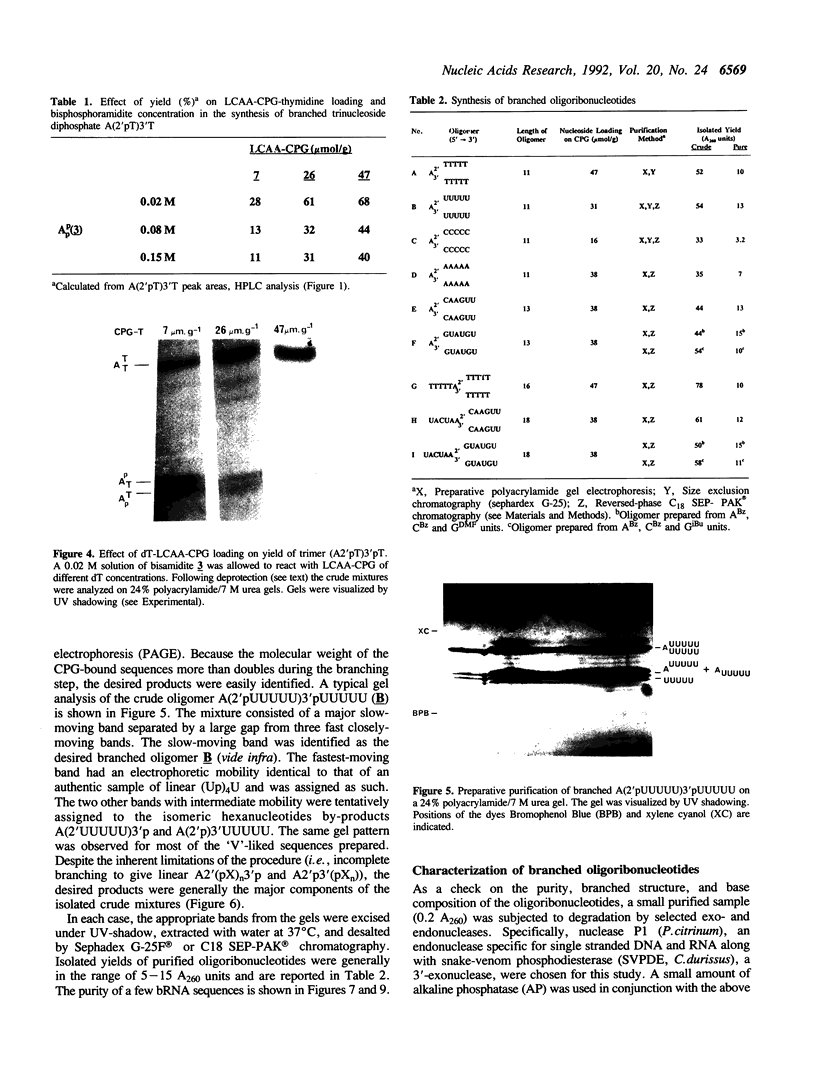
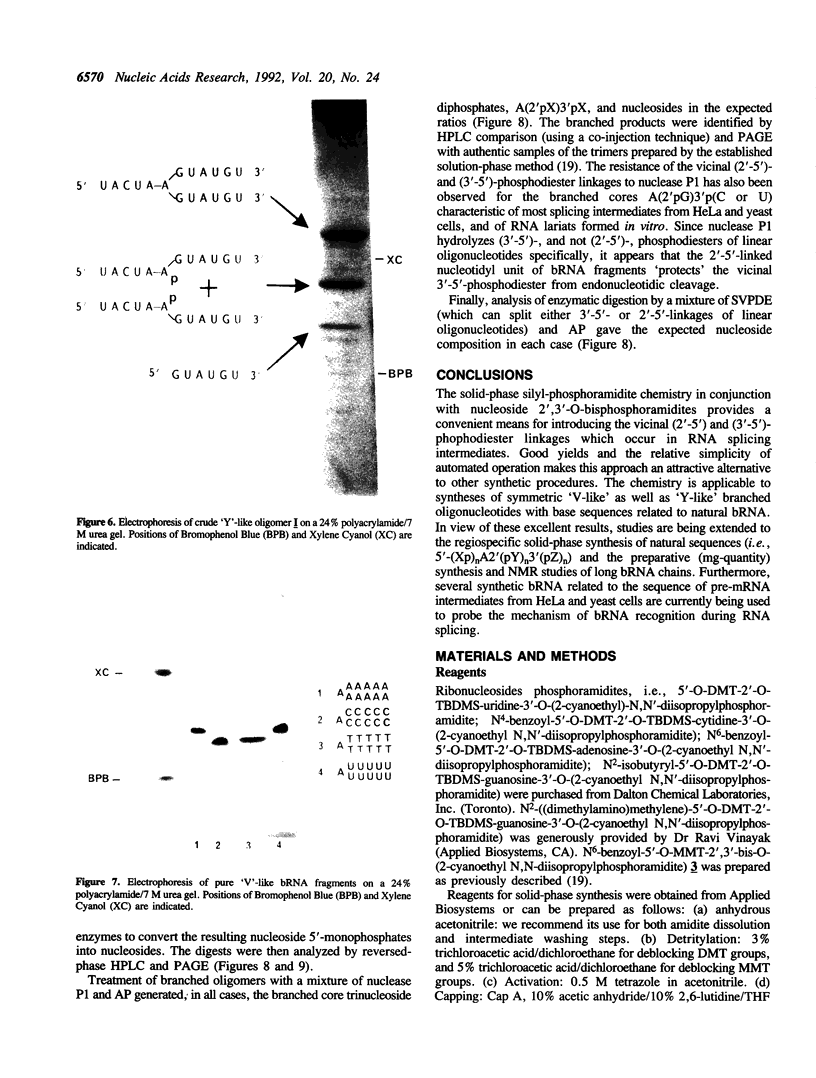
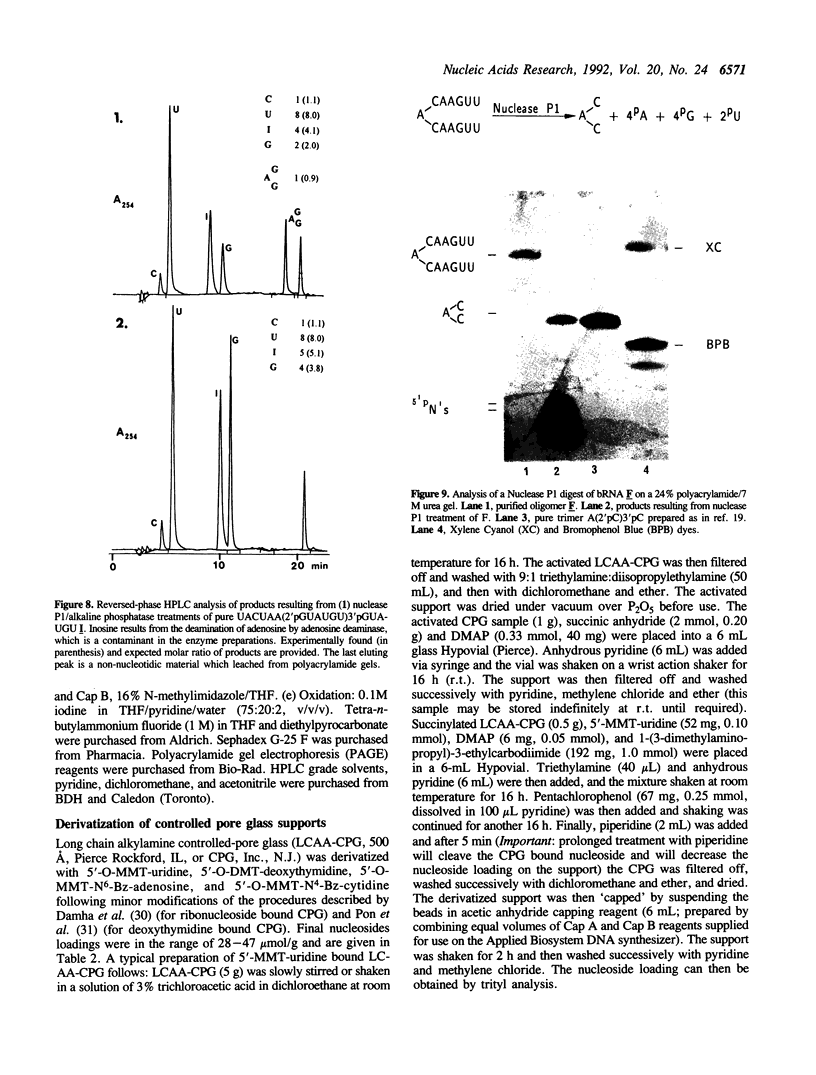
The chemical synthesis of oligoribonucleotides containing vicinal (2'-5')- and (3'-5')-phosphodiester linkages is described. The solid-phase method, based on silyl-phosphoramidite chemistry, was applied to the synthesis of a series of branched RNA [(Xp)nA2' (pN)n3'(pN)n] related to the splicing intermediates derived from Saccharomyces cerevisiae rp51a pre-messenger RNA. The branched oligonucleotides have been thoroughly characterized by nucleoside and branched nucleotide composition analysis. Branched oligoribonucleotides will be useful in the study of messenger RNA splicing and in determining the biological role of RNA 'lariats' and 'forks' in vivo.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Damha M. J., Giannaris P. A., Zabarylo S. V. An improved procedure for derivatization of controlled-pore glass beads for solid-phase oligonucleotide synthesis. Nucleic Acids Res. 1990 Jul 11;18(13):3813–3821. doi: 10.1093/nar/18.13.3813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damha M. J., Ogilvie K. K. Conformational properties of branched RNA fragments in aqueous solution. Biochemistry. 1988 Aug 23;27(17):6403–6416. doi: 10.1021/bi00417a032. [DOI] [PubMed] [Google Scholar]
- Fouser L. A., Friesen J. D. Mutations in a yeast intron demonstrate the importance of specific conserved nucleotides for the two stages of nuclear mRNA splicing. Cell. 1986 Apr 11;45(1):81–93. doi: 10.1016/0092-8674(86)90540-4. [DOI] [PubMed] [Google Scholar]
- Hartmuth K., Barta A. Unusual branch point selection in processing of human growth hormone pre-mRNA. Mol Cell Biol. 1988 May;8(5):2011–2020. doi: 10.1128/mcb.8.5.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornig H., Aebi M., Weissmann C. Effect of mutations at the lariat branch acceptor site on beta-globin pre-mRNA splicing in vitro. Nature. 1986 Dec 11;324(6097):589–591. doi: 10.1038/324589a0. [DOI] [PubMed] [Google Scholar]
- Kierzek R., Kopp D. W., Edmonds M., Caruthers M. H. Chemical synthesis of branched RNA. Nucleic Acids Res. 1986 Jun 25;14(12):4751–4764. doi: 10.1093/nar/14.12.4751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird P. W. Trans splicing in trypanosomes--archaism or adaptation? Trends Genet. 1989 Jul;5(7):204–208. doi: 10.1016/0168-9525(89)90082-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogilvie K. K., Usman N., Nicoghosian K., Cedergren R. J. Total chemical synthesis of a 77-nucleotide-long RNA sequence having methionine-acceptance activity. Proc Natl Acad Sci U S A. 1988 Aug;85(16):5764–5768. doi: 10.1073/pnas.85.16.5764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pon R. T., Damha M. J., Ogilvie K. K. Modification of guanine bases by nucleoside phosphoramidite reagents during the solid phase synthesis of oligonucleotides. Nucleic Acids Res. 1985 Sep 25;13(18):6447–6465. doi: 10.1093/nar/13.18.6447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pon R. T., Usman N., Damha M. J., Ogilvie K. K. Prevention of guanine modification and chain cleavage during the solid phase synthesis of oligonucleotides using phosphoramidite derivatives. Nucleic Acids Res. 1986 Aug 26;14(16):6453–6470. doi: 10.1093/nar/14.16.6453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pon R. T., Usman N., Ogilvie K. K. Derivatization of controlled pore glass beads for solid phase oligonucleotide synthesis. Biotechniques. 1988 Sep;6(8):768–775. [PubMed] [Google Scholar]
- Reed R., Maniatis T. Intron sequences involved in lariat formation during pre-mRNA splicing. Cell. 1985 May;41(1):95–105. doi: 10.1016/0092-8674(85)90064-9. [DOI] [PubMed] [Google Scholar]
- Reese C. B., Skone P. A. Action of acid on oligoribonucleotide phosphotriester intermediates. Effect of released vicinal hydroxy functions. Nucleic Acids Res. 1985 Jul 25;13(14):5215–5231. doi: 10.1093/nar/13.14.5215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaringe S. A., Francklyn C., Usman N. Chemical synthesis of biologically active oligoribonucleotides using beta-cyanoethyl protected ribonucleoside phosphoramidites. Nucleic Acids Res. 1990 Sep 25;18(18):5433–5441. doi: 10.1093/nar/18.18.5433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp P. A. Splicing of messenger RNA precursors. Science. 1987 Feb 13;235(4790):766–771. doi: 10.1126/science.3544217. [DOI] [PubMed] [Google Scholar]
- Sund C., Földesi A., Yamakage S., Chattopadhyaya J. Synthesis of branched nona and deca-RNA modelling the lariat formed in pre-mRNA processing reaction (splicing). Nucleic Acids Symp Ser. 1991;(24):9–12. [PubMed] [Google Scholar]
- Vijayraghavan U., Parker R., Tamm J., Iimura Y., Rossi J., Abelson J., Guthrie C. Mutations in conserved intron sequences affect multiple steps in the yeast splicing pathway, particularly assembly of the spliceosome. EMBO J. 1986 Jul;5(7):1683–1695. doi: 10.1002/j.1460-2075.1986.tb04412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace J. C., Edmonds M. Polyadenylylated nuclear RNA contains branches. Proc Natl Acad Sci U S A. 1983 Feb;80(4):950–954. doi: 10.1073/pnas.80.4.950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T., Ogilvie K. K., Pon R. T. Prevention of chain cleavage in the chemical synthesis of 2'-silylated oligoribonucleotides. Nucleic Acids Res. 1989 May 11;17(9):3501–3517. doi: 10.1093/nar/17.9.3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Rooij J. F., Wille-Hazeleger G., Burgers P. M., van Boom J. H. Neighbouring group participation in the unblocking of phosphotriesters of nucleic acids. Nucleic Acids Res. 1979;6(6):2237–2259. doi: 10.1093/nar/6.6.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]