Abstract
Given that oesophageal cancer (OC) is common in Malawi and its outcome is so dismal, would it be pragmatic to promptly mitigate the effects of smoking, alcohol and aflatoxins rather than seek a higher degree of local evidence for their role in OC?
We retrospectively analysed a total of 13,217 OC and Kaposi's sarcoma (KS) cases as recorded in the Malawi National Cancer Registry from 1985 to February, 2006. We found no OC clustering to suggest a role for culturally variable habits like smoking, alcohol, maize use and maize storage in the country. It may be that drinking and eating hot foods physically damages the oesophageal mucosa, this is in line with work recently reported from Asia. We also found that OC numbers have risen in line with KS (and HIV) suggesting a link between these conditions.
Introduction
Oesophageal cancer (OC) has been known to be unusually common in Malawi1–3 with poor survival rates. It is believed that kachasu (a local hard liquor), maize beer, smoking4 and aflatoxins from poorly stored maize increase the risk of OC. Would it be pragmatic to promptly mitigate the effects of any such factors rather than seek a higher degree of local evidence for their role?
We retrospectively analysed cases of OC in Malawi by district, as recorded in the Malawi National Cancer Registry (MNCR), over a period of twenty years. The MNCR has no populationbased cancer registration covering the whole country. We integrated our analysis of OC with data of Kaposi's sarcoma (KS), to serve as internal control for the analysis. This also allowed testing the suspicion amongst clinicians that the prominence of KS in Malawi would merely be a reflection of the fact that it is mainly diagnosed without histological confirmation whereas diagnosis of other malignancies such as OC require histological diagnosis.
Findings and discussion
There were a total of 38,305 cancer cases recorded in the MNCR during the period under study from 1985 to February 2006. The analysis included 3,022 OC and 10,195 KS cases.
As the table of cases by district of residence (in order of frequency) shows, the volume of cases reflects distance of district of residence to urban Blantyre with the exception of those districts which have central hospitals. For instance Lilongwe has more cases than the trend would suggest it should. Also in all district, there are more cases of KS than cases of OC. Analysis of personal habits e.g. smoking and drinking among OC and KS cases was not helpful as these were recorded only for 11.1% of 3,022 OC and 16.5% of 10,195 KS cases. The difference in availability of this information between the two groups was statistically significant (p<0.005) and so they could not be reliably compared. Maize storage habits, smoking and alcohol usage are unlikely to be uniform across the country, yet our analysis did not show any evidence of clustering of OC. These findings seem to add weight to existing doubts about aetiological roles of smoking, alcohol and aflatoxins in OC in Malawi. Aditionally, it remains puzzling as to why these three factors should contribute to a cancer that in the HIV era is outranked only by KS in males and only by cervical cancer and KS in females without comparable high rates of lung cancer (smoking) and liver cancer (alcohol, aflatoxins) in the country.
Table.
OC And KS cases by district of residence
| District Of Residence | Ca Oesophagus | % within residence (diagnosis) |
KS | % within residence (diagnosis) |
Total | % of Total |
| Blantyre (urban) | 504 | 16.8 (18) | 2500 | 83.2 (26.6) | 3004 | 24.6 |
| Blantyre (rural) | 317 | 22.7 (11.3) | 1082 | 77.3 (11.5) | 1399 | 11.5 |
| Lilongwe | 392 | 28.8 (14) | 970 | 71.2 (10.3) | 1362 | 11.2 |
| Zomba | 372 | 32.3 (13.3) | 778 | 67.7 (8.3) | 1150 | 9.4 |
| Thyolo | 124 | 12.8 (4.4) | 848 | 87.2 (9) | 972 | 8 |
| Chiradzulu | 118 | 18.8 (4.2) | 510 | 81.2 (5.4) | 628 | 5.1 |
| Mulanje | 122 | 19.7 (4.4) | 498 | 80.3 (5.3) | 620 | 5.1 |
| Mzimba | 105 | 27.9 (3.8) | 271 | 72.1 (2.9) | 376 | 3.1 |
| Mangochi | 128 | 34 (4.6) | 248 | 66 (2.6) | 376 | 3.1 |
| Machinga | 118 | 36.4 (4.2) | 206 | 63.6 (2.2) | 324 | 2.7 |
| Ntcheu | 69 | 26.4 (2.5) | 192 | 73.6 (2) | 261 | 2.1 |
| Chikwawa | 85 | 32.6 (3) | 176 | 67.4 (1.9) | 261 | 2.1 |
| Mwanza | 29 | 14.2 (1) | 175 | 85.5 (1.9) | 204 | 1.7 |
| Nsanje | 41 | 23.4 (1.5) | 134 | 76.6 (1.4) | 175 | 1.4 |
| Dedza | 38 | 28.6 (1.4) | 95 | 71.4 (1) | 133 | 1.1 |
| Kasungu | 22 | 17.2 (.8) | 106 | 82.8 (1.1) | 128 | 1 |
| Dowa | 26 | 24.8 (.9) | 79 | 75.2 (.8) | 105 | 0.9 |
| Phalombe | 24 | 23.3 (.9) | 79 | 76.7 (.8) | 103 | 0.8 |
| Balaka | 23 | 24.7 (.8) | 70 | 75.3 (.7) | 93 | 0.8 |
| Rumphi | 37 | 43.5 (1.3) | 48 | 56.5 (.5) | 85 | 0.7 |
| Salima | 27 | 32.5 (1) | 56 | 67.5 (.6) | 83 | 0.7 |
| Mchinji | 14 | 18.4 (.5) | 62 | 81.6 (.7) | 76 | 0.6 |
| Karonga | 6 | 9 (.2) | 61 | 91 (.6) | 67 | 0.5 |
| Nkhatabay | 5 | 12.5 (.2) | 35 | 87.5 (.4) | 40 | 0.3 |
| Nkhotakota | 9 | 23.1 (.3) | 30 | 76.9 (.3) | 39 | 0.3 |
| Nchisi | 16 | 39 (.6) | 25 | 61 (.3) | 41 | 0.3 |
| Mozambique | 7 | 18.4 (.3) | 31 | 81.6 (.3) | 38 | 0.3 |
| Not Known | 12 | 31.6 (.4) | 26 | 68.4 (.3) | 38 | 0.3 |
| Chitipa | 6 | 27.3 (.2) | 16 | 72.7 (.2) | 22 | 0.2 |
| Zambia | 0 | 0 (0) | 3 | 0 (0) | 3 | 0 |
| Zimbabwe | 0 | 0 (0) | 1 | 0 (0) | 1 | 0 |
| Total | 2796 | 22.9 (100) | 9411 | 77.1 (100) | 12207 | 100 |
In this analysis, the majority of OC cases were squamous cell carcinomas (SCC). The highest rates of SCC of the oesophagus are said to occur in Asia, Africa and Iran. In Iran5–6, Russia and South Africa ingestion of very hot foods and beverages has been associated with SCC of the oesophagus. We suggest that this could also be the main risk factor for OC in Malawi. Across Malawi cooked food is often eaten straight from the “fire place”. This habit may be associated with diffuse oesophagitis which is identifiable by endoscopy.
Equal percentages of the two cancers were diagnosed by histology but the majority of OC cases (73%) were diagnosed by clinical investigation while the majority of KS cases (72%) were diagnosed on clinical basis only. Since both diagnoses are thus based on visual inspection in the majority of cases, we propose that the prominence of KS over OCs in Malawi is likely to be a true reflection of the incidence of the two cancers.
Our arguments cast doubt on the wisdom of adopting hasty preventive measures on account of perceived prominence of OC over all other malignancies in Malawi.
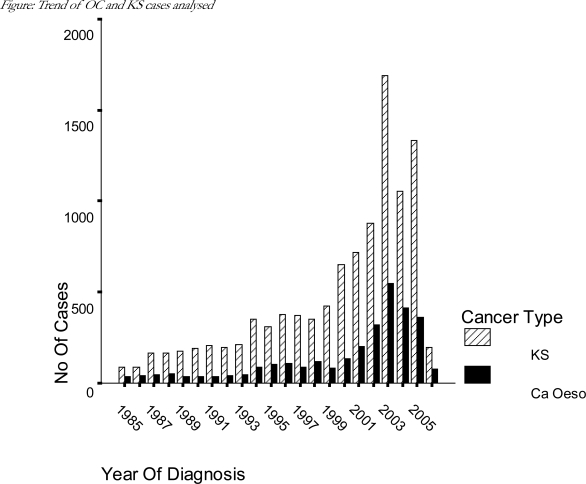
In terms of the trends for the two cancers over the years, the figure shows that there was no evidence of the expected drop in numbers of KS cases from around the year 2004 following the roll out of HAART in Malawi in this analysis. The similarity in trends between OC and KS is puzzling. One troubling explanation would be that the overall trend in cases over the study period is due to changes in data collection effort rather than a reflection of true case trends. This would explain why OC and KS cases increased and decreased in tandem over the years. Another possible explanation could be that there is a hitherto unknown link between the two diseases in Malawi such as an infectious agent perhaps. A third possibility is that KS could be presenting with “isolated” oesophageal lesions. While it is common practice to diagnose OC by endoscopy alone without histological confirmation, one doubts that morphological diagnoses such as SCC or AC could have been made as conclusively as was the case in the registraty. OCs diagnosed only by clinical investigation were recorded as SCCs (58.4%), malignant neoplasm (23.6%), carcinoma not otherwise specified (16.4%) and adenocarcinoma (1.2%). Therefore we believe that the possibility exists that OC cases diagnosed clinically or by endoscopy could include significant numbers of KS lesions. There were three oesophageal KS cases in this analysis one each diagnosed histologically, clinically and by clinical investigation.
Figure.
Trend of OC and KS cases analysed
This was a retrospective analysis and a prospective study on clinical (including HIV status) and demographic characteristics of OC in Malawi would be valuable.
References
- 1.Banda LT, Parkin DM, Dzamalala CP, Liomba NG. Cancer incidence in Blantyre, Malawi 1994–1998. Tropical Medicine & International Health. 2001;6(4):296–304. doi: 10.1046/j.1365-3156.2001.00707.x. [DOI] [PubMed] [Google Scholar]
- 2.Wapnick S, Zanamwe L N D, Chitiyo M, Mynors JM. Cancer of the Esophagus in Central Africa. Chest. 1972;61:649–654. doi: 10.1378/chest.61.7.649. [DOI] [PubMed] [Google Scholar]
- 3.Hutt MSR. Malawi: register of tumour pathology. 1976–1980. In: Parkin DM, editor. Cancer Occurrence in Developing Countries. Lyon: IARC; 1986. pp. 63–66. [Google Scholar]
- 4.Sammon AM. Carcinogens and endemic squamous cancer of the oesophagus in Transkei, South Africa. Environmental initiation is the dominant factor; tobacco or other carcinogens of low potency or concentration are sufficient for carcinogenesis in the predisposed mucosa. Med Hypotheses. 2007;69(1):125–131. doi: 10.1016/j.mehy.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 5.Ghadirian P. Thermal irritation and esophageal cancer in northern Iran. Cancer. 1987;60(8):1909–1914. doi: 10.1002/1097-0142(19871015)60:8<1909::aid-cncr2820600840>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 6.Islami F, Pourshams A, Nasrollahzadeh D, Kamangar F, Fahimi S, Shakeri R, et al. Tea drinking habits and oesophageal cancer in a high risk area in northern Iran: population based case-control study. BMJ. 2009;338:b929. doi: 10.1136/bmj.b929. [DOI] [PMC free article] [PubMed] [Google Scholar]