Abstract
The effect of treatment with either oxamniquine or praziquantel on S.mansoni specific IFN-gamma, IL-4 , IL-5 and IL-10 was compared on PBMC which were collected pretreatment, 6 and 18 weeks post treatment. Using sandwich ELISA on the supernatants harvested from the PBMC stimulation by crude S. mansoni SEA and SWAP antigens after 5 days the levels of PBMC proliferation and cytokine production were similar according to treatment with either praziquantel or oxamniquine. Before treatment, infected groups showed low ratios, of IL-4:IFN-gamma, IL-5:IFN-gamma and IL-10:IFN-gamma, indicating that IFN-gamma was high in the infected individuals. The general increase in immuno-modulation was observed post-treatment with elevated immune reactivity and cytokine production in both treatment groups. Treatment induced significant increases in levels of IL-4 (p<0.05), IL-5 (p<0.0001) and IL-10 (p<0.05) cytokines 6 and 18 weeks after treatment. There were no significant differences in the increase in IL-4, IL-5 and IL-10 between children treated with praziquantel or oxamniquine. Pre-treatment IFN-gamma and IL-5 levels were positively correlated with infection (p<0.001), while post treatment IL-4 cytokine levels were negatively correlated with baseline infection status (p<0.001). The results suggest that treatment-induced immune responses are similar for both commonantischistosome drugs praziquantel or oxamniquine having similar and immunizing effect.
Introduction
Schistosome infections under various field and experimental settings have suggested that hosts develop some acquired immunity to the parasite1–5. In humans this specific acquired immunity develops gradually over several years6, 7, and results of one study seem to suggest that treatment with praziquantel speeds up the development of schistosomespecific acquired immunity8. Praziquantel or oxamniquine treatment of schistosome-infected people alters parasite specific cellular and humoral responses particularly in children (8–10). These treatment-induced changes have been associated with resistance to re-infection11–14. The main cytokines which mark each subset are IL-4 and IFN-γ and these have a reciprocal cross-inhibitory effect. The relative importance of these functionally distinct T cell subsets and their contribution to protective immunity has yet to be fully established for the different schistosomes affecting humans during acute infection and post treatment. However, from recent reports, protection (against schistosome infection??) has been associated with Th2 responses, while Th1 responses cannot be related to susceptibility but a developmental stage after exposure15–17. A range of cytokines are now documented to be produced by the effector T helper cells. Furthermore, observations that the regulatory arm of the immune system develops with sustained exposure to schistosomes and their products suggest that they are likely to play a role during the establishment and maintenance of the infected condition. This re-infection study showed the cytokine profiles that appear before treatment and the change in the period following treatment with either praziquantel or oxamniquine. The knowledge thus gained should be helpful in understanding how the immune response is modulated during infection with S.mansoni and following treatment with either praziquantel or oxamniquine. Recently we showed that ratios of IL-4:IFN-γ increased in S. haematobium-infected children after treatment with praziquantel15,16. The study also showed a significant increase in cell proliferation after treatment. This suggests that treatment-induced immune changes are more complicated involving much more than just cytokine increases or decreases.16
Previous studies have examined factors, which affect the changes in immune responses following treatment and how they affect re-infection rates4, 5, 11, 12, 15–19. These studies mostly dealt with praziquantel or oxamniquine and not many compared the effect of the commonly used drugs in S.mansoni and in mixed schistosome infections. However, to date, there have been no studies of the nature of treatment-induced immune changes. Characterisation of these changes is important for a better understanding of how schistosome-specific acquired immunity develops and is essential for vaccine development and schistosomiasis control. We compared the cell-mediated immune changes in Zimbabwean subjects treated with two types of drugs which have different modes of action. Praziquantel acts by interfering with the Ca2+ uptake, causing muscle contraction and worm paralysis20. This causes tegumental damage and eventually worm death. Oxamniquine acts by binding to worm DNA causing an irreversible inhibition of nucleic acid synthesis20, 21. The two drugs were used to determine if there is any drug related differences in the changes in immune responses in treated children.
Methods
Study Ethical Approval
The Medical Research Council of Zimbabwe approved the study protocol. The Provincial and District Medical Directors and the District Health Executive Board gave permission to conduct the study. Parents and guardians of the participants agreed that the informed verbal consent obtained was sufficient. Treatment was offered to all infected participants after initial screening and to all participants at the end of the study.
Study site and sample collection
The study was conducted between 2002 and 2004 and recruited individuals attending school in Chiredzi, South Eastern Zimbabwe, where S.haematobium and S. mansoni is endemic. A total of 410 school going individuals aged between 6 and 18 years were surveyed after the aims of the study had been explained to them. Only participants infected with S. mansoni were divided into groups based on their sex and age before being randomly allocated to one treatment, with either praziquantel or oxamniquine. From these 148 children were selected for inclusion into the cell mediated immunity study cohort. The inclusion criteria were that each participant was expected to provide two stool samples on any of 3 consecutive days for S. mansoni detection, at least 2 urine samples on three consecutive days for S. haematobium detection. Each participant was also expected to be willing to have a blood sample taken for cell-mediated immunity assays both before treatment and at 6 and 18 weeks after treatment. Fifty-five children (29 male, 26 female) received the recommended dose (whats the recommended dose for this drug??) of oxamniquine . Sixty-five children (31 male, 34 female) received the recommended dose of praziquantel, 40mg/kg body weight. Twenty eight children (12 male, 16 female) who were neither infected with S. mansoni nor S. haematobium become the negative controls for both treatments after the screening process. Those absent on treatment days were treated at the second field visit, six weeks later when parasitology and blood samples were collected as before. Three replicate slides were prepared for each stool sample and processed following the Kato-Katz procedure to detect any S. mansoni eggs while the urine filtration method was used to detect any S. haematobium eggs in urine samples22, 23. At the same time a blood sample was collected for cellular assays, the cells were processed and preserved in liquid nitrogen according to methods by Hviid et al., (24). Observations on cellular immune responses were made on PBMC, which were collected pre-treatment, 6 and 18 weeks post treatment after stimulation with S. mansoni worm and egg antigens and PHA, observing possible proliferation and the profiles of cytokine production over 5 days incubation period.
PBMC proliferation assays
On the day of analysis, the cryopreserved PBMC were rapidly transferred asceptically into 10 ml washing media (RPMI 1640). The cells were washed by centrifugation at 250g for 10 minutes to remove the DMSO and resuspended in 10 ml of complete media containing RPMI 1640, gentamycin, 10 % AB serum, HEPES, non essential amino acids and sodium pyruvate. Cells were enumerated and viability determined using tryphan blue (0.2 % w/v) stain before incubation. Cells were cultured in a ml of medium in a 24 well tissue culture plates. The cells from each individual had unstimulated cells as a negative control and cells stimulated with 10 µg/ml phytohaemaglutinin (PHA) as positive control. Crude S. mansoni antigens SEA and SWAP were used to study specific stimulation of the cells at 15 µg/ml. The plates were incubated in a 5 % CO2 humidified incubator at 37°C for 5 days. The cells were harvested, while cell free supernatants were collected and stored at −70 °C before assaying for cytokines. After harvesting the supernatants, the cells were instantly harvested and used to enumerate the cell numbers from the resuspended cell pellet in 1 ml of RPMI 1640. The method of determining cell proliferation is comparable to the other proliferation assays25 using calorimetric or radioactive incorporation. Cell enumeration method avoided further incubation after supernatant collection. The resulting proliferation was expressed as stimulation index as follows: Stimulation Index (SI) = Difference of mean antigen/mitogen cell count (post-minus pre-culture) divided by difference of mean control cell culture (post-minus pre-culture).
Cytokine detection
Cytokine detection of IL-4, IL-5, IL-10 and IFN-γ was performed on the supernatants harvested from the PBMC stimulation after 5 days of incubation15–17. Samples were thawed and all 4 cytokines assayed at the same time for each sample. IFN-γ, IL-4, IL-5, IL10 cytokine assays were conducted using capture ELISAs with antibody sets from R&D following previously published protocols16,17. All assays were conducted in duplicate. Absorbance was read at 405 nm with 630 nm as reference wavelength on a DIAS Multiscan reader (Dynatech Laboratories). The absorbance minus background levels was interpolated from a standard curve to obtain cytokine concentrations in pg/ml (within 15 pg/ml – 2000 pg/ml range) taking into account the dilution factor of the culture supernatants.
Statistical Analyses
All statistical analyses were performed using the statistical package SPSS (SPSS, Chicago). Comparison is made of the 2 groups initially infected then treated with the group initially uninfected and untreated throughout the study time points. To determine if there were significant differences between treatment groups at each time point (treated with oxamniquine or praziquantel) Mann-Whitney Test was performed. Kruskall Wallis comparison was performed between untreated, treated with oxamniquine or praziquantel at each follow-up time point. All p values were checked using a sequential Bonferoni procedure and were only reported as significant if they satisfied the procedure.
Results
Parasitology
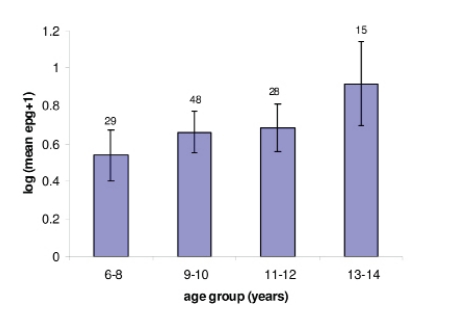
All treated children were confirmed egg negative for both S. mansoni and S. haematobium at 6 weeks examination. Schistosomiasis infection prevalence among the screened school-going participants was 83.2% (341), before treatment with either praziquantel or oxamniquine. For S. mansoni the prevalence was 67.9% (278) with arithmetic mean infection of 68 epg (please write epg in full the first time you introduce it) (95% CI, 26.1–163.9) whilst S.haematobium the prevalence was 65.6% (269) with arithmetic mean infection of 56.6 ep (shouldn't this be epg too??) 10ml (95% CI, 16.8–103.6) of urine. After treatment, the prevalence of schistosomiasis in untreated children was 33.3% and 33% after 6 weeks and 18 weeks, respectively. For S. mansoni the prevalence were 22% and 24.5%, respectively, whilst for S.haematobium it was 22.3% and 18.3%. Pre-treatment infection intensity was positively correlated with age but the correlation was not statistically significant. Mean infection intensity of the study cohort across the age groups is shown in Figure 1. The cohort was equally distributed to the 2 treatment groups, while the uninfected were used as control for each of the treatment group.
Figure 1.
Age-infection intensity distribution among the study cohort examined for both S. mansoni and S. haematobium before treatment with either praziquantel or oxamniquine.
Cellular proliferations
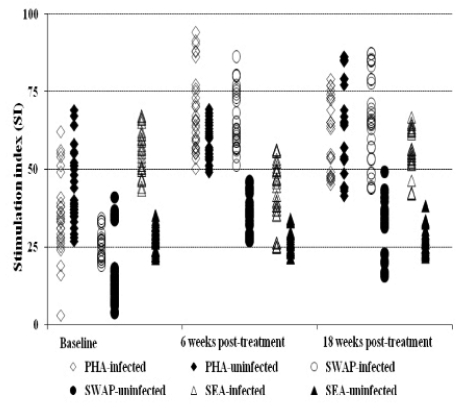
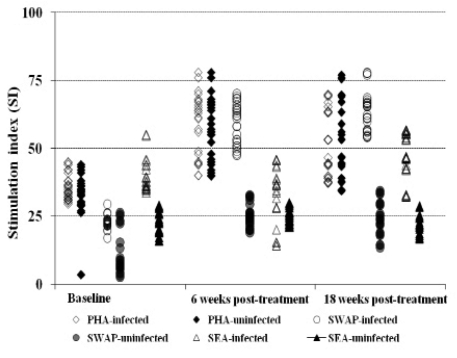
The general proliferation showed that prior to treatment the reactivities are low toward the worm antigen (26.24 ± 4.6 and 65.63± 9.3 for praziquantel; 22.95±2.32 and 59.20 ± 7.38 for oxamniquine), at baseline and 6 weeks post treatment, respectively, whilst high toward the egg antigen (Figure 2a & 2b). However, post treatment with either drug, the immune responses toward the worm is elevated whilst that toward the egg antigen remained high compared to the uninfected and untreated group in each treatment group (Figure 2a & 2b). The general trend in proliferation was an increase in the responses towards the worm antigen post treatment and also slightly elevated proliferation toward the eggs antigen in infected and treated between 6 and 18 weeks post treatment time points.
Figure 2a.
Comparison of proliferation index of PBMC collected pre and post-treatment with praziquantel. The comparison post treatment is between individuals once infected but treated (n=65) and the non treated uninfected (n=28) groups
Figure 2b.
Comparison of proliferation index of PBMC collected pre- and post-treatment with oxamniquine. The comparison post treatment is between individuals once infected but treated (n=55) and the non treated uninfected (n=28) groups.
Cytokine profiles
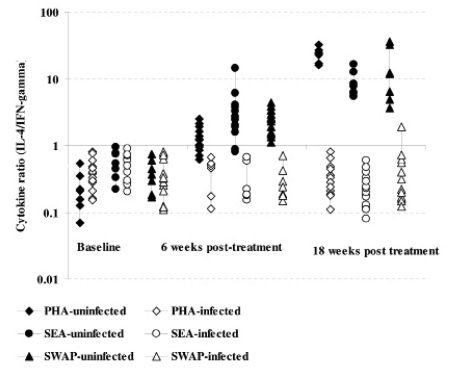
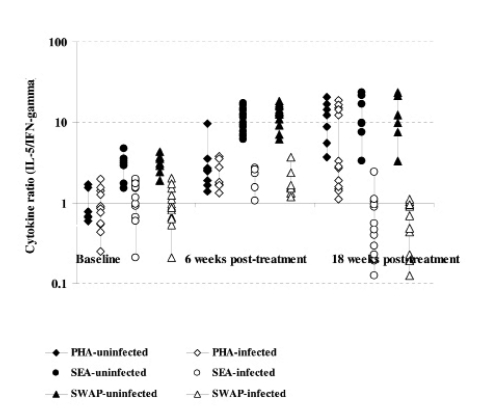
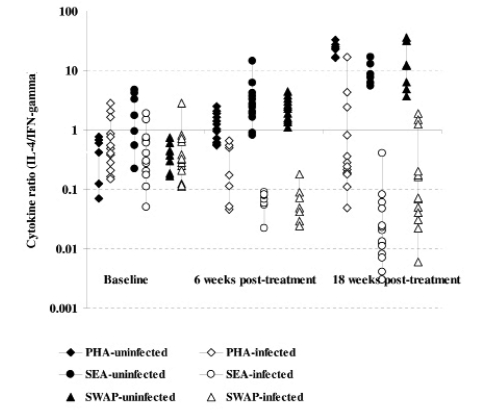
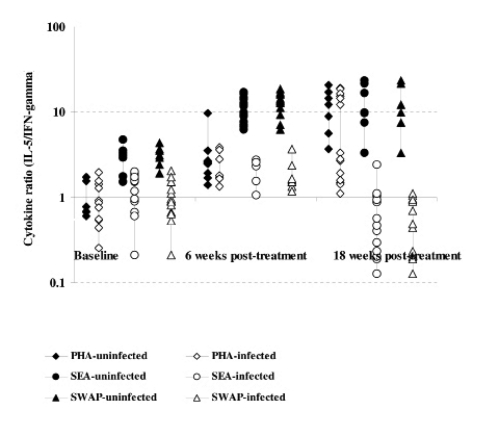
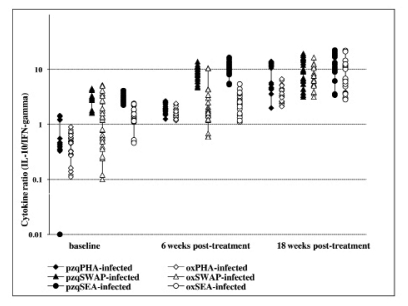
Cytokine production after S.mansoni antigen stimulation was low before treatment in both infected and uninfected groups. However, after treatment the initially infected and treated produced high levels of both IL-4 and IL-5 compared to IFN-γ (Figure 3a & b and 4a & b). On changes of IFN-γ cytokine trends, response to egg antigens was insignificant for both pre- and post-treatment times (χ2 = 5.121; df = 2; p = 0.077) while for worm antigen stimulation there was a significant change in IFN-γ showing a decreasing trend with time (χ2 = 7.366; df = 2; p = 0.025). There was no significant change in other cytokines as compared between treatment groups using Mann-Whitney test; while there was a general increasing trend pre- and post-treatment (baseline and 18 weeks: U=365.0; z = −2.677; p=0.007 and U= 410.5; z = −2.154; p=0.031) for IL-4 and IL-5, respectively. The general trend in cytokine profiles was similar for both drugs, praziquantel and oxamniquine treatment using IL-10: IFN-γ ratios (Figure 5). Using Spearman's correlations for posttreatment time points for the different cytokines; a strong correlation was observed between the drugs at p<0.01 level; for IL-4 (n = 116; rho = 0.711); for IL-5 (n = 115; rho = 0.625); IL-10 (n=116; rho=0.733) and for IFN-γ (n=113; rho=0.697).
Figure 3a.
Comparison of cytokine ratios (IL-4:IFN-γ) among the infected and uninfected as determined in culture supernatants from PBMC proliferation after 5 days stimulation with S.mansoni antigens and PHA at baseline, 6 and 18 weeks post treatment with praziquantel. The comparison post treatment is between individuals once infected but treated (65) and the non-treated uninfected (28) groups.
Figure 3b.
Comparison of cytokine ratios (IL-5:IFN-γ) among the infected and uninfected as determined in culture supernatants from PBMC proliferation after 5 days stimulation with S.mansoni antigens and PHA at baseline, 6 and 18 weeks post treatment with praziquantel. The comparison post treatment is between individuals once infected but treated (65) and the non-treated uninfected (28) groups.
Figure 4a.
Comparison of cytokine ratios (IL-4:IFN-γ) among the infected and uninfected as determined in culture supernatants from PBMC proliferation after 5 days stimulation with S.mansoni antigens and PHA at baseline, 6 and 18 weeks post treatment with oxamniquine. The comparison post treatment is between individuals once infected but treated (55) and the non-treated uninfected (28) groups.
Figure 4b.
Comparison of cytokine ratios (IL-5:IFN-γ) among the infected and uninfected as determined in culture supernatants from PBMC proliferation after 5 days stimulation with S.mansoni antigens and PHA at baseline, 6 and 18 weeks post treatment with oxamniquine. The comparison post treatment is between individuals once infected but treated (55) the non-treated uninfected (28) groups.
Figure 5.
Comparison of cytokine ratios (IL-10:IFN-γ) among infected as determined by ELISAs in culture supernatant from PBMC proliferation after 5 days stimulation with S.mansoni antigens and PHA at baseline, 6 and 18 weeks post treatment with either praziquantel or oxamniquine. The comparison post treatment is between individuals once infected but treated with praziquantel (65) or oxamniquine (55) groups.
Discussion
It is now widely accepted that treating schistosome-infected individuals alters the schistosome-specific cellular (4–6, 9–12, 26–31) and humoral immune responses (5–6). We reported that treatment accelerated the development of acquired humoral immunity in S. haematobium-infected children (9). The changes are due to increased antigen presented to the immune system following treatment. Treatment with antihelminthic drugs kills adult schistosomes. Praziquantel acts quickly and kills worms by inducing worm paralysis (20), while oxamniquine acts more slowly, binding to the worm DNA and RNA stopping protein synthesis (21). Treatment induced death of worms results in an introduction of antigens not normally available to the immune system (20, 21, 29, 32), considering the immune evasion mechanisms of the schistosomes. The overall increase in proliferation and in levels of cytokines are likely to be responding to this antigen increase (30,31). Such increases in antibody and proliferation levels have been reported from studies of S. mansoni, S. haematobium and S. japonicum infected children of similar age groups (29, 32–36). Also increases in proliferation and cytokine production post treatment with praziquantel have been reported before (15, 16), but to our knowledge to date, no study has looked at the effect of both drugs on the cell-mediated immune system at the same time.
Participants in this study had the highest infection intensities, which are attributable to cumulative infection and the development of indices of protective immunity. In schistosomiasis, it has been reported that there is a correlation between age and infection intensity29, 32–36. Therefore, it is not surprising that there is a low IFN-γ production compared to other cytokines at baseline as immune responses are mounted in response to infection, especially IFN-γ which is pro-inflammatory and is known to be involved the initial immune response against schistosome rinfection15, 16. As infection is eliminated by treatment with either praziquantel or oxamniquine, regardless of both drugs having different acting mechanism, antigens become available to the system and this contributes to cytokine production. In turn the resulting cytokine increase leads to increased immune cells proliferation resulting in an even higher level of cytokine production resulting in a better control of the parasites. This pattern was more apparent in response to worm antigens compared to the response to egg antigens. It is therefore not surprising that pre-treatment proliferation and cytokine levels of children in both drug groups are low for the worm antigen that is believed to have immune evasion mechanism while post treatment the drugs would kill the worms and make the antigens available and become recognised by the immune response. The response towards the egg antigens remained elevated but not significantly changed throughout the study, indicating that these antigens maybe in constant recognition by the immune cells, as the eggs stimulate the immune responses during each laying process. Such intense cytokine response to eggs is likely to occur in cases where there is granuloma formation and liver fibrosis.
The individuals in the area had high infection intensities, and on testing PBMC samples for proliferative responses to schistosome-specific stimulation, infected individuals, showed higher responses before treatment compared to uninfected individuals, suggesting the possibility of the individuals trying to establish an acquired protective immunity that normally develops with age after second decade of life (1–6). The infected participants showed an immunomodulation of their responses resulting in cellular unresponsiveness that has been reported before. This seems to occur when infections are intense and as the parasite and the host defences establish an apparent equilibrium enabling continued coexistence. Infected participants from the area were found to have lower proliferative responses to both worm and egg antigen stimulation before treatment as compared to post treatment. A possible interpretation of these data is that antigens became available for immune recognition after treatment, and thus treatment may have promoted the development of a protective immune response χ. At 6 and 18 weeks after treatment, there was a general increase in proliferative responses in the infected group. The PBMCs from participants treated with either praziquantel or oxamniquine showed similar patterns of proliferation and production of cytokines when compared with the uninfected and untreated participants in the area who, in turn had very low levels of proliferation and cytokine production. These data may seem to suggest that infected subjects in the area might have been at different stages of acute infection but still depicted a similar immune response in which the deleterious effects of cellular proliferation is modulated, probably by the effects of IL-4 and IL10 which are considered as anti-inflammatory38, 39. Such an interpretation is consistent with the age/prevalence and age/intensity data from different transmission areas. However, treatment with either praziquantel or oxamniquine revealed similar effects on the immune response.
Both praziquantel and oxamniquine caused the modulation of immune responses, a similar cytokine pattern to that shown for oxamniquine of the raised IFN-γ in those who were infected, during all the examination times, post treatment40–42. In the same individuals before treatment, raised levels of IL-4 and IL-5 were also observed, which is consistent with the dominance of IL-4, IL-5 later in chronic infections and a controlling role of IL-10 as reported by other investigators in S.japonicum40. The data are indicative of the counter-balancing of IL-4 and IFN-γ, opposing or down regulating each other's effects, especially before treatment was administered. IL-4, IL-5 and IL-10 were markedly raised in infected and treated children after treatment. Some investigators27 have reported similar results of restored and increased IL-4 levels post treatment from S.haematobium infected patients, while another study (28) which worked on S.mansoni infected patients showed elevated levels of IL-5 in those who were said to be resistant to infection. Similar effects wer shown in the post treatment scenario. It is reasonable to suggest therefore that these raised levels of IL-4, IL-5 and IL-10 indicate that both drugs had some kind of an immunising effect modulating the immune system. The PBMCs produced a mixture of cytokines suggesting that both Th1 and Th2 cell phenotypes were involved during infection. All the subjects produced a pattern of Th2-like cytokine response post treatment with either drug. The infected subjects showed the balance tipping towards the Th1-like response before treatment that could have contributed to the individuals with the Th1-like cytokine phenotype being either susceptible to or succumbing to the infection.
Although it has been proposed that predominant levels of IFN-γ may be associated with susceptibility to schistosome infection, the findings of this study seem to suggest that IFN-γ may be involved in some early stages of the immune response to schistosome infection15, 16. IFN-γ is generally involved in delayed-type hypersensitivity reactions dominating in individuals having acute infections. During this period, the immune response is involved in antigen processing and mobilisation of cell-mediated immune system in which IFN-γ plays a key regulatory role40, 41. The Th1-like cytokine phenotype observed in this study cannot be considered as inducing susceptibility to schistosome infection but may possibly indicate a regulatory role of Th1-like cytokines in the early responses to infection40. However, in this present study, it was observed that when a Th1-like phenotype dominates, the individuals were infected. Perhaps due to lack of the effectors which mediate protection and are marked by a Th2-like phenotype. IL-4 and IL-5 which mark the Th2-like response are known to stimulate B cells to selectively mature and proliferate for production of a particular antibody isotypes, which could ultimately be involved in ADCC reactions resulting in multicellular parasite killing and preventing any further invasion. The Th1 cytokine IFN-γ was found to exist during infection but the cytokine persisted at lower levels in treated individuals indicative of a dynamic balance which probably begins with increased levels of IFN-γ, a Th1 cytokine; and Th2-like responses dominating later in response to infection (15), with this shift in response phenotype being enhanced by both praziquantel and oxamniquine treatment41.
As reported in several studies and reviews for both S.mansoni and S.haematobium infections, Th2-like responses are considered protective and the data presented in this study shows that this aspect of an immune response can be accelerated by using anti-schistosomes. Infection with S. mansoni in individuals in their early second decade may exist in an acute phase such that levels of proliferation to schistosome-specific antigen stimulation remain raised (references). These data may be helpful in future vaccine programmes administered to individuals living in different mixed schistosome transmission areas and with co-distribution of schistosome drugs.
The study showed major similarity in both cell proliferation and cytokine production before and after treatment in participants treated with either of the two drugs. This result suggests that the development of acquired immunity to schistosomes is related more to the antigens they have been exposed to rather than any other age-related process, which would not be altered by treatment. Two age-related factors have been suggested to affect immune responses in children the first one being hormonal changes at puberty and the second being age-related maturity of the immune system (34–37, 43). If the latter was the case, and children were unable to mount some of the immune responses due to inability to recognize some of the epitopes, for instance, then administered treatment would not have altered their ability to mount the observed immune responses.
The results suggest that before treatment the infected individuals had been exposed to adequate antigens to mount immune responses to some tegumental or sub-tegumental and internal antigens so that treatment only augments the response slightly. However, the uninfected individuals had barely been exposed to such antigens so that treatment greatly increased the levels of these antigens available to the immune system. Work on vaccine candidates against schistosome infection has suggested that antigens mostly associated with resistance to infection are located within or beneath the worm tegument, thereby making it difficult for the immune system to access them in live worms44. Since worms have a long life span (3–7 years)45, it may take several years for the antigens to be released in sufficient amounts for immune responses to be mounted and modulated.
Conclusion
This study provides the first indication of a relationship between treatment-induced cellular changes and pre-treatment cytokine levels as well as an alteration of cytokine profile following treatment with commonly used anti-schistosome drugs. The results show the relationships between treatmentinduced changes in levels of IL-4, IL-5, IL-10 and IFN-γ related to the level of acquired immunity before treatment, and that the relationship between the Th1 and Th2 cytokine levels can be altered by treatment. The level of acquired immunity before treatment is related to experience of parasite antigens, this support the hypothesis that acquired immunity to schistosomiasis develops as a function of experience of parasitic antigens and that treatment speeds up the development of acquired immunity to tegumental or sub-tegumental and internal antigens. Both anti-schistosome drugs, praziquantel and oxamniquine, show similar effect on the host immune responses at least up to three months post treatment. This could be important when applying vaccines to population living in endemic areas where both drugs may be of use.
Acknowledgements
We are grateful for the co-operation of the Ministry of Health, Zimbabwe, the Provincial Medical Director of Masvingo, and the residents of the study area. We are also grateful for the assistance by the technical staff at the Blair Research Institute.
The study received support from WHO UNDP TDR Project ID 990001 and The Wellcome Trust Travel Grant to TM for data analysis with FM.
References
- 1.Hagan P, Blumenthal UJ, Dunne D, Simpson AJG, Wilkins HA. Human IgE, IgG4 and resistance to reinfection with Schistosoma haematobium. Nature. 1991;349:243–245. doi: 10.1038/349243a0. [DOI] [PubMed] [Google Scholar]
- 2.Hagan P. Reinfection, exposure and immunity in human schistosomiasis. Parasitol Today. 1992;8:12–16. doi: 10.1016/0169-4758(92)90303-j. [DOI] [PubMed] [Google Scholar]
- 3.Rihet P, Demeure CE, Bourgois A, Prata A, Dessein AJ. Evidence for an association between resistance to Schistosoma mansoni and high anti-larval IgE levels. Eur J Immunol. 1991;21:2679–2686. doi: 10.1002/eji.1830211106. [DOI] [PubMed] [Google Scholar]
- 4.Dunne DW, Butterworth AE, Fulford AJC, Kariuki HC, Langley JG, Ouma JH, Capron A, Pierce RJ, Sturrock RF. Immunity after treatment of schistosomiasis mansoni: association between IgE antibodies to adult worm antigens and resistance to reinfection. Eur J Immunol. 1992;22:1483–1494. doi: 10.1002/eji.1830220622. [DOI] [PubMed] [Google Scholar]
- 5.Dunne DW, Butterworth AE, Fulford AJC, Kariuki HC, Langley JG, Ouma JH, Capron A, Pierce RJ, Sturrock RF. Immunity after treatment of schistosomiasis mansoni: association between IgE antibodies to adult worm antigens and resistance to reinfection. Eur J Immunol. 1992;22:1483–1494. doi: 10.1002/eji.1830220622. [DOI] [PubMed] [Google Scholar]
- 6.Butterworth AE, Capron M, Cordingley JS, Dalton PR, Dunne DW, Kariuki HC, Kimani G, Koech D, Mugambi M, Ouma JH, Prentice MA, Richardson BA, Arap-Siongok TK, Sturrock RF, Taylor DW. Immunity after treatment of human schistosomiasis mansoni. ii. Indentification of resistant individuals, and analysis of their immuneresponses. Trans R Soc Trop Med Hyg. 1985;79:393–408. doi: 10.1016/0035-9203(85)90391-8. [DOI] [PubMed] [Google Scholar]
- 7.Woolhouse MEJ, Taylor P, Matanhire D, Chandiwana SK. Acquired immunity and epidemiology of Schistosoma haematobium. Nature. 1991;351:757–759. doi: 10.1038/351757a0. [DOI] [PubMed] [Google Scholar]
- 8.Woolhouse MEJ, Mutapi F, Ndhlovu PD, Chandiwana SK, Hagan P. Exposure, infection and immune responses to Schistosoma haematobium in young children. Parasitol. 2000;120(1):37–44. doi: 10.1017/s0031182099005156. [DOI] [PubMed] [Google Scholar]
- 9.Mutapi F, Ndhlovu PD, Hagan P, Spicer JT, Mduluza T, Turner CMR, Chandiwana SK, Woolhouse MEJ. Chemotherapy accelerates the development of acquired immune responses to Schistosoma haematobium infection. J Infect Dis. 1998;178(1):289–293. doi: 10.1086/517456. [DOI] [PubMed] [Google Scholar]
- 10.Webster M, Fallon PG, Fulford AJC, Butterworth AE, Ouma JH, Kimani G, Dunne DW. Effect of praziquantel and oxamniquine treatment on human isotype responses to Schistosoma mansoni: elevated IgE to adult worm. Parasite Immunol. 1997;19:333–335. doi: 10.1046/j.1365-3024.1997.d01-211.x. [DOI] [PubMed] [Google Scholar]
- 11.Grogan JL, Kremsner PG, van Dam GJ, Metzger W, Mordmuller B, Deelder AM, Yazdanbakhsh M. Antischistosome IgG4 and IgE responses are affected differentially by chemotherapy in children versus adults. J Infect Dis. 1996;173:1242–1247. doi: 10.1093/infdis/173.5.1242. [DOI] [PubMed] [Google Scholar]
- 12.Correa-Oliveira R, Rodrigues CI, Martins-Filho OA, Carvalho QC, Lambertucci JR, Renan Cunha-Melo J, Soares Silveira A, Prata A, Wilson A, Gazzinelli G. Analysis of the effects of treatment of human Schistosoma mansoni infection on the immune response of patients from endemic areas. Acta Trop. 2000;77(1):141–146. doi: 10.1016/s0001-706x(00)00127-3. [DOI] [PubMed] [Google Scholar]
- 13.Mutapi F, Burchmore R, Mduluza T, Midzi N, Turner CM, Maizels RM. Age-Related and Infection Intensity-Related Shifts in Antibody Recognition of Defined Protein Antigens in a Schistosome-Exposed Population. J Infect Dis. 2008;198(2):167–175. doi: 10.1086/589511. [DOI] [PubMed] [Google Scholar]
- 14.Dumont M, Mone H, Mouahid G, Idris MA, Shaban M, Boissier J. Influence of pattern of exposure, parasite genetic diversity and sex on the degree of protection against reinfection with Schistosoma mansoni. Parasitol Res. 2007;101:247–252. doi: 10.1007/s00436-007-0476-0. [DOI] [PubMed] [Google Scholar]
- 15.Mutapi F, Winborn G, Midzi N, Taylor M, Mduluza T, Maizels RM. Cytokine responses to Schistosoma haematobium in a Zimbabwean population: contrasting profiles for IFN-gamma, IL-4, IL-5 and IL-10 with age. BMC Infect Dis. 2007;7:139. doi: 10.1186/1471-2334-7-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mduluza T, Ndhlovu PD, Midzi N, Spicer JT, Mutapi F, Mary C, Paris CP, Turner CMR, Chandiwana SK, Woolhouse MEJ, Dessein AJ, Hagan P. Contrasting cellular responses in schistosoma haematobium infected and exposed individuals from areas of high and low transmission in Zimbabwe. Immunol Let. 2003;88(3):249–256. doi: 10.1016/s0165-2478(03)00088-9. [DOI] [PubMed] [Google Scholar]
- 17.Couissinier-Paris P, Dessein AJ. Schistosoma-specific helper T cell clones from subjects resistant to infection by Schistosoma mansoni are Th0/2. Eur J Immunol. 1995;25:2295–2302. doi: 10.1002/eji.1830250827. [DOI] [PubMed] [Google Scholar]
- 18.Feldmeier H, Gastl GA, Poggensee U. Relationship between intensity of infection and immunomodulation in human schistosomiasis. II. NK cell activity and in vitro lymphocyte proliferation. Clin Exp Immunol. 1985;60:234–240. [PMC free article] [PubMed] [Google Scholar]
- 19.Hagan P, Blumenthal UJ, Chaudri M, Greenwood BM, Hayes RJ, Hodgson J, Kelly C, Knight M, Simpson AJG, Smithers SR, Wilkins HA. Resistance to reinfection with Schistosoma haematobium in Gambian children: analysis of their immune responses. Trans R Soc Trop Med Hyg. 1987;81:938–946. doi: 10.1016/0035-9203(87)90359-2. [DOI] [PubMed] [Google Scholar]
- 20.Cioli D, Pica-Mattoccia L, Archer S. Antischistosomal drugs: past, present … and future? Pharmacol Ther. 1995;68(1):35–85. doi: 10.1016/0163-7258(95)00026-7. [DOI] [PubMed] [Google Scholar]
- 21.Bickle QD, Andrews BJ. Resistance following drug attenuation (Ro 11–3128 or oxamniquine) of early Schistosoma mansoni infections in mice. Parasitol. 1985;90(2):325–338. doi: 10.1017/s0031182000051027. [DOI] [PubMed] [Google Scholar]
- 22.Mott KE, Baltes R, Bambagha J, Baldassini B. Field studies of the reusable polyamide filter for detection of S.haematobium eggs by urine filtration. Propernmedlizin and Parasitol. 1982;33:227–228. [PubMed] [Google Scholar]
- 23.Peters PA, El Alany M, Warren KS, Mahmoud FA. Quick Kato smear for field quantification of S.mansoni eggs. Am J Trop Med Hyg. 1980;29:217–219. doi: 10.4269/ajtmh.1980.29.217. [DOI] [PubMed] [Google Scholar]
- 24.Hviid L, Theander TG, Jakobsen PH. Cell-Mediated Immune Responses to soluble Plasmodium falciparum antigens in residents from an area of unstable malaria transmission in The Sudan. APMIS. 1990;98:594–604. doi: 10.1111/j.1699-0463.1990.tb04976.x. [DOI] [PubMed] [Google Scholar]
- 25.Sorensen SF, Andersen V, Giese J. Studies on the quantitation of lymphocytes response in vitro. Acta Pathol Microbiol Scand. 1969;72(2):259–270. doi: 10.1111/j.1699-0463.1969.tb03256.x. [DOI] [PubMed] [Google Scholar]
- 26.Colley DG, Barsoum IS, Dahawi MSS, Gamil MF, Habib M, Alamy MA. Immune response and immunoregulation in relation to human schistosomiasis in Egypt. III. Immunity and longitudinal studies of in vitro responsiveness after treatment. Trans R Soc Trop Med Hyg. 1986;80:952–958. doi: 10.1016/0035-9203(86)90268-3. [DOI] [PubMed] [Google Scholar]
- 27.Grogan JL, Kremsner PG, Deelder AM, Yazdanbakhsh M. Elevated proliferation and interleukin-4 release from CD4+ cells after chemotherapy in human Schistosoma haematobium infection. Eur J Immunol. 1998;26:1365–1370. doi: 10.1002/eji.1830260628. [DOI] [PubMed] [Google Scholar]
- 28.Roberts M, Butterworth AE, Kimani G, Kamau T, Fulford AJC, Dunne DW, Ouma JH, Sturrock RF. Immunity after treatment of human schistosomiasis: Association between cellular responses and resistance to reinfection. Infect Immun. 1993;61:4984–4993. doi: 10.1128/iai.61.12.4984-4993.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Joseph S, Jones FM, Walter K, Fulford AJ, Kimani G, Mwatha JK, Kamau T, Kariuki HC, Kazibwe F, Tukahebwa E, Kabatereine NB, Ouma JH, Vennervald BJ, Dunne DW. Increases in human T helper 2 cytokine responses to Schistosoma mansoni worm and worm-tegument antigens are induced by treatment with praziquantel. J Infect Dis. 2004;190(4):835–842. doi: 10.1086/422604. [DOI] [PubMed] [Google Scholar]
- 30.Tweyongyere R, Mawa PA, Ngom-Wegi S, Ndibazza J, Duong T, Vennervald BJ, Dunne DW, Katunguka-Rwakishaya E, Elliot AM. Effect of praziquantel treatment during pregnancy on cytokine responses to schistosome antigens: results of a randomized, placebo-controlled trial. J Infect Dis. 2008;198(12):1870–1879. doi: 10.1086/593215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martins-Leite P, Gazzinelli G, Alves_oliveira LF, Gazzinelli A, Malaquias LC, Correa-Oliveira R, Teixeira-Carvalho A, Silveira AM. Effect of chemotherapy with praziquantel on the production of cytokines and morbidity associated with schistosomiasis mansoni. Antimicrob Agents Chemother. 2008;52(8):2780–2786. doi: 10.1128/AAC.00173-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Joseph S, Jones FM, Kimani G, Mwatha JK, Kamau T, Kazibwe F, Kemijumbi J, Kabatereine NB, Booth M, Kariuki HC, Ouma JH, Vennervald BJ, Dunne DW. Cytokine production in whole blood cultures from a fishing community in an area of high endemicity for Schistosoma mansoni in Uganda: the differential effect of parasite worm and egg antigens. Infect Immun. 2004;72:728–734. doi: 10.1128/IAI.72.2.728-734.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mduluza T, Ndhlovu PD, Midzi N, Mary C, Paris CP, Turner CMR, Chandiwana SK, Woolhouse MEJ, Dessein AJ, Hagan P. T cell clones from Schistosoma haematobium infected and exposed individuals lacking distinct cytokine profiles for Th1/Th2 polarisation. Mem Inst Oswaldo Cruz. 2001;96:89–101. doi: 10.1590/s0074-02762001000900013. [DOI] [PubMed] [Google Scholar]
- 34.Ottesen EA, Hiatt RA, Cheever AW, Sotomayor RZ, Neva FA. The acquisition and loss of antigen-specific cellular immune responsiveness in acute and chronic schistosomiasis in man. Clin Exp Immunol. 1978;33:38–47. [PMC free article] [PubMed] [Google Scholar]
- 35.Wilson S, Jones FM, Mwatha JK, Kimani G, Booth M, Kariuki HC, Vennervald BJ, Ouma JH, Muchiri E, Dunne DW. Hepatosplenomegaly is associated with low regulatory and Th2 responses to schistosome antigens in childhood schistosomiasis and malaria coinfection. Infection and Immunity. 2008;76:2212–2218. doi: 10.1128/IAI.01433-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gryseels B, Stelma F, Talla I, Polman K, Van Dam G, Sow S, Diaw M, Sturrock RF, Decam C, Niang M, Doehring-Schwerdtfeger E, Kardorff R. Epidemiology, immunology and chemotherapy of Schistosoma mansoni infections in a recently exposed community in Senegal. Trop Geogr Med. 1995;46:209–219. [PubMed] [Google Scholar]
- 37.Watanabe K, Mwinzi PN, Black CL, Muok EM, Karanja DM, Secor WE, Colley DG. T regulatory cell levels decrease in people infected with Schistosoma mansoni on effective treatment. Am J Trop Med Hyg. 2007;77:676–682. [PMC free article] [PubMed] [Google Scholar]
- 38.Herbert DR, Orekov T, Perkins C, Finkelman FD. IL-10 and TGF-beta redundantly protect against severe liver injury and mortality during acute schistosomiasis. J Immunol. 2008;181(10):7214–7220. doi: 10.4049/jimmunol.181.10.7214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Teixeira-Carvalho A, Martins-Filho OA, Peruhype-Magalhaes V, Silveira-Lemos D, Malaquias LC, Oliveira LF, Silveira AM, Gazzinelli A, Gazzinelli G, Correa-Oliveira R. Acta Trop. 2008;108(2–3):139–149. doi: 10.1016/j.actatropica.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 40.Arnaud V, Li J, Wang Y, Fu X, Mangzhi S, Luo X, Hou X, Dessein H, Jei Z, Xin-Ling Y, He H, Mcmanus DP, Li Y, Dessein A. J Infect Dis. 2008;198(3):418–426. doi: 10.1086/588826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.De Souza JR, Morais CN, Aroucha ML, Miranda PJ, Barbosa CS, Domingues AL, Carvalho Junior LB, Abath FG, Montenegro SM. Treatment of human acute schistosomiasis with oxamniquine induces an increase in interferon-gamma responses to Schistosoma mansoni antigens. Mem Inst Oswaldo Cruz. 2007;102(2):225–228. doi: 10.1590/s0074-02762007005000002. [DOI] [PubMed] [Google Scholar]
- 42.Wilson S, Jones FM, Mwatha JK, Kimani G, Booth M, Kariuki HC, Vennervald BJ, Ouma JH, Muchiri E, Dunne DW. Hepatosplenomegaly associated with chronic malaria exposure: evidence for a pro-inflammatory mechanism exacerbated by schistosomais. Parasite Immunol. 2009;31(2):64–71. doi: 10.1111/j.1365-3024.2008.01078.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gazzinelli G, Lambertucci JR, Katz N, Rocha RS, Lima MS, Colley DG. Immune responses during human schistosomiasis mansoni, XI. Immunological status of patients with acute infections and after treatment. J Immunol. 1985;135:2121–2127. [PubMed] [Google Scholar]
- 44.Capron A, Dessaint JP, Capron M, Ouma JH, Butterworth AE. Immunity to schistosomes: progress toward vaccine. Science. 1987;238:1065–1072. doi: 10.1126/science.3317823. [DOI] [PubMed] [Google Scholar]
- 45.Fulford AJ, Butterworth AE, Ouma JH, Sturrock RF. A statistical approach to schistosome population dynamics and estimation of the life-span of Schistosoma mansoni in man. Parasitol. 1995;110(3):307–316. doi: 10.1017/s0031182000080896. [DOI] [PubMed] [Google Scholar]